Abstract
Few studies have examined the effects of self-managed lifestyle behavioral adjustment on cognitive status. This study aimed to explore the association between self-managed behavioral changes and transitions in cognitive status. The Hubei Memory and Aging Cohort Study was a prospective cohort study conducted from 2018–2023 in rural and urban areas. Home-dwelling adults aged ≥65 years completed neuropsychological, lifestyle, clinical, and cognitive assessments. The Cox regressions and cubic splines were used to assess the risk of incident cognitive impairment, and latent class analysis was used to group participants based on behavioral patterns and assess transitions in cognitive status. Among 2477 participants with a mean of 2.02 (SD, 1.25) years of follow-up were included in the study. Participants with low and intermediate compared with high baseline behavioral risk exhibited a reduced risk of incident cognitive impairment. At follow-up, those who maintained stable healthy behaviors or positively adjusted them had a 54% (HR, 0.46 [95% CI, 0.34–0.62]) and 84% (0.16 [0.07–0.35]) lower risk of developing cognitive impairment, respectively, compared with those who maintained unhealthy behaviors. The standard and reinforced behavioral adjustment patterns exhibited a 37% (0.63 [0.22–1.79]) and 77% (0.23 [0.05–0.97]) reduction in the risk of incident cognitive impairment, respectively, compared with the basic pattern. Optimal cognitive gains were attributed to positive adjustments in social networks, physical exercise, cognitive activity, and sleep health. Older adults who maintained healthy behaviors or positively adjusted their unhealthy behaviors exhibited a reduced risk of incident cognitive impairment. Positive behavior modification brought greater cognitive improvement to all participants and more pronounced effects for those with dementia.

Similar content being viewed by others
Introduction
Between 1990 and 2017, the number of life years lost and deaths caused by cognitive impairment, mainly Alzheimer’s disease, more than doubled in China [1]. A considerable time window exists before symptoms of cognitive impairment appear, during which time lifestyle behaviors and other risk factors are modifiable [2, 3]. However, efforts to develop an approach for reducing the risk of cognitive impairment that is sufficiently intensive to be effective and scalable in the real world have thus far been unsuccessful.
Previous studies have detailed the healthy behaviors associated with a reduced risk of cognitive impairment, as well as the effects on cognitive function of one or a combination of lifestyle behaviors, including smoking, alcohol intake, physical exercise, cognitive activity, and eating patterns [2, 4,5,6,7]. Several large-scale multi-___domain randomized controlled trials (RCTs) conducted mainly in high-income countries have shown that simultaneously targeting several modifiable lifestyle risk factors in individuals without dementia is a promising strategy [8]. However, translating the interventions from these RCTs into daily life across multiple population groups is challenging. Notably, most current research has been concentrated in certain regions or populations, with a scarcity of studies targeting the Chinese people or other underrepresented groups [9]. Additionally, no known risk assessment of lifestyle behaviors currently exists for different age groups based on the risk weight of behavioral indicators for cognitive impairment, especially within the Chinese context. The type-dependent effects of behavioral adjustments on all cognitive stages also require further consideration, as such investigations remain insufficient.
Therefore, we evaluated the association between self-managed lifestyle behavior adjustments and the onset of cognitive impairment and changes in cognitive status among participants in the Hubei Memory and Aging Cohort Study (HMACS) a large prospective cohort study designed to better understand the risk factors for cognitive impairment in the Chinese context. We hypothesized that actively managing lifestyle behaviors would reduce the risk of cognitive decline and improve cognitive function.
Methods
Study design and participants
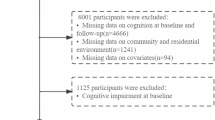
The HMACS (ChiCTR1800019164) enrolled home-dwelling adults aged 65 years and older living in Hubei, China. Details of the study design have been reported previously [5, 6, 10]. Baseline data were collected between 2018 and 2022. At the beginning of the study and every 1–2 years thereafter, each participant underwent a standardized clinical assessment. Each participant received at least two personalized health instructions and life-based health education at baseline and during the follow-up period. In total, 2477 participants with available follow-up measurements were included in the current analysis (Fig. 1). Of these, 1391 did not have cognitive impairment at baseline. The Ethics Committee of the School of Medicine at the Wuhan University of Science and Technology approved the study protocol. All participants provided written informed consent.
