Abstract
Background
Irritable bowel syndrome is common in children and exhibits a high placebo response. This study was to explore the placebo response rate and its influencing factors in children with irritable bowel syndrome.
Methods
A systematic search was performed on Pubmed, Embase, MEDLINE, Cochrane Library, CNKI, Wanfang, and CBM from database inception to March 2022. Randomized controlled trials of irritable bowel syndrome in children were included in the study. The primary outcome was the placebo response rate of improvement.
Results
Thirteen studies were included, with 445 patients in the placebo group. The rate of improvement and abdominal pain disappearance were 28.2% (95% CI, 16.6–39.9%) and 5% (95% CI, 0–18.4%). The placebo response based on the abdominal pain score was 0.675 (95% CI, 0.203–1.147). The mode of administration (P < 0.01), dosing schedule (P < 0.01), and clinical outcome assessor (P = 0.04) have a significant impact on the magnitude of placebo effect.
Conclusions
The placebo response rate for pediatric irritable bowel syndrome was 28.2%. In clinical trials, reducing dosing frequency, selecting appropriate dosage forms, and using patient-reported outcomes can help mitigate the placebo effect.
Impact
-
This is the first meta-analysis to assess the placebo response rates for improvement and disappearance in children with IBS.
-
The finding suggested that the mode of administration, dosing schedule, and clinical outcome assessor could potentially influence the magnitude of the placebo effect in children with IBS.
-
This study would provide a basis for estimating sample size in clinical trial design with a placebo control.
Similar content being viewed by others
Introduction
Irritable bowel syndrome (IBS), a functional abdominal pain disorder (FAPD) that causes abdominal discomfort or pain related to bowel movements or changes bowel habits, is one of the most common FAPDs in children.1 A study has reviewed the global epidemiology of pediatric FAPDs and has shown a high prevalence of IBS (8.8%; 95% CI, 6.2–11.9%).2 Another study has also shown a high rate of IBS in Asian children (12.41%; 95% CI, 9.87–14.95%).3 It is hypothesized that several factors play a role in the development of IBS. These include visceral hypersensitivity, central sensitization, early life events, gut flora dysbiosis, food, psychological factors, and genetics.1,4
IBS is a chronic functional condition that currently lacks effective treatment options. Therefore, treatment goals are to alleviate abdominal discomfort or global symptoms while improving the patients’ quality of life.5,6,7,8 The Rome Foundation Subcommittee for Pharmacological Clinical Trials in Children with IBS9 recommended using a placebo as a control group in clinical trials when evaluating the efficacy of new medications. It has the advantage of reflecting the test drug’s absolute efficacy while reducing sample size. However, the use of a placebo has been shown to cause a placebo response in various diseases, including migraine, arthritis, asthma, depression, Parkinson’s disease, and attention-deficit/hyperactivity disorder.10 The placebo response has a significant effect on relieving pain.11 Several studies12,13,14,15,16,17 have shown a high placebo response for IBS in adults. The placebo response rates ranged from 16.2% to 42.6%, and were associated with diagnostic criteria, number of office visits, frequency of intervention, treatment duration, and overall treatment effect.12,13,14,15,16,17
There has been no study on the placebo response of IBS in children. An analysis of abdominal pain-related functional gastrointestinal disorders (AP-FGIDs) in children showed a placebo response of 41% (95%CI, 34–49%) for symptom relief and 17% (95% CI, 8–32%) for pain disappearance.18 AP-FGIDs were simplified to FAPDs in the Rome IV criteria, and it was important to distinguish the various subtypes clearly.19 Different types of FAPDs are all involved in the alteration of the brain-gut axis. However, their pathological mechanisms are not identical, so it is necessary to separate the various types of FAPDs for study.20 In this study, we aimed to estimate the placebo response and influencing factors for IBS in children. Its significance lies in providing a foundation for the sample size calculation, minimizing the placebo response in trials, and maximizing it in clinical practice.21
Methods
This meta-analysis protocol was registered on PROSPERO (CRD 42022322310). We reported this study according to 2020 Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA).
