Abstract
Background
To analyze the clinical characteristics and outcomes of children with severe neurological symptoms associated with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection during the Omicron pandemic in China.
Methods
This study used a questionnaire to obtain data from pediatric intensive care unit (PICU) centers in seven tertiary hospitals in Northeast China from December 1, 2022, to January 31, 2023.
Results
A total of 255 patients were confirmed to have SARS-CoV-2 infection, and 45 patients (17.65 %) were included in this study. Of these, seven (15.6%) patients died, and the median time from admission to death was 35 h (IQR, 14–120 h). Twenty (52.6%) survivors experienced neurological sequelae. Patients with platelet counts lower than 100 × 109/L had a higher incidence of complications such as multiple organ dysfunction, mechanical ventilation rate, and mortality. Cranial magnetic resonance imaging (MRI) always reveals cerebral tissue edema, with some severe lesions forming a softening site.
Conclusion
Children infected with SARS-CoV-2 often exhibit severe neurological symptoms, and in some cases, they may rapidly develop malignant cerebral edema or herniation, leading to a fatal outcome. An early decrease in platelet count may associated with an unfavorable prognosis.
Impact
-
Since early December 2022, China has gradually adjusted its prevention and control policy of SARS-CoV-2; Omicron outbreaks have occurred in some areas for a relatively short period. Due to the differences in ethnicity, endemic strains and vaccination status, there was a little difference from what has been reported about children with SARS-CoV-2 infection with severe neurological symptoms in abroad. This is the first multicenter clinical study in children with nervous system involvement after acute SARS-CoV-2 infection in China, and helpful for pediatricians to have a more comprehensive understanding of the clinical symptoms and prognosis of such disease.
Similar content being viewed by others
Introduction
The SARS-CoV-2 variant B.1.1.529 was first reported in South Africa on November 24, 2021, and was later named the “Omicron” variant. With more than 30 mutations in its spike protein, this mutant strain rapidly triggered a significant global pandemic. Studies have shown that the Omicron variant is more infectious than other SARS-CoV-2 variants, although its clinical symptoms are relatively mild.1,2
Since early December 2022, China gradually adjusted its prevention and control policy for SARS-CoV-2; the Omicron variant caused sporadic outbreaks in certain regions for a limited duration, leading to a high number of reported cases. In addition to pulmonary symptoms, some newly infected children presented with severe neurological symptoms, such as continuous convulsions, disturbances of consciousness, hemiplegia, and delayed paralysis. The pathophysiology of COVID-19-related neurological involvement remains unclear and mainly includes the following three aspects: (1) SARS-CoV-2 may directly infect the central nervous system via the blood–brain barrier or through the cerebral nerves in a trans-synaptic manner;3,4,5 (2) SARS-CoV-2 can activate human macrophages, inducing inflammatory storms and immune-related damage;6,7 and (3) SARS-CoV-2 can cause hypercoagulability and multiple organ dysfunction (MODS), leading to neurological complications.8,9,10
This study retrospectively collected information on all pediatric intensive care unit (PICU) patients in Northeast China from December 2022 to January 2023, screened patients with critical neurological diseases related to COVID-19, and analyzed their clinical characteristics, imaging data, treatment, and outcomes. This study aimed to improve the ability of pediatricians to recognize neurological complications caused by the Omicron variant of COVID-19.
General information and methods
Data collection
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University (Approval No. 2023PS434K), and patient consent was not required because of the retrospective nature of this study. A questionnaire was used to collect data from PICU centers in seven tertiary hospitals in Northeast China (Shengjing Hospital of China Medical University, Shengyang Children’s Hospital, Dalian Women and Children’s Medical Group, The First Hospital of Jilin University, Changchun Children’s Hospital, Harbin Children’s Hospital, and Inner Mongolia Medical University Affiliated Hospital). Data were collected from patients admitted to these PICUs from December 1, 2022, to January 31, 2023. The questionnaire is presented in Supplementary File 1.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) age 28 days to ≤18 years; (2) positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) and/or antigen/antibody test; (3) admission with primary neurological symptoms, including drowsiness, severe headache, convulsions, disturbance of consciousness, hemiplegia, or delayed paralysis, among others; and (4) a Glasgow Coma Scale (GCS) score ≤ 13 within 24 h of admission.
