Abstract
A subgroup of patients with schizophrenia is believed to have aberrant excess of glutamate in the frontal cortex; this subgroup is thought to show poor response to first-line antipsychotic treatments that focus on dopamine blockade. If we can identify this subgroup early in the course of illness, we can reduce the repeated use of first-line antipsychotics and potentially stratify first-episode patients to intervene early with second-line treatments such as clozapine. The use of proton magnetic resonance spectroscopy (1H-MRS) to measure glutamate and Glx (glutamate plus glutamine) may provide a means for such a stratification. We must first establish if there is robust evidence linking elevations in anterior cingulate cortex (ACC) glutamate metabolites to poor response, and determine if the use of antipsychotics worsens the glutamatergic excess in eventual nonresponders. In this study, we estimated glutamate levels at baseline in 42 drug-naive patients with schizophrenia. We then treated them all with risperidone at a standard dose range of 2-6 mg/day and followed them up for 3 months to categorize their response status. We expected to see baseline “hyperglutamatergia” in nonresponders, and expected this to worsen over time at the follow-up. In line with our predictions, nonresponders had higher glutamate than responders, but patients as a group did not differ in glutamate and Glx from the healthy control (HC) group before treatment-onset (F1,79 = 3.20, p = 0.046, partial η2 = 0.075). Glutamatergic metabolites did not change significantly over time in both nonresponders and responders over the 3 months of antipsychotic exposure (F1,31 = 1.26, p = 0.270, partial η2 = 0.039). We conclude that the use of antipsychotics without prior knowledge of later response delays symptom relief in a subgroup of first-episode patients, but does not worsen the glutamatergic excess seen at the baseline. Given the current practice of nonstratified use of antipsychotics, longer-time follow-up MRS studies are required to see if improvement in symptoms accompanies a dynamic shift in glutamate profile.
Similar content being viewed by others
Introduction
Stratification of patients with psychosis based on distinct mechanisms of the underlying pathophysiology will allow us to develop treatments that are effective for a particular subgroup. This also addresses the problem of trial-and-error pharmacological practice whereby all patients, irrespective of the strata that they may belong to, are given the same treatment first, and only after enduring repeated treatment failures, get singled out for clozapine treatment. Stratification is a worthy goal to aim for in the treatment of a heterogeneous condition such as psychosis, where the introduction of clozapine occurs only after an unacceptably long delay [1], despite a substantial number of patients (25-33%) being the clozapine-deserving subgroup from the outset [2]. Nevertheless, the basis on which this stratification can be done successfully at the illness onset before treatment trials are set in motion remains elusive.
The measurement of glutamate using magnetic resonance spectroscopy (MRS) is a promising approach, as this neurochemical quantification approach does not involve injectable tracers, and is a relatively short imaging procedure that is tolerated even by antipsychotic naive subjects [3]. Numerous prior studies have indicated glutamatergic alterations in schizophrenia [4, 5], with higher levels of ACC (in a pregenual, ventral voxel) but not caudate glutamate indicating poor short term response [6,7,8]. Nevertheless, studies examining antipsychotic naïve participants report absent [9, 10] or unexpectedly opposite [11] association with subsequent response. These discrepancies may relate to differences in voxel placement, as prior studies have chosen only a single voxel either in perigenual ACC or dorsal ACC. These two ACC regions belong to functionally distinct networks (pACC has “default-mode network” affiliation while dACC has “salience network” affiliation). An increase in glutamate levels may have different physiological effects. This needs to be resolved as glutamate is a “prescriptive predictor” that is highly suited for stratification approaches [12] with the second-line treatment clozapine strongly suspected to have a glutamate-mediated mechanism of action [13, 14]. Further, glutamate-based novel treatments are being tested in a number of clinical trials, wherein in vivo glutamate-based stratification may be of notable value [15, 16].
The eventual goal of stratification is to improve effectiveness and tolerability of treatments that we offer. While some studies have suggested that such stratification may not be needed [17, 18], others [19] indicate that patients who do not show a positive response to antipsychotics may indeed worsen with exposure to antipsychotics. Some observations have suggested that antipsychotic treatment introduces variability in glutamate levels [20], while such an effect was not observed in a larger mega-analysis [21]. While cross-sectional studies cannot provide conclusive proof in this regard, it is critical to test whether a putative maker of nonresponse worsens with the exposure to a treatment that is offered as a one-size fits all intervention. In the current study, we address these issues by (1) investigating if early nonresponders have a glutamatergic excess compared to responders and HC at 2 different voxels in ACC; (2) testing the assumption that antipsychotic treatment worsens the pathophysiology of glutamate excess in the subgroup of later nonresponders. Furthermore, we recruited a treatment-naive sample, despite the limits these places on overall sample size and generalizability, as this is the target population for which the eventual application of glutamate-based pragmatic precision psychiatry is intended.