Inclusion criteria
Randomized controlled trials with parallel or crossover designs were included in this study. The population consisted of children under 18 years old diagnosed with irritable bowel syndrome. The control arm used a placebo, and another used any other treatment, including pharmacological or dietary interventions. The trials had to assess the placebo response rate of improvement or disappearance in IBS symptoms and pain scores.
Exclusion criteria
We excluded duplicate publications and documents that were not available in full-text. Clinical observations, reviews, conference abstracts, meta-analyses, study protocols, animal experiments, pharmacological investigations, and pharmacokinetic studies were all excluded from the study. Trials with a sample size of less than 15, missing data or those in which participants concurrently used other medications during the placebo period were also excluded. Literature that was not in either Chinese or English was omitted from consideration.
Information sources and search strategy
We systematically searched Pubmed, Embase, MEDLINE, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform, and China Biology Medicine disc (CBM) from database inception to March 2022. RCTs from published systematic reviews or Meta-analyses were searched manually, and those that met the requirements were included. “Irritable bowel syndrome”, “placebo” and “children” were used as search terms, and the specific search strategy is provided in Supplemental Table S1 (online).
Literature screening
Two researchers (L.L.C., X.L.) independently screened the literature and reviewed titles and abstracts to determine which studies should be included based on inclusion and exclusion criteria. After reading the complete text and cross-checking, the literature was further reviewed and information extracted. If there were any issues, they were resolved by consultation or a third party (Q.H.C.).
Data extraction and quality assessment
The investigators (L.L.C., X.L., W.C.S., Z.H.Z.) collected data from the included studies in a data extraction form. The following details were taken from each study: (1) basic study information, such as study title, first author, year of publication, year of research beginning, study site, kind of trial design, and number of centers; (2) study literature characteristics, such as sample size, diagnostic criteria, mean or median age, the proportion of female participants, intervention, dose type, dosing frequency, number of visits, treatment duration, introduction period, endpoint definition of symptom relief; (3) important factors to consider when assessing the possibility of bias; (4) the primary outcome was the placebo response rate of improvement in IBS symptoms, and the secondary outcomes were the rate of abdominal pain disappearance and pain score. The risk of bias in included studies was assessed by two reviewers (L.L.C., X.L.) with the revised Cochrane risk of bias tool.22 The assessed domains included randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result and overall bias. If there were any disagreements on the evaluation, the third researcher (Q.H.C.) resolved them.
Data synthesis and statistical analysis
The data were statistically combined and analyzed using the “meta” package in R 4.1. The untransformed proportion of the dichotomous variables was standardized by log transformation, logit transformation, arcsine transformation, and Freeman-Tukey Double arcsine transformation. The Shapiro-Wilk test was used to test the normality of the untransformed proportion and the four transformed rates. The rate closest to or following a normal distribution (with the largest P value) was selected for meta-analysis to calculate the pooled placebo response.23 The placebo response rate was calculated using the Standardized Mean Difference (SMD) between baseline and treatment completion for continuous variables. The interval estimates for each effect size were expressed using the 95% confidence interval (CI), and the P value less than 0.05 indicated statistically significant. The heterogeneity in the included studies was assessed with the Q test (α = 0.1) in combination with I2. The random-effects model was used to calculate the pooled effect sizes.
Subgroup analyses were performed on the rate of improvement or disappearance according to the following variables: trial ___location, study design, number of centers, diagnostic criteria, mode of administration, Dosing schedule, run-in period, outcome based on different definitions, proportion assigned to placebo, sex, clinical outcome assessors, and whether allocation concealment was reported.
