Abstract
During the neonatal period, many genetic disorders present and contribute to neonatal morbidity and mortality. Genomic medicine—the use of genomic information in clinical care— has the potential to significantly reduce morbidity and mortality in the neonatal period and improve outcomes for this population. Diagnostic genomic testing for symptomatic newborns, especially rapid testing, has been shown to be feasible and have diagnostic and clinical utility, particularly in the short-term. Ongoing studies are assessing the feasibility and utility, including personal utility, of implementation in diverse populations. Genomic screening for asymptomatic newborns has also been studied, and the acceptability and feasibility of such an approach remains an active area of investigation. Emerging precision therapies, with examples even at the “n-of-1” level, highlight the promise of precision diagnostics to lead to early intervention and improve outcomes. To sustainably implement genomic medicine in neonatal care in an ethical, effective, and equitable manner, we need to ensure access to genetics and genomics knowledge, access to genomic tests, which is currently limited by payors, feasible processes for ordering these tests, and access to follow up in the clinical and research realms. Future studies will provide further insight into enablers and barriers to optimize implementation strategies.
Similar content being viewed by others
Introduction
Exome sequencing was first used to identify the cause of a Mendelian disorder in 2009, and there have since been significant advances in genomic medicine in the clinical and research realms [1]. Broadly speaking, genomics is a field of biology that studies the genome (i.e., all of our DNA) and genomic medicine is an emerging field of medicine that uses genomic information in clinical care. The United Kingdom (UK) National Health Service (NHS) defines genomic medicine as “the use of genomic information and technologies to determine disease risk and predisposition, diagnosis and prognosis, and the selection and prioritization of therapeutic options” [2]. Similarly, the United States (US) National Human Genome Research Institute (NHGRI) defines genomic medicine as “using genomic information about an individual as part of their clinical care (e.g., for diagnostic or therapeutic decision-making) and the health outcomes and policy implications of that clinical use” [3]. Thus, genomic medicine involves screening, diagnostic, therapeutic, and outcome aspects across human health and disease.
Although genomic medicine has the potential to impact many clinical areas, this review will focus on the progress and challenges for implementing genomic medicine in neonatal care, ranging from its potential in newborn screening, diagnostics, and therapeutics. The neonatal period is defined as the first four weeks of life for term newborns (or up to 44 weeks postmenstrual age for preterm newborns). Early diagnosis followed by early treatment of genetic disorders is critical to optimizing the benefits of genomic medicine, and newborns arguably represent the population with the greatest potential impact of genomic medicine on lifelong health [4]. As discussed below, many genetic disorders present in the neonatal period and contribute to significant morbidity and mortality. In addition, many questions remain about genomic screening of newborns to identify and potentially prevent later-onset diseases. In this review, we discuss the unique aspects of neonatal genomics, the various facets of and utility of genomic medicine in the neonatal period, and progress and challenges for implementation of genomic medicine in neonatal care.
The importance of genomic medicine in neonatal care
The time of birth is a unique period of transition from the intrauterine to extrauterine environment. At delivery, the newborn is separated from the placental and maternal environment and undergoes adaptations to extrauterine life, including changes in gene expression. In most newborns this transition is smooth, and they receive routine care and are discharged home. A subset of them may carry disease-causing variants for childhood or adult onset genetic disorders, which could be identified in the neonatal period and acted on if treatments are available. Genomic screening in pre-symptomatic newborns is an active area of research and has important ethical, legal, and social implications, as reviewed below.
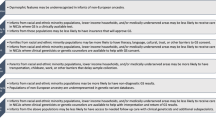
For other newborns, the transition is difficult due to factors such as prematurity, infections, hypoxia, and genetic disorders, which may require specialized care in neonatal intensive care units (NICUs). Genetic disorders contribute substantially to morbidity and mortality in this period [5], with long-lasting burdens on families and the healthcare system. In fact, as advances in obstetrics and neonatal care have led to reductions in infant mortality from other factors, the category that includes many identified or suspected genetic conditions (“congenital malformations, deformations, and chromosomal abnormalities”), is now reported as the leading cause of infant mortality in the US [6, 7]. Multiple studies, reviewed below, have demonstrated the utility of genomic diagnostic testing in symptomatic newborns, and therefore, the implementation of genomic medicine has the potential to have a large impact on their care (Fig. 1).
