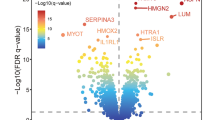
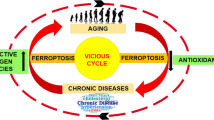
Abstract
Necrosis is uncontrolled cell death that marks the irreversible threshold of biological degeneration. Rooted in the Greek nekros (death), it is a pivotal mechanism underlying numerous diseases, including cancer, as well as renal, cardiac, neuronal, and hepatic disorders, and more broadly, the aging process. Despite its profound impact on morbidity and mortality, necrosis remains untreatable and has long been viewed as a chaotic, unavoidable aspect of biology. This review examines the mechanisms of necrosis and outlines its far-reaching impact on health, as revealed by emerging evidence. Furthermore, we explore its potential as a game-changing therapeutic target. Inhibiting necrosis could revolutionize treatments for acute and chronic age-related conditions like cancer, kidney disease, cardiovascular disease (including heart attacks and strokes), and neurodegeneration, while also preserving resilience—and even slowing aging itself. Beyond Earth, where microgravity, cosmic radiation, and oxidative stress accelerate cellular decline, targeting necrosis may also hold the key to preserving astronaut resilience and health on long-duration space missions, offering insights that could reshape human longevity both on and off the planet.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kerr JF. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol. 1965;90:419–35.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57.
Mortimore GE, Pösö AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr. 1987;7:539–64.
Virchow R. Cellular-pathologie. Archiv f. pathol Anat. 1855;8:3–39.
Virchow R, Chance FN. Cellular pathology as based upon physiological and pathological histology: twenty lectures delivered in the pathological institute of Berlin [luring the Months of February, March and April, 1858]: P. Blakiston, Son & Company; 1880.
Overholtzer M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9.
Dixon. SJea. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4.
Liu Y. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci (PNAS). 2013;110:20364–71.
Sun Q, et al. Competition between human cells by entosis. Cell Res. 2014;24:1299–310.
Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;455–465:455–65.
Khalid N, Azimpouran M Necrosis. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Mahzad Azimpouran declares no relevant financial relationships with ineligible companies. StatPearls Publishing LLC; 2024.
Fleckenstein A, Janke J, Döring HJ, Leder O. Myocardial fiber necrosis due to intracellular Ca overload-a new principle in cardiac pathophysiology. Recent Adv Stud Card Struct Metab. 1974;4:563–80.
Spiteller G. Are lipid peroxidation processes induced by changes in the cell wall structure and how are these processes connected with diseases? Med Hypotheses. 2003;60:69–83.
England K, Cotter T. Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep. 2005;10:237–45.
Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503.
Bailey DM. Oxygen, evolution and redox signalling in the human brain; quantum in the quotidian. J Physiol. 2019;597:15–28.
Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–76.
Kern C, Faragher R, Woods T, Bonventre J, Jenkins A, & Stebbing J. The Architecture of Aging: How Ageing Drives Disease (Blueprint Theory). (2024). Preprints. https://doi.org/10.20944/preprints202310.1387.v4
Majno G, La Gattuta M, Thompson TE. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch für pathologische Anat und Physiologie und für klinische Med. 1960;333:421–65.
Ruffolo PR. The pathogenesis of necrosis. I. Correlated light and electron microscopic observations of the myocardial necrosis induced by the intravenous injection of papain. Am J Pathol. 1964;45:741–56.
Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. J Lipid Mediators Cell Signal. 1996;14:15–23.
Yamashima T, Saido TC, Takita M, Miyazawa A, Yamano J, Miyakawa A, et al. Transient brain ischaemia provokes Ca2+, PIP2 and calpain responses prior to delayed neuronal death in monkeys. Eur J Neurosci. 1996;8:1932–44.
Yamashima T, Oikawa S. The role of lysosomal rupture in neuronal death. Prog Neurobiol. 2009;89:343–58.
Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23.
Park W, Wei S, Kim B-S, Kim B, Bae S-J, Chae YC, et al. Diversity and complexity of cell death: a historical review. Exp Mol Med. 2023;55:1573–94.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
Didelot C, Lanneau D, Brunet M, Joly A-L, Thonel AD, Chiosis G, et al. Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem. 2007;14:2839–47.
Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14:43–64.
Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: feel the stress. J Immunol. 2017;198:1395–402.
Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B. 1979;205:581–98.
Gould SJ, Vrba ES. Exaptation—a missing term in the science of form. Paleobiology. 1982;8:4–15.
Darwin C. Origin of the species. In: British Politics and the environment in the long nineteenth century. Routledge; 1859. p. 47–55.
