Abstract
Addiction is considered a chronic disorder that requires long-term treatment. Early identification of predictors of outcome may enable better and early adjustment of treatment. Daily fluctuations of craving have been shown to predict substance use within hours, making it a major target for treatment. The objective of this study was to examine whether trajectory and temporal dynamics of craving, at the initiation of outpatient addiction treatment, were associated to long-term substance use outcome. An Ecological Momentary Assessment study collected craving intensity changes and substance use during the first 14-days of treatment, followed by prospective regular follow-ups for 5 years or more to assess long-term outcome. Analysis investigated whether individual differences in craving trajectory (linear trend) and dynamics (inertia, variability and instability) predicted 5+ years follow-up outcome: substance use (1 day or more of primary substance use/past 30 days) versus abstinence. Thirty-nine participants were enrolled in addiction clinic in Bordeaux, France. Results showed that substance use at 5+ years was significantly associated with slower decrease of craving intensity (p < 0.001), and a lower craving inertia (p = 0.038), i.e. tendency to persist from one moment to the other, compared to abstinence status. Conversely, craving intensity was not found associated with substance use/abstinence at follow-up. Results suggest that a slower decrease in craving at treatment initiation could express a greater resistance to treatment. This resistance may have many mechanisms, among which a persistent reactivity to cues – as suggested by lower inertia – that could constitute a vulnerability to use and a valuable indicator of long-term outcomes.
Similar content being viewed by others
Introduction
Addiction, or Substance Use Disorder (SUD), characterized by a dysregulation in the control of substance use is associated with frequent relapses despite efforts to cut-down or abstain from substance use [1, 2]. The objective of SUD treatment is to achieve stable abstinence or a significant reduction of use through the reduction of relapse frequency over time [3]. On average, people achieve stable abstinence after several treatment episodes, and long-duration treatment episodes are preferable to repeated but shorter episodes [4,5,6]. To that end, it could be interesting to examine whether early treatment response could be a marker of long-term outcome (5 years or more) to provide the possibility of adjusting treatment early.
Craving, that may be defined as a strong and unwanted desire to use a substance, is considered to be an important trigger of relapse across addictions [3, 7,8,9,10]. Added as a SUD diagnostic criterion in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [2, 3, 11], it has been demonstrated to be central in networks of SUD criteria [12]. Craving is considered a therapeutic target of choice for reducing the frequency of relapse and thus improve the outcomes of SUD treatment [3, 13, 14]. Beyond being a diagnostic criterion, craving is primarily a dynamic state, which may vary in intensity and frequency from day to day in the same individual, under the influence of internal and environmental factors [15, 16]. Such phenomena are particularly well captured by Ecological Momentary Assessment (EMA). EMA allows measurement of individual momentary states, by using mobile technology, in the natural environment and in real time, through repeated measurements [17]. EMA has been adapted and validated for the study of craving in SUD [18]. Several EMA studies reported a positive association between an increase in craving at a given time, and an increase in the likelihood of reporting substance use in the following hours, confirming that craving was an important trigger for relapse in daily life [8, 10, 19,20,21,22], even for medium term intervals (12 months or less). EMA studies investigating the trajectory of craving at the beginning of treatment reported generally an increase of craving intensity on quit day [23, 24], followed by a decrease during the first few weeks after cessation [25, 26] and interestingly, changes in craving at the initiation of treatment were different between individuals who remained abstinent compared to those who relapsed at short-term (1 to 4 months) [25, 26], suggesting that these initial craving trajectories could be informative about short-term responses. However, it remains to be explored for longer-term outcomes, especially because addiction is recognized as a chronic and persistent relapsing condition, even several years after quitting [5].
Beyond measuring intensity at any given moment, literature data highlight the interest of taking into account the dynamic variability of symptoms over time [27] that can be characterized by features, such as inertia, variability or instability. Inertia (or temporal dependency) refers to the tendency to persist from one moment to the other [28, 29]. Variability reflects the overall amplitude of changes, and instability can be defined as the magnitude of symptom changes from one moment to the next [29], and is conceptualized as the combination of variability and inertia. These metrics have been used in EMA studies to capture dynamics of affect in mood disorders [29,30,31,32], or insight in obsessive-compulsive disorders [33], and more recently, in substance use disorders [21, 34,35,36,37]. However, to date, craving dynamics and their impact on treatment responses have never been studied. Identifying early indicators of disorder progression is particularly important to adjust treatment intensity and duration accordingly, to better prevent relapse and increase treatment effectiveness, as for many other chronic diseases [38,39,40].
The objective of this study was to examine whether time-dependent changes (trajectory), dynamic fluctuations (inertia, instability, variability) of craving intensity, and within-person association between craving and substance use, in daily-life, at the initiation of outpatient addiction treatment, were associated with the long-term substance use/abstinence outcome at 5 years and more. We hypothesized that a more rapid decrease of craving at the beginning of treatment would be predictive of higher probability for abstinence at 5 years or more.