Diagnostic genetic testing, including genomic sequencing, can occur prenatally for a fetus suspected to have a genetic disorder, or at delivery or postnatally for a newborn suspected to have a genetic disorder. For an asymptomatic newborn, newborn screening soon after birth can detect a group of intervenable early-onset conditions and genomic newborn screening has the potential to detect many more conditions. Created with Biorender.com.
Diagnostic genomic testing in the neonatal period
Mendelian or monogenic disorders that present in the neonatal period range from disorders that lead to dysmorphic features and congenital anomalies, such as seen in Trisomy 21, DiGeorge syndrome (22q11.2 deletion), and CHARGE syndrome (CHD7 variants), to disorders that lead to dysfunction of one or more organ systems, such as seen in neuromuscular disorders and epileptic encephalopathies. Although the former may be easier to recognize on physical exam as associated with a suspected genetic disorder, it is important to remember that a genetic disorder may not only present with a nonspecific phenotype during the neonatal period, but that not all features of a genetic disorder may manifest during early age. Thus, an underlying genetic disorder may be suspected in any symptomatic newborn with non-specific or unexplained findings and may warrant diagnostic genetic testing.
In addition, common neonatal pathologies may have underlying genetic contributions. In rare cases, a newborn presenting with a common phenotype may have an underlying monogenic disorder (e.g., a disease-causing variant associated with defective surfactant protein B or C presenting with respiratory failure or a bleeding diathesis presenting with intraventricular hemorrhage). More broadly, combinations of genetic and environmental risk factors may contribute to the development of pathologies unique to premature neonates such as necrotizing enterocolitis (NEC): a role for genetics in NEC was suggested by twin studies and candidate associated variants have emerged from genomic studies [8]. Although this review will focus on monogenic conditions in neonates, future studies with increased sample sizes and adequate power will likely continue to illuminate the genetic contribution to common neonatal conditions include those associated with prematurity.
When taking care of a newborn whose symptoms may have a genetic explanation, a clinician has multiple options for diagnostic testing to investigate a suspected underlying genetic condition, and choosing an appropriate genetic test is an important step. Neonatal clinicians are usually most familiar with chromosome-level testing, such as karyotype, fluorescence in-situ hybridization (FISH), and chromosomal microarray (CMA). Traditionally, these are the first genetic tests performed in a sequential genetic diagnostic workup. In cases where a newborn’s presentation strongly suggests a specific chromosomal disorder, such as pathognomonic features for Trisomy 13, 18, or 21, or 22q11 deletion, a chromosome-level test, particularly with rapid turnaround time (TAT), may be the appropriate choice [9].
Gene-level testing ranges from single gene and gene panel tests, which typically cover tens to hundreds of genes, to exome sequencing (ES), which covers the coding regions (exons) of the genome, and genome sequencing (GS), which covers the entire genome. Although single gene and gene panel tests have traditionally been performed concurrently with or as the next step after non-diagnostic chromosome-level tests, a substantial body of research has demonstrated the utility of genomic sequencing tests (ES or GS), as the first-line genetic testing in newborns with suspected genetic disorders [4, 5, 10,11,12,13,14,15,16,17,18,19,20,21,22]. This has been supported by society guidelines; for example, the American College of Medical Genetics and Genomics recently published an evidence-based guideline recommending genomic sequencing for infants with one or more congenital anomalies [23].
