Abstract
The high comorbidity of major depressive disorder (MDD) with other diseases has been well-documented. However, the pairwise causal connections for MDD comorbid networks are poorly characterized. We performed Phenome-wide Mendelian randomization (MR) analyses to explore bidirectional causal associations between MDD (N = 807,553) and 877 common diseases from FinnGen datasets (N = 377,277). The inverse variance weighting method was the primary technique, and other methods (weighted median and MR-Egger) were used for sensitivity analyses. Our MR analyses showed that the genetic liability to MDD is causally associated with the risks of 324 disease phenotypes (average b: 0.339), including 46 psychiatric and behavioral disorders (average b: 0.618), 18 neurological diseases (average b: 0.348), 44 respiratory diseases (average b: 0.345), 40 digestive diseases (average b: 0.281), 18 circulatory diseases (average b: 0.237), 37 genitourinary diseases (average b: 0.271), 66 musculoskeletal and connective diseases (average b: 0.326), 22 endocrine diseases (average b: 0.302), and others. In a reverse analysis, a total of 51 genetic components predisposing to various diseases were causally associated with MDD risk (average b: 0.086), including 5 infectious diseases (average b: 0.056), 11 neurological diseases (average b: 0.106), 14 oncological diseases (average b: 0.108), and 5 psychiatric and behavioral disorders (average b: 0.114). Bidirectional causal associations were identified between MDD and 15 diseases. For most MR analyses, little evidence of heterogeneity and pleiotropy was detected. Our findings confirmed the extensive and significant causal role of genetic predisposition to MDD in contributing to human disease phenotypes, which were more pronounced than those seen in the reverse analysis of the causal influences of other diseases on MDD.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is widely acknowledged as a significant global mental health challenge [1, 2]. With a lifetime prevalence of 10 percent, MDD ranks first in prevalence among all mental illnesses and is one of the leading contributors to the global morbidity burden [3]. MDD, a prototypical example of a severe mental illness, is characterized by a multifaceted etiology that encompasses both genetic and environmental determinants. In addition to exploring the biological mechanisms of MDD, addressing its long-term management and rehabilitation remains a top priority. The rising prevalence of depressive comorbidities in clinical practice complicates treatment and recovery [4]. These conditions lead to numerous adverse outcomes, including functional dependence [5], poor quality of life [6], and shortened life expectancy [7], thus, creating significant economic burdens for families and society. Further exploration is needed to understand the challenges posed by comorbid cases of MDD.
As the global standards of health care gradually improve, comorbid and multi-morbid case histories are becoming more prevalent. Epidemiological evidence suggests that 75 percent of people aged 65-74 years are multi-morbid, with an even higher prevalence of co-morbidity detected in cohorts older than 75 years [8]. Among adults aged 18-65 years, a population-based survey showed that the prevalence of comorbid states ranges from 7 to 35 percent [9]. Due to its high prevalence, MDD became one of the most common comorbid conditions among adults, especially in patients with cardiovascular diseases (e.g., coronary artery disease) [10], metabolic diseases (e.g., diabetes mellitus) [11], skin, muscle and connective tissue diseases (e.g., microvascular dysfunction) [12] as well as rheumatological and immunological diseases (e.g., arthritis) [13]. In addition, the results of a meta-analysis of 12 longitudinal studies and 16 cross-sectional surveys showed that older people with chronic conditions had a higher risk of depression when compared to those without chronic conditions (RR: 1.53, 95 percent confidence interval (CI): 1.20-1.97) [14]. On the other hand, numerous studies have confirmed that individuals with depression have a markedly increased risk of becoming comorbid with epilepsy [15], hypertensive disorders [16], thyroid dysfunction [17], and pain [18]. All indications point to the possibility of MDD interacting with other disorders, or even being bi-directionally associated with such. Nonetheless, the causal relationships between MDD and other conditions remain unclear.
The most common instrument for disentangling relationships between human conditions, so-called observational studies, faces challenges in evaluating the significance of uncovered effects due to the influence of confounding factors and reverse causation, which hinder directional inferences. In contrast, the Mendelian randomization (MR) analysis framework investigates causal links between different exposures and outcomes [19] by leveraging comprehensive sets of genetic variants, with an emphasis on that collected in GWAS databases. In recent years, discussions regarding causal associations between MDD and other diseases, such as schizophrenia [20], prostate cancer [21], inflammatory bowel disease [22], atopic diseases [23], and osteoporosis [24] have become widespread. However, numerous diseases have yet to be validated in relation to MDD. Bidirectional MR studies facilitate the development of clinical guidelines by discerning the causal pathophysiological drivers for MDD and its common co-morbidities.
To comprehensively explore the causal implications of genetic predisposition to MDD on the risks of developing other disorders, we compiled a list of disease phenotypes from the FinnGen database (version R9). As proxies for MDD, the genome-wide association study (GWAS) results from the Psychiatric Genomics Consortium (PGC) were extracted. Subsequently, two-sample bidirectional MR analyses were conducted to assess causal connections between MDD and a range of disease phenotypes at the genetic level.
Methods
Study design and data source
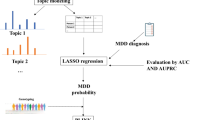
Figure 1 presents the study design. Phenome-wide, bidirectional two-sample MR analyses were combined to explore causal associations between MDD and the health conditions profiled in the FinnGen Biobank (R9). Three assumptions were made regarding these MR analyses. Firstly, the genetic variants employed as instrumental variables exhibited a close and direct relationship with the exposure. Secondly, these genetic variants should not be associated with any potential confounding factors, preserving the integrity of the analysis. The final assumption stated that the chosen genetic variants influenced the outcome exclusively through the mediation of known risk factors, ruling out any alternative pathways [25].
A total of 877 phenotypes were included. With adjusted p-values at the significance level (FDR < 0.05), genetically predicted depression was associated with a total of 324 disease phenotypes. In turn, a total of 51 genetically predicted disease phenotypes were associated with depression. Among these, bidirectional causal associations with depression were detected for 15 disease phenotypes. MR, Mendelian randomization; FDR, False discovery rate.
The dataset for MDD was sourced from the Psychiatric Genomics Consortium [26] and was originally combined by a meta-analysis of data from 807,553 individuals (246,363 cases and 561,190 controls) in the three largest genome-wide association studies of depression [27,28,29].