Methods
Participants
We identified 70 drug-naïve patients with first-episode schizophrenia (FES) who were about to begin treatment with risperidone from the Second Xiangya Hospital, and 41 healthy controls (HC) matched with age, education level and gender in Changsha, Hunan, China between 2017 and 2022. During this period, from hospital records we estimate approximately 3800 patients were seen at the respective clinics; of these only those who satisfied our inclusion criteria (drug naive at baseline, no substance use, no major medical illnesses or head injury, prescribed risperidone monotherapy by the treating clinician, able to give informed consent) were referred to the research team (n = 150); of this, n = 70 provided informed consent and completed baseline scanning. Notably, the treatment was not assigned by the research team; but a convenience sample of those who were about to start risperidone were recruited for the study. Diagnostic assessments for FES patients were completed by two experienced senior psychiatrists, based on the DSM-IV criteria [22]. 14 were noncompliant with the treatment plan (8 FES stopped taking antipsychotics against medical advice, 6 received other interventions within the 12-week period) and another 14 dropped out due to unwillingness to continue participating in this study for undisclosed reasons. In the end, 42 FES completed 12 weeks of risperidone at 2–6 mg/day (titrated as per clinical tolerance) and then were followed up. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale [23] at baseline and 12-week follow-up. All HC had no current or past history of DSM-IV Axis I disorder themselves or a family history of mental disorder. All subjects are right-handed, and no contaminant medications were used. 87.14% of subjects are of the ethnic Han background. Participants were excluded if they satisfied substance abuse or dependence criteria, had any known major physical illness, a history of prior antipsychotic exposure, or head trauma resulting in a sustained loss of consciousness for over 5 minutes or more, any contraindications for MRI.
All participants provided written informed consent to this study, which was approved by the Ethics Committee of the Second Xiangya Hospital (No. S008, 2012) and carried out in accordance with the Declaration of Helsinki. As there was no active treatment assignment, the study was deemed observational and not an interventional trial. The study procedures were registered in the Chinese Trial Registry (ChiCTR1800014844).
1H-magnetic resonance spectroscopy
MRS was acquired within 2 days after enrollment and before starting the first dose of risperidone for FES (baseline scan), and 12 weeks later (follow-up scan) to assess metabolites levels at two different voxels of the ACC. HC were scanned only at a single time point. As described in our previous study [11, 24], we measured metabolites before antipsychotic treatment using a 3 T MRI scanner (Siemens, Skyra, Germany) with 16 channel head coils at the Magnetic Imaging Centre of Hunan Children’s Hospital. A 10x20x20mm voxel of interest (VOI) was placed in anterior cingulate cortex (pACC) at a ventral and perigenual site (i.e., likely Default Mode Network - DMN site) and 20×20×20mm VOI was placed in at a dorsal ACC (dACC) site (Salience Network - SN site) (Fig. S1). The perigenual ACC site was identified on the anatomical MRI as the cortex directly rostral to the genu of the corpus callosum. We placed the MRS voxel here such that the posterior wall was directly adjacent and did not include the principal medial sulcus. This placement captures a central DMN node, as shown by a detailed anatomical analysis of the functional organization of the cingulate cortex [25]. The second voxel was placed on the dorsal ACC (anterior mid-cingulate) region identified as the cortex immediately above the cingulate sulcus, with anterior wall of the MRS voxel lying on an axis that cuts through the posterior border of genu of the corpus callosum perpendicular to AC-PC orientation (as in Dou et al. study [26]). A positional query of these voxels on neurosynth.org confirmed Default Mode and Salience Network associations on the meta-analytical maps for the perigenual and dorsal ACC voxels respectively. Every scan was examined post-acquisition and repeated if spectral linewidth was unsatisfactory or changed notably during the acquisition. 1H-MRS spectra used standard point-resolved spectroscopy sequence (PRESS) (svs_se; NEX 80; TR = 3000 ms; TE = 30 ms; spectral bandwidth = 1200 Hz), and pre-saturation pulses of variable power radiofrequency pulses with optimized relaxation delays were used for suppression of the water signal. Water unsuppressed spectra were acquired in the same voxel locations and used the sequence with NEX = 8. T1-weighted anatomical images were acquired using 3-dimensional magnetization-prepared fast gradient echo sequences for voxel tissue segmentation (TR = 2530 ms; TE = 2.33 ms; gap = 0.5 mm; flip angle = 7o; FOV = 256× 256 mm; number of excitations (NEX) = 1; slice thickness = 1.0 mm; and and number of slices = 192).
SPM 12 segment tool (FIL Wellcome Department of Imaging Neuroscience, London, UK) was used to segment T1 to extract the gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) fraction of VOIs. All spectra were analyzed by LCModel version 6.3–1B (http://www.lcmodel.com/lcmodel.shtml), using the standard LCModel basis set, acquired using gamma PRESS at 3 Tesla and 30 ms, and containing below metabolites: L-alanine, aspartate, creatine, phosphocholine, γ-aminobutyric acid, glucose, glutamate, glutamine, glycerophosphocholine, guanidinoacetate, phosphocreatine, L-lactate,myo-inositol, N-acetylaspartate, N-acetylaspartylglutamate, scyllo-inositol, taurine, -CrCH2, lipids (Lip: (Lip09, Lip13a, Lip13b, Lip20), macromolecules (MM: MM09, MM12, MM14, MM17 and MM20). The metabolite concentrations were corrected for tissue fraction and were estimated with internal reference to water for absolute metabolite quantification (measured in mM unit). The primary metabolites of interest were glutamate (Glu) and glutamate + glutamine (Glx). Data were excluded if the Cramer-Rao lower bounds (CRLB) exceeded 20% for either of these metabolites, as this is conventionally considered as an indicator of higher uncertainty in spectral quantification and used in prior studies (STRATA collaboration) investigating glutamtergic basis of treatment response [6].