Gould SJ. A developmental constraint in cerion, with comments on the definition and interpretation of constraint in evolution. Evolution. 1989;43:516–39.
Wanless IR, Shiota K, editors. The pathogenesis of nonalcoholic steatohepatitis and other fatty liver diseases: a four-step model including the role of lipid release and hepatic venular obstruction in the progression to cirrhosis. Seminars in liver disease. Thieme Medical Publishers, Inc.; 2004.
Fang Y. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595.
Zhang L, Zha Z, Qu W, Zhao H, Yuan J, Feng Y, et al. Tumor necrosis as a prognostic variable for the clinical outcome in patients with renal cell carcinoma: a systematic review and meta-analysis. BMC Cancer. 2018;18:1–13.
Bredholt G, Mannelqvist M, Stefansson IM, Birkeland E, Bø TH, Øyan AM, et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget. 2015;6:39676.
Leek R, Landers R, Harris A, Lewis C. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–5.
Jimenez RE, Wallis T, Visscher DW. Centrally necrotizing carcinomas of the breast: a distinct histologic subtype with aggressive clinical behavior. Am J Surg Pathol. 2001;25:331–7.
Investigators CN, Fisher ER, Sass R, Fisher B. Pathologic findings from the national surgical adjuvant project for breast cancers (protocol no. 4) X. Discriminants for tenth year treatment failure. Cancer. 1984;53:712–23.
Ammirante M, Luo J-L, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5.
Yamamoto A, Huang Y, Krajina BA, McBirney M, Doak AE, Qu S, et al. Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7. Proc Natl Acad Sci USA. 2023;120:e2214888120.
Zhao Y, Fu X, Lopez JI, Rowan A, Au L, Fendler A, et al. Selection of metastasis competent subclones in the tumour interior. Nat Ecol Evol. 2021;5:1033–45.
Lee G, Yoon S, Ahn B, Kim H-R, Jang SJ, Hwang HS. Blood vessel invasion predicts postoperative survival outcomes and systemic recurrence regardless of ___location or blood vessel type in patients with lung adenocarcinoma. Ann Surg Oncol. 2021;28:7279–90.
van den Boogaard WMC, Komninos DSJ, Vermeij WP. Chemotherapy side-effects: not All DNA damage is equal. Cancers. 2022;14:627
Bray ER, Lin RR, Li JN, Elgart GW, Elman SA, Maderal AD. Immune checkpoint inhibitor associated epidermal necrosis, beyond SJS and TEN: a review of 98 cases. Arch Dermatol Res. 2024;316:233.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78.
Bailey DM, Rimoldi SF, Rexhaj E, Pratali L, Salinas Salmòn C, Villena M, et al. Oxidative-nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest. 2013;143:444–51.
Christidi E, Brunham LR. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12:339.
Teicher BA, Holden SA, Al-Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50:3339–44.
Teicher BA, Lazo JS, Sartorelli AC. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81.
Strese S, Fryknäs M, Larsson R, Gullbo J. Effects of hypoxia on human cancer cell line chemosensitivity. BMC Cancer. 2013;13:1–11.
Sun S, Lee D, Lee NP, Pu JK, Wong ST, Lui W, et al. Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide. J Neuro-Oncol. 2012;109:467–75.
Wouters A, Pauwels B, Lardon F, Vermorken JB. Implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist. 2007;12:690–712.
Baker AF, Koh MY, Williams RR, James B, Wang H, Tate WR, et al. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1α-induced gene in pancreatic cancer. Pancreas. 2008;36:178-186.
Fyles AW, Milosevic M, Wong R, Kavanagh M-C, Pintilie M, Sun A, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–56.
Höckel M, Vorndran B, Schlenger K, Baußmann E, Knapstein PG. Tumor oxygenation: a new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol Oncol. 1993;51:141–9.
Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7.
Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24.
Toustrup K, Sørensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–31.
Franks SE, Solocinski K, Schlom J, Hodge J. Exploiting tumor necrosis with novel immunotherapies. J Immunol. 2020;204:170.23-.23.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer cell. 2012;21:297–308.
Lycan TW, Pardee TS, Petty WJ, Bonomi M, Alistar A, Lamar ZS, et al. A phase II clinical trial of CPI-613 in patients with relapsed or refractory small cell lung carcinoma. PLoS ONE. 2016;11:e0164244.
Alistar A, Desnoyers R, D’Agostino R, Pasche B. CPI-613 enhances FOLFIRINOX response rate in stage IV pancreatic cancer. Ann Oncol. 2016;27:vi228.