GWAS data for all other phenotypes were obtained from the R9 FinnGen database, with a total sample size of 377,277 individuals (166,407 men and 210,870 women), and a total of 2,272 disease phenotypes profiled [30]. We selected a total of 904 disease and health-related phenotypes with Ncase > 2000 (Table S2). We conducted a rigorous screening of the GWAS summary dataset to exclude a set of specific labels, such as depression-related medications (e.g., ANTIDEPRESSANTS), mode of reimbursements (e.g., HYPOTHY_REIMB), depression itself, or emotion-related phenotypes (e.g. F5_DEPRESSIO), descriptive non-disease traits (e.g., BMI_IRN), and some other conditions out of scope of this study (e.g., O15_DELIV_SPONT). In cases where traits overlapped, priority was given to retaining the trait with the largest sample size. Following the filtering process, a total of 877 phenotypes from 41 disease categories were retained (Table S1).
Two-sample MR analysis
The primary MR analyses were conducted utilizing the inverse variance weighting approach (IVW), supplemented by the weighted median (WM) and MR-Egger techniques. We used a non-zero intercept in this regression model as an indicator of imbalanced horizontal pleiotropy [31]. The validity of IVs was called into question when the MR Egger intercept significantly deviated from zero. Additionally, we evaluated the heterogeneity of the MR analyses using Cochran’s Q test and the I2 statistic, thereby enhancing the robustness of our investigation [32].
Significant correlations were identified by applying a stringent False Discovery Rate (FDR) threshold of less than 0.05, primarily based on the IVW method. To ensure the robustness of our IVs, we selected single nucleotide polymorphisms (SNPs) from the GWAS dataset based on their genome-wide significance (p < 5.00E−08). In cases of an absence of significant variants at the genome-wide level or when the number of suitable variants is limited to 10 or less, the thresholds for IVs selection within the MR pipeline should be relaxed to a p-value threshold of 1.00E−05. Subsequently, these SNPs were further refined within a 10 Mb window, employing a clumped r2 cut-off value of 0.001. For each MR analysis, we systematically removed SNPs that were not present in the resultant dataset, SNPs with intermediate allele frequencies, and palindromic SNPs. This rigorous curation of the IVs ensured the quality and reliability of our MR analyses.
All statistical analyses in this study were performed using the R software package TwoSampleMR (version 0.5.7).
Results
Causal effects of MDD on other disorders
With adjusted p-values at the significance level (FDR < 0.05), genetically predicted depression was associated with a total of 324 disease phenotypes (Fig. 2a, c & Table 1). In turn, a total of 51 genetically predicted disease phenotypes were associated with depression at FDR < 0.05 (Fig. 2b, c & Table 2). Among these, bidirectional causal associations with depression were detected for 15 genetically predicted disease phenotypes at FDR < 0.05 (Fig. 1).
a Volcano plot of MDD on other diseases in the FinnGEN database. b Volcano plot of other diseases in the Finnish database on MDD. The Beta value is used as the X-axis and -lg(FDR-Value) is used as the Y-axis. The FDR-Values were cut off at the 1.00E-5 level. The red dotted line indicates the level of statistical significance (FDR <0.05). c Violin plot of the MR and reverse MR analysis. The Beta value is used as the Y-axis and groups are used as the X-axis. Group 1: the Beta value plot of MDD on other diseases in the FinnGEN database. Group 2: the Beta value plot of other diseases in the Finnish database on MDD. FDR false discovery rate.
In this study, MR analyses yielded a total of 320 positive and 4 negative causal effects of the genetic predisposition to MDD on other disorders (Table 1). The majority of associations were associated with diseases of the musculoskeletal system and connective tissue (66/133), diseases of the digestive system (40/97), psychiatric and behavioral disorders (46/59), diseases of the genitourinary system (37/126), diseases of the respiratory system (44/69), diseases of the nervous system (18/40), endocrine nutritional and metabolic disorders (15/42), and diseases of the circulatory system (18/74). Among psychiatric disorders, MDD had the greatest causal impact on personality disorders (OR = 2.33~3.18), followed by a category of “any mental disorder or suicide (or attempt); psychiatric complications of pregnancy, partum or puerperium; nerve system disorders; hyperkinetic disorders; eating disorders; suicide or other intentional self-harm” (OR > 2.00). Another large causal effect of MDD was on fibromyalgia (OR = 1.70~3.19), followed by a total of 11 dermatoskeletal and connective tissue disorders including rheumatism, radiculopathy, trochanteric bursitis, ankylosing spondylitis, etc (OR > 1.50). Among digestive disorders, more pronounced causal effects of MDD were seen on dental caries and temporomandibular joint disorders (OR > 1.50). Among the categories of mental and behavioral disorders, the most significantly causally affected by MDD were emotionally unstable personality disorder (OR = 2.39~3.82), along with a group of nine other disorders (OR > 2.00), including mixed and other personality disorders, other and unspecified mood [affective] disorders, other anxiety disorders, and persistent mood disorders, etc. Four genitourinary disorders that were significantly affected by the genetic component of MDD were unspecified/other endometriosis, adenomyosis (Endometriosis of the uterus), neuromuscular dysfunction of the bladder, and inflammatory diseases of the uterus (OR > 1.50). Among the diseases of the nervous system, the genetic component of MDD affected six disorders: migraine without aura and triptan purchases, migraine without aura, other headache syndromes, migraine with aura and triptan purchases, brachial plexus disorders, and Other and unspecified nerve root and plexus disorders, also in other diseases (OR > 1.50). Among the endocrine and nutritional metabolism category, a total of five diseases were significantly and causally affected by MDD: ovarian dysfunction, familial hypercholesterolemia with ischemic heart disease, obesity, obesity due to excess calories, as well as disorders of lipoprotein metabolism and other lipemias (OR > 1.50). Among circulatory disease, only post-myocardial infarction status and non-ruptured cerebral aneurysm categories were significantly and causally affected by a genetic predisposition to MDD (OR > 1.50) (Fig. 2a and 3a).