Meta-regression analyses for categorical variables were similar to the results of subgroup analyses. Consequently, the univariate meta-regression analysis was only performed for the following variables: study initiation year (inferred from the mean of the time from start to publication in other studies if unavailable), age (substituted with the mean or median age of the placebo group or overall age if the average or median age of the IBS placebo group was unavailable), run-in period (assumed to be absent if not mentioned), dosing schedule (calculated as the average dosing frequency if different dosing regimens existed), duration of therapy, and the number of visits. Sensitivity analyses were performed after excluding crossover trials.
Results
Study selection
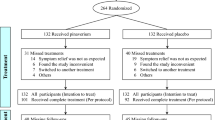
The search strategy generated 912 relevant articles, and 597 articles remained after duplication checking. After initial screening by reading the title and abstract, 74 articles that met the inclusion criteria were further assessed. Thirteen RCTs24,25,26,27,28,29,30,31,32,33,34,35,36,37 were finally included after re-screening by reading the full text (Fig. 1).
Study characteristics
A total of 445 children were randomly allocated to the placebo group in the 13 studies included. Nine studies reported the proportion of participants with symptom resolved. Three studies provided the percentage of participants with pain disappearance, and five studies assessed pain scores to measure pain severity. The main characteristics of the included studies are provided in Table 1.
Risk of bias in studies
Ten studies were estimated to have some concerns from the randomization process,24,26,27,28,30,31,32,33,34,35,37 and two due to deviations from the intended interventions.32,36 Two studies were rated as having a high risk of missing outcome data bias,35,37 and one had some concerns.36 The outcome measurement bias for all studies was low risk. Eight studies did not report the registration protocol,24,25,26,27,28,30,31,37 and the selection of the reported result was estimated to have some concerns. Two studies35,37 were assessed as having a high risk of the overall risk of bias, and ten24,25,26,27,28,30,31,32,33,34,36 had some concerns. The assessment results of risk bias in the included study are shown in Supplemental Figs. S1–2 (online).
Pooled outcomes
Placebo response rate of improvement
The pooled proportion of improvement reported in the nine studies was 28.2% (95% CI, 16.6–39.9%), ranging from 0–45.2%, with significant heterogeneity among studies (I2 = 88%, P < 0.01) (Fig. 2a). Subgroup analyses were performed to explore factors influencing the placebo response according to different study characteristics (Table 2). The mode of administration, dosing schedule, and clinical outcome assessor had a significant effect on the placebo response size. The liquid form of placebo may result in a lower placebo response (6.7%) compared to the solid form of placebo. The placebo responses were 38.1%, 30.6%, and 21.7% for powder, capsule, and chewable tablet, respectively. Considering that the powder needed to dissolve in water when taken, we calculated the pooled placebo response rate was 27.7% (95% CI, 5.3–50.2%) when combined with the liquid, with no significant difference between solid and liquid (P = 0.66). The placebo response gradually increased with increasing dosing schedule (P < 0.01), with 9.2%, 41.3%, and 42.9% for once, twice, and three times daily, respectively. One study had a placebo response rate of 33.9%, which used different dosing regimens (once or twice a day) for different age groups. The placebo response was lowest when patient was the outcome assessor (17.8%). The placebo response was 27.3%, 42.9%, and 44.9% when the patient or parent, physician, and parent assessed the outcome. Although the diagnostic criteria and the proportion of participants assigned to placebo did not reach the traditional level of statistical significance, there was a tendency to influence the size of the placebo effect. Studies using Rome II as the diagnostic criteria had higher placebo response rates than Rome III (31.6% vs 14.1%), and a study using Manning or Rome II as the diagnostic criteria had a placebo response rate of 42.9%. Most of the studies were two-arm trials with a placebo response rate of 26.3%. Only one study had less than 50% placebo enrollment, with a placebo response rate of 45.2%. Subgroup analyses of the placebo response according to trial ___location, study design, number of centers, run-in period, duration of therapy, sex, improvement rate based on different definitions, and reported allocation concealment did not reach a statistically significant level of difference.
Meta-regression with subgroup analysis yielded consistent results that the dosing schedule was associated with a placebo response of improvement (b = 0.23, P < 0.001) (Table 3). No significant correlation was seen for study initiation year, age, run-in period, duration of therapy, and the number of visits.
