Abstract
Scientists were chasing an incretin hormone, and when GLP-1 was finally discovered, we found that it had a pronounced satiety effect, slowed down gastric emptying, and actually reduced postprandial insulin response. These mechanisms are the basis for the highly efficacious GLP-1 analogues that today offer safe and effective treatment in millions of people living with obesity. Moreover, the combined GLP-1 mechanisms of weight loss and delayed carbohydrate absorption may also be the key drivers of remission of type 2 diabetes and reduced cardiovascular events found by GLP-1 analogues.
Similar content being viewed by others
Development of a rigorous methodology to measure appetite and food intake in humans
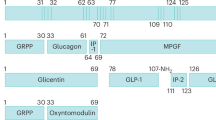
With the discovery of GLP-1 in 1986 simultaneously by Jens Juul Holst and Joel Habener a cascade of important physiological discoveries followed [1] (Fig. 1). Their identification of the amino acid sequence of the biologically active GLP-1 hormone laid the groundwork for drugs in the management of type 2 diabetes. Gut peptide hormones such as GLP-1 could potentially also have an impact on appetite regulation by effects on gastric emptying and direct CNS effects, but we needed a robust methodology that could be used to measure acute effect of putative hormones on subjective appetite and spontaneous food intake. We* developed a study design using visual analogue scales (VAS) for measurement of subjective appetite recordings during a fixed breakfast meal test, and combined it with a subsequent ad libitum lunch meal test that measured consumed energy. The putative satiety effect of a compound would be seen as an effect on satiety VAS during the breakfast meal and on the spontaneous caloric intake at the lunch meal. VAS had been mainly used to measure subjective sensations (e.g. pain) and studies of appetite lacked validated measures. In an initial pilot study, we demonstrated the need for more comprehensive validation [2].
Together with John Blundell, we developed the protocol: on two different test days we examined 55 healthy individuals and recorded their appetite sensations before breakfast and every 30 min during the 4.5 hr postprandial period under the same conditions [3]. We also examined if diet standardization the days prior to the test days influenced the results. The study showed the validity of quantification of hunger, satiety, and prospective food consumption measured by VAS as well as measurement of ad libitum energy consumption and showed that the appetite recordings during the breakfast meal predicted the energy intake during the lunch meal [3]. These results provided us with the essential methodology to design the GLP-1 infusion study and provided information on the estimated number of participants needed for adequate statistical power. This VAS paper is today a classic methods paper in this field [3] and has been cited 2,382 times.
*In this article “we” is used as a reference to the group of scientists, dieticians, and technical staff in my department who contributed to own studies cited, and the key collaborating scientists from other labs including Jens Juul Holst and John Blundell. Contributors from my own department are mentioned in the Acknowledgment.
Discovery of GLP-1 as a satiety hormone
In 1994 I took up the idea with Holst that GLP-1 could also be a mediator of meal-induced satiety, and we designed a study to infuse GLP-1 into normal human volunteers. Meanwhile, in January 1996 the first rodent study suggested that GLP-1 could be a central regulator of feeding behavior and satiety based on intracerebroventricular injection studies [4], but soon thereafter the results of other rodent studies using peripherally injected GLP-1 argued against an appetite effect [5].
The study was conducted in 1995–1996, and we used commercially available synthetic, human GLP-1 (7–36 amide) [6]. We conducted the trial in 20 young, healthy, normal-weight men in a placebo-controlled, randomized, blinded, crossover design. Infusion of GLP-1 or saline was initiated simultaneously with the consumption of the breakfast test meal. VAS were used to assess appetite sensations, and at lunchtime an ad libitum test meal was offered. We found that, after the energy-fixed breakfast, GLP-1 markedly enhanced satiety and fullness compared with placebo (Fig. 2) [6]. Furthermore, spontaneous energy intake at the ad libitum lunch was reduced 12% by GLP-1 infusion (Fig. 2). We were quite excited by the findings, as this study was the first to demonstrate a physiological role of GLP-1 in appetite control and energy intake in humans.
The pivotal findings of the study in which we infused GLP-1 in human subjects showed that it enhanced satiety reduced hunger during a fixed breakfast meal and reduced spontaneous energy intake by 12% during an ad libitum lunch meal [6].
An invention was submitted for a patent on 12 November 1996 [7], and the scientific article was submitted for publication in the Journal of Clinical Investigation 20 June 1997 and published 1st February 1998 [6]. The experimental set-up and execution of the studio are reproduced in a short video with actors (https://www.youtube.com/watch?v=4vbsEpC7Hsw).