a The OR value plot of the effects of other disorders on MDD in the two-sample MR analysis. The causal impact of other disorders on depression is characterized by a wide range of disorders with strong and significant differences in their contributions, with the more prominent ones being emotionally unstable personality disorder (OR = 3.02), personality disorders (more control exclusions) (OR = 2.66), mixed and other personality disorders (OR = 2.46), any mental disorder, or suicide (or attempt), or psychic disorders complicating pregnancy, partum or puerperium or nerve system disorders (OR = 2.44), fibromyalgia (OR = 2.33), panic disorder (OR = 2.30), hyperkinetic disorders (OR = 2.22), other and unspecified mood [affective] disorders (OR = 2.17), other anxiety disorders (OR = 2.16). KRA_PSY_ANYMENTAL_SUICID_PREG_NERV_EXMORE: any mental disorder, suicide (or attempt), or psychic disorders complicating pregnancy, partum or puerperium or nerve system disorders (more control exclusions); F5_BEHEMOCHILD: behavioural and emotional disorders with onset usually occurring in childhood and adolescence; F5_OTHERSUB: mental and behavioral disorders due to multiple drug use and use of multiple drugs and use of other psychoactive substances; VWXY20_INTENTI_SELF_P_EXPOS_OTHER_UNSPE_CHEMIC_NOXIO_SUBST: intentional self-poisoning by and exposure to other and unspecified chemicals and noxious substances. The β-values were cut off at the 0.50 level. b The OR value plot of the effects of MDD on other disorders in the 2-sample MR analysis. The causal impact of depression on other disorders is characterized by a relatively limited variety of disorders with restricted and non-significant differences in contribution, with the more prominent ones being haemangioma and lymphangioma, any site (OR = 1.41), benign neoplasm of the eye and adnexa (OR = 1.31), and benign lipomatous neoplasm of other sites/unspecified (OR = 1.30).
Causal effects of other diseases on MDD
A total of 48 positive and 3 negative causal correlations were identified in the MR analyses of the causal effects of other diseases on MDD (Table 2). Most associations are causally connected to neoplastic conditions associated with MDD (14/64), diseases of the nervous system (11/40), and certain infectious and parasitic diseases (5/31). Among the neoplastic conditions, the most prominent causal impacts on MDD were detected for hemangioma and lymphangioma, at any site (OR = 1.33 ~ 1.50). Among the neurological disorders having a causal impact on MDD were the inflammatory diseases of the central nervous system (OR = 1.13~1.36). Certain infectious and parasitic diseases have also exhibited a causal relationship with MDD, with a category “Other predominantly sexually transmitted diseases, not elsewhere classified” (OR = 1.19 ~ 1.38) (Figs. 2b and 3b).
MR sensitivity analyses
For each bidirectional MR analysis with significant causal associations revealed, we assessed the hypothesis that IVs exhibit no pleiotropy. The MDD dataset comprised 83 IVs, while other diseases had varying numbers of IVs, ranging from 10 to 241. Sensitivity analyses performed within the framework of 2-sample MR and the MR-Egger regression pointed to limited evidence of heterogeneity (I2 < 0.80). Additionally, there were some indications of pleiotropic effects, with intercept values falling below the 0.06 threshold (Tables S3). On the other hand, both the primary (IVW) and sensitivity analyses (WM and Egger) demonstrated concordant directional effects, pointing to the robustness of the primary results (Tables S4). Similarly, in the sensitivity analyses of the reverse 2-sample MR and MR-Egger regression, little heterogeneity was observed (I2 < 0.80), with no evidence of pleiotropic effects (intercepts < 0.03) (Table S5). The directions of the effects between the main and sensitivity analyses were consistent, and the main results were robust (Table S6).
Discussion
In this study, we report the results of phenome-wide MR analysis of a total of 877 non-depression-related disease phenotypes from the FinnGen database. In 2-sample MR analyses, a total of 324 genetic signatures of disease phenotypes were causally associated with MDD. Research indicates that MDD significantly influences various systemic diseases, encompassing conditions such as bone-muscle-skin-related diseases, oncological diseases, infectious diseases, and traumatic injuries. Additionally, it extends its causal impact to psycho-neurological disorders, cardio-cerebral vascular diseases, respiratory disorders, endocrine-related diseases, digestive disorders, and genitourinary disorders. Conversely, genetic liability to MDD causally affects infectious diseases, tumor-related disorders, traumatic injuries, and others, in addition to mental and behavioral disorders, neurological disorders, circulatory disorders, and reproductive disorders. In a reverse 2-sample MR analysis, the genetic susceptibility to 51 phenotypes was causally affected by MDD. Intersections of these two sets of causal associations produced a total of 15 disease phenotypes with a bidirectional causal link to MDD.
Among other disorders causally contributing to MDD, the most prominent were those affecting the musculoskeletal system and connective tissue. Many of these conditions are associated with a range of painful symptoms (e.g., fibromyalgia, low back pain, pain in joints, limb pain, and thoracic spine pain, etc.). Notably, depression is commonly associated with somatic symptoms. Likewise, pain clinics commonly encounter patients exhibiting depressive symptoms. One observational study involving 5,372 community-dwelling older adults found that individuals experiencing pain were notably more likely to exhibit depressive symptoms [33]. Another prospective survey conducted in the United States indicated that the presence of depressive symptoms predicted the future onset of chronic musculoskeletal pain [34]. Furthermore, a recent systematic review and meta-analysis, which included five cohort studies and six cross-sectional studies, demonstrated that back pain could be a predictor for depressive episodes (OR = 2.07) [35]. Depression is closely associated with somatic pain, whereas the link between migraine and depression in children is well documented [36]. Similarly, adults with migraine often present with anxiety and depressive symptoms [37]. Furthermore, depression is an independent risk factor for poor migraine prognosis [38]. This bidirectional relationship was further confirmed in the present study. Additionally, depression and pain share a descending pathway within the autonomic nervous system (ANS) [18]. Dysfunctions of ANS encompass the circulatory system (e.g., cardiac arrhythmias) [39], that of the digestive system (e.g., gastroesophageal reflux) [40], and metabolic disorders (e.g., obesity) [41]. This study effectively demonstrates the significant causal implications of genetic predisposition to these disorders on the development of depression. Together with pain, these ANS dysfunctions may have an impact on MDD through a shared mechanistic pathway, which is possibly related to pain.
Indeed, multiple lines of evidence suggest the potential for a bidirectional causal relationship between depression and pain. The present study found further evidence for one kind of pain-related condition, cervicobrachial syndrome. This finding, however, needs replication and validation through larger-scale longitudinal studies.