National Kidney Foundation. Aging and kidney disease. 2024. https://www.kidney.org/aging-and-kidney-disease#:~:text=Back%20to%20Top,rest%20of%20the%20general%20population.
Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Investig. 2011;121:4210–21.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–43.
Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–83.
Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Ren Physiol. 2001;281:F887–F99.
Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57.
Gao J, Xiong A, Liu J, Li X, Wang J, Zhang L, et al. PANoptosis: bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Ther. 2024;31:970–83.
WHO. The top 10 causes of death. 2024. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424.
Bailey DM, Raman S, McEneny J, Young IS, Parham KL, Hullin DA, et al. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radic Biol Med. 2006;40:591–600.
Bailey DM, Morris-Stiff G, McCord JM, Lewis MH. Has free radical release across the brain after carotid endarterectomy traditionally been underestimated? Significance of reperfusion hemodynamics. Stroke. 2007;38:1946–8.
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83.
Krishna M. Patterns of necrosis in liver disease. Clin Liver Dis. 2017;10:53–6.
Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–65.
Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121.
Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. Canad Med Assoc J. 2005;172:899–905.
Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–30.
Homma H, Tanaka H, Fujita K, Okazawa H. Necrosis links neurodegeneration and neuroinflammation in neurodegenerative disease. Int J Mol Sci. 2024;25:3636.
Siddappaji KK, Gopal S. Molecular mechanisms in Alzheimer’s disease and the impact of physical exercise with advancements in therapeutic approaches. AIMS Neurosci. 2021;8:357–89.
Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583–95.
Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther. 2011;17:514–24.
Kung HC, Lin KJ, Kung CT, Lin TK. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson’s disease. Biomedicines. 2021;9:918.
Betzer C, Lassen LB, Olsen A, Kofoed RH, Reimer L, Gregersen E, et al. Alpha-synuclein aggregates activatecalcium pump SERCA leading to calcium dysregulation. EMBO Rep.2018;19:e44617.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–78.
Woods T, Palmarini N, Corner L, Barzilai N, Maier AB, Sagner M, et al. Cities, communities and clinics can be testbeds for human exposome and aging research. Nat Med. 2025;1-3.
Argentieri MA, Amin N, Nevado-Holgado AJ, Sproviero W, Collister JA, Keestra SM, et al. Integrating the environmental and genetic architectures of aging and mortality. Nat Med. 2025;31:1016–25.
Widjaja AA, Lim W-W, Viswanathan S, Chothani S, Corden B, Dasan CM, et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature. 2024;632:157–65.
Faragher RGA, Heidari N, Ostler EL. Therapeutic opportunities presented by modulation of cellular senescence. Subcell Biochem. 2023;102:175–93.
Bailey DM. Decoding the space integrome: personalized countermeasures for a mission to Mars. Exp Physiol. 2025;1–4.
Lv H, Yang H, Jiang C, Shi J, Chen RA, Huang Q, et al. Microgravity and immune cells. J R Soc Interface. 2023;20:20220869.
Cohen–Jonathan E, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83.
Siew K, Nestler KA, Nelson C, D’Ambrosio V, Zhong C, Li Z, et al. Cosmic kidney disease: an integrated pan-omic, physiological and morphological study into spaceflight-induced renal dysfunction. Nat Commun. 2024;15:4923.
Riley DA, Ellis S, Slocum GR, Sedlak FR, Bain JL, Krippendorf BB, et al. In-flight and postflight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. J Appl Physiol (1985). 1996;81:133–44.
Montesinos CA, Khalid R, Cristea O, Greenberger JS, Epperly MW, Lemon JA, et al. Space radiation protection countermeasures in microgravity and planetary exploration. Life. 2021;11.
Bailey DM, Lanéelle D, Trihan J-E, Marchi N, Stacey BS, Tamiya K, et al. Gravitational transitions increase posterior cerebral perfusion and systemic oxidative-nitrosative stress: implications for neurovascular unit integrity. Neuroscience. 2020;441:142–60.
Panesar SS, Fernandez-Miranda JC, Kliot M, Ashkan K. Neurosurgery and manned spaceflight. Neurosurgery. 2020;86:317–24.
Ding Y, Zou J, Li Z, Tian J, Abdelalim S, Du F, et al. Study of histopathological and molecular changes of rat kidney under simulated weightlessness and resistance training protective effect. PLoS ONE. 2011;6:e20008.
Li Q, Mei Q, Huyan T, Xie L, Che S, Yang H, et al. Effects of simulated microgravity on primary human NK cells. Astrobiology. 2013;13:703–14.