In September 1998, Näslund et al. failed to detect an effect of GLP1 on food intake [8]. However, that study was done in only 6 subjects, raising concerns about the lack of statistical power to detect a clinically relevant effect. In 1999, Gutzwiller et al. showed a dose-dependent reduction in food intake with GLP-1 infusion in 16 healthy male subjects [9]. Subsequently, we showed that GLP-1 induces satiety equally well in people with obesity [10]. These effects could be at least partially attributed to the slowing of gastric emptying. By 2000, seven studies had been published on the acute effect of peripheral GLP-1 administration on ad libitum energy intake. Four found energy intake to be significantly reduced, whereas the remaining studies failed to find an effect – again potentially due to limited statistical power and also low GLP-1 infusion rates. We therefore conducted an individual participant data analysis, pooling raw data from the existing studies [11]. Six infusion studies provided us with 147 participants, and we found that GLP-1 reduced energy intake dose-dependently in both lean and overweight participants (a mean of 13%, P < 0.001, Fig. 3) [11]. There was also a dose-response effect on the reduction in emptying rate.
Data obtained from n = 147 volunteers following infusions with GLP-1 or saline. Adopted from [11].
In close collaboration with Jens Juul Holst, we conducted a series of infusion studies on the satiety effects of other gut hormones including GLP-2 and PYY. Beyond contributing to the conceptual knowledge of appetite and energy balance regulation in humans [12], we also made potentially clinically important discoveries, such as why dietary protein provides greater immediate satiety than the other macronutrients and why milk is more satiating than sugar-sweetened soft drinks (both dietary exposures increase GLP-1 relative to their comparators) [13].
From physiology to pharmacology
Our discovery of GLP-1 as a satiety hormone was patented in collaboration with the pharmaceutical company Novo Nordisk A/S in November 1996 [7] (I receive no royalty or other payments). Mads Krogsgaard Thomsen, at the time R&D executive in the Diabetes Care Division of Novo Nordisk, undertook the initiative to synthesize long-acting GLP-1 analogues (Fig. 1), and the first GLP-1 analogue brought into clinical development was liraglutide, a once-daily injection. I led a phase II dose-ranging trial on liraglutide, and we found that over 20 weeks the 3.0 mg/d dose had an optimal profile of effectiveness and tolerability [14]. The trial, extended for 2 years, found that completers maintained a 7.8 kg weight loss from baseline [15]. In the phase III liraglutide program (SCALE study) ~4000 participants with obesity were studied for up to three years [16].
To date, liraglutide, and the more effective once-weekly semaglutide, have been approved in the US and Europe for the treatment of obesity, with unprecedented weight loss of up to 15–20%. Presently, 19 different GLP-1 analogues are in development for the treatment of obesity by almost as many companies. In the last three months of 2023, more than 9 million people were treated by GLP-1 analogues in the US, with anticipation of continued rapid growth in usage.
The importance of GLP-1 therapies for type 2 diabetes and CVD
Today it is well established that excessive body fat together with lack of regular physical activity, together with a genetic predisposition, are the main causes of type 2 diabetes, contributing as well to a substantial proportion of cardiovascular diseases, cancers, and other chronic degenerative diseases. A large body of evidence has demonstrated that complete remission of type 2 diabetes can be achieved by a major weight loss. Until recently, the only effective treatment for obesity was bariatric surgery, and a century of drug development for obesity can be essentially described as a failure until now. GLP-1 analogues have completely changed this bleak therapeutic picture. Moreover, GLP-1 analogues cause a reduction in cardiovascular events in non-diabetic individuals [17]. The mechanisms responsible for these effects are not entirely clear, but the slowing of gastric emptying may be the key driver.
How do GLP-1 analogs confer health benefits?
An apparent paradox is that whereas GLP-1 increases glucose-stimulated insulin secretion in the isolated pancreas – fulfilling the definition of an incretin – but it lowers insulin secretion in physiologically relevant conditions [6, 10, 18]. In our discovery studies GLP-1 enhanced satiety during a meal but lacked the insulinotropic effect in the natural setting of people consuming mixed meals (Fig. 4).
We found this paradox to be explained by GLP-1 delaying gastric emptying and the rate of carbohydrate absorption after a meal, biological actions that dominant direct effects on insulin secretion at the B-cell, both in both normal-weight individuals and in non-diabetic individuals with obesity [6, 10] (Fig. 5). Despite all major review articles highlighting GLP-1 as an incretin hormone [18], meal-test studies and studies using GLP-1 analogues actually show the opposite.
GLP-1 inhibits gastric emptying in human individuals as shown by the retarded absorption of ingested paracetamol [10].
