Abstract
Background
Single-cohort studies have identified distinct neurobehavioral profiles that are associated with prenatal and neonatal factors based on the NICU Network Neurobehavioral Scale (NNNS). We examined socioeconomic, medical, and substance use variables as predictors of NNNS profiles in a multi-cohort study of preterm and term-born infants with different perinatal exposures.
Methods
We studied 1112 infants with a neonatal NNNS exam from the Environmental influences on Child Health Outcomes (ECHO) consortium. We used latent profile analysis to characterize infant neurobehavioral profiles and generalized estimating equations to determine predictors of NNNS profiles.
Results
Six distinct neonatal neurobehavioral profiles were identified, including two dysregulated profiles: a hypo-aroused profile (16%) characterized by lethargy, hypotonicity, and nonoptimal reflexes; and a hyper-aroused profile (6%) characterized by high arousal, excitability, and stress, with low regulation and poor movement quality. Infants in the hypo-aroused profile were more likely to be male, have younger mothers, and have mothers who were depressed prenatally. Infants in the hyper-aroused profile were more likely to be Hispanic/Latino and have mothers who were depressed or used tobacco prenatally.
Conclusions
We identified two dysregulated neurobehavioral profiles with distinct perinatal antecedents. Further understanding of their etiology could inform targeted interventions to promote positive developmental outcomes.
Impact
-
Prior research on predictors of neonatal neurobehavior have included single-cohort studies, which limits generalizability of findings.
-
In a multi-cohort study of preterm and term-born infants, we found six distinct neonatal neurobehavioral profiles, with two profiles being identified as dysregulated.
-
Hypo- and hyper-aroused neurobehavioral profiles had distinct perinatal antecedents.
-
Understanding perinatal factors associated with dysregulated neurobehavior could help promote positive developmental outcomes.
Similar content being viewed by others
Introduction
Neonatal neurobehavior is an indicator of long-term developmental outcomes and may be particularly critical for infants at risk for poor outcomes due to adverse prenatal conditions or preterm birth.1,2,3 Identifying infants with atypical neurobehavioral patterns could be one way to screen infants who could benefit from early intervention and prevention services. Additionally, early identification of infants with atypical neurobehavioral patterns could help researchers and clinicians pinpoint which perinatal exposures impact very early neurobehavior.
The NICU Network Neurobehavioral Scale (NNNS) is a standardized neonatal neurobehavioral exam that assesses neurologic integrity, motor and behavioral functioning, and signs of stress and abstinence.4 The NNNS exam has been used in prior research with at-risk infants, including infants born premature,5,6,7,8 infants with prenatal substance exposure,1,9,10,11,12,13,14,15,16,17 and infants without any known risk factors.18,19,20 These prior studies have identified distinct neurobehavioral profiles, including two different dysregulated profiles (i.e., hyper- and hypo-aroused profiles). Both the hypo- and hyper-aroused profiles have been shown to be associated with long-term cognitive and behavioral outcomes.1,19,21
Prior studies in single cohorts have identified pre- and perinatal factors associated with dysregulated neurobehavior as measured by the NNNS. These factors include measures of neurological integrity (e.g., abnormal cranial ultrasound,22 brain volume20), prenatal medical complications,5 prenatal mood disorders,5 newborn medical morbidities (e.g., sepsis),6 demographic risk factors (e.g., low socioeconomic status [SES], minority race/ethnicity),6 and type and dose of substance exposure.10,12 However, nearly all prior studies have investigated these associations in single cohorts of infants with similar characteristics, such as full-term, healthy infants or infants born preterm. As such, it is unknown whether the same set of NNNS profiles would be observed in a large sample representing infants across the gestational age (GA) spectrum or whether pre- and perinatal factors are associated with neurobehavioral profiles across a heterogeneous cohort of infants. Expanding on prior single-cohort findings in a large, heterogeneous cohort allows for replication of prior work and an assessment of the stability of previously reported neurobehavioral patterns.