The exclusion criteria were as follows: (1) laboratory examination (pathogen culture, RT-PCR, antibody test, and/or antigen test) reminiscent of a recent infection with other agents that may cause severe neurological illness; and (2) some non-infectious diseases that may cause neurological symptoms, such as febrile convulsion, neuroinflammatory diseases, poisoning, metabolic disease, cerebrovascular malformation, hyperventilation, and trauma.
Quality assessment
Data were evaluated by two researchers (C.F.L. and T.Z.), and any doubts regarding the data were checked and confirmed at least twice by the reporting unit. Patients who did not meet the inclusion criteria were also excluded (Fig. 1).
Grouping and statistical analysis
Based on patient prognosis, we divided them into survivor and death groups for comparison. To explore valuable early laboratory indicators, patients were grouped according to platelet (PLT) levels (PLT < 100 × 109/L or PLT ≥ 100 × 109/L) within 24 h of admission, and disease severity and prognosis were compared between the groups. Neurological sequelae were defined as newly acquired neurological dysfunction associated with SARS-CoV-2 infection (newly acquired speech or motor dysfunction; Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC) scores worsened compared to admission).
The count data are expressed as percentages (%). The chi-squared test was used to compare groups, and Fisher’s exact test was used for qualitative data that could not be analyzed using the chi-squared test. Quantitative data are expressed as mean ± standard deviation. The Kruskal–Wallis test was used to compare differences between the data groups. SPSS software (v17.0; SPSS Inc., Chicago, Ill.) was used for statistical analysis. Statistical significance was set at P < 0.05.
Results
Patient characteristics
A total of 605 patients were admitted to the seven PICUs between December 1, 2022, and January 31, 2023, of whom 255 (42.15%) were confirmed to have SARS-CoV-2 infection and 45 (17.65%) had severe neurological symptoms associated with SARS-CoV-2 infection (Fig. 1). Details of the data are provided in Supplementary File 2. Among the 45 patients, 26 were male, the median age was 38 months, and the median body weight was 15.9 kg. Only eight patients received SARS-CoV-2 vaccination, and the median time from vaccination to admission was 9 months (IQR, 9–12 months). Furthermore, 39 (86.7%) patients were healthy before admission, and the median time that patients had experienced symptoms before admission was 24 h. The median GCS score within 24 h of admission was 7 points. At the conclusion of the study, 7 individuals (15.6%) died, and 5 of those deaths were attributed to cerebral hernia. Of the 38 survivors, 20 (52.6%) experienced neurological sequelae.
Laboratory results showed that the median white blood cell (WBC) count was 6.4 × 109/L, and the proportion of neutrophil granulocytes was 68.1%. The C-reactive protein (CRP) concentration was 3.55 mg/L, while the procalcitonin (PCT) level was 1.21 ng/mL, and the PLT count was 168 × 109/L. Besides affecting the nervous system, this mutant strain also readily impacted the liver, myocardium, and coagulation systems, particularly in cases resulting in fatality. Patients in the death group had higher levels of PCT and blood lactic acid and lower PLT counts than did those in the survivor group (Table 1).
Lumbar puncture was performed in 35 patients, and the median time from lumbar puncture to admission was 4 h. Owing to the rapid progression of the disease and cerebral hernia, only 2 patients in the death group underwent lumbar puncture. The median pressure of cerebrospinal fluid (CSF) was 13 mmHg (IQR, 10–20 mmHg), and most CSF tests were unremarkable. Only two patients had elevated CSF-WBC counts (not more than 50 × 106/L), and 13 (37.1%; maximum, 7.63 g/L) patients had elevated CSF-protein levels. In the original 10 patients, a thorough re-examination was conducted for lumbar puncture; their CSF-WBC count and protein levels were near normal.