Statistical analysis
All statistical analysis was completed using SPSS 26 (IBM Corp. IBM SPSS Statistics for Windows, Version 26.0). Patients were divided into responders and nonresponders based on whether the percent PANSS total score reduction was above or below 50% from the baseline [27]. The percent PANSS total score reduction was calculated after subtraction of minimum possible scores [28]:
Thus, higher (and positive) change scores indicate higher improvement, while negative scores indicate worsening over time. Demographic variables, MRS quality metric and tissue volume were compared across groups with the use of General Linear Model and Chi-squared tests for continuous and dichotomous variables, respectively. Binary logistic regression assessed if baseline Glu and Glx level predicted later treatment response. The accuracy of prediction was evaluated by estimating the area under the receiver-operating curve (ROC). Linear regression determined continuous relationships between baseline Glu and Glx and the change in PANSS total score and subscales over 12 weeks. A mixed-measures ANOVAs were conducted with group (HC, responders, nonresponders) as the between-subject variable, voxel (pACC, dACC) and glutamate metabolites (Glu, Glx) as a within-subject variable to assessed the effect of group and applied post-hoc tests with Bonferroni correction for group effect tests. A repeated measures ANOVA assessed the treatment effect with group (responders, nonresponders) as the between-subject variable, voxel (dACC, pACC), time (baseline, 12 weeks) and glutamate metabolites (Glu, Glx) as a within-subject variable.
Results
At 12 weeks, 27 patients were classified as responders and 15 were classified as nonresponders, approximately 64% response rate in keeping with the STRATA study data [6]. One nonresponder was excluded as CRLB exceeded 20% for glutamate at pACC voxel. As shown in Table 1, the three groups were matched in demographic characteristics in the final dataset. Responders and Nonresponders did not differ in symptom burden at baseline, duration of psychosis, or risperidone dosage at 12 weeks. While both Responders and Nonresponders showed an improvement in symptom burden over 12 weeks, nonresponders had significantly higher symptom burden than Responders at 12 weeks across all symptom domains. In particular, nonresponders showed no notable improvement in negative symptoms between the time points (t = -0.41, p = 0.688).
As shown in Table S1, dropouts and included FES in the study have not significantly difference in age, education, gender, and symptom burden. The results of group effects for MRS quality metric and tissue volume are shown Table S2 in the supplement material, and we have also reported information about the concentrations of common metabolites (See Table S3).
Baseline glutamate metabolites and prediction of response
As shown in Table 2 and Fig. 1, a repeated measure ANOVA showed the main effect of group (F1,79 = 3.20, p = 0.046, partial η2 = 0.075), voxel (F1,79 = 78.68, p < 0.001, partial η2 = 0.499) and for glutamate metabolites (F2,79 = 422.83, p < 0.001, partial η2 = 0.843). Nonresponders had higher glutamate metabolites levels compared to responders, there are no significant differences in glutamate and Glx between HC group and the two patient groups, and pACC voxel had higher levels compared to dACC voxel. There were no group by voxel (F2,79 = 0.82, p = 0.446, partial η2 = 0.020) or group by glutamate metabolite (F2,79 = 0.49, p = 0.613, partial η2 = 0.012) interactions. Logistic regression analysis shown that both higher Glu in pACC (B = -0.18; S.E. = 0.08; Wald = 4.59; df = 1; P = 0.032; Exp(B) = 0.84; 95%CI (0.71,0.99)) and higher Glx in dACC (B = -0.24; S.E. = 0.11; Wald = 4.30; df = 1; P = 0.038; Exp(B) = 0.79; 95%CI (0.64,0.99) at baseline significantly predicted nonresponder status at 12 weeks. Higher Glx in pACC (B = -0.11; S.E. = 0.06; Wald = 3.28; df = 1; P = 0.070; Exp(B) = 0.90; 95%CI (0.80,1.00)) and higher Glu in dACC (B = -0.26; S.E. = 0.13; Wald = 3.82; df = 1; P = 0.051; Exp(B) = 0.77; 95%CI (0.62,1.02) had associations with nonresponder status in the same direction but these relationships were not statistically significant.
As shown in Fig. S2, ROC analysis returned comparable area under curve for both Glu and Glx at both voxels (0.71 for pACC Glu; 0.68 for PACC Glx; 0.70 for dACC Glu; 0.72 for dACC Glx) for nonresponders. Glu in pACC was associated with an overall accuracy of 68.3% (proportion of correct predictions), sensitivity of 71.4 % (proportion of nonresponders correctly predicted based on baseline Glu), specificity 77.8% (proportion of responders correctly predicted to respond). Glx in dACC was associated with an overall accuracy of 61.9%, sensitivity of 85.7%, specificity 63%.