After excluding one crossover trial, the pooled placebo response of the remaining studies was 27.7% (95% CI, 14.6%-40.7%), and the heterogeneity was high (I2 = 88%, P < 0.01). After excluding a study involving children with comorbid autism spectrum disorder and IBS, the pooled placebo response of the remaining studies was 26.3% (95% CI, 14–38.5%), and the heterogeneity was high (I2 = 88%, P < 0.01). There was no significant difference compared with before exclusion, suggesting that the above analysis results were stable (Figs. S3 and S4 in the supplement online).
Pain disappearance rate
The pooled proportion of subjects reporting pain disappearance in the three studies was 5% (95% CI, 0–18.4%), ranging from 0-14%, with significant heterogeneity among studies (I2 = 72%, P = 0.03) (Fig. 2b). subgroup analysis and Meta-regression were not performed due to the small number of included studies.
Pain score
Five studies reported results for scoring the severity of abdominal pain with a placebo response of 0.675 (95% CI, 0.203–1.147). There was significant heterogeneity among studies (I2 = 73%, P < 0.01) (Fig. 2c).
Discussion
In this study, the first meta-analysis of the placebo response in children with IBS, the pooled relief rate was 28.2% (95% CI, 16.6–39.9%), and the pooled abdominal pain disappearance rate was 5% (95% CI, 0–18.4%). The placebo response, as measured by abdominal pain scores, was 0.675 (95% CI, 0.203–1.147), indicating a significant difference compared with baseline.38 Compared with the results of a previous meta-analysis of adults with IBS before 2010,12,13,14,15 the pediatric population appeared to have a lower placebo response; however, the difference in placebo response between adults and children was not significant when compared to studies published in recent years.17 This difference may be due to various potential factors, including the deepening understanding of IBS, updated diagnostic criteria, and improved trial design and placebo manufacturing technology, which contributed to the placebo effect size gradually approaching the actual condition. Some studies suggested that the placebo response was higher in children than in adults, which differed from the results of our study. The possible explanation was that most children with IBS were adolescents, and the placebo effect decreased with age.39,40,41 However, this study found no significant association between age and placebo response. The placebo response for AP-FGIDs was 41% (95% CI, 34%-49%), which is higher than our finding, suggesting that the placebo response of AP-FGIDs may vary between diseases.18
Our study evaluated both the rate of improvement and abdominal pain disappearance. From the results, the placebo effect was found to be lower for the latter outcome. However, in clinical trials, it is more recommended to use the rate of abdominal pain improvement as the primary outcome measure. This is because using overly stringent outcome measures would be challenging to achieve, significantly increasing the difficulty of the trial. The Rome committee recommended an improvement of ≥30% in abdominal pain as the primary endpoint, and the reduction should exceed the Reliable Change Index (RCI) for that sample.9 In some of the included trials, the response definition for abdominal pain relief was set as a minimum reduction of 50%.25,29,33,34 Although this threshold of 50% is easier for children and parents to understand compared to 30%, it has lower sensitivity. This may result in some children who experience relief in abdominal pain perceiving themselves as having a negative result in the clinical trial.42
In the subgroup analysis, the mode of administration was found to have a substantial effect on the placebo response, with only 6.7% for the liquid placebo. A review of the original article revealed that the trial used fruit juice as the placebo control.31 We supposed children had no therapeutic experience due to the non-drug-like appearance, reducing the placebo response. Based on this hypothesis, it is advisable to consider selecting taste profiles that are more palatable and acceptable to children in order to minimize the placebo effect during trial design. However, to ensure the medication compliance of children, the implementation of the blind method, and the stability of the efficacy of active drugs, it is also necessary to carefully consider how to select the appropriate dosage form.
The higher the dosing frequency, the more pronounced the placebo response, which is consistent with previous findings.12,17 Patients’ expectations of receiving therapy may rise due to more frequent treatment and thus enhancing the treatment effect.