Subjects and methods
Sample
Participants were patients (18–65 years-old), starting treatment for a DSM-5 alcohol, tobacco, cannabis, or opiate use disorder (of at least moderate severity) in an outpatient addiction treatment clinic in Bordeaux, France. The participants received standard comprehensive care, consisting of individual behavioral treatment focused on relapse prevention and psychosocial support, combined when available with pharmacotherapy [41]. Inpatient or detoxification treatment before outpatient treatment was not mandatory nor recommended, but it cannot be ruled out that some patients received such treatment. After establishing a target quit date according to the patient’s personal goals, full abstinence was encouraged as an outcome, with no negative consequences for the patient if this goal was not met.
Study design and procedure
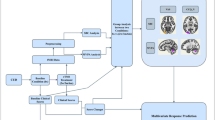
Data was collected at treatment initiation with a 14-days EMA study [18] from May 2009 to July 2013, and by follow-up interviews conducted in the framework of the ADDICTAQUI cohort [42]. At inclusion, participants were assessed using an interview inspired by the Mini International Neuropsychiatric Interview–Plus (MINI) [43], adapted for DSM-5 criteria, to explore diagnoses of current psychiatric disorders, including SUD. Number of endorsed SUD criteria defined MINI addiction severity (from 0 to 11). Substance-use characteristics and multi-dimensional addiction severity were assessed using a validated French version of the Addiction Severity Index (ASI) [44], modified to take into account tobacco addiction [44, 45]. The Interviewer Severity Ratings (ISR) from the drug/alcohol/tobacco sections of the ASI were used to assess the ASI addiction severity (from 0 to 9). When multiple SUD co-occurred, the primary substance explored in EMA assessments was determined according to the main problematic substance reported by the individual, and on which the treatment was focused. The EMA protocol was previously described in detail [7, 18, 46]. Following a training session, each participant received a personal digital assistant (PDA) to carry for 14 days, programmed to administer four electronic surveys per day, between 8:00 am and 11:00 pm. The signal schedule was chosen for participants to accommodate their usual sleep schedule. Signal times were fixed for each individual but randomized across participants in the sample using 20 distinct signaling programs. Financial compensation was provided as a function of the number of electronic surveys completed, with a maximum of 100 euros for participants who completed 75% or more of the electronic assessments. Participants in the EMA study were also included in the ADDICTAQUI Cohort, and were re-assessed with the ASI every 6 months (follow-up interviews), regardless of their status in treatment, and continued to be assessed for as long as they accepted to remain in the cohort [42]. The database for this study is based solely on individuals included in the ADDICTAQUI Cohort who had at least one follow-up 5 years or more after their inclusion in the EMA study. Both studies (ADDICTAQUI cohort and EMA study) were approved by French biomedical research regulations and ethical committees (EMA study: CPP: SOOM III/DC-2009/01; CNIL: DR-2015-408; ADDICTAQUI cohort: RIPH 2 G: 01.00002.000102; CPP N° national 2020-A03570-39), and all participants provided written informed consent.
Measures
Craving and substance use in daily life
At each electronic survey (EMA study), participants were asked to rate the maximum level of craving (i.e., the desire to use the primary substance) that they felt since the previous assessment on a 7-point scale (from 1 no desire to 7 extreme desire), if they had used this primary substance since the previous assessment, followed by questions concerning the use of any other psychoactive substances during that time period.
Long-term substance use/Abstinence status at 5+ years follow-up
Primary substance use was collected at the last follow-up assessment available (ADDICTAQUI Cohort) with the ASI. When several follow-ups were available after 5 years, the latest was considered for this study. Participants were classified into 2 categories according to the status of their primary substance use at 5+ years follow-up, regardless of potential periods of sustained abstinence: Long-term substance use or Abstinence status. Long-term substance use was defined as non-abstinence at the last assessment, i.e.,: at least 1 day of use of the primary substance in the past 30 days.
Analysis strategy
To test our hypotheses, we explored the association between craving trajectory and dynamics during the first 14 days of treatment (captured with the EMA study), and the long-term substance use status at 5+ years follow-up (captured with ADDICTAQUI Cohort follow-ups). Hierarchical linear and nonlinear modeling (HLM) [47] was used to account for the multilevel structure of the data that involved within-person variance for craving and substance use, as well as between-person variance for clinical and sociodemographic characteristics.
Prior to model estimation, participants were compared according to the substance use status at 5+ years follow-up, on initial characteristics and severity. Then, the craving intensity variance during the EMA study was explored by intraclass correlation (ICC), and a value of 0.56 indicated that 44% of variance accounted for the within-person variance. Then, the variance of craving was assessed at 3 different levels: the person level (level 3), the day level (level 2) and the assessment level (level 1) [26]. For this, a three-level unconditional model was estimated (see Supplemental Material), and the variance of craving was greatest at the person-level, that is why we performed subsequent analysis at this level.
