Abstract
Background
Hutchinson-Gilford progeria syndrome (HGPS) and progeroid laminopathies (PL) are extremely rare genetic diseases with extremely poor prognoses. This study aims to investigate the epidemiological and genotypic characteristics of patients with HGPS/PL in China.
Methods
Using a cross-sectional study design, general characteristics and genotypic data of 46 patients with HGPS/PL from 17 provinces in China were analyzed.
Results
Among the 46 patients with HGPS/PL, 20 patients are HGPS, and the rest are PL; the identified total prevalence of HGPS/PL is 1/23 million. Among 42 patients with gene reports, 3 carried compound heterozygous mutations in the ZMPSTE24 while the other 39 carried LMNA mutations. Among PL, LMNA c.1579 C > T homozygous mutation was the most common. The onset of classic genotype HGPS is skin sclerosis in the first month after birth. The primary clinical manifestations of PL patients include skin abnormalities, growth retardation, and joint stiffness. The median age of onset for PL was 12 (6,12) months.
Conclusions
In China, the identified total prevalence of HGPS/PL is 1/23 million. 92.8% of the genetic mutations of HGPS/PL were located in LMNA, and the rest in ZMPSTE24. Most patients of HGPS/PL have skin abnormalities as the earliest manifestation. Compared to PL, the classic genotype HGPS starts earlier.
Impact statement
-
Hutchinson-Gilford progeria syndrome (HGPS) and progeroid laminopathies (PL) are extremely rare genetic diseases with extremely poor prognoses. To date, there is a paucity of epidemiological data related to HGPS/PL in China.
-
This study first examined the genotypic, phenotypic, and prevalence characteristics of 40–50% of the cases of HGPS/PL in mainland China through a collaborative international registry effort.
-
In China, the identified total prevalence of HGPS/PL is 1/23 million. 92.8% of the genetic mutations of HGPS/PL are located in LMNA.
-
LMNA c.1579 C > T homozygous mutations are the most common form of gene mutations among the Chinese PL population.
Similar content being viewed by others
Introduction
Hutchinson-Gilford progeria syndrome (HGPS) and progeroid laminopathies (PL) are extremely rare genetic diseases.1 HGPS has a birth incidence of approximately 1/4-1/8 million, with no sex or racial predilection. The Progeria Research Foundation (PRF) calculates the point prevalence of HGPS at 1 in 18 million to 1 in 20 million living individuals, with an estimated worldwide case number of 350–400.2 This is determined by extrapolating from identified cases from the United States of America (USA) contained in the PRF International Progeria Registry since the USA case capture is considered complete in the USA.2
The pathogenic mechanism of HGPS/PL is mainly due to mutations of lamin A(LMNA) genes3,4 or other regulatory genes in the lamin A synthesis pathway (e.g., Zmpste24). Generally, all diseases resulting from the synthesis of abnormal nuclear lamin A proteins or the Zmpste24 lamin A processing protein can be referred to as PL,5 while HGPS is a disease resulting from over-production of the disease-causing abnormal lamin A protein called progerin. HGPS is caused by single base mutations that enhance the use of an internal splice site within exon 11. Classic genotype HGPS (90% of cases) stems from an LMNA exon 11 mutation (c.1824C > T ; p.G608G); non-classic genotype HGPS (10% of cases) stems from several different mutations in LMNA exon 11 or intron 11 splice site region, all of which generate progerin.
Both HGPS and PL are characterized by the premature and rapid onset of natural aging as their distinguishing clinical features.1 The main symptoms of HGPS include sclerodermatous skin changes, failure to thrive, alopecia, loss of subcutaneous fat, progressive joint contractures, bone dysplasia, nail dystrophy, tooth dysplasia, and progressive development of conductive hearing loss,6,7 insulin resistance, and progressive atherosclerosis leading to strokes and heart failure. Without progerin-targeted therapy, the average lifespan of patients with classic genotype HGPS is only 14.5 years, but death has occurred at as young an age as 4 years old. There are different phenotypes presented in PL patients, including dilated cardiomyopathy, congenital and lethal genodermatosis, mandibular hypoplasia, lipodystrophy, etc.,8 and the different clinical phenotypes co-exist in most cases.9,10
The pathogenesis and pathological characteristics of HGPS and PL have numerous similarities with the natural process of human physiological aging.8,11 Therefore, the research of HGPS and PL may be helpful to the prevention and treatment of cardiovascular and cerebrovascular diseases in the elderly, and may even delay the natural process of human physiological aging, which has important scientific value and social significance.