The current study describes NNNS profiles and examines socioeconomic, medical, and substance use variables as potential predictors of these profiles in a sample of newborns spanning the GA spectrum (i.e., 22–43 weeks). We leveraged data from the Environmental influences on Child Health Outcomes (ECHO) Program, a multi-cohort study funded by the National Institutes of Health that brings together diverse cohorts of infants with varying characteristics and exposures from around the United States (U.S.). Specifically, we analyzed data from five cohorts who collected neonatal NNNS data, including two cohorts of infants born very premature (<33 weeks GA).
Methods
Study population
The ECHO Program was launched in 2016 to investigate the influence of environmental exposures on child health and development.23,24,25 Of the 69 extant cohorts within ECHO (https://www.nih.gov/echo/pediatric-cohorts), five collected data on neonatal neurobehavior using the NNNS exam (Table 1). The current analyses include data from these five cohorts, which represent 10 recruitment sites and 1112 mother–infant pairs. Of the five cohorts, two were comprised of infants born very preterm (<33 weeks GA), and three were comprised of infants mostly born at term (≥37 weeks GA). This resulted in a sample of infants with wide-ranging GA (mean = 29.9 weeks, standard deviation [SD] = 5.26 weeks, range = 22–43 weeks). Overall, 67% were born at <30 weeks; 12% were born between 30 and 37 weeks; and 21% were born at 37 weeks or later. For each cohort contributing data, the local Institutional Review Board reviewed and approved all data collection procedures, and cohort participants provided written informed consent.
Measures
NICU Network Neurobehavioral Scale
The NNNS is a standardized newborn neurobehavioral assessment that examines neurological integrity, motor and behavioral functioning, and signs of stress and abstinence. It has previously been used in studies of at-risk and healthy infants1,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 with documented internal consistency and validity.26 The NNNS results in 12 summary scores indexing attention, handling, self-regulation, arousal, excitability, lethargy, hypertonicity, hypotonicity, non-optimal reflexes, asymmetric reflexes, quality of movement, and signs of stress and abstinence. Prior studies have used these summary scores to create discrete, mutually exclusive profiles of infants who share similar patterns of neurobehavior.1,6,7,18,19,20,27,28,29 We utilized latent profile analysis (LPA) to estimate these neurobehavioral profiles for infants included in this ECHO-wide analysis.
Maternal and neonatal variables
We examined associations between infants’ NNNS profile membership and several pre- and perinatal variables that were available and harmonized at the time of the analysis. These variables included maternal demographic, medical, and substance use characteristics and neonatal demographic and medical characteristics (Table 2). Maternal and neonatal variables were collected via standardized maternal interviews and medical record abstraction according to each cohort’s protocol. We utilized harmonized data from cohort-specific protocols along with data collected via the standard ECHO-wide protocol.24,30 Variable definitions are provided in Supplementary Table 1. Some variables were not included in final models due to multicollinearity (e.g., infant NICU status was redundant with cohort and GA) or other estimation issues (e.g., the unique effect of being large for gestational age was unable to be estimated due to low prevalence).
Statistical analysis
Statistical analyses proceeded in three steps. First, we used LPA to classify infants into mutually exclusive groups based on the 12 NNNS summary scores. We fit LPA models with different numbers of profiles and compared the fit of each model. To determine the best-fitting model, we used the following criteria: (1) the majority of solutions met statistical convergence criteria; (2) lower Bayesian Information Criteria (BIC) values indicated goodness of fit alongside model parsimony; (3) higher entropy and higher average class probabilities indicated high classification accuracy; (4) non-significant Lo–Mendell–Rubin (LMRT) and bootstrapped likelihood ratio (BLRT) tests indicated that a model with k profiles fit significantly better than a model with k−1 profiles; and (5) all profiles contained 5% or more of the sample. All LPA models were run in Mplus 8.8. Correlated residuals were allowed for summary scales that had items in common. Using the best-fitting LPA solution, we described the mean pattern of NNNS summary scores in each profile. A plot of mean scores by profile was generated to aid interpretation.
After identifying the latent NNNS profiles, we conducted univariate analyses using generalized estimating equations (GEEs) to compare individual neonatal and maternal characteristics across the profiles. Our GEE models included a logit link function to account for a categorical outcome. We accounted for nesting of children within the 10 recruitment sites using an exchangeable correlation matrix at the cohort-site level.