In addition to the utility aspects discussed below, there are several reasons genomic sequencing represents an optimal genetic test for most newborns with suspected genetic disorders. First, accurate phenotyping may be difficult in a symptomatic newborn due to both critical illness and the fact that not all clinical features of a given genetic disorder may manifest in the neonatal period [24]. Second, newborns have limited blood volume, and sequential workup with multiple genetic tests requires repeated blood draws with larger total required blood volume. Third, rapid TAT is important for sick newborns, their families, and their treating clinicians, and rapid ES/GS can provide results on the order of a few days to a couple of weeks (and more recently even on the order of hours) [25]. Moreover, ES/GS is often performed as a trio test with the proband and biological parents versus single gene and gene panel tests that are usually performed as a singleton test with only the proband; trio testing may enhance variant interpretation and diagnostic yield [23] and shorten the diagnostic odyssey. Thus, ES/GS represents a single genetic test that requires minimal sample volume and can rapidly identify the majority of disease-causing variants in symptomatic newborns. Of note, there are still limitations to the types of variants that can be detected by current clinically accredited genomic sequencing tests. For example, methylation defects often cannot be detected by short-read genomic sequencing, and specific methylation testing may be required in cases with clinical features suggestive of imprinting conditions, such as Prader-Willi syndrome. However, newer long-read genomic sequencing technologies hold promise for overcoming these limitations.
When implementing genomic medicine in neonatal care, the selection process of symptomatic newborns for diagnostic genomic testing is an important facet which may be limited by the need for genetics expertise to appropriately evaluate newborns and furthermore by the nonspecific presentation of many genetic disorders in the neonatal period. Using a phenotype-driven approach has been shown to have high diagnostic yield [16], while more inclusive approaches may have comparatively lower overall diagnostic yield but may more fully capture the heterogenous landscape of neonatal-onset genetic diseases (moving towards genotype-driven) [9]. In an ideal situation, clinical geneticists and genetic counselors would be readily available to collaborate with neonatologists and rapid genomic sequencing could be ordered based on clinical decision making. In the real world, there is a shortage of clinical geneticists and genetic counselors, neonatal clinicians have limited training in genetics and genomics, and access to diagnostic genomic sequencing is often restricted by institutional, insurance, and/or healthcare system policies, mainly due to cost [26,27,28,29,30,31]. Thus, as discussed further below, clinical decision making that a symptomatic newborn warrants diagnostic genomic sequencing and site-specific policies that dictate access to such sequencing are both relevant aspects of the selection process and potential implementation barriers. Research studies that provide access to these tests for symptomatic newborns in the short term and provide evidence for policy changes in the long term are critical to enabling broader implementation.
After identifying a symptomatic newborn with a suspected genetic disorder, selecting an appropriate genetic test, and having the access to order that test, a clinician needs to provide pre-test counseling and obtain consent from the biological parents (or other legal caregivers). For genomic sequencing, in addition to explaining the test and the potential types of primary results, it is important to counsel parents on the potential of secondary and/or incidental findings and implications for future data privacy, discrimination, and related aspects, in accordance with site-specific policies. If the family consents, samples need to be collected and transported to the testing facility, which may be “in house” for institutions that have the resources to perform such testing or “sent out” to commercial vendors. A clinician then needs to interpret the results and return the results to the family with post-test counseling and coordination of appropriate short and long term follow up. Similar to aspects of the selection process, in the real world, the ordering process and follow up are often implementation barriers.
Follow up in the clinical setting and research opportunities are both important for newborns with genetic conditions. For newborns with diagnostic findings, follow up in the clinical setting with genetics as well as with additional relevant subspecialities is indicated for disease-specific management. In addition, follow up in the research setting may be offered for available studies, such as natural history studies and clinical trials of emerging precision therapies. For newborns with inconclusive or non-diagnostic findings, clinical follow up with genetics is indicated for monitoring for additional phenotypes, reanalysis, and additional testing, while follow up in the research setting can provide additional reanalysis, functional studies of VUS, or biobanking of samples for future studies, as examples. Over the coming years, integration of multiple “omics” technologies, such as transcriptomics, epigenomics, proteomics and metabolomics [32], will likely further increase the diagnostic yield for such genetic disorders. Integration between clinical genomic testing and relevant research studies will maximize the benefits of genomic medicine implementation for newborn patients.