To examine the hypothesis that the substance use status at 5+ years was associated with difference in time-dependent evolution (trajectory) of craving at the beginning of treatment, we employed a random coefficient regression model to assess the influence of time (day in study) on craving intensity, then we extracted individual coefficients, and finally these indices were tested in a logistic regression to determine their association with long-term substance use status. Then, the inertia, variability, and instability metrics of craving were calculated at the person-level. The craving inertia was obtained by means of average within-person autocorrelation (AR1) of craving at time t and craving at time t-1, the craving variability by the within-person Standard Deviation (SD), and the craving instability by the within-person root mean square of successive differences (rMSSD) [48, 49]. To explore if the long-term use status was associated with difference in craving dynamics at the beginning of treatment, associations between long-term use status and each craving metrics were tested separately in different logistic regression models, while controlling each model for the individual average craving intensity. Additionally, we explored if the long-term substance use status was associated with differences in the craving-use relationship at beginning of treatment. For this, the prospective association between craving at one time, and primary substance use at the next assessment (controlled for initial substance use) was explored in a multi-level model, then we extracted individual coefficients, and finally these indices were tested in a logistic regression to determine their association with long-term substance use status.
In all HLM analyses, continuous variables were group-centered (i.e., centered around the individual’s own mean) for within-person variables, and grand-centered (around the sample average) for between-person variables. Time-lagged analyses examined within-day (and not across-day) associations. Missing data were handled by excluding that observation from analyses. Adjustment for potential confounding factors (sex, age and pharmacological treatment) was included in model testing for influence on the 5+ year use status, if the factor was significantly associated with the predictor. Influence of such factors (sex, age, substance type and pharmacological treatment) on craving dynamics, craving/days and craving/use associations were explored by a non-parametric test (Kruskal-Wallis Test for categorical variables and linear regression for continuous variables). Influence of substance type is described in Supplemental material (Table S2 and Result S1). Due to a restricted number of assessments occurring on the starting and ending day of the study, and their overlap with investigator contact, only data collected between days 2 and 13 were analyzed. An alpha level of .05 was used in all analyses. Analyses were not pre-registered, and results should be considered exploratory. All analyzes were performed on JMP Pro (15.0), R (4.1.0) and HLM (8.0).
Results and discussion
Sample description
Among the 159 participants who were included both in ADDICTAQUI Cohort and EMA study, only those with an available follow-up assessment at 5+ years were selected to explore long-term outcomes. This sample (N = 39) was compared with participants with no follow-up available at 5 years or more (N = 120), and they were not significantly different, on baseline socio-demographic characteristics (age: p = 0.621, sex: p = 0.767), or on ASI addiction severity (p = 0.562). Nonetheless, they differed on primary substance, and were more frequently in the alcohol group and less frequently in the opiate group (p = 0.047) compared to the 120 subjects not included in this analysis.
The average long-term follow-up time was 6.5 years (SD = 12.7) after treatment initiation (when EMA study was conducted), and 51% (n = 20) of participants reported at least one day of primary substance use in the past 30 days (and were considered as long-term substance use group) (Table 1). At inclusion, the long-term substance use group and the abstinence group were not significantly different on ASI addiction severity (respectively 6.5 (SD = 0.61) vs. 6.5 (SD = 0.70); Wilcoxon: Z = 0.16; χ2 = 0.03, p = 0.854), or MINI addiction severity (respectively 6.05 (SD = 1.23) vs. 6 (SD = 1.63) criteria; Wilcoxon: Z = −0.13; χ2 = 0.02, p = 0.885) and number of days of use in past 30 days. On the contrary, at follow-up, ASI addiction severity was significantly higher in the long-term substance use group compared to the abstinence group (respectively 3.5 (1.8) vs. 0.9 (1.0); Wilcoxon: Z = −4.04; χ2 = 16.47, p < 0.0001), they reported an average of 16.6 days of use, and a majority of them (70%, n = 14) had regular use (more than 8 times in the last month, SD = 12.5; Min-Max=1–30; median= 15.5, versus 0 days for the abstinence group; Wilcoxon: Z = −5.69; χ2 = 32.57, p < 0.0001). Among all available follow-up assessments from treatment initiation to long-term follow-up (number comparable in both groups), the long-term substance use group also reported the use of the primary substance more frequently, and higher addiction severity (see Supplemental Material Results S2 and Table S3). At inclusion, among the 1497 assessments completed (corresponding to an overall response rate of 79.9%), craving episode (non-null intensity) and primary substance use were reported respectively in 67% and 34% of assessments (Table 2). Use of primary substance, other substances, and craving (intensity and episodes) were not significantly different according to long-term use status (Table 2).
Craving trajectory at treatment initiation and association with long-term substance use status
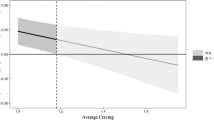
Increasing time in the first 14 days of treatment was significantly associated with a decrease in craving intensity (γ = −0.05; SE = 0.02; df = 38; T-ratio = −2.60; p = 0.013), and this association was not influenced by age (β = 0.001, p = 0.871), sex (χ2 = 0.579, df = 1, p = 0.447), treatment (χ2 = 0.801, df = 1, p = 0.370) or substance type (χ2 = 0.509, df = 3, p = 0.917). The long-term substance use group had a slower decrease of craving compared to the abstinence group (β = 8.99; SE = 4.3; Z = 2.07; p < 0.001) (Fig. 1).