One of the possible mechanisms to connect digestive diseases and mental disorders is through changes in the niche ecology of the gut, which may involve the function or composition of bacteria, viral, fungal, archaea, and protozoa species [42]. Distinct species of individual human gut are shaped by a multitude of factors, including direct perturbations of the intestinal milieu as well as lifestyle, and dietary choices [43]. While the framework for studying the microbiota-gut-brain axis (MGBA) originated from observations of patients with inflammatory bowel disease and irritable bowel syndrome (IBS) [44, 45], it further expanded into the phenomenon of depression and other psychiatric conditions [46]. In particular, the impacts of gut microbes on depressive behavior have been validated in a well-established rat model [47]. The most likely culprit that connects the microbiota with the pathophysiology of depression is the microbial synthesis of serotonin and its role in ensuring the continuity of the gut barrier [48, 49]. The mechanistic connections between various phenotypes of digestive disorders and MDD, as found in this study, likely occur through variations in gut microbiota. Specifically, it typically initiates with abnormalities in genetic composition affecting homeostasis in the host, followed by changes in metabolite abundance, which leads to disruption of intestinal barrier integrity, translocation of toxic metabolites into the circulation, and contributes to chronic inflammatory responses that ultimately culminate in the development of depressive comorbidities [50]. Shared genetic profiles of depression and metabolic traits provide convincing evidence for the above theories [51, 52]. Furthermore, kynurenine levels have been strongly associated with the severity of depression, indicating its potential as a predictive marker for disease progression [53]. The involvement of metabolites in digestive disorders such as inflammatory bowel disease has been extensively described [54]. Collectively, these findings underscore the potential role of the gut microbiome and its metabolites as critical links between MDD and digestive disorders.
Currently, mental illnesses continue to be diagnosed symptomatically, with no objective genetic or soluble biomarker validated so far [55]. Biochemical boundaries between MDD and other mental or neurological disorders are not well-defined. The 5-hydroxytryptamine (5-HT) deficiency hypothesis of MDD put forward by Coppen in 1967 may also be relevant to other psychiatric conditions. Recently, this view has been strengthened in a large number of studies [56,57,58]. 5-HT, a monoamine neurotransmitter, is released in both the central nervous system (5%) and the peripheral nervous system (95%) [59]. Genetic variants and changes in expression levels of 5-HT receptors and transporters [60] as well as overactivity of presynaptic autoreceptors [61] are implicated in a wide range of psychiatric or neurological disorders, such as depression, panic disorder, schizophrenia, and migraine [62,63,64,65]. Association of depression with other psychiatric or neurological disorders at the genetic level has proceeded [66,67,68,69,70]. Accordingly, this study demonstrated that both psychiatric and neurological disorders have a causal influence on MDD, possibly through alterations in serotoninergic signaling. Some of these interactions were bidirectional, including that with other and unspecified nonorganic psychotic disorders; with delirium, not induced by alcohol and other psychoactive substances; with other and unspecified nerve root and plexus disorders; with migraine with aura and triptan purchases; with other neurological diseases, and with nerve, nerve root and plexus disorders.
Our observations are also in line with previous findings that other psychiatric comorbidity among mental disorders is common and that many of these disorders exhibit pairwise relationships [71, 72]. For example, an analysis based on 27 national communities involving 145,990 respondents showed that, after adjusting for comorbidities, the risk of developing generalized anxiety disorder was 55 times higher in the first year after developing major depression when compared to patients without comorbidities (hazard ratio (HR) = 55.1, 95% CI = 51.0–59.6); even 15 years later, patients with major depression still had a nearly 7-fold higher risk of developing generalized anxiety disorder (HR = 6.6, 95% CI = 5.7–7.7) [73]. Additionally, a majority of antidepressants and antipsychotics used in clinical practice for various psychiatric conditions target the same set of pathways [74].
Although typical infection is only a short-term pathological process, it may have a profound effect on MDD. Multiple meta-analyses have confirmed [75,76,77] that considerable consensus exists on the increase of pro-inflammatory cytokines and acute-phase proteins, in particular c-reactive protein (CRP), in individuals with MDD compared to healthy controls [78,79,80]. As an acute phase response protein, the high sensitivity of CRP is regularly used as a biomarker of infection in clinical practice [81]. Acute stress and/or traumatic brain injury increases blood-brain barrier (BBB) permeability [82] prompting CRP to cross the BBB [83], triggering an inflammatory response as well as emotional and behavioral disorders. Naturally, dysregulation of peripheral myeloid cells, pro-inflammatory cytokines, and complement pathways may be potential complementary mechanisms of action [84]. In addition, CNS inflammation can be driven by elevated levels of CRP and its pro-inflammatory activity through the activation of microglia and astrocytes [85]. In comparison, while chronic and low-grade inflammation has been observed in only a quarter of patients with MDD, pro-inflammatory signaling pathways are still considered to have a significant role in the development of MDD [86,87,88]. Indeed, the levels of acute phase proteins and various pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, in patients with depression are higher than in the general population [89, 90]. Pro-inflammatory pathways initiate at the inflammasome, which plays a key role in the relationship between inflammation and oxidative stress [91], damaging cellular and intracellular membranes and key biomolecules [92]. Similar damage has been reported in many diseases that are likely to be comorbid with depression,such as rheumatoid arthritis [93], psoriasis [94], cancer [95], type 2 diabetes [96], and coronary heart disease [97]. For all these diseases, their causal effects on MDD were confirmed. Among these, the association between asthma and depression has been widely explored [23, 98]. A large genome-wide cross-trait analysis revealed significantly elevated levels of IFN-γ, TNF-α, and IL-17A in individuals with asthma and psychological symptoms [99]. A meta-analysis of studies on depression and asthma suggests that the two conditions are comorbid due to the sharing of immune-inflammatory pathways [100]. Specifically, environmental factors trigger a chronic airway inflammatory response, characterized by elevated inflammatory markers, resulting in asthma attacks. Asthma attacks are associated with activation of NF-κB, which impacts 5-HT1A gene expression and contributes to the development of MDD [101]. On the other hand, the causal association of several inflammatory disorders (e.g., otitis externa, conjunctivitis, rhinitis, periodontitis, cystitis, and pelvic inflammatory diseases) with MDD, though rarely discussed in observational studies, has also been well-documented.
In depressed patients, the most prevalent comorbidity is type 2 diabetes, which shares with MDD both its genetic underpinning and pathophysiological pathways [102,103,104]. In Both conditions, the neuroendocrine function of the hypothalamic-pituitary-adrenal (HPA) axis is altered. In depression, overactivation of the HPA axis leads to elevation in the levels of cortisol [105] and promotes insulin resistance, thus, shifting affected individuals toward metabolic syndrome [106]. The findings of this study contribute to supporting a causal link between diabetes and MDD. However, no evidence of a bidirectional causal association was observed. Similarly, the HPA axis was shown to be involved in stroke [107], suicide [108], epilepsy [109], and substance abuse [110]. Although inconsistent findings have also been reported [111] for each of these conditions, their causal effects on depression were confirmed in the current work.