It is worth noting that in some cases, a genetic disorder may be suspected in the fetus prior to birth (e.g., by screening or imaging) and diagnostic testing during pregnancy or at the time of delivery may be possible to expedite the genetic diagnostic odyssey. During pregnancy, invasive methods like chorionic villus sampling and amniocentesis are well established options to obtain fetal samples for diagnostic genomic testing; however, such invasive methods are often declined by or inaccessible to pregnant patients [33]. Ongoing research is optimizing non-invasive methods using cell-free fetal DNA for prenatal genomic sequencing that would require only a blood draw during pregnancy. At delivery, umbilical cord blood presents an opportunity to collect samples equivalent to neonatal peripheral blood for immediate diagnostic testing. Adams and colleagues recently described the implementation of a cord blood genetic testing program, which had high uptake, diagnostic yield, and clinical utility as well as rapid TAT for prenatally identified high-probability cases [34].
Genomic newborn screening
In contrast to diagnostic genomic testing of symptomatic infants, genomic newborn screening using sequencing can detect genetic disorders in asymptomatic newborns. Historically, newborn screening programs have followed the principles of Wilson and Jungner and have been designed to cost-effectively detect serious but treatable childhood-onset disorders in pre-symptomatic newborns with the goal of reducing morbidity and mortality from these disorders by early intervention [35, 36]. In the United States, the Advisory Committee on Heritable Disorders in Newborns and Children reports a “Recommended Uniform Screening Panel”, which includes core and secondary conditions [37]. Currently, apart from critical congenital heart disease and hearing loss, these disorders are screened for using dried blood spot samples collected from newborns. DNA sequencing methods are not typically used for primary screening, but may be used for reflex secondary testing; for example, to detect disease-causing variants associated with cystic fibrosis (CF) when there is an abnormal primary screening result for CF [38].
There has been growing interest in exploring genomic sequencing as a tool for newborn screening especially as extraction of sufficient quality and quantity of DNA from dried blood spot samples has been shown to be technically feasible [39]. While this approach would lead to detection of many more disorders, some of which may have management implications for newborns and/or their families or other utility, there is also concern that it would fundamentally change the principles underlying existing newborn screening program and could lead to detection of disease-causing variants for many currently untreatable and/or adult-onset disorders as well as a multitude of VUS and secondary/incidental findings. In addition, concerns have been raised regarding appropriate education and consent, more complex logistics and follow up, increased costs, risks regarding data privacy, discrimination, and psychosocial stress, and the potential to compromise participation in existing newborn screening programs [40].
