Abstract
The impact of center volume on outcomes in pediatric hematopoietic cell transplantation (HCT) is not well established. We retrospectively analyzed data from a nationwide registry, including 6966 pediatric patients who underwent their first allogeneic HCT at 123 centers in Japan between 2001 and 2020. Centers were categorized by transplant volume as low volume centers (C1, the smallest number of transplantation), medium-low volume centers (C2), medium-high volume centers (C3), and high volume centers (C4, the greatest number of transplantation), and outcomes were compared across these categories. The analysis revealed no statistically significant differences in HCT outcomes among center categories. The 5-year OS by center category was 66.8% (95% CI 64.4–69.0%) for C1, 66.8% (95% CI 64.5–69.0%) for C2, 67.9% (95% CI 65.6–70.2%) for C3, and 68.3% (95% CI 65.9–70.6%) for C4. These results were consistent even when analysis was restricted to malignant and nonmalignant diseases. Our findings suggest that, unlike in adult HCT, outcomes for pediatric HCT are not significantly affected by center volume. These results indicate the consistent quality of care across centers, supporting the accessibility of HCT at various institutions for pediatric patients.

Similar content being viewed by others
Introduction
Hematopoietic cell transplantation (HCT) offers curative potential for conditions such as refractory leukemia [1, 2], bone marrow failure syndromes [3], immune deficiencies [4, 5], and inborn errors of metabolism [6]. However, intensive conditioning regimens and post-transplant immune responses often result in severe, sometimes life-threatening complications, necessitating effective management strategies to improve post-transplant survival.
Several factors influencing post-transplant survival are beyond the physician’s control, such as the disease type, and the amount of residual tumor. However, other factors, including conditioning regimen selection and donor matching, can be optimized. Supportive care is also crucial for safe transplantation. Institutional practices and physician experience often shape strategies for conditioning, immunosuppression, and the management of infections and organ dysfunction, potentially leading to variability in outcomes between centers.
The “center effect” has been documented in several studies [7,8,9,10,11], with some reports suggesting that high-volume centers may achieve better outcomes, though findings are inconsistent. High-volume centers might benefit from accumulated experience, enabling better management of complications and other factors collectively reduce transplant-related mortality. Conversely, high-volume centers may also treat patients with a higher relapse risk or more complex conditions. On the other hand, low-volume centers might provide more individualized care to their small number of transplant patients, potentially contributing to improved survival rates.
Most research on center effects has focused on adult patients. Pediatric transplantation differs in its indications and disease distribution, which include not only leukemia but also bone marrow failure, primary immune deficiencies, and inborn errors of metabolism. The accumulation of pediatric transplantation experience may follow a different trajectory than in adults, and findings from adult studies may not be directly extrapolatable to pediatric patients. Few studies have examined center effects in pediatric transplantation [12,13,14,15]. A report has indicated that centers with a high volume of haploidentical transplants showed better outcomes [15], whereas another study found no correlation between transplant center volume and outcomes in intensive care settings. However, these studies are often limited by sample size and disease scope.
Therefore, to assess the center effect in pediatric transplantation and provide insights for improving outcomes, we conducted an analysis using nationwide registry data.
Methods
Study population
All data were collected using the Transplant Registry Unified Management Program, sponsored by the Japanese Society for Transplantation and Cellular Therapy and the Japanese Data Center for Hematopoietic Cell Transplantation. This registration program covers over 99% of transplants nationwide [16].
To evaluate the treatment experience of pediatric patients, we included pediatric cases aged 19 years or younger at HCT, who received their first HCT from an allogeneic donor between 2001 and 2020. The transplant registry is organized by department, and cases are registered accordingly. Therefore, we included only those departments classified as pediatric departments in this analysis, even if the patients were under 19 years of age. Additionally, a small number of facilities operate joint teams comprising pediatric and adult departments; cases from such teams were excluded from this analysis. Allogeneic transplants for solid tumors were also excluded.