Craving dynamics at treatment initiation and association with long-term substance use status
Craving dynamic parameters of inertia, variability and instability are presented in Fig. 2. These parameters were not influenced by age (β = −0.007, p = 0.074; β = 0.001, p = .985 and β = 0.01, p = 0.303 respectively), sex (χ2 = 1.92, df = 1, p = 0.166; χ2 = 0.05, df = 1, p = 0.831 and χ2 = 0.14, df = 1, p = 0.704 respectively), treatment (χ2 = 0.213, df = 1, p = 0.644; χ2 = 0.621, df = 1, p = 0.430 and χ2 = 1.08, df = 1, p = 0.299 respectively) or by substance type (χ2 = 4.90, df = 3, p = 0.179; χ2 = 0.86, df = 3, p = 0.835 and χ2 = 1.15, df = 3, p = 0.764 respectively).
A Craving inertia (AR1) for Abstinence group mean = 0.22 (SD = 0.22) vs Substance Use group mean = 0.03 (SD = 0.29). B Craving variability (SD) for Abstinence group mean = 1.43 (SD = 0.51) vs Substance Use group mean=1.19 (SD = 0.66); C Craving instability (rMSSD) for Abstinence group mean=1.75 (SD = 0.73) vs Substance Use group mean=1.61 (SD = 0.92). Violins combined with box plots depicted the data distribution, with the median shown by the dark horizontal line. Colors indicate long-term outcome groups: Substance use group (purple, N = 20) and Abstinence group (blue, N = 19). P-value: logistic regression, controlling on the individual average craving intensity.
Long-term substance use status at follow-up was not significantly associated with craving instability (β = −0.15; SE = 0.41; χ² = 0.13; p = 0.720) or craving variability (β = −0.66; SE = 0.60; χ² = 1.23; p = 0.256) at beginning of treatment (Fig. 2). By contrast, a significant association was observed with craving inertia (β = −2.78; SE = 1.42; χ² = 3.83; p = 0.034), in the direction of low inertia at treatment initiation among the long-term substance use group (Fig. 2).
The craving-use association at treatment initiation and association with long-term substance use
Prospective analysis revealed that an increase in craving intensity reported at a given time was significantly associated with a greater likelihood of reporting primary substance use at the next assessment approximately four hours later (γ = 0.15; SE = 0.04; df = 38; T-ratio = 3.11; p = 0.004). This association was found significantly influenced by age (β = 0.009, p = 0.008) and substance type (χ2 = 8.12, df = 3, p = 0.044), but not by sex (χ2 = 1.34, df = 1, p = 0.247), nor treatment (χ2 = 0.801, df = 1, p = 0.371). The long-term substance use status was not significantly associated with the craving-use association at beginning of treatment, while controlling on age and substance type (β = 0.33; SE = 1.73; Z = 0.191; p = 0.849).
Discussion
The aim of this study was to examine whether trajectory and dynamic fluctuations of craving intensity in daily life, during the first 14 days of outpatient addiction treatment, were associated with substance use/abstinence outcome at 5 years or more. Our findings show that craving intensity demonstrated an overall decrease during the first two-weeks of treatment, but this decrease was found slower among participants classified in the substance use group at 5+ years follow-up. Other studies did report that trajectories of craving or withdrawal appeared to be predictive of subsequent relapse [23, 25, 26, 50,51,52,53], but these findings were reported for short-term outcomes (one year or less) only. Our study is the first to support that craving trajectory during the first two weeks of treatment could be predictive of long-term outcome.
Exploration of craving dynamics brings interesting elements to further understand craving and its association with outcome and hints towards possible mechanisms. In this study, craving instability and variability at inclusion were not found significantly different between the substance use and the abstinence groups at 5+ years. However, interestingly, a lower craving inertia was observed in the long-term substance use group while controlling for average intensity of craving. This result suggests that, for this long-term substance use group, craving intensity at treatment initiation was more variable from moment-to-moment in daily life, while exhibiting an overall slower decrease. One hypothesis is that this lower craving inertia may reflect the persistence of reactivity to cues (and other everyday life triggers) that, in parallel, limit the overall improvement in craving (i.e.: decrease in craving intensity/frequency) that is usually experienced at beginning of addiction treatment, as observed in the abstinence group. To our knowledge, this study is the first to explore association of craving dynamics with treatment addiction outcomes and suggests that low craving inertia could represent a promising indicator for reduced treatment response and further relapse.