Baseline glutamate metabolites and continuous measures of response
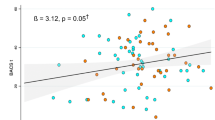
Glu and Glx in dACC and pACC at baseline were not significantly associated with the continuous measure of change in total PANSS scores over 12 weeks (P = 0.617 to 0.792). There was a significant relationship between baseline dACC Glu (Beta = -0.38; t = -2.61; P = 0.013) and Glx (Beta = -0.43; t = -3.03; P = 0.004) and change in PANSS negative subscale (Fig. 2), while other PANSS subscale changes were not significantly associated with Glu and Glx at baseline in dACC (P = 0.386 to 0.723). In the pACC, Glu and Glx at baseline were not significantly associated with the change PANSS subscale over 12 weeks (P = 0.061 to 0.565). Thus, only the dACC metabolite levels, when higher, indicated poor improvement (even worsening burden), but this association was restricted to the negative symptoms ___domain.
Degree of improvement was measured by subtracting follow-up PANSS scores from baseline scores. Larger positive values indicate a greater improvement in symptoms, while negative values along y axis indicate a worsening over time. Glu: glutamate; Glx: glutamate + glutamine; PANSS: PANSS positive and negative syndrome scale for schizophrenia. The relationships between both dACC Glu and Glx at baseline and degree of improvement in PANSS negative symptoms over 12 weeks were significant, P < 0.05.
Longitudinal changes in glutamate metabolites over 12 weeks
A repeated measure ANOVA showed that there were no significant changes in glutamate metabolite concentrations over time (F1,31 = 1.26, p = 0.270, partial η2 = 0.039), and the effect of time on glutamate metabolites also did not differ according to response status over 12 weeks (F1,31 = 0.60, p = 0.444, partial η2 = 0.019), (Table 2, Fig. 3). The main effects of the voxel (F1,31 = 50.68, p < 0.001, partial η2 = 0.620; pACC>dACC) and group (F1,31 = 5.92, p = 0.021, partial η2 = 0.160; Nonresponder>Responder) were still seen after including this longitudinal MRS data, indicating that the pattern of a high glutamatergic metabolite level in nonresponders compared to responders continued during the course of the 12 weeks of treatment, driven largely by the baseline difference.
Discussion
In this longitudinal study, we examined glutamate metabolites in the pACC and dACC in a cohort of patients with FEP undergoing risperidone treatment for 12 weeks. The main findings are (1) higher levels of MRS-based glutamatergic metabolite signal at the baseline in both pACC and dACC indicate later nonresponse, supporting our primary hypothesis (2) higher glutamatergic signal at treatment onset in the dACC relate to lack of improvement in the negative symptom burden. There is no significant change in glutamatergic metabolites signal over time, with nonresponders having a high glutamatergic metabolite level compared to responders over the 3-month treatment with risperidone. Our results confirm prior findings from the STRATA study [6] on the association of prefrontal glutamatergic excess of lack of treatment response, and extend them to indicate the specific link between hyperglutamatergia in the Salience Network (dACC voxel) and the subsequent lack of improvement or worsening of negative symptoms during the early treatment phase. In addition, we also demonstrate high glutamatergic signals in nonresponders irrespective of the MRS voxel chosen in the medial prefrontal cortex. Taken together, our results support the glutamatergic theory of early nonresponse and the potential for MRS glutamate measurement to guide decisions regarding length of antipsychotic treatment trials before considering second-line therapies (e.g., clozapine).
The association between elevated ACC glutamate metabolites and poor antipsychotic response is now well established with the constructs of treatment-resistance [7, 8, 29, 30] and nonresponse [6, 31,32,33] in early-stage illness. Our results support the view that MRS glutamate (dACC preferably) could be a potential treatment response predictor. Nevertheless, the classification accuracy achieved by the MRS measurements alone appears to be insufficient, as reflected by the within-sample accuracy values that we report (62-68%), with low predictive values especially in the first episode samples where there is a class imbalance, with most subjects being responders. Prior reports suggest that short courses of treatment (4-6 weeks) with different nonclozapine medications do not alleviate or worsen the glutamatergic excess in the nonresponding group in the ACC [6, 34] and striatum [35]. Our results concur with this interpretation over a longer period of 12 weeks; thus, a Bayesian approach that “stacks” clinical ratings of nonresponse as the first set of observations with the prefrontal glutamatergic signal from MRS as the second test, may enhance the predictive accuracy of nonresponse, reducing the lead time before second-line support is offered. A sufficiently powered diagnostic trial could demonstrate this clinical utility and demonstrate the feasibility of early intervention for Treatment-Resistant Schizophrenia.
