Abstract
Background
Sunitinib has emerged as the primary treatment for advanced or metastatic clear cell renal cell carcinoma (ccRCC) due to its significant improvement in patients’ average survival time. However, drug resistance and adverse effects of sunitinib pose challenges to its clinical benefits.
Methods
The differentially expressed genes (DEGs) associated with sunitinib sensitivity and resistance in ccRCC were investigated. Cell counting kit-8, plate colony formation, flow cytometry and subcutaneous xenograft tumor model assays were employed to explore the effects of PDZK1 on ccRCC. Further research on the molecular mechanism was conducted through western blot, co-immunoprecipitation, immunofluorescence co-localization and immunohistochemical staining.
Results
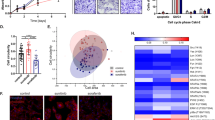
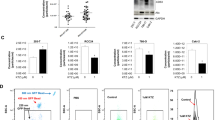
We elucidated that PDZK1 is significantly downregulated in sunitinib-resistant ccRCC specimens, and PDZK1 negatively regulates the phosphorylation of PDGFR-β and the activation of its downstream pathways through interaction with PDGFR-β. The dysregulated low levels of PDZK1 contribute to inadequate inhibition of cell proliferation, tumor growth, and insensitivity to sunitinib treatment. Notably, our preclinical investigations showed that miR-15b antagomirs enhance sunitinib cytotoxic effects against ccRCC cells by upregulating PDZK1 levels, suggesting their potential in overcoming sunitinib resistance.
Conclusions
Our findings establish the miR-15b/PDZK1/PDGFR-β axis as a promising therapeutic target and a novel predictor for ccRCC patients’ response to sunitinib treatment.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The previously published data underlying Figs. 1, 2, 7 and Supplementary Figs. 1–6 are openly available in [E-MTAB-3267] at [https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-3267]; [GSE64052] at [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64052]; [TCGA, Kidney Renal Clear Cell Carcinoma, PanCancer Atlas] at [https://www.cbioportal.org/study/summary?id=kirc_tcga_pan_can_atlas_2018]; [CPTAC, Clear Cell Renal Cell Carcinoma Discovery Cohort] at [https://pdc.cancer.gov/pdc/study/PDC000128]; [GSE210189] at [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210189]. The data underlying Fig. 5 (tissue microarrays containing 90 pairs of ccRCC and adjacent tissues) and Fig. 7 (23 human ccRCC samples from the First Hospital of Shanxi Medical University) in this study are not publicly available due to patient privacy issues. However, the data are available from the corresponding author upon reasonable request (for research only).
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74–84. https://doi.org/10.1016/j.eururo.2018.08.036.
Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17:245–61. https://doi.org/10.1038/s41581-020-00359-2.
Choueiri TK, Kaelin WG. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat Med. 2020;26:1519–30. https://doi.org/10.1038/s41591-020-1093-z.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. https://doi.org/10.1016/j.ctrv.2007.12.001.
Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol. 2019;16:621–33.
Najjar YG, Rini BI. Novel agents in renal carcinoma: a reality check. Ther Adv Med Oncol. 2012;4:183–94. https://doi.org/10.1177/1758834012443725.
Oudard S, George D, Medioni J, Motzer R. Treatment options in renal cell carcinoma: past, present and future. Ann Oncol. 2007;18:x25–31. https://doi.org/10.1093/annonc/mdm411.
Rathmell WK, Rumble RB, Van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–95. https://doi.org/10.1200/JCO.22.00868.
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3. https://doi.org/10.1038/nrdp.2017.9.
Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13:496–511. https://doi.org/10.1038/nrneph.2017.82.
Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–85. https://doi.org/10.1016/S1470-2045(19)30413-9.
Méjean A, Ravaud A, Thezenas S, Chevreau C, Bensalah K, Geoffrois L, et al. Sunitinib alone or after nephrectomy for patients with metastatic renal cell carcinoma: is there still a role for cytoreductive nephrectomy? Eur Urol. 2021;80:417–24. https://doi.org/10.1016/j.eururo.2021.06.009.
Morais C. Sunitinib resistance in renal cell carcinoma. J Kidney Cancer Vhl. 2014;1:1–11.
Dranitsaris G, Schmitz S, Broom RJ. Small molecule targeted therapies for the second-line treatment for metastatic renal cell carcinoma: a systematic review and indirect comparison of safety and efficacy. J Cancer Res Clin Oncol. 2013;139:1917–26. https://doi.org/10.1007/s00432-013-1510-5.
Ravaud A. Treatment-associated adverse event management in the advanced renal cell carcinoma patient treated with targeted therapies. The Oncologist. 2011;16:32–44. https://doi.org/10.1634/theoncologist.2011-S2-32.
Bracarda S, Iacovelli R, Boni L, Rizzo M, Derosa L, Rossi M, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol. 2015;26:2107–13. https://doi.org/10.1093/annonc/mdv315.