Finally, we conducted multivariate, adjusted models to determine the strongest predictors of NNNS profile membership. We performed multiple imputation (n = 20 datasets) to account for missing data in maternal and neonatal variables. Using the imputed datasets, we then ran a series of logistic GEE models to predict latent profile membership from the list of pre- and perinatal characteristics described earlier. As in the univariate models, we accounted for nesting of children within recruitment site. The results were pooled across imputation datasets prior to interpretation. In a final step, we entered interaction terms into the model to determine GA-specific correlates of NNNS profile membership. All interaction terms were entered simultaneously, and non-significant (p > 0.15) terms were removed from the model prior to interpretation. Significant interactions were probed at clinically relevant levels of GA.
Results
Descriptive statistics
Descriptive statistics for the sample are presented in Table 2. Overall, 80% of the infants were born preterm (<37 weeks GA; N = 886). Of the mothers, 6% identified as Asian, 25% as Black, 16% as Hispanic, 51% as White, and 8% as Other Race or Ethnicity. Most mothers (71%) were married, and approximately one quarter (28%) held a 4-year college degree. Obesity (33%) and pre-eclampsia (20%) were the most prevalent maternal medical problems. Additionally, 8.6% of mothers experienced depression; 7.7% experienced anxiety; and 15% used opioids during pregnancy.
NNNS profiles
We estimated LPA models with 1–7 profiles and compared model fit statistics (Table 3). A model with 6 profiles was determined to have the best fit based on a low BIC, high entropy, high average class probabilities, and a sufficient fraction in the smallest class (6% of the sample). The BLRT indicated that a model with 6 profiles fit significantly better than a model with 5 profiles. Despite the 7-profile solution having a lower BIC, a 6-profile solution was preferred due to convergence problems in the 7-profile solution. Entropy and average class probabilities were lower in the 7-profile solution compared with the 6-profile solution. In addition, the LMRT was not significant, and the BLRT was inconclusive due to convergence issues.
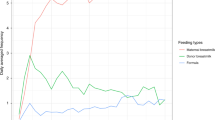
Using the results from the 6-profile solution, we next examined patterns of NNNS summary scores in each profile. Figure 1 shows the means of the standardized scores by profile while Table 4 shows the means and standard deviations of the NNNS summary scores by profile. Infants in profile 1 (blue in Fig. 1; 42%) had the lowest handling, excitability, and stress/abstinence scores. All remaining summary scores were approximately average. Infants in profile 2 (green in Fig. 1; 12%) had the highest attention and regulation scores. Infants in profile 3 (purple in Fig. 1; 16%) had average attention and regulation with somewhat elevated arousal and excitability. Infants in profile 4 (yellow in Fig. 1; 8%) required more handling, had below average regulation, and had elevated arousal and excitability. Infants in profiles 5 and 6 exhibited clinically significant dysregulated neurobehavioral phenotypes. Infants in profile 5 (orange in Fig. 1; 16%) were hypo-aroused. These infants had the highest lethargy, hypotonicity, and nonoptimal reflexes compared with all other profiles. Infants in profile 6 (black in Fig. 1; 6%) were hyper-aroused. These infants had the highest arousal and excitability, lowest regulation and quality of movement, and the most signs of stress/abstinence compared with all other profiles.
Predictors of NNNS profiles
Profiles 5 (hypo-aroused) and 6 (hyper-aroused) represent clinically relevant phenotypes given that NNNS scores fell approximately one SD above the mean for lethargy, hypotonicity, and nonoptimal reflexes (profile 5); at least one SD above the mean for handling, arousal, excitability, and stress abstinence (profile 6); and almost two SDs below the mean for regulation and quality of movement (profile 6). Therefore, subsequent analyses examined predictors of membership in these two dysregulated profiles compared with profiles 1-4, which contained infants with typical patterns of neurobehavior.
Descriptively, infants born premature were more likely to be in the hypo-aroused profile (17% vs 11%) but less likely to be in the hyper-aroused profile (4% vs 15%) compared with infants born full-term. This difference was only significant for the hyper-aroused profile (odds ratio [OR] = 0.33, 95% confidence interval [CI] = 0.15–0.74). Continuous GA was not associated with membership in the hypo- or hyper-aroused profile.