The data used in this study were obtained from the participants of the HMACS who were recruited between 2018 and 2023. During this period, a total of 2603 participants were followed up. After excluding 36 participants with severe diseases and/or significant impairment in activities of daily living, as well as 11 participants with severe mental disorders, a total of 2556 participants met the inclusion criteria of the HMACS.
Assessment of lifestyle behaviors
Participants rated seven lifestyle behaviors: social network, nicotine exposure, diet, physical activity, cognitive activity, sleep health, and mental health (Table S1). Social network was categorized as follows: at least three friends and concern from neighbors; at least three friends or concern from neighbors; fewer than three friends and no concern from neighbors [10]. Unhealthy nicotine exposure was defined as a former smoker who quit <5 years ago or a current smoker [11]. Diet was measured by dietary preference and frequency of intake of vegetables, fruits, and fish [12]. Physical activity was defined as participation in moderate activity for at least 150 min/week [13]. Cognitive activity was measured by the frequency of participating in active information-processing activities such as reading, playing chess, cards, and games, and speculating on stocks [7]. Self-reported sleep and Athens Insomnia Scale (AIS) scores were used to assess sleep status [14]. Unhealthy sleep was defined as self-reported insomnia or an AIS score >6 [15]. Participants who self-reported no depression or depression for less than two weeks and a Geriatric Depression Scale (GDS)-15 score <5 (or GDS-30 score <10) were classified as mentally healthy [16].
We calculated risk scores of lifestyle behaviors for cognition (RSLCs), which indicate an individual’s potential risk for cognitive decline or dementia. The use of RSLCs enhanced statistical power, enabled personalized assessments, and dynamically reflected health changes across the seven age-related behaviors (Table S1 and S2) [17]. RSLC cutoff values were determined using the receiver operating characteristic curve (Table S3) [18]. Risk levels of lifestyle behaviors for cognition (RLLCs) were classified as low, intermediate, and high. Behavioral adjustments during follow-up were assessed based on changes in the RLLC. Subsequently, participants were categorized into stable healthy, positively adjusted, negatively adjusted, and stable unhealthy groups, according to their changing RLLC profiles.
We used the latent class analysis (LCA) program in Mplus 7.0 to create a single indicator of positive behavioral adjustment patterns for 440 participants who adjusted their behaviors positively based on the seven lifestyle behaviors. LCA was used because of its strengths in identifying homogeneous subgroups and handing complex data [19, 20]. Each participant was assigned to one of three groups—basic, standard, or reinforced adjustment group—based on their level of behavioral modification.
Cognition evaluation and diagnosis
Participants’ cognitive status was tested using the following standardized batteries: (1) the Mini-Mental State Examination (MMSE); (2) the Montreal Cognitive Assessment-Basic (MoCA-B); and (3) a battery of cognitive tests, including the Auditory Verbal Learning Test, Trail-Making Tests A and B, forward and backward conditions of the Digit Span Test, the Boston Naming Test, the Animal Fluency Test, and the Clock Drawing Test. These tests evaluate memory, executive ability, language ability, and attention, respectively [6, 10]. Mild cognitive impairment (MCI) and dementia were diagnosed at baseline and all follow-up assessments using Petersen’s MCI criteria and the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, respectively, by an expert panel of two neurologists and two neuropsychologists with dementia expertise (eMethods) [21]. Cognitive impairment was defined as the occurrence of MCI or dementia.
Statistical analysis
Descriptive statistics were calculated for all study variables. The continuous variables were presented as median (interquartile interval) owing to a non-normal distribution as confirmed by the Kolmogorov–Smirnov test. Categorical variables were expressed as frequency (%).