Bracarda S, Sisani M, Marrocolo F, Hamzaj A, Del Buono S, De Simone V. GOAL: an inverse toxicity-related algorithm for daily clinical practice decision making in advanced kidney cancer. Crit Rev Oncol Hematol. 2014;89:386–93. https://doi.org/10.1016/j.critrevonc.2013.09.002.
Knox JJ, Barrios CH, Kim TM, Cosgriff T, Srimuninnimit V, Pittman K, et al. Final overall survival analysis for the phase II RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC. Ann Oncol. 2017;28:1339–45. https://doi.org/10.1093/annonc/mdx075.
Yamada Y, Ohno Y, Kato Y, Kobayashi R, Hayashi H, Miyahara S, et al. Optimal dose of sunitinib for long-term treatment in Japanese patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2019;84:987–92. https://doi.org/10.1007/s00280-019-03935-x.
Demlova R, Turjap M, Pes O, Kostolanska K, Jurica J. Therapeutic drug monitoring of sunitinib in gastrointestinal stromal tumors and metastatic renal cell carcinoma in adults—a review. Ther Drug Monit. 2020;42:20–32. https://doi.org/10.1097/FTD.0000000000000663.
Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang Y. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 2018;17. https://doi.org/10.1186/s12943-018-0801-5.
Neul C, Schaeffeler E, Sparreboom A, Laufer S, Schwab M, Nies AT. Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol Sci. 2016;37:904–32. https://doi.org/10.1016/j.tips.2016.08.003.
Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–9. https://doi.org/10.1093/annonc/mdx703.
Sekino Y, Teishima J, Liang G, Hinata N. Molecular mechanisms of resistance to tyrosine kinase inhibitor in clear cell renal cell carcinoma. Int J Urol. 2022. https://doi.org/10.1111/iju.15042.
Tao T, Yang X, Zheng J, Feng D, Qin Q, Shi X, et al. PDZK1 inhibits the development and progression of renal cell carcinoma by suppression of SHP-1 phosphorylation. Oncogene. 2017;36:6119–31. https://doi.org/10.1038/onc.2017.199.
Zhao C, Tao T, Yang L, Qin Q, Wang Y, Liu H, et al. Loss of PDZK1 expression activates PI3K/AKT signaling via PTEN phosphorylation in gastric cancer. Cancer Lett. 2019;453:107–21. https://doi.org/10.1016/j.canlet.2019.03.043.
Wang H, Yang W, Qin Q, Yang X, Yang Y, Liu H, et al. E3 ubiquitin ligase MAGI3 degrades c-Myc and acts as a predictor for chemotherapy response in colorectal cancer. Mol Cancer. 2022;21. https://doi.org/10.1186/s12943-022-01622-9.
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ, Sun RH, et al. Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRbeta-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 2010;20:1252–62. https://doi.org/10.1038/cr.2010.111.
Xue Y, Lim S, Yang Y, Wang Z, Jensen LDE, Hedlund E, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2012;18:100–10. https://doi.org/10.1038/nm.2575.
Shim M, Song C, Park S, Choi S, Cho YM, Kim C, et al. Prognostic significance of platelet-derived growth factor receptor-β expression in localized clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2015;141:2213–20. https://doi.org/10.1007/s00432-015-2019-x.
Carrato Mena A, Grande Pulido E, Guillén-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21:S3–11. https://doi.org/10.1097/01.cad.0000361534.44052.c5.
Qi Y, Ma Y, Peng Z, Wang L, Li L, Tang Y, et al. Long noncoding RNA PENG upregulates PDZK1 expression by sponging miR-15b to suppress clear cell renal cell carcinoma cell proliferation. Oncogene. 2020;39:4404–20. https://doi.org/10.1038/s41388-020-1297-1.
Lu L, Li Y, Wen H, Feng C. Overexpression of miR-15b promotes resistance to sunitinib in renal cell carcinoma. J Cancer. 2019;10:3389–96. https://doi.org/10.7150/jca.31676.
Lee WJ, Lee JL, Chang SE, Lee MW, Kang YK, Choi JH, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161:1045–51. https://doi.org/10.1111/j.1365-2133.2009.09290.x.
Lankhorst S, Baelde HJ, Kappers MHW, Smedts FMM, Hansen A, Clahsen-van Groningen MC, et al. Greater sensitivity of blood pressure than renal toxicity to tyrosine kinase receptor inhibition with sunitinib. Hypertension. 2015;66:543–9. https://doi.org/10.1161/HYPERTENSIONAHA.115.05435.
Narayan V, Keefe S, Haas N, Wang L, Puzanov I, Putt M, et al. Prospective evaluation of sunitinib-induced cardiotoxicity in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2017;23:3601–9. https://doi.org/10.1158/1078-0432.CCR-16-2869.