Outcome reported by physicians or parents showed a higher placebo response. Children under the age of eight were generally reported by proxy. Some children may have difficulty accurately describing their own sensations, and subjective judgments by parents or physician may introduce bias. Therefore, it is recommended to recruit children aged eight years or older in clinical trials, as they have a certain level of self-assessment ability.9 Using patient-reported outcomes can more accurately reflect the alleviation of pain, thereby avoiding the potential for erroneous feedback resulting from high expectations of pain relief from parents or physicians.
The effect of diagnostic criteria on the placebo response approached the level of a statistically significant difference. It is considered that this may be due to the smaller sample size, according to previous significant results for diagnostic criteria for IBS in adults.13 Placebo responses were lower in trials using the Rome III criteria than the Manning or Rome II diagnostic criteria, possibly because the latter’s stricter criteria narrowed the study population. The placebo response was higher in multi-arm trials compared to two-arm trials, presumably because participants had higher expectations in multi-arm trials since they thought they had a better chance of obtaining active therapy.
The significance of this study is to provide a foundation for sample size estimation and trial design. The sample size should be calculated using the predicted clinical difference in efficacy between the treatment and placebo groups. For sample size estimation, an appropriate pooled estimate can be chosen based on the trial’s outcome. The upper limit of its 95% confidence interval can also be selected, which is more conservative but substantially increases the sample size. Reducing the dosing frequency, improving the dosing form and taste, and the patient-reported outcomes are all advised ways to lessen the placebo reaction.
However, the following limitations of this study remain: (1) IBS-C and IBS-D were not discussed in the subgroup. It is advised in the Rome IV criteria published in 2016 that children with IBS be staged using the adult staging method, which has weak evidence but aids in the conduct of scientific investigations.1 Fewer studies in this review defined IBS subtypes at the results.28,31 We expect that more trials will focus on this in the future. (2) The total pain score differed from study to study. Although pain scores were transformed into SMD to reduce the variation associated with different total scores, the sensitivity varies between scores. Therefore, the results should be regarded with caution. In future trials, it is suggested that uniform measurement tools, such as the 0-100 mm VAS score or the 0-10 NRS, be employed. (3) There was significant heterogeneity among studies. The possible reasons include different inclusion criteria, severity, and characteristics. The pooled placebo response was estimated using a random-effects model, but the effect estimates are broad because of the small number of studies. (4) The study contained crossover trials,30,35 and the conventional approach is to include the data from the first phase in the meta-analysis. However, this could lead to bias and undermine the crossover design. Sensitivity analysis was performed after crossover tests were excluded, and the results were stable. (5) FAPDs was used as an inclusion criterion in many research, although the results did not include subgroup analysis of different disorders. Some of the included studies26,29,31,37 were included in the FAPDs and had small sample sizes. Even though the trials in children are more challenging to conduct, a separate clinical study for IBS is still advised. Different FAPDs subtypes have distinct characteristics, and combining them may not show their individual qualities but diminish the study’s effectiveness. (6) We excluded a study on open-label placebo treatment in children with IBS, which reported a significant 46.7% improvement rate.43 The included articles all utilized double-blind methods, making it difficult to determine the influence of open-label treatment on the placebo effect. This study also used dissolvable hyoscyamine tablets as rescue medication for pain, potentially introducing bias into the results. Although not included in our analysis, we view open-label placebo treatment as a promising approach for managing IBS in children.
Conclusion
The pooled placebo improvement and disappearance rates in randomized controlled trials in children with IBS were 28.2% and 5%, respectively. The placebo response rate of improvement may be associated with dosing frequency, mode of administration, and outcome assessor. Reducing dosing frequency, selecting appropriate dosage forms, and using patient-reported outcomes in future trials are recommended to reduce the placebo response. It is also essential to consider the differences between subtypes in the design of pediatric IBS trials. In terms of outcome indicators, it is also important to focus on stool trait changes in addition to abdominal pain symptoms.