Time-dependent Cox regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) between the RLLCs and incident cognitive impairment. There was a crossover in the survival curves for the different RLLCs when the hazard probability hypothesis was visually assessed using log mini-log plots. Thus, the statistical significance of the interactions was evaluated using likelihood ratio tests. Interactions between RLLCs and the natural logarithm (ln) of time showed that the proportional hazards hypothesis could be rejected (p < 0.001). Therefore, we constructed a time-dependent covariate based on the RLLC and fitted a time-varying HR function between the RLLC and incident cognitive impairment. Subsequently, we evaluated the minimum adjusted model using sociodemographic variables (age, sex, education, and residence) as covariates. In the fully adjusted model, additional covariates included hypertension, diabetes, hyperlipidemia, coronary heart disease, and cerebrovascular disease. In the multivariate-adjusted Cox model, a restricted cubic spline (RCS) with three knots was used to demonstrate a non-linear correlation shape. The Akaike information criterion was used to determine optimal linear fit. Likelihood ratio tests were used to examine potential non-linear associations between baseline and follow-up RSLC as a continuous variable and incident cognitive impairment by age group.
We evaluated four main trends in RLLC: stable healthy, positively adjusted, negatively adjusted, and stable unhealthy. Furthermore, we added a more detailed analysis of the nine types of changes in RLLC. To make the HRs more comparable, the stable unhealthy and intermediate-intermediate groups were used as reference categories. Cognitively normal survival at 6 years was analyzed by examining the RLLC trend using the Kaplan-Meier method, with subgroups compared using the log-rank test. Owing to different RLLC evaluation criteria in different age groups, we used RCS to demonstrate the risk of incident cognitive impairment in relation to changes in RSLC after excluding specific participants across age groups during the follow-up period.
Next, logistic regression was used to investigate the relationship between changes in the RLLCs and positive cognitive transitions. Improvement and deterioration in cognitive status were initially categorized as transitioning from cognitive impairment to normal cognition and from normal cognition to cognitive impairment, respectively. To further clarify the impact of changes in RLLC on the cognitive function of participants with different cognitive statuses, we divided participants into three groups based on their baseline cognitive diagnosis: normal cognition, MCI, and dementia. For each group, we analyzed the associations between changes in RLLC and cognitive status at follow-up, with the stable unhealthy group as the reference category in all analyses. The models were also adjusted for all variables.
To evaluate the impact of different patterns of positive behavioral adjustment on incident cognitive impairment, we classified the seven behavioral indicators into two categories based on whether they were actively adjusted for or included in the model. A stepwise approach was used to fit the model, progressively incorporating each indicator until all seven models were established. Model evaluation details are provided in the eMethods and Table S4 in the Supplement. Based on the practical significance and number of participants in each category, we identified three positive behavior adjustment patterns: basic, standard, and reinforcement adjustment. Further analysis of the HRs of incident cognitive impairment for these three groups was undertaken in relation to those who were stable healthy. Associations between the risk of cognitive impairment at follow-up and behavioral adjustment patterns were assessed to explore the long-term effects of each adjustment pattern on the prevalence of cognitive impairment.
In the sensitivity analysis, we evaluated the effects of unmeasured potential confounders on the robustness of the results using Van der Weele and Ding’s e-value method. This method was used to estimate odds ratios (ORs) for any unmeasured confounder with substantial effects on the occurrence and reversal of incident cognitive impairment [22]. To accurately estimate associations between long-term stable RLLCs and risk of cognitive impairment, we excluded participants with changes in RLLC during follow-up and repeated the previous analyses. All analyses and visualizations were performed using R software (Version 4.2.1). All P-values were two-sided, and p < 0.05 was considered significant.
Results
A total of 2477 participants (mean age 72.65 [SD, 5.68] years, 54.7% women) from the HMACS were analyzed for cognitive transitions over 5250 person-years of follow-up. The mean follow-up time was 2.02 years (Table 1). Participants with low, intermediate, and high behavioral risks accounted for 48.9, 42.2, and 8.9% of the sample, respectively. Higher behavioral risks were linked to older age, female sex, vascular diseases, and poorer cognition. Lower baseline behavioral risk and health behavior improvements were correlated with urban residence and higher education. Of 1391 participants with normal cognition at baseline, 547 (55.4% women) developed cognitive impairment during follow-up, and 474 had low RLLC at baseline (51.7% women) (Tables S5–S7).