To date, there is a paucity of epidemiological data related to HGPS and PL in China. In this study, we aimed to provide a resource for geographical distribution, prevalence, and genotypic characteristics of identified patients with HGPS and PL in China, and to disseminate information that will lead to the discovery of new cases, clinical care, and treatment options.
Subjects and methods
Subjects and institutional approval
The subjects were recruited from three patient registry sources between August 1st, 2020 and March 31st, 2023: (1) the Chinese Organization for Rare Disorders (CORD); (2) The Progeria Research Foundation (PRF); and (3) Children’s Hospital, Zhejiang University School of Medicine. This study has been approved by the Medical Ethics Committee of Children’s Hospital, Zhejiang University School of Medicine (Approval No.: 2021-IRBAL-108). Use of identifiable information was permitted with the patient or parental written consent prior to the study (see supplementary file).
Methods
Data collection
Clinical phenotypes and genotypic data such as sex, birthplace, genotype, age of onset, and primary clinical characteristics were obtained from the outpatient or inpatient information of all subjects.
Regional distribution map design
The regional distribution map is designed by Adobe Illustrator software.
Statistical analysis
Data were tested for normal distribution using the Kolmogorov–Smirnov test, Quantile–Quantile Plot, and Histogram. Normally distributed continuous data are mainly represented as mean ± standard deviation, data that are not normally distributed are expressed as the median and interquartile range (IQR), and the categorical variables are described as absolute count and percentage. The population of each province presented in this study was derived from the Bulletin of the Seventh National Population Census (No. 3). All statistical calculations were performed using GraphPad Prism 9.0 (California).
Results
Point prevalence of HGPS and PL and regional distribution characteristics of patients with HGPS and PL in China
A total of 46 patients with HGPS (N = 20, 19 living,1 deceased on March 29th, 2022) and PL (N = 26) from 17 provinces of China were enrolled in this study. The geographical distribution characteristics of patients with HGPS and PL in China are shown in Fig. 1 and the prevalence of HGPS and PL in 17 provinces on the Chinese mainland is summarized in Table 1. The identified case prevalence of HGPS is 1/53 million in these 17 provinces on the Chinese mainland; when including only provinces with identified cases, the prevalence is 1/40 million, with the top three provinces being Hainan, Zhejiang, and Guangxi. The identified prevalence of PL is 1/39 million in these 17 provinces on the Chinese mainland; when including only provinces with identified cases, the prevalence is 1/30 million, with the top three provinces being Hainan, Guangxi, and Anhui; the identified total prevalence of HGPS and PL is 1/23 million, with the top three provinces being Hainan, Guangxi, and Anhui.
Early clinical and genotypic characteristics of patients
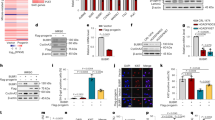
Among the 46 patients, there were 23 males and 23 females, with a male-to-female ratio of 1:1; one female patient with HGPS died at the age of 21 due to heart failure. Phenotypes relevant to diagnosis are shown in Fig. 2. The genotypes of all study subjects and their ages of onset and primary manifestations are detailed in Table 2. Twenty patients were diagnosed with HGPS, including 17 patients with classic genotype HGPS, all of whom had onset within the first month of life and whose primary clinical manifestations were cutaneous sclerosis on retrospective chart review; 3 with non-classic genotype HGPS, with onset at 1 week, 1 month, and 5 years of age, whose primary clinical presentations on retrospective chart review were cutaneous sclerosis, dyspnea, and growth retardation, respectively. The median age of diagnosis for the 20 patients with HGPS was 12 (3.5, 44) months. The median age of onset for the 26 patients with PL was 12 (6,12) months; however, the median age of diagnosis for patients with PL was 42 (24,87.3) months. 3 patients had compound heterozygous mutations in the ZMPSTE24 gene while 39 patients had exonic or intronic mutations in the LMNA gene. Among PL patients, LMNA c.1579 C > T homozygous mutation was the most common, including two siblings, whose onset age was between 1 and 2 years old and whose primary clinical manifestations were skin abnormalities and growth retardation.
a, b are the skin manifestations of the same HGPS patient at 1 month and 3 months of age; c shows skin pigmentation in a patient carrying LMNA c.1453_1454delins AG heterozygous mutation; d shows the clinical characteristics of a patient with PL carrying LMNA c.1579 C > T homozygous mutation: She is 17 years and 11 months old, with a height of only 115 cm, which is equivalent to the height of a healthy child at the age of 5 years and 9 months, with alopecia and mandibular retraction. e, f are the general characteristics of the same PL patient with ZMPSTE24 compound heterozygous mutation at the age of 3 months and 6 years: At the age of 3 months, the patient had sclerodermatous skin and stiffness of both ankle & knee joints; At the age of 6 years, joint stiffness was further aggravated, and the skin was rough with pigmentation and mandibular retraction.
