Abstract
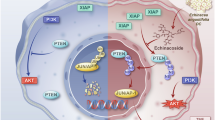
PTEN tumor suppressor opposes the PI3K/Akt signaling pathway in the cytoplasm and maintains chromosomal integrity in the nucleus. Nucleus–cytoplasm shuttling of PTEN is regulated by ubiquitylation, SUMOylation and phosphorylation, and nuclear PTEN has been proposed to exhibit tumor-suppressive functions. Here we show that PTEN is conjugated by Nedd8 under high glucose conditions, which induces PTEN nuclear import without effects on PTEN stability. PTEN neddylation is promoted by the XIAP ligase and removed by the NEDP1 deneddylase. We identify Lys197 and Lys402 as major neddylation sites on PTEN. Neddylated PTEN accumulates predominantly in the nucleus and promotes rather than suppresses cell proliferation and metabolism. The nuclear neddylated PTEN dephosphorylates the fatty acid synthase (FASN) protein, inhibits the TRIM21-mediated ubiquitylation and degradation of FASN, and then promotes de novo fatty acid synthesis. In human breast cancer tissues, neddylated PTEN correlates with tumor progression and poor prognosis. Therefore, we demonstrate a previously unidentified pool of nuclear PTEN in the Nedd8-conjugated form and an unexpected tumor-promoting role of neddylated PTEN.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
For IP-MS, all raw data and search results have been deposited to the PRIDE database (http://www.iprox.org/index) with the accession number: PXD011368 (Supplementary information, Data S1); PXD021544 (Supplementary information, Data S2); PXD011395 (Supplementary information, Data S3). The authors declare that all the relevant data supporting the findings of this study are available within the article and its Supplementary information files, or from the corresponding author on reasonable request.
Change history
29 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41422-021-00470-4
References
Song, M. S., Salmena, L. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 (2012).
Naguib, A. et al. PTEN functions by recruitment to cytoplasmic vesicles. Mol. Cell 58, 255–268 (2015).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Salmena, L., Carracedo, A. & Pandolfi, P. P. Tenets of PTEN tumor suppression. Cell 133, 403–414 (2008).
Hollander, M. C., Blumenthal, G. M. & Dennis, P. A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 11, 289–301 (2011).
Lee, J. O. et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 (1999).
Lee, Y. R., Chen, M. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 19, 547–562 (2018).
Shen, W. H. et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 (2007).
Puc, J. & Parsons, R. PTEN loss inhibits CHK1 to cause double stranded-DNA breaks in cells. Cell Cycle 4, 927–929 (2005).
Li, A. G. et al. Mechanistic insights into maintenance of high p53 acetylation by PTEN. Cell 23, 575–587 (2006).
Ying, H. et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov. 1, 158–169 (2011).
Wang, J. et al. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res. 65, 6601–6611 (2005).
Song, M. S. et al. Immunohistochemical tumor-suppressive complex in a phosphatase-independent manner. Cell 144, 187–199 (2011).
Trotman, L. C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Song, M. S. et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455, 813–817 (2008).
Bassi, C. et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341, 395–399 (2013).
Kerscher, O., Felberbaum, R. & Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Ann. Rev. Cell Dev. Biol. 22, 159–180 (2006).
Enchev, R. I., Schulman, B. A. & Peter, M. Protein neddylation: beyond cullin-RING ligases. Nat. Rev. Mol. Cell Biol. 16, 30–44 (2015).
Mendoza, H. M. et al. NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J. Biol. Chem. 278, 25637–25643 (2003).
Cope, G. A. et al. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science 298, 608–611 (2002).
Soucy, T. A. et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 (2009).
Abidi, N. & Xirodimas, D. P. Regulation of cancer-related pathways by protein NEDDylation and strategies for the use of NEDD8 inhibitors in the clinic. Endocr. Relat. Cancer 22, T55–T70 (2015).
Zhou, L. & Jia, L. Targeting Protein Neddylation for Cancer Therapy. Adv. Exp. Med. Biol. 1217, 297–315 (2020).
Finicle, B. T., Jayashankar, V. & Edinger, A. L. Nutrient scavenging in cancer. Nat. Rev. Cancer 18, 619–633 (2018).
Hu, Q. et al. LncRNAs-directed PTEN enzymatic switch governs epithelial-mesenchymal transition. Cell Res. 29, 286–304 (2019).
Liang, H. et al. PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab. 19, 836–848 (2014).
Liang, H. et al. PTENβ is an alternatively translated isoform of PTEN that regulates rDNA transcription. Nat. Commun. 8, 14771 (2017).
Xie, P. et al. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat. Commun. 5, 3733 (2014).
Ohki, Y. et al. The mechanism of poly-NEDD8 chain formation in vitro. Biochem. Biophys. Res. Commun. 381, 443–447 (2009).
Keuss, M. J. et al. Unanchored tri-NEDD8 inhibits PARP-1 to protect from oxid-ative stress induced cell death. EMBO J. 38, e100024 (2019).
Hori, T. et al. Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene 18, 6829–6834 (1999).
Zhuang, M., Guan, S., Wang, H., Burlingame, A. L. & Wells, J. A. Substrates of IAP ubiquitin ligases identified with a designed orthogonal E3 ligase, the NEDDylator. Mol. Cell 49, 273–282 (2013).
Van Themsche, C., Leblanc, V., Parent, S. & Asselin, E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J. Biol. Chem. 284, 20462–20466 (2009).
Lloyd, C. T. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Lee, Y. R. et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 364, eaau0159 (2019).
Maddika, S. et al. WWP2 Is an E3 ubiquitin Ligase for PTEN. Nat. Cell Biol. 13, 728–733 (2011).