To be noticed, in this study, the 5+- years substance use status was not found significantly associated with the strength of the daily life craving-use association at treatment initiation, suggesting that individuals who are less resistant to craving at treatment initiation (i.e., who have a stronger craving-use link), were not necessarily at greater risk of long-term substance use. In the same way, more intense craving at treatment initiation was not found associated with a greater risk of substance use at 5+ years. This result is consistent with a previous study among inpatients in residential opioid treatment [26]. On the contrary, a previous study on alcohol reported that patients who relapsed after 12 months had significantly higher craving intensity at the end of a 3-month treatment episode [54]. However, in this previous study, craving was assessed after a period of 1 month of abstinence. Two studies on tobacco also showed that greater craving intensity at treatment intake was associated with less abstinence at one month [53], and 6 months [50].
Some limitations must be acknowledged. The first one concerned how long-term substance use outcome was operationalized, based on primary substance use reported at one time point, 5+ years after treatment intake. This may not reflect the overall outcome over the 5-years period, nor relapse or remission, that are dynamic processes [55]. We cannot rule out that some participants in the abstinence group may have relapsed at some point before this date, and that some participants in the substance use group may have experienced only a brief relapse. However, we controlled, among all available follow-up assessments from treatment initiation to long-term follow-up, that the long-term substance use group reported use of the primary substance more frequently, and had higher addiction severity compared to the abstinence group (see Supplemental Material). Nonetheless, we cannot exclude that different results could have been obtained by considering a continuous substance use measure, or different time periods for substance use measures. However, the long-term substance use group is clinically significantly different from the abstinence group on addiction severity and substance use. Although both groups presented a decrease in addiction severity and substance use compared to baseline, the substance use group is using on average every other day with important severity. Overall, these results support a significant clinical distinction between the 2 groups. Nonetheless, it is still possible that some participants from the long-term substance use group may have regained controlled use [56]. Another potential limitation to consider is that the different groups of substances were pooled. Indeed, the literature suggests that craving for different substances share similar neurobiological substrates, and similarly influence substance use and relapse, thus legitimizing lumping craving data from different substance use disorders [9, 10, 12, 46, 57]. Furthermore, additional analyses in our sample confirmed that craving dynamics and craving trajectory were not modified by substance groups (see Supplemental Material). Another limitation is that only linear trends were tested in craving trajectory. It would be interesting to explore other more complex pattern trajectories such as quadratic and cubic growth [20]. Finally, the main limitation of this study is the small sample size, which consists of only 39 participants. Therefore, our findings need to be replicated with a larger sample size in other population and should be interpreted with caution.
Results of the present work illustrate the utility of properly capturing craving dynamics and trajectory over time at treatment initiation, as they could provide promising indicators of long-term outcomes, more effectively than craving intensity only. Indeed, if immediate craving intensity is logically associated with initiation of substance use in short intervals of time, on the contrary, over longer periods of time, it is rather the way in which craving is modulated by the environment, the treatment, or managed by the participant, which is a more stable characteristic, that is more likely to provide information on what an individual might experience in a more distant future. Such prognostic markers of long-term outcomes exist for other chronic conditions in medicine and psychiatry [58], but remain to be determined for SUD. Such indicators may help clinicians to anticipate and provide adjustment in treatment provided (frequency, duration) and resource allocation, to reduce the increased risk of poorer outcome. From a therapeutic point of view, mobile technologies, such as smartphone apps, could offer accessible, easy-to-use, and autonomous measures of symptom dynamics in daily life [59,60,61], that could also be used to detect the most appropriate moment to propose intervention when needed [62].
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Witkiewitz K, Marlatt GA. Modeling the complexity of post-treatment drinking: It’s a rocky road to relapse. Clin Psychol Rev. 2007;27:724–38. https://doi.org/10.1016/j.cpr.2007.01.002
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). (American Psychiatric Pub, 2013).
Auriacombe M, Serre F, Denis C, Fatseas M. in The Routledge Handbook of the Philosophy and Science of Addiction (eds H Pickard & S.H. Ahmed) Ch. 10, 132-44 (2018).
Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–84. https://doi.org/10.1146/annurev.clinpsy.3.022806.091455
Dennis ML, Scott CK, Funk R, Foss MA. The duration and correlates of addiction and treatment careers. J Subst Abus Treat. 2005;28:S51–62. https://doi.org/10.1016/j.jsat.2004.10.013
Dennis M, Scott CK. Managing addiction as a chronic condition. Addict Sci Clin Pr. 2007;4:45–55. https://doi.org/10.1151/ascp074145
Serre F, Fatseas M, Denis C, Swendsen J, Auriacombe M. Predictors of craving and substance use among patients with alcohol, tobacco, cannabis or opiate addictions: Commonalities and specificities across substances. Addictive Behav. 2018;83:123–9. https://doi.org/10.1016/j.addbeh.2018.01.041
Cavicchioli M, Vassena G, Movalli M, Maffei C. Is craving a risk factor for substance use among treatment-seeking individuals with alcohol and other drugs use disorders? A meta-analytic review. Drug Alcohol Depend. 2020;212:108002. https://doi.org/10.1016/j.drugalcdep.2020.108002
Vafaie N, Kober H. Association of Drug Cues and Craving With Drug Use and Relapse: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022. https://doi.org/10.1001/jamapsychiatry.2022.1240
Serre F, Fatseas M, Swendsen J, Auriacombe M. Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug Alcohol Depend. 2015;148C:1–20. https://doi.org/10.1016/j.drugalcdep.2014.12.024
Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 Criteria for Substance Use Disorders: Recommendations and Rationale. Am J Psychiatry. 2013;170:834–51. https://doi.org/10.1176/appi.ajp.2013.12060782
Gauld C, Baillet E, Micoulaud-Franchi J-A, Kervran C, Serre F, Auriacombe M. The Centrality of Craving in Network Analysis of Five Substance Use Disorders. Drug Alcohol Depend. 2023;109828. https://doi.org/10.1016/j.drugalcdep.2023.109828
O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31.