Data availability
The raw data and code can be obtained directly from the author.
References
Drossman, D. A. ROME IV: Functional Gastrointestinal Disorders/Disorders of Gut-Brain Interaction (The Rome Foundation, Raleigh, 2016).
Korterink, J. J., Diederen, K., Benninga, M. A. & Tabbers, M. M. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS ONE 10, e0126982 (2015).
Devanarayana, N. M. et al. Epidemiology of irritable bowel syndrome in children and adolescents in Asia. J. Pediatr. Gastroenterol. Nutr. 60, 792–798 (2015).
Thapar, N. et al. Paediatric functional abdominal pain disorders. Nat. Rev. Dis. Prim. 6, 89 (2020).
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry irritable bowel syndrome-clinical evaluation of drugs for treatment. https://www.fda.gov/media/78622/download (2012).
European Medicines Agency. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. https://www.ema.europa.eu/en/evaluation-medicinal-products-treatment-irritable-bowel-syndrome (2014).
China Food and Drug Administration. Clinical Research Guidance for New Drug of Chinese Medicine in Irritable Bowel Syndrome 2017. https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=c2b78e36b58535dfb0a1150be64d9470 (2017).
Lacy, B. E. et al. ACG clinical guideline: management of irritable bowel syndrome. Am. J. Gastroenterol. 116, 17–44 (2021).
Saps, M. et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol. Motil. 28, 1619–1631 (2016).
Munnangi, S., Sundjaja, J. H., Singh, K., Dua, A. & Angus, L. D. Placebo effect. In StatPearls (StatPearls Publishing, Treasure Island, FL, 2022).
Colloca, L. The placebo effect in pain therapies. Annu Rev. Pharmacol. Toxicol. 59, 191–211 (2019). Jan 6.
Pitz, M., Cheang, M. & Bernstein, C. N. Defining the predictors of the placebo response in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 3, 237–247 (2005).
Patel, S. M. et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol. Motil. 17, 332–340 (2005).
Dorn, S. D. et al. A meta-analysis of the placebo response in complementary and alternative medicine trials of irritable bowel syndrome. Neurogastroenterol. Motil. 19, 630–637 (2007).
Ford, A. C. & Moayyedi, P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 32, 144–158 (2010).
Barberio, B., Savarino, E. V., Black, C. J. & Ford, A. C. Placebo response rates in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: meta-analysis. Clin. Gastroenterol. Hepatol. S1542-3565, 00905–00908 (2021).
Bosman, M. et al. The placebo response rate in pharmacological trials in patients with irritable bowel syndrome: a systematic review and meta-analysis[J]. Lancet Gastroenterol. Hepatol. 6, 459–473 (2021).
Hoekman, D. R. et al. The placebo response in pediatric abdominal pain-related functional gastrointestinal disorders: a systematic review and meta-analysis. J. Pediatr. 182, 155–163.e7 (2017).
Hyams, J. S. et al. Functional disorders: children and adolescents. Gastroenterology S0016-5085, 00181–00185 (2016).
Rajindrajith, S., Zeevenhooven, J., Devanarayana, N. M., Perera, B. J. C. & Benninga, M. A. Functional abdominal pain disorders in children. Expert Rev. Gastroenterol. Hepatol. 12, 369–390 (2018).
Enck, P. & Klosterhalfen, S. Placebos and the placebo effect in drug trials. Handb. Exp. Pharmacol. 260, 399–431 (2019).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Luo, M. et al. Meta analysis of single rate in R software. J. Evid. Based Med. 13, 181–184 (2013).
Kline, R. M., Kline, J. J., Di Palma, J. & Barbero, G. J. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J. Pediatr. 138, 125–128 (2001).
Bauserman, M. & Michail, S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J. Pediatr. 147, 197–201 (2005).
Gawrońska, A., Dziechciarz, P., Horvath, A. & Szajewska, H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol. Ther. 25, 177–184 (2007).