Ethics approval and consent to participate
All study procedures complied with the Helsinki Declaration. The study was devised by the Complication Working Group of the Japanese Society for Transplantation and Cellular Therapy, and approved from the Data Management Committee of the Japanese Data Center for Hematopoietic Cell Transplantation (#20-70). The study was also approved by the Institutional Review Board of the University of Tokyo Hospital (#2022273NI). All patients provided informed consent for the use of their clinical data for research purposes.
Statistical analysis
Center experience was defined based on the number of allogeneic HCT performed during the 20-year period, by which centers were divided into four groups using quartiles. Institutions were categorized as low volume centers (C1, the smallest number of transplantation), medium-low volume centers (C2), medium-high volume centers (C3), and high volume centers (C4, the greatest number of transplantation). For malignant/non-malignant specific analysis, institutions were re-categorized according to the number of transplantations performed for malignant diseases as low volume centers (malignant-C1/NM-C1, the smallest number of transplantation for malignant/non-malignant diseases), medium-low volume centers (malignant-C2/NM-C2), medium-high volume centers (malignant-C3/NM-C3), and high volume centers (malignant-C4/NM-C4, the greatest number of transplantation for malignant/non-malignant diseases).
The median follow-up time was estimated using the Reverse Kaplan-Meier method, where censoring events were treated as failures. The probability of overall survival (OS) was estimated using Kaplan-Meier methods. Cumulative incidence curves were used in a competing-risk setting to calculate the probability of non-relapse mortality and relapse. Multivariate analysis was performed using the Cox proportional-hazard regression model. A two-sided p value of less than 0.05 was considered to be significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics [17]. Data preparation was conducted using a script provided by Drs. Yoshinobu Kanda and Junya Kanda [18].
Results
Transplantation and institutions
The study included 6966 patients who underwent transplantation at 123 centers (Table 1). C1 comprised centers performing ≤63 allo-HCT over 20 years (≤3.2 HCT/year), while C4 included centers performing ≥227 allo-HCT ( ≥ 11.4 HCT/year). Age distribution across categories is shown in Supplementary Fig. 1.
Centers with higher transplant volumes, such as C3 and C4, had a relatively higher proportion of non-malignant diseases, with fewer umbilical cord blood transplants and more non-myeloablative transplants.
Outcome of HCT
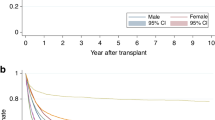
The median follow-up period for surviving patients was 7.2 years, with an overall 5-year OS of 67.4% (95% confidence interval [CI]; 66.3–68.6%). The 5y-OS by center category was 66.8% (95% CI 64.4–69.0%) for C1, 66.8% (95% CI 64.5–69.0%) for C2, 67.9% (95% CI 65.6–70.2%) for C3, and 68.3% (95% CI 65.9–70.6%) for C4, with no statistically significant differences (p = 0.85) (Fig. 1). Early post-transplant survival rates did not consistently correlate with center experience. The 100-day OS was 90.9% (95% CI 89.4–92.1%) for C1, 92.1% (95% CI 90.8–93.3%) for C2, 92.9% (95% CI 91.6–94.0%) for C3, and 91.6% (95% CI 90.1–92.9%) for C4. Similar trends were observed for transplants conducted after 2011, with no significant differences in OS by center category (Supplementary Fig. 2).
No clear differences were observed in the distribution of causes of death across center categories (Supplementary Fig. 3). A small number of patients had late events at later than 10 years from HCT. Cause of these late death were predominantly secondary malignancies, with C3 and C4 including patients with underlying genetic diseases such as Fanconi anemia.