Discussion
To the best of our knowledge, this study is the first report investigating the epidemiological characteristics of HGPS and PL in China. We collected those cases from 17 provinces on the Chinese mainland, about one-fifth of PRF-registered cases, with a median province-specific case-identified prevalence of 1/53 million for HGPS and 1/39 million for PL, and a median combined case-identified prevalence of 1/23 million for both HGPS and PL, with Hainan Province having the highest case-identified prevalence of both HGPS and PL. Since HGPS does not have ethnic or sex biases, these case-identified prevalence rates and the worldwide disease prevalence is 1/18–20 million living individuals,2 this information can be used to anticipate the number of unregistered cases in a region and stimulate efforts to identify those cases.
HGPS and PL are ultra-rare and poorly identified by physicians who have no experience with these diseases. This study shows that the average delayed diagnosis time in HGPS patients is about 33 months, and the longest is nearly 20 years; the average delayed diagnosis time in PL patients is about 40 months, and the longest is 12 years. Many patients living in remote areas had to go to many hospitals in many provinces before identification. They were often misdiagnosed as neonatal scleredema, hypertonia, skin disease of unknown cause, etc. Children’s Hospital, Zhejiang University School of Medicine in Zhejiang Province is the referral center for almost all children with complicated and undiagnosed childhood diseases in China, and has been able to gather information on 50-60% of anticipated cases in mainland China, and serves as a central source of information on clinical care for these children, which includes the Chinese-translated Progeria Research Foundation Clinical Care Handbook.12 Meanwhile, as the prevalence of HGPS is estimated by PRF without sex and race differences, we can take the prevalence of HGPS in China as an estimate of the potential number of patients in other provinces: the HGPS prevalence in Zhejiang Province is 1/22 million (this prevalence is extremely close to that of HGPS estimated by PRF), so it is estimated that there are 65-70 patients with HGPS in China. In this study, 20 HGPS patients were collected (the male-to-female ratio is about 1:1, accounting for about 1/3 of all estimated cases), indicating that we still need to actively look for about 2/3 of other HGPS patients.
HGPS was first proposed in 1886 by Dr. Jonathan Hutchinson and in 1897 by Dr. Hastings Gilford until it became clear in 2003 that HGPS was caused by mutations in the LMNA gene.3,4 The first HGPS cohort study, which included approximately half of the global patients with classic genotype HGPS (15 cases), was reported in 2008. All patients had growth retardation and skin abnormalities and were diagnosed at 3.5 months to 4 years old.13 Gordon et al. demonstrated that 90% of HGPS cases are classic genotype HGPS.1 In this study, 85% of enrolled cases were classic genotype HGPS with all patients having skin sclerosis as the first clinical manifestation within the first month of life. One case of LMNA c.1968+5 G > C point mutations HGPS had mild clinical manifestations compared with the classic genotype, while the other cases of LMNA c.1822G > A point mutations HGPS patients developed severe skin stiffness and growth retardation at an earlier age (1 week after birth), which are not consistent with the literature,1,4 which may be related to the geography, race, and also to the degree of metabolic disorder after LMNA gene mutation.14 Patients with HGPS caused by LMNA c.1968+3-1968+6 del have severe respiratory distress in the first month of life as the first clinical manifestation, which has not been reported in the literature.