In this study, the most pronounced causal influence on MDD was observed for emotionally unstable personality disorder (OR = 3.02, 95%CI:2.39-3.82). The exploration of the association between personality traits and MDD dates back to Hippocrates’ humor theory. Among more modern, but still empirical models of personality are Eysenck’s three dimensions (i.e., neuroticism, psychoticism, and extraversion) and the Big Five model of personality (extraversion, neuroticism, conscientiousness, agreeableness, and openness) [112]. Notably, an in-depth discussion of the relationship between neuroticism, characterized by emotional instability, emotional impulsivity, and emotional hypersensitivity, and MDD has been initiated earlier [20, 113]. Also, an earlier Meta-analysis pooling 175 studies hinted toward a robust and significant correlation between MDD and high scoring for neuroticism (Cohen’s d = 1.54) [114]. Some other studies examined the relationship between the two, [115, 116] with available evidence showing similarities in the structural characteristics of the brain in neuroticism and depression reinforcing and strengthening these conclusions [117]. The current study reaffirms the robust causal effect of personality disorders on MDD.
Our previous studies have examined in depth the mechanisms of genetic-level interactions between insomnia and depression [118]. Insomnia and depression share a large amount of genetic variation and display bidirectional causal associations with one another [118]. The effects of the genetic susceptibility to insomnia on depression were distinctly higher than that of depression on insomnia.
The present study indicated that the effects of depression on other disorders were more prominent than the reverse when considered genetically, mainly in the form of a broader spectrum of diseases, and a higher magnitude of the the effect sizes. The genetic variance profile, derived from the multigene overlap analysis, provides a compelling explanation for this phenomenon. Our previous study showed the presence of about 158,000 causal genetic variants in MDD, whereas cardiovascular disease exhibits a relatively lower number of causal variants [119]. For instance, atrial fibrillation is associated with around 0.5 million causal variants, whereas heart failure has 28,000 causal variants. In short, the greater number of genetic variants influencing depression implies a higher susceptibility to more potent causal effects compared to other disorders.
The comprehensive results of our study showed a substantial degree of overlap with the findings from the recently published large-scale meta-analysis [120]. An in-depth exploration of the complex pathophysiological mechanisms underlying depression, encompassing genetic, psychological, social, and biological factors, unveils its intricate interaction with various coexisting conditions (Fig. 4). Psychosocial factors should not be underestimated [121, 122]. Education has been shown to be a strong predictor of depression [123]. Additionally, personal income significantly influences depression risk, with low-income individuals facing a much higher likelihood of depression compared to those with higher incomes [124]. Notably, these factors also contribute to other conditions, such as type 2 diabetes [125], hypertension [126], and stroke [127], which share common comorbidities with depression. One possible explanation is that less-educated or lower-income people are more likely to be under greater social stress, which would probably lead to changes in neurotransmitter and hormone levels, ultimately contributing to comorbid depression. The most effective strategy for addressing the comorbidities of depression should rely on integrated interventions that include psychological, social, behavioral, and pharmacological dimensions.
The numerous risk factors involved such as genetics, childhood trauma, individual traits, stress, socially undesirable environments, and unhealthy behavioral patterns may contribute to co-morbid depression in those with medical disorders. The potential link between depression and medical disorders is established through one or more pathway mechanisms, encompassing behavioral, biological, and psychological factors. The figure was drawn by Figdraw.
The primary strength of our study lies in utilizing data from the newly released Finnish database to integrate extensive disease phenotypes, revealing comorbidities associated with depression through hypothesis-free MR-PheWAS analyses. Additionally, upon identifying robust causal associations between MDD and other disorders at the genetic level, this study pinpointed disease phenotypes exhibiting bi-directional causal influences on MDD through inverse MR analysis. Several limitations of the MR approach need acknowledgment. Firstly, although pleiotropy is a concern, the impact of this bias should be minimal due to the overall consistency observed in the sensitivity analyses and the fact that the regression intercepts are all small in the MR-Egger analysis. Secondly, the filtering criteria were relaxed to obtain a larger number of IVs, which may have introduced bias into the results. Thirdly, caution should be exercised when applying the conclusions of this study to other populations, as the data were derived from the GWAS database for European ancestry. Fourth, some conditions with relatively small sample sizes remained within the scope of analysis, potentially increasing the risk of random error in the results. Thus, further validation through preclinical and clinical studies is necessary.
Conclusions
This phenome-wide MR study reveals significant bidirectional causal relationships between MDD and a variety of disease phenotypes. These results provide valuable insights into the genetic underpinnings of MDD and its comorbidities, offering a foundation for future research and potential clinical interventions. In the course of clinical practice, physicians should be adept at identifying and promptly diagnosing and treating comorbid depression.
Data availability
The sources of data and the methods for data processing are comprehensively explained in the Materials and Methods section and the Supplementary Tables. All data used in this study were publicly accessible. Should you require additional information, please don’t hesitate to reach out to the corresponding author for further clarification.
References
Monroe SM, Harkness KL. Major depression and its recurrences: life course matters. Annu Rev Clin Psychol. 2022;18:329–57.
Lee S-Y, Lee JP, Lee J, Park JY, Kim EY. Association between depressive symptoms and the risk of all-cause and cardiovascular mortality among US adults. Prog Neuropsychopharmacol Biol Psychiatry. 2023;125:110755.
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50.
Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: A systematic review and meta-analysis. J Affect Disord. 2017;221:36–46.
Rizzuto D, Melis RJF, Angleman S, Qiu C, Marengoni A. Effect of chronic diseases and multimorbidity on survival and functioning in elderly adults. J Am Geriatr Soc. 2017;65:1056–60.
Makovski TT, Schmitz S, Zeegers MP, Stranges S, Van Den Akker M. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res Rev. 2019;53:100903.
Tazzeo C, Zucchelli A, Vetrano DL, Demurtas J, Smith L, Schoene D, et al. Risk factors for multimorbidity in adulthood: A systematic review. Ageing Res Rev. 2023;91:102039.
Britt HC, Harrison CM, Miller GC, Knox SA. Prevalence and patterns of multimorbidity in Australia. Med J Aust. 2008;189:72–77.
Pefoyo AJK, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415.
Kozela M, Bobak M, Besala A, Micek A, Kubinova R, Malyutina S, et al. The association of depressive symptoms with cardiovascular and all-cause mortality in Central and Eastern Europe: Prospective results of the HAPIEE study. Eur J Prev Cardiol. 2016;23:1839–47.
Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diab Care. 2001;24:1069–78.
Engin B, Keçici AS, Uzun AÖ, Yalçın M. Psychiatric comorbidity, depression, and anxiety levels and quality of life of the patients with mycosis fungoides. Dermatol Ther 2020; 33. https://doi.org/10.1111/dth.13922.
Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology. 2013;52:2136–48.
Chang-Quan H, Xue-Mei Z, Bi-Rong D, Zhen-Chan L, Ji-Rong Y, Qing-Xiu L. Health status and risk for depression among the elderly: a meta-analysis of published literature. Age Ageing. 2010;39:23–30.
Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41.
Li Z, Li Y, Chen L, Chen P, Hu Y. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine. 2015;94:e1317.
Gorkhali B, Sharma S, Amatya M, Acharya D, Sharma M. Anxiety and depression among patients with thyroid function disorders. J Nepal Health Res Counc. 2020;18:373–8.
Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433.
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Prim. 2022;2:6.
Liu S, Rao S, Xu Y, Li J, Huang H, Zhang X, et al. Identifying common genome-wide risk genes for major psychiatric traits. Hum Genet. 2020;139:185–98.
Chen X, Kong J, Diao X, Cai J, Zheng J, Xie W, et al. Depression and prostate cancer risk: A Mendelian randomization study. Cancer Med. 2020;9:9160–7.
Luo J, Xu Z, Noordam R, Van Heemst D, Li-Gao R. Depression and inflammatory bowel disease: a bidirectional two-sample Mendelian randomization study. J Crohn’s Colitis. 2022;16:633–42.
Cao H, Li S, Baranova A, Zhang F. Shared genetic liability between major depressive disorder and atopic diseases. Front Immunol. 2021;12:665160.
Baurecht H, Welker C, Baumeister SE, Weidnger S, Meisinger C, et al. Relationship between atopic dermatitis, depression and anxiety: a two‐sample Mendelian randomization study. Br J Dermatol. 2021;185:781–6.
Thompson SG, Burgess S. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. 2015th ed. Chapman and Hall/CRC: London, UK.
Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Howard DM, Adams MJ, Shirali M, Clarke T-K, Marioni RE, Davies G, et al. Author Correction: Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun. 2021;12:2012.
Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48:1031–6.
eQTLGen, 23andMe, the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Bowden J, Del Greco M F, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48:728–42.
Landi F, Onder G, Cesari M, Russo A, Barillaro C, Bernabei R. Pain and its relation to depressive symptoms in frail older people living in the community: an observational study. J Pain Symptom Manag. 2005;29:255–62.
Magni G, Marchetti M, Moreschi C, Merskey H, Luchini SR. Chronic musculoskeletal pain and depressive symptoms in the national health and nutrition examination I. Epidemiologic follow-up study. Pain. 1993;53:163–8.
Amiri S, Behnezhad S, Azad E. Back pain and depressive symptoms: A systematic review and meta-analysis. Int J Psychiatry Med. 2020;0:1–15.
Lee H, Kim S, MC Chang. Associations between headache (migraine and tension-type headache) and psychological symptoms (depression and anxiety) in pediatrics: a systematic review and meta-analysis. Pain Physician. 2023;26:E617–E626.
Alwhaibi M, Balkhi B, AlRuthia Y. Anxiety and depression and health-related quality of life among adults with migraine: a National Population-Based Study. Front Public Health. 2023;11:1241800.
Molgat CV, Patten SB. Comorbidity of major depression and migraine-a Canadian population-based study. Can J Psychiatry. 2005;50:832–7.
Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–21.
Huang W, Shu C, Chou K, Wang Y, Hsu Y, Ho C, et al. Evaluating the autonomic nervous system in patients with laryngopharyngeal reflux. Otolaryngol-head Neck surg. 2013;148:997–1002.
Russo B, Menduni M, Borboni P, Picconi F, Frontoni S. Autonomic nervous system in obesity and insulin-resistance—the complex interplay between leptin and central nervous system. IJMS. 2021;22:5187.
Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36.
Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S.
Si J-M. Intestinal microecology and quality of life in irritable bowel syndrome patients. WJG. 2004;10:1802.
Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21:147–59.
Zheng S, Zhu Y, Wu W, Zhang Q, Wang Y, Wang Z, et al. A correlation study of intestinal microflora and first‐episode depression in Chinese patients and healthy volunteers. Brain Behav. 2021;11:e02036.
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
Kim Y-K, Shin C. The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr Neuropharmacol. 2018;16:559–73.
Hayes CL, Dong J, Galipeau HJ, Jury J, McCarville J, Huang X, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep. 2018;8:14184.
Sonali S, Ray B, Ahmed Tousif H, Rathipriya AG, Sunanda T, Mahalakshmi AM, et al. Mechanistic insights into the link between gut dysbiosis and major depression: an extensive review. Cells. 2022;11:1362.
Kang J, Castro VM, Ripperger M, Venkatesh S, Burstein D, Linnér RK, et al. Genome-wide association study of treatment-resistant depression: shared biology with metabolic traits. AJP. 2024;181:608–19.
Postolache TT, Del Bosque‐Plata L, Jabbour S, Vergare M, Wu R, Gragnoli C. Co‐shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am J Med Genet Pt B. 2019;180:186–203.
Setoyama D, Kato TA, Hashimoto R, Kunugi H, Hattori K, Hayakawa K, et al. Plasma metabolites predict severity of depression and suicidal ideation in psychiatric patients-a multicenter pilot analysis. PLoS One. 2016;11:e0165267.
Li G, Lin J, Zhang C, Gao H, Lu H, Gao X, et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. 2021;13:1968257.
Cao H, Wang J, Baranova A, Zhang F. Classifying major mental disorders genetically. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110410.
Sadkowski M, Dennis B, Clayden RC, ElSheikh W, Rangarajan S. et al. The role of the serotonergic system in suicidal behavior. NDT. 2013;9:1699–1716.
Pawluski JL, Li M, Lonstein JS. Serotonin and motherhood: From molecules to mood. Front Neuroendocrinol. 2019;53:100742.
Gasparini CF, Smith RA, Griffiths LR. Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J Headache Pain. 2017;18:20.
Pourhamzeh M, Moravej FG, Arabi M, Shahriari E, Mehrabi S, Ward R, et al. The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol. 2022;42:1671–92.