The incidence of grade II–IV acute GVHD at day 100 was 35.0% (95% CI, 32.7–37.3%) for C1, 35.5% (95% CI 33.2–37.7%) for C2, 34.8% (95% CI 32.5–37.0%) for C3, and 36.8% (95% CI 34.4–39.1%) for C4 (p = 0.45) (Supplementary Fig. 4A). The incidence of grade III–IV acute GVHD at day 100 was 13.8% (95% CI 12.2–15.5%) for C1, 15.6% (95% CI 14.0–17.3%) for C2, 14.5% (95% CI 12.8–16.2%) for C3, and 14.9% (95% CI 13.2–16.7%) for C4 (p = 0.56). The incidence of chronic GVHD at 1 year was 23.4% (95% CI 21.4–25.5%) for C1, 20.1% (95% CI 18.3–22.1%) for C2, 20.1% (95% CI 18.2–22.1%) for C3, and 17.7% (95% CI 15.8–19.7%) for C4 (p < 0.001) (Supplementary Fig. 4B).
Outcomes of HCT for malignant diseases
In an analysis restricted to malignant diseases (Supplementary Table 1), no statistically significant differences in OS were observed between center categories. The 5-year OS was 61.4% (95% CI, 58.5–64.2%) for malignant-C1, 60.2% (95% CI, 57.3–63.0%) for malignant-C2, 61.3% (95% CI, 58.3–64.1%) for malignant-C3, and 59.7% (95% CI, 56.7–62.5%) for malignant-C4 (p = 0.44) (Fig. 2a). The 5-year disease free survival was 56.4% (95% CI, 53.5–59.2%) for malignant-C1, 56.8% (95% CI, 53.8–59.6%) for malignant-C2, 58.8% (95% CI, 55.8–61.6%) for malignant-C3, and 55.3% (95% CI, 52.4–58.2%) for malignant-C4 (p = 0.28) (Fig. 2b).
Patients who underwent transplantation at low volume centers (malignant-C1, the smallest number of transplantation), medium-low volume centers (malignant-C2), medium-high volume centers (malignant-C3), and high volume centers (malignant-C4, the greatest number of transplantation) are compared. a Overall survival, (b) disease free survival, (c) cumulative incidence of relapse, and (d) cumulative incidence of non-relapse mortality.
The 5-year cumulative incidence of relapse was 31.1% (95% CI, 28.4–33.7%) for malignant-C1, 27.9% (95% CI, 25.3–30.5%) for malignant-C2, 27.6% (95% CI, 25.0–30.3%) for malignant-C3, and 29.4% (95% CI, 26.7–32.0%) for malignant-C4 (p = 0.25) (Fig. 2c). The 5-year cumulative incidence of non-relapse mortality was 12.6% (95%CI, 10.7–14.6%) for malignant-C1, 15.4% (95%CI, 13.3–17.5%) for malignant-C2, 13.6% (95%CI, 11.6–15.7%) for malignant-C3, and 15.3% (95%CI, 13.3–17.5%) for malignant-C4 (p = 0.02) (Fig. 2d).
When analyzing data restricted to patients with HCT for CR1 of ALL or AML, the survival curves were nearly identical across center categories (Supplementary Fig. 5). The 5-year OS was 73.6% (95%CI, 68.7–77.9%) for malignant-C1, 78.1% (95%CI, 73.6–81.9%) for malignant-C2, 77.7% (95%CI, 73.0–81.6%) for malignant-C3, and 74.7% (95%CI, 69.8–78.9%) for malignant-C4 (p = 0.72). The 5-year cumulative incidence of relapse was 19.9% (95%CI, 16.0–24.2%) for malignant-C1, 18.6% (95%CI, 14.9–22.7%) for malignant-C2, 18.9% (95%CI, 15.0–23.1%) for malignant-C3, and 21.0% (95%CI, 17.0–25.4%) for malignant-C4 (p = 0.84). The 5-year cumulative non-relapse mortality was 11.5% (95%CI, 8.4–15.1%) for malignant-C1, 8.7% (95%CI, 6.1–11.7%) for malignant-C2, 9.0% (95%CI, 6.3–12.2%) for malignant-C3, and 9.1% (95%CI, 6.4–12.3%) for malignant-C4 (p = 0.74).