Philpott D, Corbett R, Turnbill C, Harrison G, Leaffer D, Black S, et al. Cosmic ray effects on the eyes of rats flown on Cosmos No. 782, experimental K-007. Aviat Space Environ Med. 1978;49:19–28.
Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, et al. The NASA Twins Study: a multidimensional analysis of a year-long human spaceflight. Science. 2019;364:eaau8650.
da Silveira WA, Fazelinia H, Rosenthal SB, Laiakis EC, Kim MS, Meydan C, et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell. 2020;183:1185–201. e20
Kern C, Siew K. Beyond the horizon: why space biology is the next great innovation opportunity. Exp. Physiol. n/a(n/a).
Braithwaite SA, van der Kaaij NP. New techniques for optimization of donor lungs/hearts. Anesthesiol Clin. 2019;37:639–60.
Ruaengsri C, Bethencourt DM, Koyano T, Shudo Y. Heart preservation techniques for transplantation 2023. Cardiology and Cardiovascular Medicine. IntechOpen; 2024.
Chen J, Liu X, Hu Y, Chen X, Tan S. Cryopreservation of tissues and organs: present, bottlenecks, and future. Front Vet Sci. 2023;10:1201794.
Baust JG, Gao D, Baust JM. Cryopreservation: an emerging paradigm change. Organogenesis. 2009;5:90–6.
Amer MH, Rose FRAJ, Shakesheff KM, Modo M, White LJ. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. npj Regen Med. 2017;2:23.
Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–66.
Mitrousis N, Fokina A, Shoichet MS. Biomaterials for cell transplantation. Nat Rev Mater. 2018;3:441–56.
Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165:268–77.
Zhang H, Chen H, Wang W, Wei Y, Hu S. Cell survival and redistribution after transplantation into damaged myocardium. J Cell Mol Med. 2010;14:1078–82.
Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11.
Masson-Meyers DS, Tayebi L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J Tissue Eng Regen Med. 2021;15:747–62.
Rademakers T, Horvath JM, van, Blitterswijk CA, LaPointe VLS. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J Tissue Eng Regen Med. 2019;13:1815–29.
Bhaduri A, Andrews MG, Mancia Leon W, Jung D, Shin D, Allen D, et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature. 2020;578:142–8.
Nickels SL, Modamio J, Mendes-Pinheiro B, Monzel AS, Betsou F, Schwamborn JC. Reproducible generation of human midbrain organoids for in vitro modeling of Parkinson’s disease. Stem Cell Res. 2020;46:101870.
Soriano J, Mora-Espí I, Alea-Reyes ME, Pérez-García L, Barrios L, Ibáñez E, et al. Cell death mechanisms in tumoral and non-tumoral human cell lines triggered by photodynamic treatments: apoptosis, necrosis and parthanatos. Sci Rep. 2017;7:41340.
Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng. 2012;114:577–81.
Dias S, Shmelkov SV, Lam G, Rafii S. VEGF165 promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood, J Am Soc Hematol. 2002;99:2532–40.
Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495–504.
Sun D, Wang W, Wang X, Wang Y, Xu X, Ping F, et al. bFGF plays a neuroprotective role by suppressing excessive autophagy and apoptosis after transient global cerebral ischemia in rats. Cell Death Dis. 2018;9:172.
Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–39.
Esco MA, Wang Z, McDermott ML, Kurpakus-Wheater M. Potential role for laminin 5 in hypoxia-mediated apoptosis of human corneal epithelial cells. J cell Sci. 2001;114:4033–40.
Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma Y-C, Cowan CW, et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev cell. 2002;3:631–43.
Clift MJ, Fytianos K, Vanhecke D, Hočevar S, Petri-Fink A, Rothen-Rutishauser B. A novel technique to determine the cell type specific response within an in vitro co-culture model via multi-colour flow cytometry. Sci Rep. 2017;7:434.
Goval J-J, Thielen C, Bourguignon C, Greimers R, Dejardin E, Choi YS, et al. The prevention of spontaneous apoptosis of follicular lymphoma B cells by a follicular dendritic cell line: involvement of caspase-3, caspase-8 and c-FLIP. Haematologica. 2008;93:1169–77.
Ferrada L, Barahona MJ, Salazar K, Vandenabeele P, Nualart F. Vitamin C controls neuronal necroptosis under oxidative stress. Redox Biol. 2020;29:101408.
Chapman S, McDermott DH, Shen K, Jang MK, McBride AA. The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res Ther. 2014;5:1–11.
Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607.
Williams AB, Schumacher B. p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med. 2016;6:a026070.
Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014;125:293–9.
Pickkers P, Murray PT, Ostermann M. New drugs for acute kidney injury. Intensive Care Med. 2022;48:1796–8.