In our study, patients with higher dACC glutamatergic MRS signal at baseline had a notable lack of improvement of negative symptoms, with even worsening of these symptoms over treatment seen in those with the highest baseline levels. While a specific relationship between prefrontal MRS glutamate signal and negative symptoms was not observed in a recent meta-analysis [36], our observation is consistent with the putative mechanistic relationship between hyperglutamatergia and negative symptoms proposed by Goff & Coyle [37], and pursued by several pharmacological studies on glutamate modulation to date [32, 38]. Further, in a study that modeled regional excitation/inhibition balance, Limongi et al. [39]. observed a relationship between higher dACC 7-Tesla glutamate signal and a disinhibition effect within the Salience Network, especially in patients with a higher burden of social withdrawal, a prominent negative symptom in early psychosis. Salience Network is seen as a bridge that integrates glutamatergic cognitive control models with dopaminergic prediction error signals [40]. More recent work in treatment-resistant subjects indicate that ACC glutamate could influence subcortical substrates of reward learning, thus providing further mechanistic link to negative symptoms [41]. This is also consistent with several other observations based on genetic [42] and structural imaging data [43] linking glutamate aberrations to the broader construct of negative symptoms.
In our FES sample, nonresponse was largely driven by lack of improvement in negative symptom burden. In contrast to established schizophrenia, patients with FES show more robust reduction in negative symptoms with available treatments [44]. While it is likely that some of this improvement may arise from secondary negative symptoms (i.e., due to improved extrapyramidal side effects, reduced substance use, diminishing positive symptoms), in our sample this pseudo specificity is unlikely. We excluded patients who were using substances, in particular cannabis, which is a major contributor to expressive deficits in FES [45]. Higher glutamate levels does not relate to improvement in positive symptoms; thus, the observed glutamate-negative symptom improvement cannot be explained by the secondary effects of that positive symptoms may have on negative symptoms. Finally, drug-naive patients later received the antipsychotic risperidone in increasing doses over 3 months; any dose-related extrapyramidal effect is likely to have resulted in a trajectory of increasing burden of secondary negative symptoms. This was not the case in our sample (there was an overall reduction in negative symptoms, especially in responders). Nevertheless, as we did not use a specific scale that identifies primary negative symptoms alone [46], we cannot fully rule out the influence of higher dACC glutamate on the secondary negative symptom burden.
In our study, we observed that pACC had higher glutamatergic level than dACC. This observation is supported by Dou et al. (2013) who assessed the local Glu concentrations for four architectonically different cingulate subregions (pregenual ACC, dorsal anterior/ mid-CC, rostral posterior CC, caudal posterior CC) using 7 T MRS. They found that the pregenual ACC had significantly higher Glu concentration than all other regions [26]. This is likely linked to an antero-posterior gradient of AMPA receptor distribution, which affects glutamate turnover, with highest levels in pACC compared to other cingulate regions [47, 48]. Such a region-specific metabolic heterogeneity with higher pACC glutamate values have been replicated using 3 T MRS more recently [49], further supporting our findings.
We do not see a significant change in glutamatergic signal over 3 months of treatment, in keeping with our prior report of a lack of notable changes over 6 months period [50]. Systematic reviews and meta-analyses suggest that antipsychotic treatment may decrease glutamatergic metabolite levels [4, 36, 51, 52], while Egerton et al. [51], noted an almost equal split between studies that reported significant glutamate decrease in at least one brain region and studies reported no glutamate reduction, some studies showing no longitudinal glutamate reduction in the ACC after 4 weeks [53], 6 weeks [54,55,56,57], 4 months [54], 6 months [50, 58], 9 months [56], 12 months [59] and 80 months [60]. Our findings are more consistent with these recent studies as well as the lack of progressive ACC glutamate change reported by Egerton et al. [6], Badari et al. [54], and suggested that antipsychotic medications may have limited effects on metabolite levels in these regions. However, we cannot rule out such a change occurring over longer follow-up periods in first-episode samples. In a recent 4 years follow-up of medicated patients with early-phase schizophrenia (2 years of treatment exposure at the baseline), Wang et al. noted a reduction in 7-Tesla MRS glutamate in dACC but this reduction also occurred in matched healthy controls followed up over the same duration, albeit at a somewhat slower rate in the absence of schizophrenia. Concurrent PET/MRS studies also indicate the possibility that antipsychotics may affect the relationship between dopamine and ACC glutamate without altering the glutamate concentrations per se [61]. Further longitudinal studies that begin at the treatment-naive stage of psychosis, will be required to clarify the effect of long-term antipsychotic exposure on glutamate trajectory.