After adjusting for confounders, we found a reduced risk of incident cognitive impairment in participants with low (HR, 0.49 [95% CI, 0.29–0.83]) and intermediate (HR, 0.47 [95% CI, 0.30–0.74]) compared with high baseline RLLC (Table S8). A U-shaped correlation linked baseline RSLC to the risk of cognitive impairment, with upslope correlation lines at follow-up (Fig. 2A).
A Histogram showing participant counts across the range of risk score of lifestyle behaviors for cognition (RSLC). Non-linear relationships between RSLC and incidence of cognitive impairment. Data were HR (solid line) and 95%CI (shadow area) from cox regression analysis with restricted cubic splines. The RCSs illustrated a U-shaped correlation between either a lower or higher baseline RSLC score and an increased risk of cognitive impairment. Conversely, upslope correlation lines were evident at follow-up. B Kaplan-Meier survival curves showing the overall survival according to the change trend of risk level of lifestyle behaviors for cognition. C Heatmap showing the HRs (see eTable 6) of incident cognitive impairment in different RLLC changes.
Maintaining an unhealthy lifestyle profile resulted in a higher incidence rate of cognitive impairment (238.53/1000 person-years). In comparison, healthier lifestyles and maintained healthy profiles exhibited lower incidence rates (31.41 and 86.63/1000 person-years, respectively). Stable healthy and positively adjusted groups had a 54% (HR, 0.46 [95% CI, 0.34–0.62]) and 84% (HR, 0.16 [95% CI, 0.07–0.35]) lower risk of developing cognitive impairment, respectively, than the stable unhealthy group. Conversely, the negatively adjusted group exhibited increased risk by 55% (HR, 1.55 [95% CI, 1.27–1.90]) (Fig. 2B; Table 2). These findings suggest a significantly decreased risk of cognitive impairment for participants who either improved their RLLC or maintained a low RLLC, irrespective of baseline RLLC, in contrast to those who maintained an intermediate RLLC (Table S9; Fig. 2C). There was a non-linear positive correlation at follow-up between changes in RSLC and risk of incident cognitive impairment (Figure S1).
In secondary analyses, stable healthy behaviors were significantly associated with promoting cognitive reversal (OR, 4.28 [95% CI, 2.69–6.82]) and preventing the development of cognitive impairment (OR, 0.36 [95% CI, 0.24–0.54]) compared with the stable unhealthy group. Stronger effects were observed in the positive lifestyle adjustments group (improvement: OR, 7.86 [95% CI, 5.37–11.52]; decline: OR, 0.06 [95% CI, 0.03–0.15]). Conversely, negative lifestyle adjustment significantly hindered cognitive recovery (OR, 0.05 [95% CI, 0.01–0.33]), and was associated with a substantially increased risk of cognitive deterioration (OR, 4.80 [95% CI, 3.21–7.18]) (Table S10). These results were consistent across analyses subdividing cognitive status transitions among the normal cognition, MCI, and dementia groups (Fig. 3). Among those with dementia at baseline, only those with positive adjustment experienced significant cognitive improvements (OR, 7.05 [95% CI, 2.12–23.46]) (Table S11).
The basic adjustment group changed social networks, diet, cognitive activities, and sleep behaviors. More participants in the standard adjustment group made positive changes, especially in physical activity. The reinforced adjustment group further augmented these changes, with a notable increase in active modifications across social networks, physical activity, cognitive activity, and sleep (Table S12). Compared with the stable health behavior group, the risk of incident cognitive impairment was reduced by 77% in the reinforced adjustment group (HR, 0.23 [95% CI, 0.05–0.97]) and 37% in the standard adjustment group (HR, 0.63 [95% CI, 0.22–1.79]) (Table S13). The prevalence of cognitive impairment across all three active adjustment patterns was substantially higher than that in the reference group (Table S14).
Sensitivity analysis demonstrated a low likelihood that unmeasured confounding factors could undermine the robustness of these findings (Table S15). Furthermore, after excluding participants with changes in RLLC, lower baseline RLLC was significantly associated with a reduced risk of incident cognitive impairment (Table S16).