Several published and ongoing research studies are investigating the benefits, risks, and feasibility of implementing genomic newborn screening. As one example, the BabySeq study in the United States was a randomized clinical trial designed to investigate the medical, behavioral, and economic impacts of adding genomic sequencing to existing newborn screening in neonatal care [41]. The study had low enrollment rates, with 10% of 3860 eligible approached families attending an enrollment session, and 67% of those deciding to enroll [42]. Major reasons for decline by families were lack of interest in research and study logistics; other reasons included concerns about privacy and insurability. Of 159 newborns who underwent genomic sequencing, results revealed unanticipated childhood-onset disease risk in 9.4%, carrier status for recessive disease in 88%, and pharmacogenomics variants in 5% [43]. In addition, results revealed actionable adult-onset disease risk in 3.5% of newborns whose parents consented to receive those results. Follow up surveys found no persistent negative psychosocial effect on families whose newborns had genomic sequencing [44]. The ongoing BabySeq2 study is a randomized clinical trial that aims to enroll 500 participants and implement GS for screening in a diverse cohort of healthy infants (NCT05161169). Also in the US, the ongoing GUARDIAN study aims to enroll 100,000 newborns born at New York Presbyterian Hospitals and use genomic sequencing to screen for over 250 conditions that are not part of standard newborn screening in the state of New York. The screening focuses on newborn conditions that have treatments (although not necessarily cures) available (Group 1) with an option for parents to also have their newborn screened for additional conditions that do not currently have treatments available (Group 2) [45]. In the UK, Genomics England and the NHS are currently designing and running a study that aims to enroll 100,000 newborns and perform genomic sequencing. The study will expand newborn screening from the nine conditions currently screened for in the UK to many more conditions, focusing on conditions chosen based on four guiding principles: genetic variant(s) cause the condition and can be reliably detected, undiagnosed individuals generally have debilitating symptoms, early interventions improve outcomes, and the interventions are equitably accessible [46]. Over the next decade, these and other similar studies will provide important insights into best principles for implementing genomic newborn screening and the benefits and risks of doing so. A recent systematic review of genomic newborn screening studies noted that the current literature primarily reflected participants who identified as White, female, and of higher socioeconomic status, highlighting the need for more diverse participants [40].
From neonatal diagnosis to therapy
Early diagnosis of genetic disorders in the neonatal period opens the door to early intervention if effective treatments are available, and early intervention is critical to improving outcomes for early onset disorders. Moreover, advances in fetal diagnostics also open the door to advances in fetal therapeutics. Precision therapies may be tailored to the impacted pathway, gene, and/or specific variant of an individual patient. Currently, several examples exist in clinical practice; for example, sodium channel blockers are used in newborns with epilepsy due to gain-of-function variants in sodium channels like SCN2A but avoided in those with loss-of-function variants in these channels [47]. Over the past decade, a growing number of exciting examples have emerged, including at the “n-of-1” level, that are bridging the research to the clinical setting. For example, spinraza, an anti-sense oligonucleotide, was approved by the US Food and Drug Administration in 2016 for the treatment of spinal muscular atrophy [48, 49]. Kim and Hu and colleagues described in 2019 the first “n-of-1” anti-sense oligonucleotide therapy developed for an individual patient with Batten disease [50]. Currently, many research and industry groups are working on preclinical studies and clinical trials involving ASOs, gene editing (e.g., CRISPR-based), and viral vectors approaches [51]. As with precision diagnostics, equitable access to such therapies and payor funding are important aspect of implementation of genomic medicine in neonatal care.
Utility of genomic medicine in neonatal care
The utility of genomic medicine in neonatal care, which can be thought of as the usefulness (benefits and potentially risks) of genomic medicine for newborns and their families, has primarily been studied through the lens of diagnostic genomic testing in symptomatic newborns. Multiple studies have looked at the diagnostic and clinical utility of ES/GS in this population [5, 9,10,11,12,13,14,15,16,17,18,19,20,21,22, 52,53,54]. A recent review by Kingsmore and Cole analyzed 31 studies of rapid genomic sequencing in critically ill infants and children (2874 total) and found a weighted average diagnostic yield of 36% (range 19–83%) [4]. Clinical utility has also been reported in many of these studies; however, investigators have primarily used study-specific approaches to measure clinical utility and have focused on short-term clinical utility. Kingsmore and Cole reported a weighted average change of management rate of 27% (range 7–63%) based on 29 studies and a weighted average change in outcome rate of 18% (range 8–30%) based on 10 studies [4]. In addition to clinical utility, which is typically measured from the clinician’s perspective, personal utility, which is measured from the patient’s (or in the case of a newborn, the parent’s) perspective, is important to assess. Personal utility can be thought of as the subjective value of genomic medicine for the patient and their family [55]. Kohler and colleagues performed a systematic review of personal utility in genomic testing and identified four main domains to assess: affective, cognitive, behavioral, and social [56]. A few studies have looked at personal utility, although again investigators have primarily used study-specific approaches, limiting the generalizability of conclusions that can be drawn [57].