Clinically, PL includes atypical Werner syndrome (AWS), type A Mandibuloacral dysplasia (MADA), type B Mandibuloacral dysplasia (MADB), Restrictive Dermopathy (RD) and Atypical Progeria Syndrome (APS). The literature was often presented in the form of case reports and reviews. This study completed the first cross-sectional survey of PL in China. As indicated, LMNA c.1579 C > T homozygous mutations are the most common form of gene mutation among the Chinese PL population. To date, 12 cases of LMNA c.1579 C > T homozygous mutations have been reported,15,16,17,18,19,20 of which 4 families have 10 people from China (5 of them are included in this study), all of which are recessive, and the parents of patients are carriers of LMNA c.1579 C > T gene mutations, suggesting that genetic counseling and prenatal screening in this population is very important. The clinical characteristics that result from ZMPSTE24 gene mutations are MADB and RD. In most cases, ZMPSTE24 gene compound heterozygous mutations (loss of function of one allele, missense mutations of another gene) cause MADB. Loss-of-function mutations in the ZMPSTE24 double allele are associated with lethal RD, which leads to the death of patients within one week after birth. However, Schaflinger et al. reported a novel pure combined shift code mutation called NM_005857.5:.28_29insA, p. (Leu10Tyrfs*37), in ZMPSTE24 gene, which causes not lethal RD but MADB.21 In this study, three patients with ZMPSTE24 gene complex heterozygous mutations showed MADB with a postnatal onset of 1 to 2 months, with skin stiffness and joint stiffness as the primary clinical manifestations.
Lonafarnib is a farnesyltransferase inhibitor, which was approved for use by prescription in the United States in Nov, 202022,23 for patients with HGPS or ZMPSTE24 gene compound heterozygous or homozygous mutations, which can increase the weight of patients, improve the vascular stiffness, vascular compliance, bone structure, and hearing level of patients while reducing the risk of cardiovascular events and stroke, and prolong the lifespan of patients.24,25 Currently, Children’s Hospital, Zhejiang University School of Medicine has successfully passed the Lonafarnib Managed Access Program (MAP) and applied for the free administration of Lonafarnib for patients with HGPS and processing-deficient PL in China who meet the indications for Lonafarnib (ten patients have received free treatment with Lonafarnib for 1–10 months each to date). However, almost all patients with Lonafarnib developed gastrointestinal reactions of varying degrees, and there are many PL patients who are not the indicated population for the treatment of Lonafarnib. Hence, the discovery of new treatments that improve symptoms and lifespan for those with HGPS and PL through clinical treatment trials is essential. Therefore, more patients with HGPS and PL in China need to be identified to maximize clinical trial enrollment for these ultra-rare conditions.
There are several limitations of this study. Although we report epidemiological and genotypic characteristics of patients with HGPS/PL in China, the patients mainly come from the eastern and southern regions of China, and we still need to actively seek patients from the western and northern regions of China. In addition, this is only a cross-sectional study, and we need to observe the characteristics of these children vertically in order to better understand the disease characteristics of Chinese children with HGPS/PL.
Conclusions
The median total identified case prevalence of HGPS and PL in 17 provinces of China is 1/23 million. In China, the ratio of male and female in HGPS/PL patients is 1:1. The onset of classic genotype HGPS is featured by skin sclerosis in the first month after birth, and the onset of non-classic genotype HGPS occurs sooner or later. The primary clinical manifestations of PL patients include skin abnormalities, growth retardation, and joint stiffness. LMNA c.1579 C > T homozygous mutations are the most common form of gene mutations among the Chinese PL population, which usually occurs at the age of 1–2 years old. The complex heterozygous mutations of the ZMPSTE24 gene occur in 1–3 months after birth, with skin abnormalities and joint stiffness as the primary clinical manifestations.
This study examined genotypic, phenotypic, and prevalence characteristics of 40–50% of the cases of HGPS/PL in mainland China through a collaborative international registry effort that facilitated clinical care and progeria-specific drug treatments for qualified patients. Continued search for more patients with HGPS/PL will be helpful for us to study the natural medical history of children with HGPS/PL, and also for us to conduct clinical trials of new drugs.
Data availability
The data analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gordon, L. B., Brown, W. T., & Collins, F. S. in GeneReviews(®) (eds. Adam, M. P. et al.) (University of Washington, Seattle. Copyright © 1993–2023, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved., 1993).
Leslie, B. & Gordon, M. D. PRF by the Numbers. (The Progeria Research Foundation., 2013). Accessed February 15, 2023).
De Sandre-Giovannoli, A. et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 300, 2055 (2003).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 (2003).
Coppedè, F. Mutations involved in premature-ageing syndromes. Appl. Clin. Genet. 14, 279–295 (2021).
Guardiani, E. et al. Otologic and audiologic manifestations of Hutchinson-Gilford progeria syndrome. Laryngoscope 121, 2250–2255 (2011).
Gordon, L. B. et al. Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation 130, 27–34 (2014).
Neilan, E. G. Laminopathies, other progeroid disorders, and aging: common pathogenic themes and possible treatments. Am. J. Med. Genet. A 149a, 563–566 (2009).