The results for the multivariate analyses adjusting for GA and all other neonatal and maternal characteristics are shown in the right-hand panel of Table 5. GA was not associated with profile membership after adjusting for all other predictors.
Adjusted analyses showed that boys were more likely to be in the hypo-aroused profile compared with girls (OR = 1.37; 95% CI = 1.14–1.64). Maternal age at birth was negatively associated with membership in the hypo-aroused profile (OR = 0.97, 95% CI = 0.94–0.99). Maternal depression was positively associated with membership in the hypo-aroused profile (OR = 1.51, 95% CI = 1.06–2.51).
Infants in the hyper-aroused profile were more likely to be of Hispanic/Latino ethnicity (OR = 1.67, 95% CI = 1.04–2.68). Similar to the hypo-aroused profile, maternal depression was associated with a greater likelihood of being in the hyper-aroused profile (OR = 2.50, 95% CI = 1.02–6.15). Finally, infants born to mothers who used tobacco prenatally were more likely to be in the hyper-aroused profile (OR = 1.73, 95% CI = 1.01–2.95).
Because the sample was primarily comprised of children born at <30 weeks GA, we ran sensitivity analyses where we replaced continuous GA with a dichotomous variable indicating whether infants were born at <30 weeks GA. Model findings were very similar. Infant sex, maternal age, and maternal depression continued to be associated with membership in the hypo-aroused profile. Hispanic/Latino ethnicity was associated with the hyper-aroused profile, and maternal depression and tobacco use were marginally associated (ps = 0.06). Full results from these sensitivity models are presented in Supp. Table 2.
A secondary investigation of GA-specific predictors indicated a significant interaction between continuous GA and opioid use (GA × Opioid: b = 0.15, p = 0.001). Opioid use was associated with an increased likelihood of being in the hypo-aroused profile but only for children born at a later GA. For example, opioid exposure was not significantly associated with an increased likelihood for infants born at 30 weeks GA (OR = 1.24; 95% CI = 0.80–1.94) but was associated with an increased likelihood for infants born at 37 weeks GA (OR = 3.52; 95% CI = 1.78–6.95).
Analysis of the hyper-aroused profile indicated a significant interaction between continuous GA and White race (GA × White race: b = 0.15, p = 0.001). No significant association was found between race and likelihood of being in the hyper-aroused profile for infants born at 30 weeks GA (OR = 0.74, 95% CI = 0.41–1.33), but White race was associated with a lower likelihood of being in the hyper-aroused profile for infants born at 27 weeks GA (OR = 0.47, 95% CI = 0.25–0.93).
Discussion
The current study aimed to identify neonatal neurobehavioral profiles and predictors of profile membership in a heterogeneous cohort of infants with different GAs and perinatal exposures. We found evidence for six distinct neurobehavioral profiles, with two dysregulated profiles similar to those reported in prior work. Infants in the hypo-aroused profile were lethargic and hypotonic, whereas children in the hyper-aroused profile were highly aroused and excitable, with poor regulation and quality of movement and many signs of stress and abstinence. We identified infant and maternal characteristics that were associated with membership in each of these two atypical profiles, with some effects that were contingent on infant GA.
Prior studies investigating neonatal neurobehavior using the NNNS have also identified the presence of hypo- and hyper-aroused profiles. For example, the hyper-aroused profile has been identified in samples of drug-exposed infants2,28,29 and term, healthy infants,18,19,20,27 with similar patterns of atypical scores for arousal, excitability, hypertonia, quality of movement, regulation, and stress signs. The hypo-aroused profile has also been seen in prior studies of term, healthy infants19,20,27 and premature infants,6,7 which is typically characterized by high lethargy and hypotonicity along with non-optimal reflexes. While the magnitude of extreme scores may vary based on the study population, it is striking that qualitatively similar profiles of infants have emerged across these different investigations. The current analyses of infants with different GAs and exposures confirm that these profiles can be observed across a range of infants who vary in terms of their risk for neurobehavioral impairment.