Of the 45 patients, 11 had PLT counts < 100 × 109/L within 24 h of admission. When compared with the higher PLT count (≥100 × 109/L) group, we found that the lower PLT group had a higher risk of complications with MODS, higher rate of mechanical ventilation (MV), higher incidence of mortality, and higher rate of experienced neurological sequelae (Table 2).
Neurological imaging
Forty (88.9%) patients underwent cranial computed tomography (CT) in the early stages of the disease; half of the CT scans showed abnormal findings. Reduced parenchymal density and ventricle compression were the most common pathological changes, suggesting severe edema of the cerebral tissue.
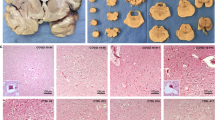
Most patients (38 cases) underwent MRI during hospitalization; diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) sequences were helpful for early detection of lesions (19 had DWI sequence alone, 7 had FLAIR sequence alone, and 12 had both sequences). Approximately 25 (65.8%) cases showed abnormalities on MRI (14 had abnormal DWI alone, three had abnormal FLAIR alone, eight had abnormalities on both sequences). Only one patient showed a hemorrhage lesion, others were characterized by multiple site and lobe edema, and we did not find arterial ischemic stroke or cerebral sinovenous thrombosis change. Lesions appeared at various locations in the brain tissue, including the bilateral para-ventricular, basal ganglia, frontal, parietal, and occipital lobes, cerebellum, and brain stem. Some MRIs showed symmetrical necrotic encephalopathy-like changes,11,12 with some severe lesions leading to the formation of softened areas (Fig. 2). Thirty patients underwent ambulatory electroencephalogram monitoring, and 24 (80%) showed abnormalities. The most notable abnormal manifestations included slow-wave activity, spike-and-wave complexes, and sharp-and-wave complex discharges, with the frequency of these abnormal patterns diminishing as the patient’s condition improved. Patients with neurological sequelae required long-term rehabilitation training, including speech and motor functions.
a Case 4, survival, 190 h after symptoms, MRI appeared with multiple edema lesions, including bilateral para-ventricular, basal ganglia, frontal, parietal, occipital, cerebellum and brain stem. b Case 14, survival, 10d after symptoms, MRI appeared with multiple lesions of cells edema, including left temporal lobe, bilateral ventricles, and semi-oval center. c Case 20(C1–C2), Case 36(C3–C5), all survived. Head CT and MRI showed symmetrical concentric circle lesions of bilateral thalamus in the early stage of disease. d Case 23, dead, 22 h after symptoms, CT scan showed brain parenchymal density decreased, the border between gray and white tissues was not clear, and a few spot-like high-density shadows were seen in bilateral brain; cerebral sulcus, cerebral fissure and cistern were narrowed; brain stem thickened, foramen magnum hernia was suspected. e Case 37, survival, 15 h after symptoms, MRI showed cerebral cortex extensively edema, bilateral basal ganglia and thalamic region swelling, bilateral ventricle enlarged. f Case 24, survival, F1–F2(40 h after symptoms), CT showed multiple low-density shadows in bilateral thalamus, lateral ventricle and left semi-oval center; F3–F4(6d after symptoms) MRI showed multiple lesions in bilateral thalamus and left frontal and parietal lobes; F5–F6(28d after symptoms) MRI showed some severe lesions forming a softening site. g Case 39, survival, 27 h after symptoms, MRI showed brain cortical diffused edema, bilateral basal ganglia and thalamic regions swelling.