There is no consensus on how the utility of genomic medicine in the neonatal period should be measured, although such consensus is critical to harmonize study approaches and compare study findings, provide robust evidence for policy decisions, and provide best practices for equitable implementation [58]. A recent systematic review by Callahan and colleagues analyzed data from 21 studies of ES/GS in critically ill infants (1654 total) [57]. The authors reported substantial heterogeneity in how studies measured and reported utility but identified five common categories reported: change in treatment, redirection of care, screening or referral, prognostic information, and reproductive information. They noted that most studies did not report on personal utility, utility of non-diagnostic results, or disutility—all of which are important to understand for implementation. Consensus approaches for measurement of utility of genomic medicine are urgently needed and are being developed. It will be important to assess utility not only in the short term but also in the long-term, which may include economic aspects as well [59].
Enablers and barriers to implementation of genomic medicine in neonatal care
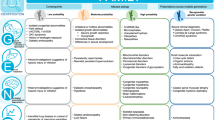
Genomic medicine in neonatal care is a process that begins with selection of a newborn for genomic testing, followed by ordering a genomic test and continued follow up after genomic test results return (Fig. 2). Selection of a newborn for genomic testing involves making the decisions of which patient will benefit from such testing and what test is appropriate for them. This can be achieved by having access to clinical geneticists and genetic counselors, and having multidisciplinary collaboration between neonatologists, clinical geneticists, genetic counselors, and other subspecialists as relevant. However, many neonatal care settings do not have ready access to clinical geneticists and genetic counselors and many neonatologists have limited education in genetics and genomics and are uncomfortable with the genomic medicine process, highlighting the need for education of non-genetic providers and infrastructure like virtual medicine to increase access to genetics providers [60].
This figure summarizes considerations discussed in the text for implementation of genomic medicine in neonatal care in three main parts of the genomic medicine process: 1) selection of newborns for genetic testing, 2) ordering genetic testing, and 3) returning results of genetic testing and follow up. Created with Biorender.com.
Access to order a given test depends on policies at an institutional, insurance, and/or healthcare system level. Although there are a few examples of recent payor funding for rapid genomic testing in critically ill infants (e.g., NHS Genomic Medicine Service in the UK and Michigan Medicaid coverage in the US), in many neonatal care settings, genomic testing is not available or depends on approval by the payor (e.g., an institutional committee) [61]. Recent surveys in the US demonstrate that genomic sequencing is not available on a regular basis in NICUs, and that access to this technology is usually restricted even in level IV NICUs [61, 62]. Equitable access to genomic sequencing will require coverage by both public and private payors. Evidence for clinical utility is central to payor decision making about coverage and often linked by payors to medical necessity, highlighting the need for standardized measures for reporting the clinical utility of genomic sequencing and for payors to recognize expanded aspects of clinical utility that are valued by patients and their families (e.g. informational utility) [63, 64]. If insurance payors do not cover the cost of genomic sequencing for an admitted newborn, either the institution or the family are potential payors. In most cases, “out-of-pocket” payment by the family is not a feasible option. In many institutions where genomic sequencing is an orderable test, access to order the test is often restricted [62]. For example, approval may be required by an institutional committee based on determination of severity of illness and likelihood of the test results to change management.
Ordering a genomic test is often more complicated than ordering other tests in neonatal care. A clinician needs to coordinate and carry out pre-test counseling and consent with the parents (or other caregivers) of the newborn, ensure appropriate samples are collected from the newborn and often the biological parents and transported to the laboratory, and provide the laboratory with clinical information to help with analysis. Returning the results of a genomic test involves interpreting the results provided by the laboratory, which often requires additional research regarding detected variant(s) and associated genes and diseases, providing post-test counseling to the parents, and then coordinating appropriate short and long term follow up. These logistics require time, effort, and knowledge that are often in short supply in acute neonatal care settings. Furthermore, the level of resources and infrastructure needed to carry this out are often only available in large referral institutions.