Distinguishing between different types of dysregulated neurobehavior is important for at least two reasons. First, understanding whether infants are hypo- or hyper-aroused could lead to the development of personalized interventions with strategies that are tailored to the specific needs of these infants (for example, teaching caregivers different strategies to support up- or down-regulation of arousal and excitability). Existing NICU interventions do not specifically target neurobehavioral dysregulation, nor does the standard of care in most NICUs involve a standardized neurobehavioral exam such as the NNNS. Thus this information could be used to improve upon existing interventions for high-risk neonates prior to and immediately following NICU discharge, especially since the efficacy of many existing interventions have not been robustly supported by randomized control trials.31,32 Second, infants with different types of dysregulated neurobehavior may be at risk for different downstream outcomes (e.g., cognitive versus behavioral problems, internalizing versus externalizing disorders), and appropriate supports following NICU discharge (e.g., early screening, referrals) could look different depending on these risks.
Predictors of atypical profiles included demographic, medical, and substance use variables. Infants in the hypo-aroused profile were more likely to be male, have mothers of a younger age, and have mothers who were depressed prenatally. Others have similarly reported a preponderance of males in hypotonic profiles,20,29 with hypotonia possibly representing neurodevelopmental immaturity. Suggestive links between maternal depression and hypotonic profiles and/or characteristics of the hypotonic profile (i.e., high lethargy) have also been reported.5,19,33,34 Links between maternal age and NNNS scores are novel. Older maternal age may be protective because of its association with greater educational and career attainment, financial and social resources, access to high quality care during pregnancy, social support during pregnancy, and socioemotional skills and maturity.35 However, these findings do not necessarily generalize to samples that would be considered of advanced maternal age given the relatively low proportion of women above age 35 (24%) or age 40 (7%) in the sample.
Infants in the hyper-aroused profile were more likely to be Hispanic/Latino and have mothers who were depressed or used tobacco prenatally. Sociodemographic factors, including minority race/ethnicity, have been associated with atypical NNNS scores.6,36 This association is likely due to the widespread, pernicious impacts of institutional racism in the U.S. For example, oppressed groups are afforded less access to healthy foods, experience greater financial and other stressors, and are exposed to more crime and pollution, all of which may affect fetal central nervous system development and newborn neurobehavior.37,38 Further, issues unique to members of the LatinX community, such as immigration-related stressors,39 may explain why we found a main effect of Hispanic/Latino ethnicity and not Black race. As discussed before, maternal depression has been associated with atypical neurobehavior, although these links have been more apparent for hypo- rather than hyper-arousal. Finally, tobacco use, either on its own or in combination with other prenatal drug exposures, has been shown to be associated with a greater likelihood of hyper-arousal in infants.1,29,40,41
Our sample was primarily comprised of premature infants, with small cell sizes for some GA categories and several prenatal and perinatal predictors within each latent profile. Therefore, we were limited in our ability to test GA-specific effects. However, our findings suggest that neurobehavioral effects associated with opioid exposure may be more pronounced among term infants than preterm infants. Indeed, prior studies have shown that the clinical presentation of neonatal opioid withdrawal syndrome (NOWS) is markedly different in preterm versus term infants42,43 to the extent that different NOWS diagnostic tools may be needed for preterm infants. For example, hyperexcitability is considered to be a hallmark presentation of NOWS, yet it may not be as common a presentation in preterm newborns with NOWS, whereas other symptoms such as poor feeding may be more common.42 Conversely, we found that maternal race was more predictive of neurobehavior in infants born at extremely low GA. These findings suggest that belonging to a non-oppressed racial/ethnic group may be protective for very premature infants, a trend that has been noted in the recent literature.44 It is likely that lower maternal stress,45 better access to high quality prenatal and neonatal healthcare,46 and relatively better socioeconomic position47 may provide some advantage for White premature infants.
Interestingly, no significant associations were observed between GA and NNNS profile membership after adjusting for potentially confounding infant and maternal characteristics. These findings suggest that factors associated with prematurity may predict profile membership rather than prematurity per se. Prior studies have found poorer neurodevelopment in preterm versus full-term infants based on NNNS summary scales, but these studies did not estimate NNNS profiles nor did they account for the wide number of factors considered in the present study.36,48 Follow-up multicohort studies with preterm and term-born children are needed to untangle the associations among prenatal risk factors, neonatal illness, and neonatal neurobehavior.