Discussion
This study investigated how self-management adjustments affect cognitive status transitions. A low baseline behavioral risk score in older adults significantly reduced the risk of incident cognitive impairment. Furthermore, maintaining healthy behaviors lowered the incidence of cognitive impairment and improved cognitive performance in those without dementia. Positive behavioral modifications provided short-term cognitive benefits among persons with dementia. Additionally, positive adjustments in social networks, physical exercise, cognitive activity, and sleep yielded the most significant cognitive gains.
Consistent with previous research [23,24,25,26], our results demonstrate that a combination of healthy behaviors correlates with better cognitive function. To date, the complex mechanism underlying the relationship between lifestyle behaviors and cognitive impairment is yet to be fully understood. However, factors that may play a role in the association include oxidative stress, inflammation, vascular factors, neurotoxicity, and psychosocial processes [7]. Physical activity, cognitive activity, and socialization can increase brain volume and cognitive reserve [27,28,29,30]. Moreover, physical activity is beneficial in relieving psychological stress and improving the status of cardiovascular factors that increase the risk of cognitive impairment, such as hypertension and hyperglycemia [31]. Healthy diet patterns also play a role in promoting metabolic health [32]. The gut-brain axis maintains brain health by maintaining the integrity of the neural membrane and upregulating neurotrophic factors [33, 34]. Sleep disorders increase β-amyloid (Aβ) load by inducing systemic inflammation [35,36,37]. This suggests that sleep disorders are not only a consequence of cognitive impairment as commonly assumed, but may be driven by inflammation, potentially contributing to the development of Alzheimer’s dementia [38]. An association also exists between Aβ dynamics and depression, which may be an important reason why patients with depression are at a much higher risk of developing cognitive impairment [39].
Notably, this study uniquely quantified risk by assigning weights to behaviors related to cognitive impairment and categorized them into levels of behavioral risk, thereby providing a more accurate measure of the impact of these behaviors on cognitive health. Other factors like sex, residence, and chronic diseases may affect this relationship [7, 40]. However, our main results remained significant after adjusting for such factors, reinforcing lifestyle behavior as an independent risk factor for cognitive impairment. We also found a U-shaped relationship between baseline behavioral risk and risk of incident cognitive impairment. This finding may be related to participants’ behavioral adjustments due to increased health education and enhanced self-health awareness [41].
Few studies have explored the effects of self-managed behavioral risk changes on cognition. We found that positive behavior adjustments or maintaining healthy behaviors reduced cognitive impairment risk and improved cognitive function. Such self-management has effects on cognitive improvement similar to those of other interventions [8, 42], which may delay further cognitive impairment by reducing exposure to risk factors [43, 44]. Positive behavior adjustment is a dynamic process involving self-awareness and self-adjustment [2, 45], which also enhances feelings of mastery, reduces stress, and reduces psychological risk factors for cognitive decline [46]. Long-term cognitive benefits of maintaining healthy behaviors may be reflected in a lower risk of cognitive impairment. We found that small changes in RSLC significantly altered cognitive impairment risk, but larger changes exhibited a slight downward trend. This may be because the impact of behavioral risk changes on cognitive impairment plateau, leading to diminished future increases in risk. Additionally, high-risk individuals may have had severe health issues before the onset of impairment. Survivorship bias could also underestimate cognitive impairment risk in high-risk groups.
This study identified three behavioral adjusting patterns—basic, standard, and reinforced—and demonstrated that the reinforced pattern significantly reduced cognitive impairment risk compared with maintaining health behavior. In all three adjustment patterns, the effects of adjusting some behaviors (e.g., social networks, physical exercise, cognitive activity, sleep, and diet) on cognitive impairment were consistent with the reported efficacy of multi-___domain RCTs [47, 48]. Moreover, the reinforced adjustment group focused on adjustments in social networks, physical exercise, cognitive activity, and sleep. Despite the many suggestions for preventing dementia [2], implementing complex strategies is challenging. We recommend focusing on key behaviors, including social networks, exercise, cognitive activity, and sleep, which are relevant to overall health and relatively easy to adjust.