Bahar, R. J., Collins, B. S., Steinmetz, B. & Ament, M. E. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J. Pediatr. 152, 685–689 (2008).
Handen, B. L. et al. A double-blind, placebo-controlled trial of oral human immunoglobulin for gastrointestinal dysfunction in children with autistic disorder. J. Autism Dev. Disord. 39, 796–805 (2009).
Francavilla, R. et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 126, e1445–e1452 (2010).
Guandalini, S. et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J. Pediatr. Gastroenterol. Nutr. 51, 24–30 (2010).
Romano, C., Comito, D., Famiani, A., Calamarà, S. & Loddo, I. Partially hydrolyzed guar gum in pediatric functional abdominal pain. World J. Gastroenterol. 19, 235–240 (2013).
Kianifar, H. et al. Probiotic for irritable bowel syndrome in pediatric patients: a randomized controlled clinical trial. Electron Physician 7, 1255–1260 (2015).
Shulman, R. J. et al. Psyllium fiber reduces abdominal pain in children with irritable bowel syndrome in a randomized, double-blind trial. Clin. Gastroenterol. Hepatol. 15, 712–719.e4 (2017).
Shulman, R. J. et al. Randomized, double blind trial of psyllium fiber in children with irritable bowel syndrome (IBS). Gastroenterology 148, S120 (2015).
Giannetti, E. et al. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Gastroenterol. 51, e5–e10 (2017).
Sudha, M. R., Jayanthi, N., Aasin, M., Dhanashri, R. D. & Anirudh, T. Efficacy of Bacillus coagulans unique IS2 in treatment of irritable bowel syndrome in children: a double blind, randomised placebo controlled study. Benef. Microbes 9, 563–572 (2018).
Rahmani, P., Ghouran-Orimi, A., Motamed, F. & Moradzadeh, A. Evaluating the effects of probiotics in pediatrics with recurrent abdominal pain. Clin. Exp. Pediatr. 63, 485–490 (2020).
Schober, P., Mascha, E. J. & Vetter, T. R. Statistics from A (Agreement) to Z (z Score): a guide to interpreting common measures of association, agreement, diagnostic accuracy, effect size, heterogeneity, and reliability in medical research. Anesth. Analg. 133, 1633–1641 (2021).
Weimer, K. et al. Placebo effects in children: a review. Pediatr. Res. 74, 96–102 (2013).
Janiaud, P. et al. Is the perceived placebo effect comparable between adults and children? A meta-regression analysis. Pediatr. Res. 81, 11–17 (2017).
Gniß, S., Kappesser, J. & Hermann, C. Placebo effect in children: the role of expectation and learning. Pain 161, 1191–1201 (2020).
Mohammad, S., Pusatcioglu, C. & Saps, M. Comparison of primary efficacy endpoints recommended by regulatory agencies in children with functional gastrointestinal disorders. Gastroenterology 148, S–586 (2015)..
Nurko, S. et al. Effect of open-label placebo on children and adolescents with functional abdominal pain or irritable bowel syndrome: a randomized clinical trial. JAMA Pediatr. 176, 349–356 (2022).
Author information
Authors and Affiliations
Contributions
L.L.C. and X.L. contributed equally to this manuscript and should be considered joint first author. L.L.C. and X.L. carried out the concept, design and drafting of the manuscript, performed the acquisition, analysis, and interpretation of data, and critically revised the manuscript for important intellectual content. Q.H.C. and S.Y.H. carried out the concept, design and drafting of the manuscript. G.S.X. and Y.Z. performed the Statistical analysis. WCS and Z.H.Z. searched databases, screened articles, and extracted data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, LL., Li, X., Cai, QH. et al. Irritable bowel syndrome in children: the placebo response rate and influencing factors a meta-analysis. Pediatr Res 95, 1432–1440 (2024). https://doi.org/10.1038/s41390-023-02996-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02996-2