Shin, J. Y. & Worman, H. J. Molecular pathology of laminopathies. Annu. Rev. Pathol. 17, 159–180 (2022).
Foo, M. X. R., Ong, P. F. & Dreesen, O. Premature aging syndromes: From patients to mechanism. J. Dermatol. Sci. 96, 58–65 (2019).
Ashapkin, V. V., Kutueva, L. I., Kurchashova, S. Y. & Kireev, I. I. Are there common mechanisms between the Hutchinson-Gilford progeria syndrome and natural aging? Front. Genet. 10, 455 (2019).
Gordon, L. B. The Progeria Handbook 2nd Edition. A Guide for Families & Health Care Providers of Children with Progeria. October 26, 2013.
Merideth, M. A. et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N. Engl. J. Med. 358, 592–604 (2008).
Kreienkamp, R. & Gonzalo, S. Metabolic dysfunction in Hutchinson-Gilford progeria syndrome. Cells 9, https://doi.org/10.3390/cells9020395 (2020).
Zhu, L. Y. et al. [Clinical features and genetic diagnosis of four cases with progeria syndrome]. Zhonghua Er Ke Za Zhi 57, 636–638 (2019).
Doanh, L. H., Phuong, H. T., Phuong, N. T. T., Thu, L. T. H. & Thuong, N. V. Mandibuloacral dysplasia in a young Vietnamese girl caused by homozygous missense variant c.1579C>T in the LMNA gene with progeria and severe skin lesions. JAAD Case Rep. 16, 5–8 (2021).
Luo, D. Q. et al. Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China. BMC Pediatr. 14, 256 (2014).
Agarwal, A. K., Kazachkova, I., Ten, S. & Garg, A. Severe mandibuloacral dysplasia-associated lipodystrophy and progeria in a young girl with a novel homozygous Arg527Cys LMNA mutation. J. Clin. Endocrinol. Metab. 93, 4617–4623 (2008).
Xiong, Z. et al. Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two Chinese siblings: two case reports. J. Med. Case Rep. 7, 63 (2013).
Liang, L., Zhang, H. & Gu, X. Homozygous LMNA mutation R527C in atypical Hutchinson-Gilford progeria syndrome: evidence for autosomal recessive inheritance. Acta Paediatr. 98, 1365–1368 (2009).
Schaflinger, E. et al. An exceptional biallelic N-terminal frame shift mutation in ZMPSTE24 leads to non-lethal progeria due to possible utilization of a downstream alternative start codon. Gene 833, 146582 (2022).
Misteli, T. Farnesyltransferase inhibition in HGPS. Cell 184, 293 (2021).
Suzuki, M. et al. FDA approval summary for lonafarnib (Zokinvy) for the treatment of Hutchinson-Gilford progeria syndrome and processing-deficient progeroid laminopathies. Genet. Med. 25, 100335 (2023).
Gordon, L. B. et al. Clinical trial of the protein farnesylation inhibitors lonafarnib, pravastatin, and zoledronic acid in children with Hutchinson-Gilford progeria syndrome. Circulation 134, 114–125 (2016).
Gordon, L. B. et al. Association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson-Gilford progeria syndrome. JAMA 319, 1687–1695 (2018).
Acknowledgements
We thank the patients and their families for their participation, and we are particularly grateful to the CORD organization, the Zhejiang Sunflower Foundation, and the Zhejiang Women and Children’s Foundation for their support. This study has been supported by the Lingyan Program of the Zhejiang Provincial Department of Science and Technology, 2023C03027.
Author information
Authors and Affiliations
Contributions
J.W. conceptualized and designed the study, drafted the initial manuscript, and revised the manuscript. Q.Y., X.T. designed the data collection instruments, collected data, and carried out the initial analyses. L.B.G. critically reviewed and revised the manuscript. J.C., B.J., G.H., H.F., and J.Q. collected data, carried out the initial analyses, and analyzed imaging data. Z.L. critically reviewed and revised the manuscript for important intellectual content. M.J. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
This study has been approved by the Medical Ethics Committee of Children’s Hospital, Zhejiang University School of Medicine (Approval No.: 2021-IRBAL-108) and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from each participating individual’s guardian.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Yu, Q., Tang, X. et al. Epidemiological characteristics of patients with Hutchinson-Gilford progeria syndrome and progeroid laminopathies in China. Pediatr Res 95, 1356–1362 (2024). https://doi.org/10.1038/s41390-023-02981-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02981-9