This study has several strengths. This is the first large-scale prospective cohort study to explore the relationship between self-management, lifestyle behavioral adjustments, and cognitive transitions. Our comprehensive consideration of social health, mental health, sleep state, and daily behaviors sufficiently captures health behaviors associated with cognitive health and provides a broad perspective on lifestyle behaviors. Based on the real-world clinical experience of the HMACS group, this study has identified lifestyle behavior standards that are consistent with the habits of Chinese older adults, as well as a feasible behavioral modification plan with positive feedback from participants. Consequently, the behavior levels proposed in this study appear to be less influenced by objective factors and more resistant to strong subjective rejection. We developed an age-adjusted weighted risk score for the effects of lifestyle behaviors on cognitive impairment that fits the characteristics of the population. Compared with the traditional quantitative scoring method for the frequency of unhealthy behaviors, this method can accurately assess behavioral risk according to individual conditions. Participants with normal cognition, MCI, or dementia were included separately in the analysis, suggesting the wide-ranging significance of our results for individuals at all cognitive stages. Finally, the LCA analyses that focused on positive behavior adjustments in four lifestyle behaviors—social networks, physical exercise, cognitive stimulation, and sleep— suggest a more actionable strategy for behavior adjustment in real life.
Our study also has some limitations. First, our sample was drawn from a community cohort in Hubei province, China, and our results cannot be generalized to people living in nursing homes and patients with moderate-to-severe dementia at home. Additionally, the applicability of our results to other cultural populations should be considered after further assessment. Second, the RSLC established in this study applied only to people ≥65 years; thus, its applicability to assess risks in younger people is limited. Third, some participants with poor health were less likely to participate in the cognitive assessment. A few did not complete the assessment, which may have produced an underestimated proportion of people with unhealthy behaviors and negative self-management. Fourth, lifestyle behaviors were self-reported; thus, measurement errors may have occurred, although we extracted the data from electronic health records and then double-checked some of the records with participants over the phone in cases of unclear or contradictory information. Additionally, we adjusted some data for the years of behavior by calendar year. Fifth, although we used baseline and follow-up lifestyle behavior status and changes in lifestyle behaviors assessed using two records, and addressed time-dependent covariates, reverse causality cannot be completely excluded. Sixth, we did not eliminate the possibility that some participants made positive behavioral adjustments during follow-up because they were diagnosed with other chronic conditions that might adversely affect cognition. However, despite the potential for the effect to be masked, the benefits of positive behavioral adjustment on cognitive function were significant, suggesting that the effect of positive behavioral adjustment on cognitive function may be greater in real life. Seventh, owing to the influence of different periods and eras, the incidence and characteristics of influencing factors of cognitive impairment may change over time. Therefore, the influence of cohort effects must be further considered in future studies of cognitive impairment. Eighth, the smaller sample size remains an unavoidable limitation. We will use the data of more participants continuously collected in future studies to verify our results. Ninth, the temporal sequence between lifestyle changes and outcomes remains unclear, which restricts causal inference despite annual cognitive assessments and updated electronic health records. Future research with extended follow-up and more intensive monitoring is needed to clarify this relationship. Finally, there may be residual or unmeasured confounders in our study design, although it seems unlikely that any potentially unmeasured confounders would affect the robustness of the results.
Conclusion
The results of this study indicate that proactive self-behavioral management in later life—particularly in areas such as social engagement, physical exercise, cognitive activities, and sleep—can induce short-term improvements in cognitive status. Recommendations for specific behavioral adjustments related to dementia risk that are based on quantitative assessments provide an efficient, targeted path for public health policy implementation and personalized self-health management. Future research must explore and validate the relationship between healthy behaviors and cognitive improvement in other cultural settings.
Data availability
The datasets analyzed during the current study are not publicly available due to the metadata containing information that could compromise the patients but are available from the corresponding author on reasonable request.
References
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Lond Engl. 2019;394:1145–58.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet Lond Engl. 2020;396:413–46.
Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier WM, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement J Alzheimers Assoc. 2019;15:888–98.
Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet Lond Engl. 2017;390:2673–734.