Our study has several strengths, including the recruitment of a fully antipsychotic-naive sample, 12-week follow-up, dual voxel acquisition and a single antipsychotic exposure. Several limitations are also worth considering when interpreting these results. The separation of glutamate from other related metabolites is said to be less precise at 3 T compared to ultra-high field MRS at 7 T [62]. As the study was naturalistic, patients might have received over the counter remedies for sleep, anxiety which we could not account for. While we had sufficient power to demonstrate group effect and time effect, we were underpowered to estimate the interactions between time and group, so caution is warranted when interpreting the lack of differences between Responders and Nonresponders in the trajectory of glutamate changes. Further, we only acquired 2 time points of MRS data, given the untreated nature of our sample. We cannot exclude the possibility of progressive glutamate changes in different brain regions (as shown by Théberge et al. [63], Kraguljac et al. [34] and Goto et al. [58] in thalamus, hippocampus and basal ganglia) or in ACC over a longer time treatment or multiple time points. Glutamate measurements obtained from 1H-MRS reflect the total concentration of glutamate in relatively large voxels, and it is not possible to specifically attribute the results to glutamate neurotransmission or to glutamatergic level in specific cell types [64]. Finally, the use of clinician-based dose change introduces variability that might have influenced the ultimate treatment response; nevertheless, this ensured continued treatment compliance, and tracked the real-world titration practice that is likely to happen when MRS glutamate measures are available.
In summary, this longitudinal MRS study of glutamatergic metabolites found that first-episode schizophrenia with poor treatment response has a higher glutamatergic level that does not progressively worsen over 12 weeks risperidone treatment. While this supports the call for a stratified use of antipsychotics, longer time follow-up is required to see if improvement in symptoms accompanies a reduction in glutamate. We need larger, prospective studies on highly resolved glutamate measurements to study the effects of dopamine D2 receptor blockers. Such studies will help us advance empirically-informed drug development for treatment resistant schizophrenia by parsing therapeutically meaningful subtypes at the outset of early intervention.
References
Disorders ECPfOPToP: The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. J Clin Psych. 2003;64:2–100.
Dempster K, Li A, Sabesan P, Norman R, Palaniyappan L. Treatment resistance: a time-based approach for early identification in first episode psychosis. J Pers Med. 2021;11:711.
Gudmundson A, Koo A, Virovka A, Amirault A, Soo M, Cho J, et al. In vivo proton MR Spectroscopy of the healthy and diseased human brain. bioRxiv. 2023. https://doi.org/10.1101/2023.02.10.528046.
Nakahara T, Tsugawa S, Noda Y, Ueno F, Honda S, Kinjo M, et al. Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Mol Psych. 2022;27:744–57.
Liemburg E, Sibeijn-Kuiper A, Bais L, Pijnenborg G, Knegtering H, Van der Velde J, et al. Prefrontal NAA and Glx levels in different stages of psychotic disorders: a 3T 1H-MRS study. Sci Rep. 2016;6:21873.
Egerton A, Griffiths K, Casetta C, Deakin B, Drake R, Howes OD, et al. Anterior cingulate glutamate metabolites as a predictor of antipsychotic response in first episode psychosis: data from the STRATA collaboration. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2023;48:567–75.
Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psych. 2014;75:e11–e13.
Iwata Y, Nakajima S, Plitman E, Caravaggio F, Graff-Guerrero A. Glutamatergic neurometabolite levels in patients with ultra treatment-resistant schizophrenia: a cross-sectional 3T Proton MRS study. Biolog Psych. 2018;85:596–605.
Dempster K, Jeon P, MacKinley M, Williamson P, Théberge J, Palaniyappan L. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol Psych. 2020;25:1640–50.
Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naïve patients with psychosis. Psychol Med. 2020;50:2182–93.
Li J, Ren H, He Y, Li ZC, Tang J. Anterior cingulate cortex glutamate levels are related to response to initial antipsychotic treatment in drug-naive first-episode schizophrenia patients. Front Psych. 2020;11:553269.
Kessler RC, Luedtke A. Pragmatic precision psychiatry—a new direction for optimizing treatment selection. JAMA Psych. 2021;78:1384–90.
Hribkova H, Svoboda O, Bartecku E, Zelinkova J, Horinkova J, Lacinova L, et al. Clozapine reverses dysfunction of glutamatergic neurons derived from clozapine-responsive schizophrenia patients. Front Cell Neurosci. 2022;16:830757.
McQueen G, Sendt K-V, Gillespie A, Avila A, Lally J, Vallianatou K, et al. Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophrenia Bull. 2021;47:662–71.
Kantrowitz JT, Grinband J, Goff DC, Lahti AC, Marder SR, Kegeles LS, et al. Proof of mechanism and target engagement of glutamatergic drugs for the treatment of schizophrenia: RCTs of pomaglumetad and TS-134 on ketamine-induced psychotic symptoms and pharmacoBOLD in healthy volunteers. Neuropsychopharmacology. 2020;45:1842–50.
Dogra S, Conn PJ. Metabotropic glutamate receptors as emerging targets for the treatment of schizophrenia. Mol Pharmacol. 2022;101:275–85.
Leucht S, Chaimani A, Krause M, Schneider-Thoma J, Wang D, Dong S, et al. The response of subgroups of patients with schizophrenia to different antipsychotic drugs: a systematic review and meta-analysis. Lancet Psych. 2022;9:84–993.
McCutcheon RA, Pillinger T, Mizuno Y, Montgomery A, Pandian H, Vano L, et al. The efficacy and heterogeneity of antipsychotic response in schizophrenia: a meta-analysis. Mol Psych. 2021;26:1310–20.