This study was unique in that it incorporated a large, heterogeneous sample of neonates—the largest ever investigated with the NNNS scale—with racial/ethnic, socioeconomic, and geographic diversity. However, several limitations remain. First, although a wide range of GAs were represented in the sample, there was a preponderance of very preterm infants and a relative dearth of term infants. Additionally, although we included a large number of risk factors, not all relevant medical and demographic factors were available. Because maternal health complications were exclusionary for some of the cohorts included in this analysis, we may have been biased toward underestimating the effect of some maternal medical problems on infant neurodevelopment. Because of the nature of the harmonizing efforts in the ECHO Program, some variables were collapsed across the immediate pre-pregnancy, pregnancy, and early postnatal periods. For example, prenatal opioid use was coded as present if there was any evidence of use from 3 months before to 6 months after the target pregnancy. Therefore, these variables are best understood as representing perinatal characteristics or exposures. Finally, although we found some associations between maternal mental health and child neurobehavioral profiles, insufficient data were available regarding psychiatric medication use; therefore, we could not examine the specific effects of different psychiatric medications (e.g., selective serotonin reuptake inhibitors).
In conclusion, we found evidence for two dysregulated profiles in a large sample of preterm and term infants drawn from a national consortium of cohorts. These hypo- and hyper-aroused profiles have clinical relevance and different perinatal antecedents. Given the links between these two dysregulated profiles and later cognitive and socioemotional outcomes,1,19,21 identifying the precursors associated with these neurobehavioral profiles in infants of varying GA could identify risk and inform practices to promote positive developmental outcomes starting at the very beginning of life. Clinicians could consider screening infants exposed to adverse prenatal conditions using a neurobehavioral exam such as the NNNS to aid in the infant’s care and discharge plan.
Data availability
The datasets for this manuscript are not publicly available because, per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public for review/analysis. Requests to access the datasets should be directed to the ECHO Data Analysis Center, [email protected].
References
Liu, J. et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics 125, e90–e98 (2010).
Stephens, B. E. et al. Neurobehavioral assessment predicts motor outcome in preterm infants. J. Pediatr. 156, 366–371 (2010).
Lester, B. M. et al. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics 124, 1355–1362 (2009).
Lester, B. M. & Tronick, E. Z. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113(Pt. 2), 634–640 (2004).
Hofheimer, J. A. et al. Psychosocial and medical adversity associated with neonatal neurobehavior in infants born before 30 weeks gestation. Pediatr. Res. 87, 721–729 (2020).
McGowan, E. C. et al. Sociodemographic and medical influences on neurobehavioral patterns in preterm infants: a multi-center study. Early Hum. Dev. 142, 104954 (2020).
Zhang, X. et al. The association of prenatal exposure to intensive traffic with early preterm infant neurobehavioral development as reflected by the NICU Network Neurobehavioral Scale (NNNS). Environ. Res. 183, 109204 (2020).
Zhang, X. et al. NICU-based stress response and preterm infant neurobehavior: exploring the critical windows for exposure. Pediatr. Res. https://doi.org/10.1038/s41390-022-01983-3 (2022).
Coyle, M. G. et al. Neurobehavioral effects of treatment for opiate withdrawal. Arch. Dis. Child. Fetal Neonatal Ed. 90, F73–F74 (2005).
Coyle, M. G. et al. Neonatal neurobehavior effects following buprenorphine versus methadone exposure. Addiction 107(Suppl. 1), 63–73 (2012).
Heller, N. A. et al. Neonatal abstinence syndrome: neurobehavior at 6 weeks of age in infants with or without pharmacological treatment for withdrawal. Dev. Psychobiol. 59, 574–582 (2017).
Velez, M. L. et al. Prenatal buprenorphine exposure and neonatal neurobehavioral functioning. Early Hum. Dev. 117, 7–14 (2018).
Velez, M. L., Jansson, L. M., Schroeder, J. & Williams, E. Prenatal methadone exposure and neonatal neurobehavioral functioning. Pediatr. Res. 66, 704–709 (2009).
Camerota, M. et al. Effects of pharmacologic treatment for neonatal abstinence syndrome on DNA methylation and neurobehavior: a prospective cohort study. J. Pediatr. 243, 21–26 (2022).