More satiety hormone than incretin
From this perspective, GLP-1 is more appropriately understood as a satiety hormone, rather than an incretin. It was also an early observation that a higher dose of GLP-1 is needed to produce satiety and reduced food intake than to the antidiabetic effect, so a low dose GLP-1 analog could be used to study weight loss independent effects of GLP-1. In an 8-week study using low-dose liraglutide (0.6 mg/day) in patients with type 2 diabetes no weight or fat loss was produced, but both fasting and postprandial glucose were substantially reduced, but neither fasting nor postprandial insulin (or C-peptide) response was increased [19]. This supports that the short-term anti-diabetic effect of GLP-1 is mainly due to slowing of gastric emptying and consequently a reduced rate of carbohydrate absorption. The most common side effects of GLP-1 analogues are nausea and vomiting, which have been attributed to delayed gastric emptying [14,15,16]. The reports of increased residual gastric content after long-term treatment with GLP-1 analogues emphasize that the effect on gastric emptying is sustained, even though that it does normally not pose a risk for postoperative respiratory complications [20].
Reductions of glycemic index and hyperinsulinemia by GLP-1
GLP-1 exerts an important effect directly on the brain, and this effect is probably even more important for the analogues than for the endogenous GLP-1 released after ingestion of protein and fat. My view is that the pronounced effect of GLP-1 analogues on gastric emptying is not only an important mediator of satiety and reduced food intake but also a likely mechanism responsible for its effect on type 2 diabetes and cardiovascular disease. The reduced glycemic load of meals caused by GLP-1 is very similar to that seen when replacing a meal with a high glycemic index (GI) with that of a low GI [21] (See Fig. 6). Due to the reduced food intake total carbohydrate ingestion is also reduced, which will further reduce glycemia.
The difference between a meal with high versus low glycemic index (GI) on plasma glucose (panel A) and insulin response (panel B). The difference is very similar to the lowering effect of GLP-1 on GI, and this effect is likely to contribute to the weight-loss-independent effect of GLP-1 analogues on type 2 diabetes and cardiovascular disease in analogy with the effects of a low GI diet [21].
Consequently, on top of the beneficial effect of weight loss caused by GLP-1 analogues these drugs also cause a substantial reduction of the glycemic load (GL = carbohydrate intake X Glycemic index), which per se reduces hyperinsulinemia and causes weight loss [22], reduce diabetes risk [23], inflammation [24], and cardiovascular risk [25]. Indeed, it is well documented that regular consumption of high-glycemic index meals, compared to isoenergetic meals with the same carbohydrate content, results in higher average 24-h blood glucose and insulin levels, higher C-peptide U-excretion, and higher HgbA1c in both non-diabetic and diabetic individuals [26, 27]. When it comes to the effect of GLP-1 analogues the reduction in postprandial glucose response, and insulinemia may actually contribute to weight loss, but also be the key drivers to the anti-diabetic effect and reduction in cardiovascular disease risks. Studies with treatment with GLP-1 analogues in type 2 diabetes have found that the initial postprandial insulin is increased after treatment, and this has been misinterpreted as evidence for a GLP-1 mediated insulin stimulation [28]. However, this phenomenon is rather a restoration of the first-phase insulin response due to the concomitant weight loss of typically 5–6 kg. The restoration of first-phase insulin response is a well-established observation caused by weight loss in type 2 diabetes [29]. The overall reduction in insulin secretion is also shown by reduced levels of C-peptide in the same studies. So GLP-1 therapy reduces insulin secretion and levels, which have implications for the understanding of the mechanisms behind its effect on weight loss.
Endogenous hyperinsulinemia may cause weight gain and obesity [30], and treatment with insulin causes substantial weight gain, and may even increase cardiovascular risk [31]. Whereas general obesity and central adiposity, in particular, are the primary factors responsible for the development of insulin resistance, postprandial increases in insulin secretion causes a further expansion of the adipose tissue mass, which further worsens insulin resistance. According to this scenario, obesity and hyperinsulinemia initiate a vicious metabolic cycle leading to deterioration of glucose tolerance and eventual development of T2DM. So, reducing hyperinsulinemia by GLP-1 analogies will contribute to weight loss and improve insulin sensitivity, and glycemic control in type 2 diabetes. This novel view on GLP-1 mechanisms has recently been highlighted by Ludwig and Holst [32].
Perspectives
The pivotal discovery of GLP-1 was followed by the discovery of GLP-1 being a satiety hormone in humans acting on the brain and exerting a pronounced slowing of gastric emptying leading to weight loss. The combination of reduced carbohydrate intake and slowing of glucose absorption also reduces postprandial glycemia and insulinemia and may be the dominant weight-loss-independent effect on type 2 diabetes and cardiovascular disease. These findings provided a better understanding of the physiology of GLP-1 and the pharmacology of its analogues and have revolutionized the management of obesity and several comorbidities. For the first time in the history of obesity medications we see weight loss efficacy of a magnitude that might raise concern about excessive loss of lean body tissue due to the initial pronounced reduction in energy intake and subsequent risk of protein deficiency.