Therapeutics
The questionnaire also collected information about the treatment. For example, 43 patients were treated with 20% mannitol and/or hypertonic saline to reduce the intracranial pressure. However, methods for monitoring intracranial and cerebral perfusion pressure are not available during treatment. Thirty-nine patients were treated with glucocorticoids, and the dosage and course of treatment varied greatly between centers. The range of the initial dose converted to methylprednisolone was 4–20 [mg/kg]/day, and 19 patients (48.7%) received an initial dose of >10 [mg/kg]/day. The course of glucocorticoid treatment ranged from one to three weeks. Human immunoglobulin was administered to 35 patients, while nine patients underwent plasma exchange, with five of them receiving more than two sessions of plasma exchange. Seven patients were treated with continuous renal replacement therapy (CRRT) for renal dysfunction, and the longest treatment duration was 114 h. MV support was provided to 25 (55.6%) patients, and the duration of MV therapy was 140 h.
Owing to the lack of pharmaceutical therapeutic indications and experience in treating children, antiviral drugs for SARS-CoV-2 have rarely been used in children in China. In our investigation, some patients were treated with other antiviral drugs, such as acyclovir (8 patients), adenosine monophosphate (7 patients), and interferon (6 patients). Twenty-four patients did not receive any antiviral treatment.
Discussion
At the time of writing this article, nearly 700 million people worldwide had been infected with SARS-CoV-2 since the acute COVID-19 outbreak in 2019, resulting in approximately 4 million deaths. Existing epidemiological data have shown that children are less susceptible to SARS-CoV-2 infection than adults and often display milder symptoms.13,14,15,16,17 However, the incidence and diversity of neurological complications associated with SARS-CoV-2 infection in children are much higher than expected, and the disease distribution is significantly different from that in adults.18,19,20 Two large clinical studies from the United States of America and the United Kingdom showed that the incidence of severe neurological illness associated with SARS-CoV-2 infection in children and adolescents was 2.5–3.8%, and approximately 40% of survivors had remaining neurological sequelae. Most cases occurred weeks or months after acute infection with SARS-CoV-2 (a multisystem inflammatory syndrome in children).21,22
In comparison to MRI, cranial CT allows for more accessible early screening of life-threatening conditions, such as severe brain edema or cerebral herniation. Variations exist between our findings and the research published in JAMA Neurology by LaRovere et al. in 2022,22 especially regarding neurological presentations. These discrepancies may stem from two potential reasons. First, LaRovere et al. surveyed all children and adolescents with COVID-19 in the United States during a nine-month period in 2020, utilizing a public health registry. They collected data of 365 patients with documented neurologic involvement; of these, 43 developed life-threatening conditions clinically adjudicated to be associated with COVID-19, including severe encephalopathy (n = 15), stroke (n = 12), central nervous system infection/demyelination (n = 8), Guillain-Barré syndrome/variants (n = 4), and acute fulminant cerebral edema (n = 4). The small sample size of our study and an entirely different screening procedure may have contributed to these differences. Second, owing to the differences in epidemic prevention and control policies between China and the United States, all our patients were recently infected with SARS-CoV-2. In the study by LaRovere et al., 20 out of 43 patients (47%) received a diagnosis of multisystem inflammatory syndrome in children (MIS-C), and among these cases, a proportion exhibited neurological changes such as stroke or demyelination. In general, our study collected the data from patients with SARS-CoV-2 infection recently, while the population in the study by LaRovere et al. also included a large number of post-COVID-19 syndrome patients; the difference was pronounced in the age distribution: 12 years (IQR, 7–15 years) in the study by LaRovere et al. vs. 38 months (IQR, 23–108.5 months) in our study.
Some studies have shown that PLT counts dynamically reflect the pathophysiological changes in COVID-19 patients. Early reduction in PLT was associated with mortality in patients with COVID-19.23,24 This phenomenon is partially because of the participation of PLT in thrombotic and thromboembolic events in COVID-19 patients.25,26 Referring to previous studies,27,28 we set the PLT to less than 100 × 109/L as a cut-off point and divided the patients into two groups. PLT count < 100 × 109/L in the early stage may be a risk factor in children with severe neurological symptoms associated with SARS-CoV-2 infection.