Lester, B. M. et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 110, 1182–1192 (2002).
Jones, H. E. et al. Infant neurobehavior following prenatal exposure to methadone or buprenorphine: results from the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Subst. Use Misuse 45, 2244–2257 (2010).
Jones, H. E. et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 363, 2320–2331 (2010).
Appleton, A. A. et al. Prenatal programming of infant neurobehaviour in a healthy population. Paediatr. Perinat. Epidemiol. 30, 367–375 (2016).
Sucharew, H. et al. NICU Network Neurobehavioral Scale profiles predict developmental outcomes in a low-risk sample. Paediatr. Perinat. Epidemiol. 26, 344–352 (2012).
Parikh, A. N. et al. Neonatal Intensive Care Unit Network Neurobehavioral Scale profiles in full-term infants: associations with maternal adversity, medical risk, and neonatal outcomes. J. Pediatr. https://doi.org/10.1016/j.jpeds.2022.04.016 (2022).
McGowan, E. C. et al. Analysis of neonatal neurobehavior and developmental outcomes among preterm infants. JAMA Netw. Open 5, e2222249 (2022).
Helderman, J. et al. Association of abnormal findings on neonatal cranial ultrasound with neurobehavior at neonatal intensive care unit discharge in infants born before 30 weeks’ gestation. JAMA Netw. Open 5, e226561 (2022).
Blackwell, C. K., Wakschlag, L. S., Gershon, R. C. & Cella, D. Measurement framework for the Environmental influences on Child Health Outcomes research program. Curr. Opin. Pediatr. 30, 276–284 (2018).
Gillman, M. W. & Blaisdell, C. J. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr. Opin. Pediatr. 30, 260–262 (2018).
Jacobson, L. P., Lau, B., Catellier, D. & Parker, C. B. An Environmental influences on Child Health Outcomes viewpoint of data analysis centers for collaborative study designs. Curr. Opin. Pediatr. 30, 269–275 (2018).
Lester, B. M., Tronick, E. Z. & Berry Brazelton, T. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 113(Suppl. 2), 641–667 (2004).
Lesseur, C., Paquette, A. G. & Marsit, C. J. Epigenetic regulation of infant neurobehavioral outcomes. Med. Epigenet. 2, 71–79 (2014).
Czynski, A. J. et al. Neurodevelopmental outcomes of neonates randomized to morphine or methadone for treatment of neonatal abstinence syndrome. J. Pediatr. 219, 146.e1–151.e1 (2020).
Wouldes, T. A. & Woodward, L. J. Neurobehavior of newborn infants exposed prenatally to methadone and identification of a neurobehavioral profile linked to poorer neurodevelopmental outcomes at age 24 months. PLoS ONE 15, e0240905 (2020).
LeWinn, K. Z. et al. SPR perspectives: Environmental influences on Child Health Outcomes (ECHO) Program: overcoming challenges to generate engaged, multidisciplinary science. Pediatr. Res. https://doi.org/10.1038/s41390-021-01598-0 (2021).
Pineda, R. et al. Randomized clinical trial investigating the effect of consistent, developmentally-appropriate, and evidence-based multisensory exposures in the NICU. J. Perinatol. 41, 2449–2462 (2021).
Ohlsson, A. & Jacobs, S. E. NIDCAP: a systematic review and meta-analyses of randomized controlled trials. Pediatrics 131, e881–e893 (2013).
Marcus, S. et al. Depressive symptoms during pregnancy: Impact on neuroendocrine and neonatal outcomes. Infant Behav. Dev. 34, 26–34 (2011).
Stroud, L. R., McCallum, M. & Salisbury, A. L. Impact of maternal prenatal smoking on fetal to infant neurobehavioral development. Dev. Psychopathol. 30, 1087–1105 (2018).
Duncan, G. J., Lee, K. T. H., Rosales-Rueda, M. & Kalil, A. Maternal age and child development. Demography 55, 2229–2255 (2018).
Fink, N. S., Tronick, E., Olson, K. & Lester, B. Healthy newborns’ neurobehavior: norms and relations to medical and demographic factors. J. Pediatr. 161, 1073.e3–1079.e3 (2012).