Zhang J-J, Li L, Liu D, Hu F-F, Cheng G-R, Xu L, et al. Urban-rural disparities in the association between body mass index and cognitive impairment in older adults: a cross-sectional study in central china. J Alzheimers Dis JAD. 2021;83:1741–52.
Li L, Cheng G-R, Liu D, Hu F-F, Gan X-G, Zhang B, et al. The Hubei memory and aging cohort study: study design, baseline characteristics, and prevalence of cognitive impairments. J Alzheimers Dis JAD. 2022;85:561–71.
Zhang J-J, Wu Z-X, Tan W, Liu D, Cheng G-R, Xu L, et al. Associations among multidomain lifestyles, chronic diseases, and dementia in older adults: a cross-sectional analysis of a cohort study. Front Aging Neurosci. 2023;15:1200671.
Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Bäckman L, Havulinna S, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement J Alzheimers Assoc. 2018;14:263–70.
Ye KX, Sun L, Wang L, Khoo ALY, Lim KX, Lu G, et al. The role of lifestyle factors in cognitive health and dementia in oldest-old: a systematic review. Neurosci Biobehav Rev. 2023;152:105286.
Cheng G-R, Liu D, Huang L-Y, Han G-B, Hu F-F, Wu Z-X, et al. Prevalence and risk factors for subjective cognitive decline and the correlation with objective cognition among community-dwelling older adults in China: results from the Hubei memory and aging cohort study. Alzheimers Dement J Alzheimers Assoc. 2023;19:5074–85.
Rajkumar S, Adibah N, Paskow MJ, Erkkila BE. Perceptions of nicotine in current and former users of tobacco and tobacco harm reduction products from seven countries. Drugs Alcohol Today. 2020;20:191–206.
Zhang J, Jia L, Zhu T, Zhu H, Shu L. The relationship and interaction between triglyceride glucose index and obesity in the risk of prehypertension population: a cross-sectional study from a survey in Anhui, Eastern China. BMC Cardiovasc Disord. 2023;23:336.
Snetselaar LG, de Jesus JM, DeSilva DM, Stoody EE. Dietary guidelines for Americans, 2020–2025: understanding the scientific process, guidelines, and key recommendations. Nutr Today. 2021;56:287–95.
Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55:263–7.
Chagas J, de M, Santos EHR, Ronchi CF, Biagini AP. Sleep and life quality in frail elderly - a narrative review. Sleep Sci Sao Paulo Braz. 2023;16:102–16.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49.
Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41.
Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp J Intern Med. 2013;4:627–35.
Dougherty L, Bellows N, Dadi C. Creating reproductive health behavioral profiles for women of reproductive age in niger using cross-sectional survey data: a latent class analysis. Int J Public Health. 2023;68:1605247.
Zhang J, Zou L, Jiao C, Zhang M, Wang L, Song W, et al. Cognitive benefits of activity engagement among 12,093 adults aged over 65 Years. Brain Sci. 2020;10:967.
American Psychiatric Association. American Psychiatric Association, editors. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association, 2013.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74.
Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuzma E, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322:430–7.
Licher S, Ahmad S, Karamujić-Čomić H, Voortman T, Leening MJG, Ikram MA, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25:1364–9.
Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology. 2020;95:e374–83.
Dhana K, Barnes LL, Liu X, Agarwal P, Desai P, Krueger KR, et al. Genetic risk, adherence to a healthy lifestyle, and cognitive decline in African Americans and European Americans. Alzheimers Dement J Alzheimers Assoc. 2022;18:572–80.
Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53.
Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37:2243–57.
Boa Sorte Silva NC, Barha CK, Erickson KI, Kramer AF, Liu-Ambrose T. Physical exercise, cognition, and brain health in aging. Trends Neurosci. 2024;47:402–17.
Sharifian N, Zaheed AB, Morris EP, Sol K, Manly JJ, Schupf N, et al. Social network characteristics moderate associations between cortical thickness and cognitive functioning in older adults. Alzheimers Dement J Alzheimers Assoc. 2022;18:339–47.