McCutcheon RA, Pillinger T, Efthimiou O, Maslej M, Mulsant BH, Young AH, et al. Reappraising the variability of effects of antipsychotic medication in schizophrenia: a meta‐analysis. World Psych. 2022;21:287–94.
Kaminski J, Mascarell-Maricic L, Fukuda Y, Katthagen T, Heinz A, Schlagenhauf F. Glutamate in the dorsolateral prefrontal cortex in patients with schizophrenia: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Biol Psych. 2021;89:270–7.
Merritt K, McCutcheon RA, Aleman A, Ashley S, Beck K, Block W, et al. Variability and magnitude of brain glutamate levels in schizophrenia: a meta and mega-analysis. Mol Psych. 2023:1–10.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psych. 1998;59:22–33.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13:261–76.
Wang Q, Ren H, Li C, Li Z, Li J, Li H, et al. Metabolite differences in the medial prefrontal cortex in schizophrenia patients with and without persistent auditory verbal hallucinations: a 1H MRS study. Transl Psych. 2022;12:116.
Lopez-Persem A, Verhagen L, Amiez C, Petrides M, Sallet J. The human ventromedial prefrontal cortex: sulcal morphology and its influence on functional organization. J Neurosci : Off J Soc Neurosci. 2019;39:3627–39.
Dou W, Palomero-Gallagher N, van Tol M-J, Kaufmann J, Zhong K, Bernstein H-G, et al. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J Neurosci. 2013;33:12698–704.
Wang M, Hu K, Fan L, Yan H, Li P, Jiang T, et al. Predicting treatment response in schizophrenia with magnetic resonance imaging and polygenic risk score. Front Genet. 2022;13:848205.
Leucht S, Davis J, Engel R, Kissling W, Kane J. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scandinavica. 2009;119:7–14.
Elias M, Bloomfield M, Vincent L, Katherine B, Sudhakar S, Naresh R, et al. Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull. 2016;42:744.
Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol. 2015;18:pyu117.
Egerton A, Broberg BV, Van HN, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1 H-MRS study (OPTiMiSE). Mol Psych. 2018;23:2145–55.
Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, Mcguire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–21.
Gudmundson AT, Koo A, Virovka A, Amirault AL, Soo M, Cho JH, et al. Meta-analysis and open-source database for in vivo brain Magnetic Resonance spectroscopy in health and disease. Anal Biochem. 2023;676:115227.
Kraguljac NV, Morgan CJ, Reid MA, White DM, Jindal RD, Sivaraman S, et al. A longitudinal magnetic resonance spectroscopy study investigating effects of risperidone in the anterior cingulate cortex and hippocampus in schizophrenia. Schizophrenia Res. 2019;210:239–44.
Reyes-Madrigal F, Guma E, León-Ortiz P, Gómez-Cruz G, Mora-Durán R, Graff-Guerrero A, et al. Striatal glutamate, subcortical structure and clinical response to first-line treatment in first-episode psychosis patients. Prog Neuro-Psychopharmacol Biol Psych. 2022;113:110473.
Merritt K, McGuire PK, Egerton A, Aleman A, Block W, Bloemen OJ, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psych. 2021;78:667–81.
Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psych. 2001;158:1367–77.
Kruse AO, Bustillo JR. Glutamatergic dysfunction in Schizophrenia. Transl Psych. 2022;12:500.
Limongi R, Jeon P, Théberge J, Palaniyappan L. Counteracting effects of glutathione on the glutamate-driven excitation/inhibition imbalance in first-episode schizophrenia: a 7T MRS and dynamic causal modeling study. Antioxidants. 2021;10:75.
Menon V, Palaniyappan L, Supekar K. Integrative brain network and salience models of psychopathology and cognitive dysfunction in schizophrenia. Biol Psych. 2022;94:108–20.
Mouchlianitis ED, Vanes LD, Tracy DK, Fett A-K, Joyce D, Shergill SS. Neuroimaging glutamatergic mechanisms differentiating antipsychotic treatment-response. Sci Rep. 2023;13:8938.
Ouyang X, Pan Y, Chen X, Wu G, Cheng Y, Tan W, et al. Cortical morphological heterogeneity of schizophrenia and its relationship with glutamatergic receptor variations. Eur Psych: J Assoc Eur Psychiatrists. 2023;66:e38.
Liang L, Silva AM, Jeon P, Ford SD, MacKinley M, Théberge J, et al. Widespread cortical thinning, excessive glutamate and impaired linguistic functioning in schizophrenia: A cluster analytic approach. Front Hum Neurosci. 2022;16:954898.
Lutgens D, Joober R, Iyer S, Lepage M, Norman R, Schmitz N, et al. Progress of negative symptoms over the initial 5 years of a first episode of psychosis. Psychol Med. 2019;49:66–74.
Ihler HM, Lyngstad SH, Gardsjord ES, Widing LH, Flaaten CB, Åsbø G, et al. The trajectory of two negative symptom dimensions in first-episode psychosis and the role of cannabis use: A 10-year follow-up study. Schizophrenia Res. 2023;252:317–25.
Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psych. 2013;170:165–72.
Palomero‐Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: Evaluation of the four‐region neurobiological model. Hum Brain Mapp. 2009;30:2336–55.
Pittaluga A, Feligioni M, Longordo F, Luccini E, Raiteri M. Trafficking of presynaptic AMPA receptors mediating neurotransmitter release: neuronal selectivity and relationships with sensitivity to cyclothiazide. Neuropharmacology. 2006;50:286–96.
He J, Wang D, Ban M, Kong L, Xiao Q, Yuan F, et al. Regional metabolic heterogeneity in anterior cingulate cortex in major depressive disorder: A multi-voxel 1H magnetic resonance spectroscopy study. J Affect Disord. 2022;318:263–71.
Jeon P, Limongi R, Ford SD, Mackinley M, Dempster K, Théberge J, et al. Progressive changes in glutamate concentration in early stages of schizophrenia: a longitudinal 7-Tesla MRS study. Schizophrenia Bull Open. 2021;2:072.
Alice E, Akarmi B, Kate M, Grant MQ, Agata S, Philip MG. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal 1H-MRS studies. Front Psych. 2017;8:66.
Kubota M, Moriguchi S, Takahata K, Nakajima S, Horita N. Treatment effects on neurometabolite levels in schizophrenia: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophrenia Res. 2020;222:122–32.
de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psych. 2013;70:1057–66.
Birur B, Kraguljac NV, VerHoef L, Morgan CJ, Jindal RD, Reid MA, et al. Neurometabolic correlates of 6 and 16 weeks of treatment with risperidone in medication-naive first-episode psychosis patients. Transl Psych. 2020;10:15.
Cadena EJ, White DM, Kraguljac NV, Reid MA, Maximo JO, Nelson EA, et al. A longitudinal multimodal neuroimaging study to examine relationships between resting state glutamate and task related BOLD response in schizophrenia. Front Psych. 2018;9:632.
Merritt K, Perez-Iglesias R, Sendt K-V, Goozee R, Jauhar S, Pepper F, et al. Remission from antipsychotic treatment in first episode psychosis related to longitudinal changes in brain glutamate. NPJ Schizophrenia. 2019;5:12.
Szulc A, Galinska B, Tarasow E, Waszkiewicz N, Konarzewska B, Poplawska R, et al. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44:148–57.
Goto N, Yoshimura R, Kakeda S, Nishimura J, Moriya J, Hayashi K, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–22.
Bustillo J, Rowland L, Mullins P, Jung R, Chen H, Qualls C, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psych. 2010;15:629–36.
Aoyama N, Theberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RW, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psych. 2011;198:448–56.
Jauhar S, McCutcheon RA, Veronese M, Borgan F, Nour M, Rogdaki M, et al. The relationship between striatal dopamine and anterior cingulate glutamate in first episode psychosis changes with antipsychotic treatment. Transl Psych. 2023;13:184.
Lavigne KM, Kanagasabai K, Palaniyappan L. Ultra-high field neuroimaging in psychosis: A narrative review. Front Psych. 2022;13:2688.
Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psych. 2007;191:325–34.
Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev. 2015;51:276–95.
Funding
This work was supported by National Key Research and development plan of China (intergovernmental international scientific and technological innovation cooperation project, Grant No:2021YFE0191400), the National Natural Science Foundation of China (NSFC) (Grant No. 81871056 and 82101576), the Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2020zzts287), Science and Technology Innovation Program of Hunan Province (Grant No. 2022RC1040 (ZL)) and China Scholarship Council (Grant No. 202106370192) for LF to train at the Douglas Research Centre. LP acknowledges research support towards this work from the Canada First Research Excellence Fund, awarded to the Healthy Brains, Healthy Lives initiative at McGill University (through New Investigator Supplement to LP); Monique H. Bourgeois Chair in Developmental Disorders and Graham Boeckh Foundation (Douglas Research Centre, McGill University) and a salary award from the Fonds de recherche du Quebec-Sante ́(FRQS).
Author information
Authors and Affiliations
Contributions
XC: Supervision, Funding acquisition, Project administration. LP: Conceptualization, supervision of analysis, writing of original and revised drafts. LF: conceptualization, methodology, acquisition and analysis of data, writing of original and revised drafts. LL: conceptualization, revised drafts. Performed research (acquisition, analysis, and interpretation of data): YW, XM, LY, LO, YH, ZL, CL. All authors contributed to drafting and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
LP reports personal fees for serving as chief editor from the Canadian Medical Association Journals, speaker/consultant fee from Janssen Canada and Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Janssen Canada, Sunovion and Otsuka Canada outside the submitted work. All the other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, L., Liang, L., Wang, Y. et al. Glutamatergic basis of antipsychotic response in first-episode psychosis: a dual voxel study of the anterior cingulate cortex. Neuropsychopharmacol. 49, 845–853 (2024). https://doi.org/10.1038/s41386-023-01741-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01741-x