Dvirnik N, Belley-Cote E, Hanif H, Devereaux P, Lamy A, Dieleman J, et al. Steroids in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2018;120:657–67.
Pickkers P, Mehta RL, Murray PT, Joannidis M, Molitoris BA, Kellum JA, et al. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. Jama. 2018;320:1998–2009.
van Till JO, Nojima H, Kameoka C, Hayashi C, Sakatani T, Washburn TB, et al. The effects of peroxisome proliferator-activated receptor-delta modulator ASP1128 in patients at risk for acute kidney injury following cardiac surgery. Kidney Int Rep. 2023;8:1407–16.
Cheng SY, Wang SC, Lei M, Wang Z, Xiong K. Regulatory role of calpain in neuronal death. Neural Regen Res. 2018;13:556–62.
Dhir N, Medhi B, Prakash A, Goyal MK, Modi M, Mohindra S. Pre-clinical to clinical translational failures and current status of clinical trials in stroke therapy: a brief review. Curr Neuropharmacol. 2020;18:596–612.
Wahlgren N. The Clomethiazole Acute Stroke Study (CLASS): results of a randomised controlled study of clomethiazole versus placebo in 1360 acute stroke patients. Cerebrovasc Dis. 1997;7:24–30.
Wahlgren N, MacMahon D, De Keyser JF, Indredavik B, Ryman T. Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke. Cerebrovasc Dis. 1994;4:204–10.
Oczkowski WJ, Hachinski VC, Bogousslavsky J, Barnett H, Carruthers S. A double-blind, randomized trial of PY108-068 in acute ischemic cerebral infarction. Stroke. 1989;20:604–8.
Jensen B. BMS-204352: a potassium channel opener developed for the treatment of stroke. CNS Drug Rev. 2002;8:353–60.
Franke C, Palm R, Dalby M, Schoonderwaldt H, Hantson L, Eriksson B, et al. Flunarizine in stroke treatment (FIST): A double‐blind, placebo‐controlled trial in Scandinavia and the Netherlands. Acta Neurol Scand. 1996;93:56–60.
Overmeyer JH, Kaul A, Johnson EE, Maltese WA. Active Ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol Cancer Res. 2008;6:965–77.
Lee D, Kim IY, Saha S, Choi KS. Paraptosis in the anti-cancer arsenal of natural products. Pharmacol Ther. 2016;162:120–33.
Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–94.
Li J.Ferroptosis: past, present and future.Cell Death Dis. 2012;11:88
Li J. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88.
Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32:417–8.
Liu J, Kuang F, Kang R, Tang D. Alkaliptosis: a new weapon for cancer therapy. Cancer Gene Ther. 2020;27:267–9.
Holze C. et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat Immunol. 2018;19:130–40.
Acknowledgements
This work did not receive any funding from public, commercial, or not-for-profit organizations. Figures were created partially in BioRender. Kern, C.(2025) and partially using images under license from iStock.com.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript. CK, JVB, AWJ, KK, ER, KS, BD, HK, NG, and DMB jointly conceptualized, drafted, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
CK is CEO of LikGevity. BD is Head of R&D of LinkGevity and formerly held senior leadership positions, inc. director of scientific operations, at GlaxoSmithKline (GSK). JB holds equity in LinkGevity; is Director of Health Sciences and Technology at Massachusetts General Hospital, Harvard, USA; Chair of Renal Medicine, Harvard; Chief, Renal Division; Chief, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard; Past President, American Society of Nephrology; and Member of the Kidney Cancer Program at the Dana-Farber/Harvard Cancer Center. ER is Managing Director, Starburst Aerospace, USA and Director of NASA Human Research Program, Microsoft and Translational Research Institute Space-Health (Space-H) Program, USA. NG is CTO at LinkGevity. HK is a LIDO student at LinkGevity. DMB is Editor-in-Chief of Experimental Physiology; Chair of the Life Sciences Working Group, European Space Agency; Member of the Human Spaceflight and Exploration Science Advisory Committee to the European Space Agency; Member of the Space Exploration Advisory Committee to the UK and Swedish National Space Agencies; and Member of the National Cardiovascular Network for Wales and South-East Wales Vascular Network. DMB is also affiliated to Bexorg, Inc. (USA) focused on the technological development of novel biomarkers of cerebral bioenergetic function and structural damage in humans.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kern, C., Bonventre, J.V., Justin, A.W. et al. Necrosis as a fundamental driver of loss of resilience and biological decline: what if we could intervene?. Oncogene 44, 1893–1904 (2025). https://doi.org/10.1038/s41388-025-03431-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-025-03431-y