Valenzuela PL, Ruilope LM, Santos-Lozano A, Wilhelm M, Kränkel N, Fiuza-Luces C, et al. Exercise benefits in cardiovascular diseases: from mechanisms to clinical implementation. Eur Heart J. 2023;44:1874–89.
Frazier K, Kambal A, Zale EA, Pierre JF, Hubert N, Miyoshi S, et al. High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction. Cell Host Microbe. 2022;30:809–23.e6.
Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, et al. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024;9:37.
Wang Q, Yang Q, Liu X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell. 2023;14:762–75.
Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39:552–66.
Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52.
Irwin MR, Opp MR. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2017;42:129–55.
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–72.
Pomara N, Bruno D, Plaska CR, Ramos-Cejudo J, Osorio RS, Pillai A, et al. Plasma Amyloid-β dynamics in late-life major depression: a longitudinal study. Transl Psychiatry. 2022;12:301.
Sindi S, Kåreholt I, Ngandu T, Rosenberg A, Kulmala J, Johansson L, et al. Sex differences in dementia and response to a lifestyle intervention: evidence from Nordic population-based studies and a prevention trial. Alzheimers Dement J Alzheimers Assoc. 2021;17:1166–78.
Gan DRY, Mann J, Chaudhury H. Dementia care and prevention in community settings: a built environment framework for cognitive health promotion. Curr Opin Psychiatry. 2024;37:107–22.
Baker LD, Snyder HM, Espeland MA, Whitmer RA, Kivipelto M, Woolard N, et al. Study design and methods: U.S. study to protect brain health through lifestyle intervention to reduce risk (U.S. POINTER). Alzheimers Dement J Alzheimers Assoc. 2024;20:769–82.
Garcia-Lunar I, van der Ploeg HP, Fernández Alvira JM, van Nassau F, Castellano Vázquez JM, van der Beek AJ, et al. Effects of a comprehensive lifestyle intervention on cardiovascular health: the TANSNIP-PESA trial. Eur Heart J. 2022;43:3732–45.
Juul Rasmussen I, Frikke-Schmidt R. Modifiable cardiovascular risk factors and genetics for targeted prevention of dementia. Eur Heart J. 2023;44:2526–43.
Dunning D, Heath C, Suls JM. Flawed self-assessment: implications for health, education, and the workplace. Psychol Sci Public Interest J Am Psychol Soc. 2004;5:69–106.
Bandura A. Health promotion by social cognitive means. Health Educ Behav Off Publ Soc Public Health Educ. 2004;31:143–64.
Liao Y-Y, Chen I-H, Hsu W-C, Tseng H-Y, Wang R-Y. Effect of exergaming versus combined exercise on cognitive function and brain activation in frail older adults: a randomised controlled trial. Ann Phys Rehabil Med. 2021;64:101492.
Yaffe K, Vittinghoff E, Dublin S, Peltz CB, Fleckenstein LE, Rosenberg DE, et al. Effect of personalized risk-reduction strategies on cognition and dementia risk profile among older adults: the SMARRT randomized clinical trial. JAMA Intern Med. 2024;184:54–62.
Acknowledgements
We acknowledge all staff members of the HMACS group for their contributions in creating the study database. They all received compensation.
Funding
This research was supported by grants from Science and Technology Innovation 2030 Major Projects (2022ZD0211600), and National Natural Science Foundation of China (82371444, 82304172, 82071272, and 72174159).
Author information
Authors and Affiliations
Contributions
Dr Zeng had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: JJZ, DL; acquisition, analysis, or interpretation of data: JJZ; drafting of the manuscript: JJZ, DL, YZ; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: JJZ; visualization of the manuscript: JJZ, JL, CC; obtained funding: YZ; supervision: YZ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Ethics Committee of the School of Medicine at the Wuhan University of Science and Technology approved the study protocol (approval number: 201845). All participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
All authors have agreed to the submission and publication of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Liu, D., Liu, J. et al. Effects of self-managed lifestyle behavioral changes on cognitive impairment control in Chinese older adults: a population-based prospective study. Transl Psychiatry 15, 165 (2025). https://doi.org/10.1038/s41398-025-03365-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03365-9