Lin S-H, Lee L-T, Yang YK. Serotonin and mental disorders: a concise review on molecular neuroimaging evidence. Clin Psychopharmacol Neurosci. 2014;12:196–202.
Sharp T, Boothman L, Raley J, Quérée P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharm Sci. 2007;28:629–36.
Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18:1606–16.
Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT 1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–34.
Aznar S, Hervig ME-S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci Biobehav Rev. 2016;64:63–82.
Deen M, Hansen HD, Hougaard A, Nørgaard M, Eiberg H, Lehel S, et al. High brain serotonin levels in migraine between attacks: A 5-HT4 receptor binding PET study. NeuroImage: Clin. 2018;18:97–102.
Yang W, Han Y, He C, Zhong S, Ren F, Chen Z, et al. Association between psychiatric disorders and glioma risk: evidence from Mendelian randomization analysis. BMC Cancer. 2024;24:118.
Lv X, Xu B, Tang X, Liu S, Qian J-H, Guo J, et al. The relationship between major depression and migraine: A bidirectional two-sample Mendelian randomization study. Front Neurol. 2023;14:1143060.
Zhang F, Rao S, Cao H, Zhang X, Wang Q, Xu Y, et al. Genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder. J Clin Invest. 2022;132:e145942.
Baranova A, Zhao Q, Cao H, Chandhoke V, Zhang F. Causal influences of neuropsychiatric disorders on Alzheimer’s disease. Transl Psychiatry. 2024;14:114.
Li C, Cheng S, Chen Y, Jia Y, Wen Y, Zhang H, et al. Exploratory factor analysis of shared and specific genetic associations in depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2023;126:110781.
Plana-Ripoll O, Pedersen CB, Holtz Y, Benros ME, Dalsgaard S, De Jonge P, et al. Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry. 2019;76:259.
Kuan V, Denaxas S, Patalay P, Nitsch D, Mathur R, Gonzalez-Izquierdo A, et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English National Health Service: a population-based study. Lancet Digit Health. 2023;5:e16–e27.
McGrath JJ, Lim CCW, Plana-Ripoll O, Holtz Y, Agerbo E, Momen NC, et al. Comorbidity within mental disorders: a comprehensive analysis based on 145 990 survey respondents from 27 countries. Epidemiol Psychiatr Sci. 2020;29:e153.
Lanfumey L, Hamon M. 5-HT1 receptors. CDTCNSND. 2004;3:1–10.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86.
Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–44.
Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: A meta-analysis. J Affect Disord. 2020;277:940–8.
Jung Y-E, Kang KY. Elevated hs-CRP level is associated with depression in younger adults: Results from the Korean National Health and Nutrition Examination Survey (KNHANES 2016). Psychoneuroendocrinology. 2019;109:104397.
Chamberlain SR, Cavanagh J, De Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. 2019;214:11–19.
Osimo EF, Stochl J, Zammit S, Lewis G, Jones PB, Khandaker GM. Longitudinal population subgroups of CRP and risk of depression in the ALSPAC birth cohort. Compr Psychiatry. 2020;96:152143.
Windgassen EB, Funtowicz L, Lunsford TN, Harris LA, Mulvagh SL. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad Med. 2011;123:114–9.
Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20:1752–60.
Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25:1301–11.
Aveleira CA, Lin C-M, Abcouwer SF, Ambrósio AF, Antonetti DA. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–82.
Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O’Brien TJ, Monif M. Innate Immunity in the Central Nervous System: A Missing Piece of the Autoimmune Encephalitis Puzzle? Front Immunol. 2019;10:2066.
Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–70.
Sun W, Cao H, Liu D, Baranova A, Zhang F, Zhang X. Genetic association and drug target exploration of inflammation-related proteins with risk of major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 2024; 111165.
Suneson K, Grudet C, Ventorp F, Malm J, Asp M, Westrin Å, et al. An inflamed subtype of difficult-to-treat depression. Prog Neuropsychopharmacol Biol Psychiatry. 2023;125:110763.
Liu Y, Ho RC-M, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9.
Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87.
Roy S, Arif Ansari M, Choudhary K, Singh S. NLRP3 inflammasome in depression: A review. Int Immunopharmacol. 2023;117:109916.
Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270–6.
Fakra E, Marotte H. Rheumatoid arthritis and depression. Jt Bone Spine. 2021;88:105200.
González-Parra S, Daudén E. Psoriasis y depresión: el papel de la inflamación. Actas Dermo-Sifiliogr. 2019;110:12–19.
McFarland DC, Doherty M, Atkinson TM, O’Hanlon R, Breitbart W, Nelson CJ, et al. Cancer‐related inflammation and depressive symptoms: Systematic review and meta‐analysis. Cancer. 2022;128:2504–19.
Sánchez-Ortí JV, Correa-Ghisays P, Balanzá-Martínez V, Selva-Vera G, Vila-Francés J, Magdalena-Benedito R, et al. Inflammation and lipid metabolism as potential biomarkers of memory impairment across type 2 diabetes mellitus and severe mental disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110817.
Lin J, Yang R, Zhang Y, Hou Y, Yang H, Zhou X, et al. The mediation effects of metabolic and immune–inflammation factors on the depression–premature coronary heart disease association. J Affect Disord. 2023;331:434–41.
Gao Y, Zhao H, Zhang F, Gao Y, Shen P, Chen R, et al. The relationship between depression and asthma: a meta-analysis of prospective studies. PLoS ONE. 2015;10:e0132424.
Zhu Z, Zhu X, Liu C-L, Shi H, Shen S, Yang Y, et al. Shared genetics of asthma and mental health disorders: a large-scale genome-wide cross-trait analysis. Eur Respir J. 2019;54:1901507.
Jiang M, Qin P, Yang X. Comorbidity between depression and asthma via immune-inflammatory pathways: A meta-analysis. J Affect Disord. 2014;166:22–29.
Van Lieshout RJ, MacQueen G. Psychological factors in Asthma. All Asth Clin Immun. 2008;4:12.
Tao H, Fan S, Zhu T, You L, Zheng D, Yan L, et al. Psychiatric disorders and Type 2 diabetes mellitus: A bidirectional Mendelian randomization. Eur J Clin Invest. 2023;53:e13893.
Possidente C, Fanelli G, Serretti A, Fabbri C. Clinical insights into the cross-link between mood disorders and type 2 diabetes: A review of longitudinal studies and Mendelian randomisation analyses. Neurosci Biobehav Rev. 2023;152:105298.