Nonetheless, it is vital to acknowledge the limitations of this study. First, this was a retrospective study that used a questionnaire to collect clinical data from different institutions. Although we arranged for two experts for quality control, selection bias was unavoidable. Second, owing to the lack of relevant nationwide data, we only analyzed data from seven PICUs in northeastern China and did not obtain data from other parts of the region and other primary medical institutions. Finally, this study only analyzed patients with severe neurological conditions during the acute phase of SARS-CoV-2 infection. Other neurological diseases related to previous SARS-CoV-2 infections, such as autoimmune encephalitis and MIS-C, were not included in our study.
As SARS-CoV-2 continues to mutate and worldwide prevention and control policies adapt, the virus will likely persist for an extended period, occasionally leading to outbreaks akin to those observed with other viruses.29 The present study showed that even with the relatively mild Omicron variant, children also have a certain chance of complications with severe neurological conditions, possibly death due to malignant cerebral edema or hernia. It is worth noting that among these 45 patients, only 8 had received SARS-CoV-2 vaccination, and the time from vaccination to admission was 6 months to 1 year. Learning from successful experiences in influenza virus prevention and control,30,31 the discussion of whether SARS-CoV-2 vaccination should be incorporated into regular childhood vaccination schedules is a matter that warrants careful consideration by healthcare decision-making bodies.
Conclusions
Children infected with SARS-CoV-2 tend to present severe neurological complications. In addition to the nervous system, the liver, myocardium, and coagulation systems are readily involved. Owing to the rapid progression of the disease, certain patients may experience acute fulminant cerebral edema, leading to fatal outcome. Additionally, over half of the survivors may endure neurological sequelae. Early decrease in PLT counts (PLT < 100 × 109/L) may be a risk factor in children with nervous system involvement after acute SARS-CoV-2 infection.
Data availability
All authors agreed that the data and materials mentioned in this article are true and availability. The datasets used and/or analyzed during the current study available from the appendix.
References
Wang, L. et al. Comparison of outcomes from covid infection in pediatric and adult patients before and after the emergence of omicron. medRxiv https://doi.org/10.1101/2021.12.30.21268495 (2022).
Viana, R. et al. Rapid epidemic expansion of the SARS-cov-2 omicron variant in southern africa. Nature 603, 679–686 (2022).
Lin, J. E. et al. Neurological issues in children with covid-19. Neurosci. Lett. 743, 135567 (2021).
Schober, M. E., Pavia, A. T. & Bohnsack, J. F. Neurologic manifestations of covid-19 in children: emerging pathophysiologic insights. Pediatr. Crit. Care Med. 22, 655–661 (2021).
Lewis, A. et al. Cerebrospinal fluid in covid-19: a systematic review of the literature. J. Neurol. Sci. 421, 117316 (2021).
Stafstrom, C. E. & Jantzie, L. L. Covid-19: neurological considerations in neonates and children. Children 7. https://doi.org/10.3390/children7090133 (2020).
Franke, C. et al. High frequency of cerebrospinal fluid autoantibodies in covid-19 patients with neurological symptoms. Brain Behav. Immun. 93, 415–419 (2021).
Nordvig, A. S. et al. Potential neurologic manifestations of covid-19. Neurol. Clin. Pract. 11, e135–e146 (2021).
Smadja, D. M. et al. Covid-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis 24, 755–788 (2021).
Thakur, K. T. et al. Covid-19 neuropathology at columbia university irving medical center/new york presbyterian hospital. Brain 144, 2696–2708 (2021).
Mizuguchi, M. et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J. Neurol. Neurosurg. Psychiatry 58, 555–561 (1995).
Michael, B. D. et al. Consensus clinical guidance for diagnosis and management of adult COVID-19 encephalopathy patients. J. Neuropsychiatry Clin. Neurosci. 35, 12–27 (2023).
Garcia-Vera, C. et al. Covid-19 in children: clinical and epidemiological spectrum in the community. Eur J. Pediatr. 181, 1235–1242 (2022).
Cui, X. et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (covid-19). J. Med. Virol. 93, 1057–1069 (2021).