Moore TM, Seavey A, Ritter K, McNulty JK, Gordon KC, Stuart GL. Ecological momentary assessment of the effects of craving and affect on risk for relapse during substance abuse treatment. Psychol Addictive Behav: J Soc Psychologists Addictive Behav. 2014;28:619–24. https://doi.org/10.1037/a0034127
Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95:S189–210.
Enkema MC, Hallgren KA, Larimer ME. Craving is impermanent and it matters: Investigating craving and cannabis use among young adults with problematic use interested in reducing use. Drug Alcohol Depend. 2020;210:107957. https://doi.org/10.1016/j.drugalcdep.2020.107957
Stone AA, Shiffman S. Ecological Momentary Assessment (Ema) in Behavioral Medicine. Ann Behav Med. 1994;16:199–202. https://doi.org/10.1093/abm/16.3.199
Serre F, Fatseas M, Debrabant R, Alexandre J-M, Auriacombe M, Swendsen J. Ecological momentary assessment in alcohol, tobacco, cannabis and opiate dependence: A comparison of feasibility and validity. Drug Alcohol Depend. 2012;126:118–23. https://doi.org/10.1016/j.drugalcdep.2012.04.025
Panlilio LV, Stull SW, Kowalczyk WJ, Phillips KA, Schroeder JR, Bertz JW, et al. Stress, craving and mood as predictors of early dropout from opioid agonist therapy. Drug Alcohol Depend. 2019;202:200–8. https://doi.org/10.1016/j.drugalcdep.2019.05.026
Burgess-Hull AJ, Panlilio LV, Preston KL, Epstein DH. Trajectories of craving during medication-assisted treatment for opioid-use disorder: Subtyping for early identification of higher risk. Drug Alcohol Depend. 2022;233:109362. https://doi.org/10.1016/j.drugalcdep.2022.109362
Ellis JD, Mun CJ, Epstein DH, Phillips KA, Finan PH, Preston KL. Intra-individual variability and stability of affect and craving among individuals receiving medication treatment for opioid use disorder. Neuropsychopharmacology. 2022;47:1836–43. https://doi.org/10.1038/s41386-022-01352-y
Serre F, Gauld C, Lambert L, Baillet E, Beltran V, Daulouede JP, et al. Predictors of substance use during treatment for addiction: A network analysis of ecological momentary assessment data. Addiction. 2024. https://doi.org/10.1111/add.16658
McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115:454–66. https://doi.org/10.1037/0021-843X.115.3.454
Bujarski S, Roche DJ, Sheets ES, Krull JL, Guzman I, Ray LA. Modeling naturalistic craving, withdrawal, and affect during early nicotine abstinence: A pilot ecological momentary assessment study. Exp Clin Psychopharmacol. 2015;23:81–89. https://doi.org/10.1037/a0038861
Shiyko MP, Lanza ST, Tan X, Li R, Shiffman S. Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: differences between successful quitters and relapsers. Prev Sci: Off J Soc Prev Res. 2012;13:288–99. https://doi.org/10.1007/s11121-011-0264-z
Cleveland HH, Knapp KS, Brick TR, Russell MA, Gajos JM, Bunce SC. Effectiveness and Utility of Mobile Device Assessment of Subjective Craving during Residential Opioid Dependence Treatment. Subst Use Misuse. 2021;56:1284–94. https://doi.org/10.1080/10826084.2021.1921808
Ebner-Priemer UW, Eid M, Kleindienst N, Stabenow S, Trull TJ. Analytic strategies for understanding affective (in)stability and other dynamic processes in psychopathology. J Abnorm Psychol. 2009;118:195–202. https://doi.org/10.1037/a0014868
Trull TJ, Lane SP, Koval P, Ebner-Priemer UW. Affective Dynamics in Psychopathology. Emot Rev. 2015;7:355–61. https://doi.org/10.1177/1754073915590617
Lamers F, Swendsen J, Cui L, Husky M, Johns J, Zipunnikov V, et al. Mood reactivity and affective dynamics in mood and anxiety disorders. J Abnorm Psychol. 2018;127:659–69. https://doi.org/10.1037/abn0000378
Johns JT, Di J, Merikangas K, Cui L, Swendsen J, Zipunnikov V. Fragmentation as a novel measure of stability in normalized trajectories of mood and attention measured by ecological momentary assessment. Psychol Assess. 2019;31:329–39. https://doi.org/10.1037/pas0000661
Panaite V, Rottenberg J, Bylsma LM. Daily Affective Dynamics Predict Depression Symptom Trajectories Among Adults with Major and Minor Depression. Affect Sci. 2020;1:186–98. https://doi.org/10.1007/s42761-020-00014-w