Baranova A, Liu D, Sun W, Xu C, Chen M, Cao H, et al. Antidepressants account for the causal effect of major depressive disorder on type 2 diabetes. Prog Neuropsychopharmacol Biol Psychiatry 2024: 111164.
Mayer SE, Lopez-Duran NL, Sen S, Abelson JL. Chronic stress, hair cortisol and depression: A prospective and longitudinal study of medical internship. Psychoneuroendocrinology. 2018;92:57–65.
Le Min. Functional hypercortisolism, visceral obesity, and metabolic syndrome. Endocr Pract. 2016;22:506–8.
Zhou L, Wang T, Yu Y, Li M, Sun X, Song W, et al. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed Pharmacother. 2022;151:113146.
Berardelli I, Serafini G, Cortese N, Fiaschè F, O’Connor RC, Pompili M. The involvement of Hypothalamus–Pituitary–Adrenal (HPA) axis in suicide risk. Brain Sci. 2020;10:653.
Basu T, Maguire J, Salpekar JA. Hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Neurosci Lett. 2021;746:135618.
Cservenka A, Lahanas S, Dotson-Bossert J. Marijuana use and hypothalamic-pituitary-adrenal axis functioning in humans. Front Psychiatry. 2018;9:472.
Chu H, Wang B, Zhao X, Mu L. Epilepsy and psychiatric comorbidities: A bidirectional mendelian randomization study. J Affect Disord. 2024;350:774–83.
Digman JM. Personality structure: Emergence of the five-factor model annual review of psychology. Annu Rev Psychol. 1990;41:417–40.
Zhang F, Baranova A, Zhou C, Cao H, Chen J, Zhang X, et al. Causal influences of neuroticism on mental health and cardiovascular disease. Hum Genet. 2021;140:1267–81.
Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol Bull. 2010;136:768–821.
Yoon KL, Maltby J, Joormann J. A pathway from neuroticism to depression: examining the role of emotion regulation. Anxiety Stress Coping. 2013;26:558–72.
Chen J, Sun M, Huang C, Xiao J, Tang S, Chen Q. Pathways from neuroticism, social support, and sleep quality to antenatal depression during the third trimester of pregnancy. IJERPH. 2022;19:5602.
Joseph C, Wang L, Wu R, Manning KJ, Steffens DC. Structural brain changes and neuroticism in late-life depression: a neural basis for depression subtypes. Int Psychogeriatr. 2021;33:515–20.
Baranova A, Cao H, Zhang F. Shared genetic liability and causal effects between major depressive disorder and insomnia. Hum Mol Genet. 2022;31:1336–45.
Zhang F, Cao H, Baranova A. Shared genetic liability and causal associations between major depressive disorder and cardiovascular diseases. Front Cardiovasc Med. 2021;8:735136.
Arnaud AM, Brister TS, Duckworth K, Foxworth P, Fulwider T, Suthoff ED, et al. Impact of major depressive disorder on comorbidities: a systematic literature review. J Clin Psychiatry 2022; 83. https://doi.org/10.4088/JCP.21r14328.
Baranova A, Cao H, Zhang F. Exploring the influences of education, intelligence and income on mental disorders. Gen Psychiatr. 2024;37:e101080.
Andreu-Bernabeu Á, González-Peñas J, Arango C, Díaz-Caneja CM. Socioeconomic status and severe mental disorders: a bidirectional multivariable Mendelian randomisation study. BMJ Ment Health. 2023;26:e300821.
De Oliveira G, Cianelli R, Gattamorta K, Kowalski N, Peragallo N. Social determinants of depression among hispanic women. J Am Psychiatr Nurses Assoc. 2017;23:28–36.
Ettman CK, Cohen GH, Vivier PM, Galea S. Savings, home ownership, and depression in low-income US adults. Soc Psychiatry Psychiatr Epidemiol. 2021;56:1211–9.
Liang J, Cai H, Liang G, Liu Z, Fang L, Zhu B, et al. Educational attainment protects against type 2 diabetes independently of cognitive performance: a Mendelian randomization study. Acta Diabetol. 2021;58:567–74.
Yanagiya S, Nakamura K, Ukawa S, Tsutsumi A, Atsumi T, Tamakoshi A. Household income and the risk of incident hypertension in employees at multiple workplaces in Japan: J-HOPE. Hypertens Res. 2020;43:1445–53.
Zhang W, Li Y, Li Y, Zheng K, Zou S, Jia X, et al. Genetically predicted higher educational attainment decreases the risk of stroke: a multivariable Mendelian randomization study. BMC Cardiovasc Disord. 2022;22:269.
Acknowledgements
We greatly appreciate all investigators for sharing these data.
Funding
This study was supported by Suzhou Key Laboratory (SZS2024016); Suzhou Key Technologies Program (SKY2021063); Suzhou Clinical Medical Center for mood disorders (Szlcyxzx202109); Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116); Suzhou Science and Technology Development Program Youth Project (SKYD2023160); National Mentorship Training Program for Young Health Professionals in Suzhou (Qngg2022027).
Author information
Authors and Affiliations
Contributions
Fuquan Zhang and Xiaobin Zhang played integral roles in conceiving and designing the study. Wenxi Sun, Dongming Liu, and Hongbao Cao contributed to the statistical analysis and data interpretation and drafted the manuscript. Fuquan Zhang and Ancha Baranova provided critical revisions to the article, enhancing its intellectual content. All authors actively participated in data collection and gave their final approval for the version to be published. These authors contributed equally: Wenxi Sun, Ancha Baranova, and Dongming Liu. Fuquan Zhang and Xiaobin Zhang contributed equally to this work and shared last authorship.
Corresponding authors
Ethics declarations
Competing interests
All authors declare no competing interests.
Ethics approval and consent to participate
We adhere to the STROBE-MR guidelines when reporting our study, ensuring the transparent and comprehensive presentation of our research findings. Ethical review and approval did not apply to this study, as the datasets used for MDD and other human diseases in the MR analysis were sourced from publicly available data and studies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, W., Baranova, A., Liu, D. et al. Phenome-wide investigation of bidirectional causal relationships between major depressive disorder and common human diseases. Transl Psychiatry 14, 506 (2024). https://doi.org/10.1038/s41398-024-03216-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03216-z
This article is cited by
-
Causal associations between posttraumatic stress disorder and type 2 diabetes
Diabetology & Metabolic Syndrome (2025)
-
Gut microbiome links obesity to type 2 diabetes: insights from Mendelian randomization
BMC Microbiology (2025)