Stitzlein L, Rao P, Dudley R. Emerging oral VEGF inhibitors for the treatment of renal cell carcinoma. Expert Opin Investig Drugs. 2019;28:121–30. https://doi.org/10.1080/13543784.2019.1559296.
Hirsch L, Flippot R, Escudier B, Albiges L. Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs. 2020;80:1169–81. https://doi.org/10.1007/s40265-020-01327-7.
Song SH, Jeong IG, You D, Hong JH, Hong B, Song C, et al. VEGF/VEGFR2 and PDGF-B/PDGFR-beta expression in non-metastatic renal cell carcinoma: a retrospective study in 1,091 consecutive patients. Int J Clin Exp Pathol. 2014;7:7681–9.
Baxter RM, Secrist JP, Vaillancourt RR, Kazlauskas A. Full activation of the platelet-derived growth factor beta-receptor kinase involves multiple events. J Biol Chem. 1998;273:17050–5. https://doi.org/10.1074/jbc.273.27.17050.
Kazlauskas A, Durden DL, Cooper JA. Functions of the major tyrosine phosphorylation site of the PDGF receptor β subunit. Cell Regul. 1991;2:413–25.
Nickas ME, Bernard A, Kazlauskas A. The requirement of tyrosines 579 and 581 for maximal ligand-dependent activation of the βPDGFR is influenced by noncytoplasmic regions of the receptor. Exp Cell Res. 2001;265:80–9. https://doi.org/10.1006/excr.2001.5169.
Turner EC, Mulvaney EP, Reid HM, Kinsella BT. Interaction of the human prostacyclin receptor with the PDZ adapter protein PDZK1: role in endothelial cell migration and angiogenesis. Mol Biol Cell. 2011;22:2664–79. https://doi.org/10.1091/mbc.E11-04-0374.
Zhu W, Saddar S, Seetharam D, Chambliss KL, Longoria C, Silver DL, et al. The scavenger receptor class B type I adaptor protein PDZK1 maintains endothelial monolayer integrity. Circ Res. 2008;102:480–7. https://doi.org/10.1161/CIRCRESAHA.107.159079.
Dai C, Liang Y, Wang Y, Tiwari AK, Yan Y, Wang F, et al. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74–83. https://doi.org/10.1016/j.canlet.2009.01.027.
Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37:359–65. https://doi.org/10.1124/dmd.108.024612.
Jin J, Xie Y, Zhang JS, Wang JQ, Dai SJ, He WF, et al. Sunitinib resistance in renal cell carcinoma: from molecular mechanisms to predictive biomarkers. Drug Resist Updat. 2023;67:100929. https://doi.org/10.1016/j.drup.2023.100929.
Acknowledgements
We would like to thank Doctor Tongmei Zhang (Beijing Chest Hospital, Capital Medical University) and Doctor Jinwei Miao (Beijing Obstetrics and Gynecology Hospital, Capital Medical University) for kindly providing some clinical samples. We thank Dr. Randy Hall at Emory University (Atlanta, GA) and Dr. Michael R Beard at the Centre for Cancer Biology (Adelaide, Australia) for plasmids materials.
Funding
This study was supported by the National Natural Science Foundation of the People’s Republic of China (Grants Nos. 81772707, 81972732, 82273965); China Postdoctoral Foundation (Grants No. 2023M732409). The funding agency did not participate in the study design, data collection, analysis and interpretation, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: JH and HW; data curation: LZ, HL and HW; methodology: HW and LZ; investigation: RS and QQ; validation: XY, DF and SW; visualization: HW and WL; formal analysis: XC and YY; project administration: SW and JH; resources: TB; supervision: JH and TB; funding acquisition: JH and HW; writing—original draft: HL and HW; writing—review & editing: JH and TB.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Animal experiments were conducted in compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and approved by the Animal Use and Care Committee of Capital Medical University (approval numbers AEEI-2020-133 and AEEI-2018-201). The research involving ccRCC samples was approved by the Ethics Committee of Capital Medical University (2017SY09) and the First Hospital of Shanxi Medical University (2022HLL001).
Consent for publication
Written informed consent was obtained from all patients for the use of their tissue specimens and publication of this report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Zhang, L., Liu, H. et al. PDZK1 confers sensitivity to sunitinib in clear cell renal cell carcinoma by suppressing the PDGFR-β pathway. Br J Cancer 131, 347–360 (2024). https://doi.org/10.1038/s41416-024-02725-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02725-4
This article is cited by
-
Microbially produced imidazole propionate impairs prostate cancer progression through PDZK1
Molecular Medicine (2025)
-
PDZK1 inhibits MRP2-mediated oxaliplatin chemosensitivity in hepatocellular carcinoma
Scientific Reports (2025)
-
Trends and hotspots in targeted therapy resistance research for renal cell carcinoma: a bibliometric and visualization analysis
Discover Oncology (2025)