Outcomes of HCT for non-malignant diseases
An analysis focusing on non-malignant diseases (Supplementary Table 2) showed that bone marrow failure syndromes were the most common diagnosis in all centers. Metabolic disorders were slightly less frequent in NM-C1, and Fanconi anemia patients were transplanted more commonly transplanted in NM-C4.
Even when focusing on non-malignant diseases, no significant differences in OS were observed across center categories. The 5-year OS was 90.2% (95% CI 85.3–93.5%) for NM-C1, 90.7% (95% CI 86.2–93.8%) for NM-C2, 92.3% (95% CI 88.3–95.0%) for NM-C3, and 89.2% (95% CI 84.3–92.7%) for NM-C4 (p = 0.94) (Fig. 3).
Multivariate analysis for outcomes
Multivariate analysis focusing on both acute leukemia (Table 2) and non-malignant diseases (Table 3) showed no significant center effect on post-transplant survival. Differences in transplant outcomes may vary depending on the cell source. As donor selection criteria are often shaped by transplant experience, a potential center effect cannot be ruled out. However, in our study, the influence of transplant experience on outcomes was not evident, even after performing a multivariate analysis that accounted for cell source.
Discussion
This study did not find substantial differences in survival outcomes between high- and low-volume centers for pediatric HCT. While the study does not prove the absence of differences, and may be subject to various potential biases due to a retrospective nature, it does suggest that clinically significant differences are unlikely. These results were consistent even when the analysis was restricted to malignant or non-malignant diseases or more recent transplants.
In contrast, several studies on adult HCT have reported a center effect, including studies using the same registry data [9, 11]. The acute non-relapse mortality may reflect transplant quality. However, even when focusing on early post-transplant cumulative mortality (e.g., within 100 days), no evidence suggested that high-volume centers performed better.
Given the smaller number of pediatric HCT compared to adults, one interpretation is that pediatric transplants are managed at a comparable quality across low- and high-volume centers. While many hospitals treat pediatric hematologic and oncologic diseases, only a limited number of facilities perform allo-HCT, contributing a certain level of quality of transplantation. Additionally, the rapid dissemination of information in recent years may have contributed to standardizing care across centers. It is crucial to rigorously assess and communicate the transplantation experience from each facility as evidence, rather than allowing them to remain anecdotal.
However opposite perspective is also valid. The relatively small number of pediatric allo-HCT, even at high-volume centers suggested that center experience was still insufficient for pediatric transplantation. It may be more important to centralize more patients than at present in order to accumulate experience and improve transplantation for rare pediatric patients. Especially, it is important to acknowledge that transplantation for rare diseases require unique management skill and knowledge [19]. Additionally, rare and severe transplantation-related complications may not be encountered without a large volume of transplants. Given the limited evidence available in rare diseases and complications, a centralized approach should be preferable to enhance evidence generation. Furthermore, conducting transplants at centers that manage similar diseases can provide valuable peer support for patients and their families. The approach to transplantation should therefore be tailored, balancing centralization and decentralization according to the specific needs of each disease.
Our study has several limitations, the most important being its retrospective design, which introduce biases affecting post-transplant outcomes. However, it is practically impossible to control for the center effect in a prospective interventional study. We attempted to mitigate this limitation by increasing the number of cases analyzed. While differences may exist in more challenging transplants, such as second transplants, these were not included in the current analysis. Such cases are few in number and require innovative approaches to study. However, it can be assumed that facilities with extensive transplant experience tend to perform a higher number of second and third transplants. This study primarily assessed overall survival. However, post-transplant, patients may experience significant complications, some of which are late-onset and have a profound impact on quality of life, such as chronic GVHD. The frequency and severity of complications were only partially analyzed in this study.
In conclusion, our analysis suggests that there are no substantial differences in survival outcomes between high- and low-volume centers for pediatric transplants, indicating that consistent quality of care is maintained across centers. This is positive information for patients, and it is recommended that pediatric patients undergo transplants at the most accessible center.