Husen K, Rafaeli E, Rubel JA, Bar-Kalifa E, Lutz W. Daily affect dynamics predict early response in CBT: Feasibility and predictive validity of EMA for outpatient psychotherapy. J Affect Disord. 2016;206:305–14. https://doi.org/10.1016/j.jad.2016.08.025
Landmann S, Cludius B, Tuschen-Caffier B, Moritz S, Külz AK. Mindfulness predicts insight in obsessive-compulsive disorder over and above OC symptoms: An experience-sampling study. Behav Res Ther. 2019;121:103449. https://doi.org/10.1016/j.brat.2019.103449
Buu A, Cai Z, Li R, Wong SW, Lin HC, Su WC, et al. The association between short-term emotion dynamics and cigarette dependence: A comprehensive examination of dynamic measures. Drug Alcohol Depend. 2021;218:108341. https://doi.org/10.1016/j.drugalcdep.2020.108341
Chirokoff V, Dupuy M, Abdallah M, Fatseas M, Serre F, Auriacombe M, et al. Craving dynamics and related cerebral substrates predict timing of use in alcohol, tobacco, and cannabis use disorders. Addiction Neurosci. 2023;9:100138.
Houben M, Van Den Noortgate W, Kuppens P. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychol Bull. 2015;141:901–30. https://doi.org/10.1037/a0038822
Morawetz C, Berboth S, Chirokoff V, Chanraud S, Misdrahi D, Serre F, et al. Mood Variability, Craving, and Substance Use Disorders: From Intrinsic Brain Network Connectivity to Daily Life Experience. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8:940–55. https://doi.org/10.1016/j.bpsc.2022.11.002
Lai S, Dimko M, Galani A, Coppola B, Innico G, Frassetti N, et al. Early markers of cardiovascular risk in chronic kidney disease. Ren Fail. 2015;37:254–61. https://doi.org/10.3109/0886022x.2014.982489
Konerman MA, Yapali S, Lok AS. Systematic review: identifying patients with chronic hepatitis C in need of early treatment and intensive monitoring-predictors and predictive models of disease progression. Aliment Pharm Ther. 2014;40:863–79. https://doi.org/10.1111/apt.12921
Greenberg JL, Jacobson NC, Hoeppner SS, Bernstein EE, Snorrason I, Schwartzberg A, et al. Early response to cognitive behavioral therapy for body dysmorphic disorder as a predictor of outcomes. J Psychiatr Res. 2022;152:7–13. https://doi.org/10.1016/j.jpsychires.2022.06.001
Auriacombe M, Fatseas M. Approche transversale de la thérapeutique et des prises en charge en addictologie: principes. in Addictologie (ed M. Lejoyeux) 449-58 (2023). https://doi.org/10.1016/B978-2-294-77934-3.00051-2
Auriacombe M. ADDICTAQUI - Aquitaine Addiction Cohort: Trajectories of people with addiction (substances or behaviour) in contact with health-care system. Medical, neurobiological, sociological and psychological characteristics. Prospective multicentric, multidisciplinary study <https://epidemiologie-france.aviesan.fr/en/epidemiology/records/cohorte-addiction-aquitaine-trajectoires-de-personnes-presentant-une-addiction-aux-substances-ou-une-addiction-comportementale-en-contact-avec-le-dispositif-de-soins.-caracteristiques-medicales-neurobiologiques-sociologiques-et-psychologiques.-etude#tab_1> (2017).
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:34–57.
McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. J Subst Abus Treat. 1992;9:199–213. https://doi.org/10.1016/0740-5472(92)90062-S
Denis C, Fatséas M, Beltran V, Serre F, Alexandre J-M, Debrabant R, et al. Usefulness and validity of the modified Addiction Severity Index: A focus on alcohol, drugs, tobacco, and gambling. Subst Abus. 2016;37:168–75. https://doi.org/10.1080/08897077.2015.1036334
Fatseas M, Serre F, Alexandre J-M, Debrabant R, Auriacombe M, Swendsen J. Craving and substance use among patients with alcohol, tobacco, cannabis or heroin addiction: a comparison of substance- and person-specific cues: Cues, craving and substance use. Addiction. 2015;110:1035–42. https://doi.org/10.1111/add.12882
Raudenbush S, Bryk A, Congdon R. HLM for Windows, version 6.03. Chicago, IL: Scientific Software International (2005).
Neuman. Distribution of the Ratio of the Mean Square Sucessive Difference to the Variance. Ann Math Stat. 1941;12:367–95.
Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychol Methods. 2008;13:354–75. https://doi.org/10.1037/a0014173
Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117:94–105. https://doi.org/10.1037/0021-843X.117.1.94
Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112:3–13.
Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh W-Y, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216:569–78. https://doi.org/10.1007/s00213-011-2250-3
Cofta-Woerpel L, McClure JB, Li Y, Urbauer D, Cinciripini PM, Wetter DW. Early cessation success or failure among women attempting to quit smoking: Trajectories and volatility of urge and negative mood during the first postcessation week. J Abnorm Psychol. 2011;120:596–606. https://doi.org/10.1037/a0023755
Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol. 2004;39:357–61. https://doi.org/10.1093/alcalc/agh073
Maisto SA, Roos CR, Hallgren KA, Moskal D, Wilson AD, Witkiewitz K. Do Alcohol Relapse Episodes During Treatment Predict Long-Term Outcomes? Investigating the Validity of Existing Definitions of Alcohol Use Disorder Relapse. Alcohol Clin Exp Res. 2016;40:2180–9. https://doi.org/10.1111/acer.13173
Witkiewitz K, Wilson AD, Pearson MR, Montes KS, Kirouac M, Roos CR, et al. Profiles of recovery from alcohol use disorder at three years following treatment: can the definition of recovery be extended to include high functioning heavy drinkers? Addiction. 2019;114:69–80. https://doi.org/10.1111/add.14403
Koban L, Wager TD, Kober H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat Neurosci. 2023;26:316–25. https://doi.org/10.1038/s41593-022-01228-w
McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug Dependence, a Chronic Medical IllnessImplications for Treatment, Insurance, and Outcomes Evaluation. JAMA. 2000;284:1689–95. https://doi.org/10.1001/jama.284.13.1689.
Dulin PL, Gonzalez VM. Smartphone-based, momentary intervention for alcohol cravings amongst individuals with an alcohol use disorder. Psychol Addict Behav. 2017;31:601–7. https://doi.org/10.1037/adb0000292
Bahadoor R, Alexandre JM, Fournet L, Gellé T, Serre F, Auriacombe M. Inventory and Analysis of Controlled Trials of Mobile Phone Applications Targeting Substance Use Disorders: A Systematic Review. Front Psychiatry. 2021;12:622394. https://doi.org/10.3389/fpsyt.2021.622394
Serre F, Moriceau S, Donnadieu L, Forcier C, Garnier H, Alexandre JM, et al. The Craving-Manager smartphone app designed to diagnose substance use/addictive disorders, and manage craving and individual predictors of relapse: a study protocol for a multicenter randomized controlled trial. Front Psychiatry. 2023;14:1143167. https://doi.org/10.3389/fpsyt.2023.1143167
Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, et al. Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Ann Behav Med. 2018;52:446–62. https://doi.org/10.1007/s12160-016-9830-8
Acknowledgements
The authors express their thanks to all participants for their contribution and are grateful to all the interviewers of the ADDICTAQUI team.
Funding
Office and staff support was provided by CH Charles Perrens and University of Bordeaux. This study received financial support from the French government in the framework of the University of Bordeaux’s IdEx “Investments for the Future” program / GPR BRAIN_2030. Funding for the ADDICTAQUI cohort data collection was provided by Research Grant PHRC (2006-2014) from the French Ministry of Health, French Government Addiction Agency MILDT/MILDECA grant 2010 and 2016 to Marc Auriacombe. Funding for the EMA study was provided by Research Grant AAP-Recherche-CRA (20091301018) from the Aquitaine Regional Council to Fuschia Serre and Marc Auriacombe, French National Research Agency PRA-CNRS-CHU-Bordeaux award (2008-2010) to Melina Fatseas and CNRS ATIP award to Joel Swendsen. Emmanuelle Baillet received a PhD doctoral grant from French Cancer and Public Health Instituts (INCA-IRESP-2020-169).
Author information
Authors and Affiliations
Contributions
MA was the overall principal investigator and study supervisor, obtained funding and access to participants. MA, FS, MF and JS developed the EMA study design and methods. EB and FS conceptualized and conducted the analyses, interpreted the data, and wrote the manuscript. CR and CG helped in analysis. HG, CR and CV helped for literature review and participants inclusion. All authors undertook the critical revision of the manuscript for important intellectual content and all authors significantly contributed to the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Both studies (ADDICTAQUI cohort and EMA study) were approved by French biomedical research regulations and ethical committees (EMA study: Comité de Protection des Personnes (CPP SOOM III/DC-2009/01; CNIL: DR-2015-408; ADDICTAQUI cohort: RIPH 2 G: 01.00002.000102; Comité de Protection des Personnes CPP N° national 2020-A03570-39). All participants signed an informed consent form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baillet, E., Auriacombe, M., Romao, C. et al. Craving changes in first 14 days of addiction treatment: an outcome predictor of 5 years substance use status?. Transl Psychiatry 14, 497 (2024). https://doi.org/10.1038/s41398-024-03193-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03193-3