Moreira, A. et al. Demographic predictors of hospitalization and mortality in us children with covid-19. Eur. J. Pediatr. 180, 1659–1663 (2021).
Ranabothu, S. et al. Spectrum of covid-19 in children. Acta Paediatr. 109, 1899–1900 (2020).
Lee, P. Y. et al. Distinct clinical and immunological features of SARS-cov-2-induced multisystem inflammatory syndrome in children. J. Clin. Investig. 130, 5942–5950 (2020).
Panda, P. K. et al. Neurological complications of SARS-cov-2 infection in children: a systematic review and meta-analysis. J. Trop. Pediatr. 67. https://doi.org/10.1093/tropej/fmaa070 (2021).
Nepal, G. et al. Neurological manifestations of covid-19 associated multi-system inflammatory syndrome in children: a systematic review and meta-analysis. J. Nepal Health Res. Counc. 19, 10–18 (2021).
Abdel-Mannan, O. et al. Neurologic and radiographic findings associated with covid-19 infection in children. Jama Neurol. 77, 1440–1445 (2020).
Ray, S. T. J. et al. Neurological manifestations of SARS-cov-2 infection in hospitalised children and adolescents in the uk: a prospective national cohort study. Lancet Child Adolesc. Health 5, 631–641 (2021).
LaRovere, K. L. et al. Neurologic involvement in children and adolescents hospitalized in the united states for covid-19 or multisystem inflammatory syndrome. Jama Neurol. 78, 536 (2021).
Xiao, L., Ran, X., Zhong, Y. & Li, S. Clinical value of blood markers to assess the severity of coronavirus disease 2019. BMC Infect. Dis. 21, 1471–2334 (2021).
Zhao, X. et al. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients-indications for predictive, preventive, and personalized medical approach. EPMA J. 11, 139–145 (2020).
Gorog, D. A. et al. Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 19, 475–495 (2022).
Overmyer, K. A. et al. Large-scale multi-omic analysis of COVID-19 severity. Cell Syst. 12, 23–40 (2021).
Liao, D. et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 7, e671–e678 (2020).
Henry, B. M., de Oliveira, M., Benoit, S., Plebani, M. & Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 58, 1021–1028 (2020).
COVID-19 Forecasting Team. Past SARS-cov-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet 401, 833–842 (2023).
Tenforde, M. W. et al. Vaccine effectiveness against influenza-associated urgent care, emergency department, and hospital encounters during the 2021-2022 season, vision network. J. Infect. Dis. https://doi.org/10.1093/infdis/jiad015 (2023).
Adams, K. et al. Prevalence of SARS-cov-2 and influenza coinfection and clinical characteristics among children and adolescents aged <18 years who were hospitalized or died with influenza - united states, 2021-22 influenza season. MMWR Morb. Mortal Wkly Rep. 71, 1589–1596 (2022).
Funding
This work was supported by the Major science and technology Project of Liaoning Province (No. 2020JH1/10300001 to Chun-Feng Liu); the Natural Science Foundation of Liaoning Province (No. 2023-MS-191 to T.Z.), and 345 Talent Project of Shengjing Hospital of China Medical University (C.-F.L. and T.Z.).
Author information
Authors and Affiliations
Contributions
T.Z., and C.F.L, conceived and designed the study. T.Z., Q.F.Z., H.M.Y., P.L., P.S., Y.M.L., Z.Z., Y.Z.H., X.Y.Y., Q.Q.G.C.L.M. and Q.S., collected all the data. T.Z. analyzed the data. All authors participated in drafting or revising of the manuscript, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University (2023PS434K), and the patient consent was not required in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Zhang, QF., Yang, HM. et al. Children with severe neurological symptoms associated with SARS-CoV-2 infection during Omicron pandemic in China. Pediatr Res 95, 1088–1094 (2024). https://doi.org/10.1038/s41390-023-02904-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02904-8
This article is cited by
-
Neurological manifestations and risk factors associated with poor prognosis in hospitalized children with Omicron variant infection
European Journal of Pediatrics (2024)