Harper, J. W. Neddylating the guardian; Mdm2 catalyzed conjugation of Nedd8 to p53. Cell 118, 2–4 (2004).
Zuo, W. et al. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-β type II receptor. Mol. Cell 49, 499–510 (2013).
Hu, J., McCall, C. M., Ohta, T. & Xiong, Y. Targeted ubiquitination of CDT1 by the DDB1 CUL4A-ROC1 ligase in response to DNA damage. Nat. Cell Biol. 6, 1003–1009 (2004).
Nikolovska-Coleska, Z. et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 47, 2430–2440 (2004).
Siegel, C., Li, J., Liu, F., Benashski, S. E. & McCullough, L. D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA 108, 11662–11667 (2011).
Yang, J. R.-M. et al. Characterization of PTEN mutations in brain cancer reveals that Pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene 36, 3673–3685 (2017).
Valiente, M. et al. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J. Biol. Chem. 280, 28936–28943 (2005).
Huang, G. et al. SCCRO (DCUN1D1) promotes nuclear translocation and assembly of the neddylation E3 complex. J. Biol. Chem. 286, 10297–10304 (2012).
Jia, X. et al. Neddylation inactivation facilitates FOXO3a nuclear export to suppress estrogen receptor transcription and improve fulvestrant sensitivity. Clin. Cancer Res. 25, 3658–3672 (2019).
Beck, M. & Hurt, E. The nuclear pore complex: understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 18, 73–89 (2017).
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E. & Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 (2014).
Ikenoue, T., Inoki, K., Zhao, B. & Guan, K. L. PTEN acetylation modulates its interaction with PDZ ___domain. Cancer Res. 68, 6908–6912 (2008).
Liu, F. et al. PTEN enters the nucleus by diffusion. J. Cell Biochem. 96, 221–234 (2005).
Guan, J., Zhao, Q. & Mao, W. Nuclear PTEN interferes with binding of Ku70 at double-strand breaks through post-translational poly(ADP-ribosyl)ation. Biochim. Biophys. Acta. 1863, 3106–3115 (2016).
Malaney, P., Palumbo, E., Semidey-Hurtado, J. & Hardee, J. PTEN physically Interacts with and regulates E2F1-mediated transcription in lung cancer. Cell Cycle 17, 947–962 (2018).
Menendez, J. A. & Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 7, 763–777 (2007).
Lin, H. P. et al. Destabilization of fatty acid synthase by acetylation inhibits de novo lipogenesis and tumor cell growth. Cancer Res. 76, 6924–6936 (2016).
Perren, A. et al. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am. J. Pathol. 155, 1253–1260 (1999).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 24, 2206–2223 (2013).
Chen, J. H. et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy 11, 239–252 (2015).
Wu, Y. et al. PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxid. Redox Signal. 20, 1382–1395 (2014).
Gu, Y. et al. MLN4924, an NAE inhibitor, suppresses AKT and mTOR signaling via upregulation of REDD1 in human myeloma cells. Blood 123, 3269–3276 (2014).
Barbier-Torres, L. et al. Stabilization of LKB1 and Akt by neddylation regulates energy metabolism in liver cancer. Oncotarget 6, 2509–2523 (2015).
Crunkhorn, S. Breast cancer: FASN inhibitor increases survival. Nat. Rev. Drug Discov. 15, 532 (2016).
lwarawrah, Y. et al. Fasnall, a selective FASN inhibitor, shows potent anti-tumor activity in the MMTV-Neu model of HER2+ breast cancer. Cell Chem. Biol. 23, 678–688 (2016).
Acknowledgements
We thank Drs. Mian Wu (University of Science and Technology of China, Anhui Province, China), Xiaofeng Zheng (Peking University, Beijing, China), Mingyao Liu (East China Normal University, Shanghai, China), Qinong Ye (Beijing Institute of Biotechnology) for kindly providing materials. This work was jointly supported by the National Key R&D Program of China (2017YFA0505602), Chinese National Basic Research Programs (2015CB910401), Chinese National Natural Science Foundation Project (31670774, 31971229), Beijing Natural Science Foundation Project (Z151100003915083), Open Project Program of the State Key Laboratory of Proteomics (SKLP-O201901), Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (CIT&TCD201704097) and Beijing excellent talents training project.
Author information
Authors and Affiliations
Contributions
The project was conceived by L.Z. The experiments were designed by L.Z., P.X. and F.H. Most of the modification experiments were performed by P.X. The cell biology function experiments were contributed by Z.P., P.X., and M.D. The animal experiments were contributed by Z.P., H.L. and X.Z. The breast cancer sample collection and immunohistochemical analysis were contributed by Z.P., Y.C. and Y.T. The statistical analysis was completed by Z.P., Z.L., C.H.L. and C.-P.C. Data were analyzed by L.Z., P.X. and Z.P. The manuscript was written by L.Z. and P.X.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Xie, P., Peng, Z., Chen, Y. et al. Neddylation of PTEN regulates its nuclear import and promotes tumor development. Cell Res 31, 291–311 (2021). https://doi.org/10.1038/s41422-020-00443-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-020-00443-z
This article is cited by
-
Screening biomarkers related to cholesterol metabolism in osteoarthritis based on transcriptomics
Scientific Reports (2025)
-
PTEN neddylation aggravates CDK4/6 inhibitor resistance in breast cancer
Oncogene (2025)
-
Neddylation of RhoA impairs its protein degradation and promotes renal interstitial fibrosis progression in diabetic nephropathy
Acta Pharmacologica Sinica (2025)
-
Neddylation status determines the therapeutic sensitivity of tyrosine kinase inhibitors in chronic myeloid leukemia
Scientific Reports (2025)
-
Targeting Smurf1 to block PDK1–Akt signaling in KRAS-mutated colorectal cancer
Nature Chemical Biology (2025)