Data availability
The data of this study are not publicly available due to ethical restrictions that it exceeds the scope of the recipient/donor’s consent for research use in the registry. Data may be available from the corresponding author upon reasonable request and with permission of the JSTCT/JDCHCT.
References
Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority phase III study. J Clin Oncol. 2021;39:295–307.
Kato M. Recent progress in pediatric lymphoblastic leukemia. Int J Hematol. 2023;117:155–61.
Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428–36.
Vallee TC, Glasmacher JS, Buchner H, Arkwright PD, Behrends U, Bondarenko A, et al. Wiskott-Aldrich syndrome: a study of 577 patients defines the genotype as a biomarker for disease severity and survival. Blood. 2024;143:2504–16.
Goebel GA, de Assis CS, Cunha LAO, Minafra FG, Pinto JA. Survival after hematopoietic stem cell transplantation in severe combined immunodeficiency (SCID): a worldwide review of the prognostic variables. Clin Rev Allergy Immunol. 2024;66:192–209.
Kubaski F, Yabe H, Suzuki Y, Seto T, Hamazaki T, Mason RW, et al. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis II. Biol Blood Marrow Transpl. 2017;23:1795–803.
Frassoni F, Labopin M, Powles R, Mary JY, Arcese W, Bacigalupo A, et al. Effect of centre on outcome of bone-marrow transplantation for acute myeloid leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 2000;355:1393–8.
Loberiza FR Jr, Zhang MJ, Lee SJ, Klein JP, LeMaistre CF, Serna DS, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–87.
Kurosawa S, Fukuda T, Ichinohe T, Hashii Y, Kanda J, Goto H, et al. Center effect on allogeneic hematopoietic stem cell transplantation outcomes for B-cell acute lymphoblastic leukemia. Cytotherapy. 2024;26:1185–92.
Loberiza FR Jr, Serna DS, Horowitz MM, Rizzo JD. Transplant center characteristics and clinical outcomes after hematopoietic stem cell transplantation: what do we know?. Bone Marrow Transpl. 2003;31:417–21.
Yanada M, Yano S, Kuwatsuka Y, Kawamura K, Fukuda T, Ichinohe T, et al. The effect of center experience on allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia. Bone Marrow Transpl. 2024;59:541–9.
Page KM, Labopin M, Ruggeri A, Michel G, Diaz de Heredia C, O’Brien T, et al. Factors Associated with Long-Term Risk of Relapse after Unrelated Cord Blood Transplantation in Children with Acute Lymphoblastic Leukemia in Remission. Biol Blood Marrow Transpl. 2017;23:1350–8.
Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–71.
Zinter MS, Brazauskas R, Strom J, Chen S, Bo-Subait S, Sharma A, et al. Intensive care risk and long-term outcomes in pediatric allogeneic hematopoietic cell transplant recipients. Blood Adv. 2024;8:1002–17.
Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R, et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115:3437–46.
Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol. 2016;103:11–19.
Ono S, Takeshita K, Kiridoshi Y, Kato M, Kamiya T, Hoshino A, et al. Hematopoietic cell transplantation rescues inflammatory bowel disease and dysbiosis of gut microbiota in XIAP deficiency. J Allergy Clin Immunol Pract. 2021;9:3767–80.
Acknowledgements
We would like to thank all the staff at the participating hospitals and centers who provided valuable data from the JSHCT registry. This work was supported in part by a research grant from the National Center for Child Health and Development (2022B-10) and AMED under Grant Number JP24lk0221194.
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
MKato designed the study, analyzed data, interpreted the results, and drafted the paper; HN, KY interpreted results and edited the paper; KMatsuo, YI, MN, MO, KT, TI, YH, JK, HG, KK, MY, AS, MH, KMatsumoto, AY, and TF contributed to data management, interpreted the results, and edited the paper. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kato, M., Nakashone, H., Matsuo, K. et al. Impact of center volume on outcomes in allogeneic hematopoietic cell transplantation for children. Bone Marrow Transplant 60, 851–856 (2025). https://doi.org/10.1038/s41409-025-02569-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-025-02569-3