Taylor, J. K. Structural racism and maternal health among Black women. J. Law Med. Ethics 48, 506–517 (2020).
Kingsbury, A. M. et al. Social adversity in pregnancy and trajectories of women’s depressive symptoms: a longitudinal study. Women Birth 31, 52–58 (2018).
Kingston, D. et al. Comparison of maternity experiences of canadian-born and recent and non-recent immigrant women: findings from the Canadian Maternity Experiences Survey. J. Obstet. Gynaecol. Can. 33, 1105–1115 (2011).
Law, K. L. et al. Smoking during pregnancy and newborn neurobehavior. Pediatrics 111, 1318–1323 (2003).
Stroud, L. R. et al. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics 123, e842–e848 (2009).
Song, G. & Pak, V. M. Understanding the effects of prematurity on clinical manifestations of neonatal abstinence syndrome: a narrative literature review. J. Neonatal Nurs. 26, 319–323 (2020).
Ruwanpathirana, R. et al. Prematurity reduces the severity and need for treatment of neonatal abstinence syndrome. Acta Paediatr. 104, e188–e194 (2015).
Wallace, M. E. et al. Racial/ethnic differences in preterm perinatal outcomes. Am. J. Obstet. Gynecol. 216, 306.e1–306.e12 (2017).
Borders, A. E. B. et al. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J. Perinatol. 35, 580–584 (2015).
Willer, B. L. & Nafiu, O. O. Racial and ethnic disparities in NICU care practices. Pediatrics 148, e2021051298 (2021).
Wong, H. S. & Edwards, P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Matern. Child Health J. 17, 1689–1700 (2013).
Spittle, A. J. et al. Neurobehaviour and neurological development in the first month after birth for infants born between 32-42 weeks’ gestation. Early Hum. Dev. 96, 7–14 (2016).
Acknowledgements
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators: ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Catellier DJ; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D. ECHO Awardees and Cohorts—Icahn School of Medicine at Mount Sinai, New York, NY: Teitelbaum SL; Emory University, Atlanta, GA: Dunlop A; University of Utah, Salt Lake City, UT: Stanford J.
Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), UH3OD023320 (J.A.), UH3OD023318 (Dunlop), UH3OD023275 (M.R.K.), UH3OD023347 (B.M.L.), UH3OD023249 (Stanford), UH3OD023348 (T.M.O.). M.C. was additionally supported by a career development award from the National Institute of Mental Health (NIMH), grant K01MH129510 (M.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception and design of study: M.C., B.M.L. Acquisition of data: J.A., A.S., M.R.K., E.C., S.E.C., P.A.B., B.S.C., J.C., S.A.D., J.B.H., C.R.N., S.L.P., L.M.S. Analysis and interpretation of data: M.C., J.R.K. Drafting the article: M.C., B.M.L. Revising article critically for important intellectual content: M.C., E.C.M., J.A., A.S., M.R.K., E.C., S.E.C., P.A.B., B.S.C., J.C., L.M.D., T.M.E., J.B.H., J.A.H., J.R.K., C.M.L., C.J.M., C.R.N., M.O.S., S.L.P., S.J.S., L.M.S., X.Z., B.M.L. Final approval of the version as submitted: M.C., E.C.M., J.A., A.S., M.R.K., E.C., S.E.C., P.A.B., B.S.C., J.C., L.M.D., S.A.D., T.M.E., J.B.H., J.A.H., J.R.K., C.M.L., C.J.M., C.R.N., M.O.S., S.L.P., S.J.S., L.M.S., X.Z., B.M.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by local Institutional Review Boards, and participants gave informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camerota, M., McGowan, E.C., Aschner, J. et al. Prenatal and perinatal factors associated with neonatal neurobehavioral profiles in the ECHO Program. Pediatr Res 94, 762–770 (2023). https://doi.org/10.1038/s41390-023-02540-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02540-2
This article is cited by
-
Neurobehavioral outcomes of preterm infants: toward a holistic approach
Pediatric Research (2024)
-
Optimal presence: enhancing parent integration to maximize neurodevelopmental outcomes in preterm infants
Pediatric Research (2024)
-
The evolving model of pediatric research
Pediatric Research (2023)