The effects on the brain of GLP-1 analogues go far beyond satiety but may also change food preferences in a healthier direction, but also reduce a person’s drive for alcohol, tobacco and even opioids. Consequently, the potential for this class of drugs is enormous and may be a paradigm shift not only for the management of obesity but also for several other conditions.
References
Holst JJ. From the Incretin concept and the discovery of GLP-1 to Today’s Diabetes Therapy. Front Endocrinol. 2019;10:260.
Raben A, Tagliabue A, Astrup A. The reproducibility of subjective appetite scores. Br J Nutr. 1995;73:517–30.
Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;23:38–48.
Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72.
Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–56.
Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20.
Holst JJ; Astrup AV; Judge ME; Knudsen LB; Thim L; Wulff BS Use of GLP-peptides. Treatment of diseases associated with impaired appetite regulation e.g. obesity - by administration of the proglucagon fragment GLP-1(1-45), or its fragment, analogue or amide. Filed by Novo Nordisk A/S. Priority date: 12 November 1996. Patent EP0941114(B1)/23 February 2005.
Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–30.
Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86.
Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes. 2001;25:781–92.
Verdich C, Flint A, Gutzwiller J-P, Näslund E, Beglinger C, Hellström PM, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–9.
Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013;97:980–9.
Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes. 2000;24:288–98.
Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–16.
Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–54.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of Liraglutide in weight management. N Engl J Med. 2015;373:11–22.
Lincoff AM, SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32.
Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513–48.
Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27:1915–21.
Dixit AA, Bateman BT, Hawn MT, Odden MC, Sun EC. Preoperative GLP-1 receptor agonist use and risk of postoperative respiratory complications. JAMA. Published online April 22, 2024. https://doi.org/10.1001/jama.2024.5003.
Krog-Mikkelsen I, Sloth B, Dimitrov D, Tetens I, Björck I, Flint A, et al. A low glycemic index diet does not affect postprandial energy metabolism but decreases postprandial insulinemia and increases fullness ratings in healthy women. J Nutr. 2011;141:1679–84.
Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–13.
Livesey G, Taylor R, Livesey HF, Buyken AE, Jenkins DJA, Augustin LSA, et al. Dietary Glycemic Index and load and the risk of Type 2 Diabetes: Assessment of causal relations. Nutrients. 2019;11:1436.
Gögebakan O, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, Papadaki A, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124:2829–38.
Dwivedi AK, Dubey P, Reddy SY, Clegg DJ. Associations of Glycemic Index and Glycemic Load with cardiovascular disease: updated evidence from meta-analysis and cohort studies. Curr Cardiol Rep. 2022;24:141–61.
Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, Buckley G, et al. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr. 1987;46:968–75.
Miller JC. Importance of glycemic index in diabetes. Am J Clin Nutr. 1994;59:747S–752S.
Kuwata H, Yabe D, Murotani K, Fujiwara Y, Haraguchi T, Kubota S, et al. Effects of glucagon-like peptide-1 receptor agonists on secretions of insulin and glucagon and gastric emptying in Japanese individuals with type 2 diabetes: A prospective, observational study. J Diabetes Investig. 2021;12:2162–71.
Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, et al. Remission of Human Type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab. 2018;28:547–e3.
Gagnon Eloi, Mitchell PatriciaL, Arsenault BenoitJ. Body fat distribution, fasting insulin levels, and insulin secretion: A bidirectional mendelian randomization study. J Clin Endocrin Metab. 2023;108:1308–17.
Shi Q, Nong K, Vandvik PO, Guyatt GH, Schnell O, Rydén L, et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ. 2023;381:e074068.
Ludwig DS, Holst JJ. Childhood obesity at the crossroads of science and social justice. JAMA. 2023;329:1909–10.
Acknowledgements
I would like to thank my former PhD students and post-docs Anne Raben, Anne Flint, Camilla Verdich, Birgitte Sloth, Helle Harder, Anita Belza and Thomas Meinert Larsen for their invaluable contributions to the research described in this article. Also, a great thanks to the kitchen staff and dieticians at my old lab where we developed the methodology to measure appetite and food intake. A special thanks to the head of the metabolic kitchen at my old department (now “Department of Nutrition, Exercise and Sports”), Charlotte Kostecki, for her invaluable creativity and support, and to my former PA Tina Cuthbertson for making it possible to be head of the department and still conducting research. I would also like to thank David L Ludwig for valuable discussions about the physiology and pharmacology of GLP-1 and for careful reading and commenting on the manuscript. The views and opinions expressed in this article are those of the author and do not necessarily reflect the views or positions of the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Astrup, A. Reflections on the discovery GLP-1 as a satiety hormone: Implications for obesity therapy and future directions. Eur J Clin Nutr 78, 551–556 (2024). https://doi.org/10.1038/s41430-024-01460-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-024-01460-6