Several studies have used implementation science frameworks to study rapid genomic sequencing in critically ill infants and children. In Australia, Best and colleagues used the Consolidated Framework for Implementation Research (CFIR) to design the Australian Genomics Acute Care Program, initially implementing and evaluating rapid genomic sequencing in two hospitals and then scaling up ultra-rapid genomic testing for critically ill children to a national network [10, 65,66,67]. Common themes across interviews with stakeholders included the relative advantage of rapid genomic testing and networks and communication, while intensivists also focused on access to knowledge and genetics professionals focused on the rapid genomic testing intervention and process. In the United States, Franck and colleagues used the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability technology adoption framework to interview stakeholders involved in Project Baby Bear, a payor-funded study of implementing rapid genomic testing for critically ill infants at five centers in California [12, 68, 69]. Five key themes emerged: the importance of rapid genomic testing “champions”, the need for education, negotiation of decision-making roles and processes (e.g., around responsibility for ordering the test, returning results, and follow up), workflows and workarounds, and perceptions about rapid genomic testing. The lessons learned from Project Baby Bear in California were applied to the implementation of Project Baby Deer in Michigan. Seven sites in Michigan, including four without on-site geneticists, collaborated with an institution in California to offer rapid GS to critically ill infants and children. Project Baby Deer focused on “clinical” and “administrative” champions to improve access to this technology and used a standardized approach with inclusion and exclusion criteria and quality improvement evaluation across sites [70]. The champions prioritized payor advocacy during implementation, and Michigan then became the first state in the US with a Medicaid policy for carve-out reimbursement to hospitals for rapid GS in this population. In the UK, the NHS Genomic Medicine Service is undertaking mixed-methods research, also using the CFIR framework, to evaluate the early implementation of genomic testing in pediatric rare disease [71]. These and other ongoing implementation science studies in multiple countries will provide further insight into implementation strategies at scale, including implementation outside of historically studied academic referral centers.
Conclusions
The neonatal period is a time when many genetic disorders present and lead to substantial morbidity and mortality. A large body of evidence supports the potential of genomic medicine to improve neonatal care and outcomes. Research has demonstrated the feasibility, diagnostic, and clinical utility of diagnostic genomic testing, and measurements of utility that can be applied across studies and incorporate personal utility and additional aspects are being developed. Ongoing studies are investigating the implementation of genomic medicine in diverse populations of symptomatic newborns and the feasibility of genomic screening in asymptomatic newborns. As genomic medicine continues to advance and the cost of genomic sequencing decreases, continued research will be critical to provide evidence for policy changes and best practices to enable sustained implementation of equitable, effective, and ethical genomic medicine in neonatal care.
References
Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42:30–5.
Genomics Education Programme. Genomic medicine. 2021. https://www.genomicseducation.hee.nhs.uk/glossary/genomic-medicine/.
National Human Genome Research Institute. Genomics and medicine. 2020. https://www.genome.gov/health/Genomics-and-Medicine.
Kingsmore SF, Cole FS. The role of genome sequencing in neonatal intensive care units. Annu Rev Genomics Hum Genet. 2022;23:427–48.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra35.
Almli LM, Ely DM, Ailes EC, Abouk R, Grosse SD, Isenburg JL, et al. Infant mortality attributable to birth defects - United States, 2003-2017. MMWR Morb Mortal Wkly Rep. 2020;69:25–9.
Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021;70:1–114.
Cuna A, George L, Sampath V. Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin Fetal Neonatal Med. 2018;23:387–93.
D’Gama AM, Del Rosario MC, Bresnahan MA, Yu TW, Wojcik MH, Agrawal PB. Integrating rapid exome sequencing into NICU clinical care after a pilot research study. NPJ Genom Med. 2022;7:51.
Australian Genomics Health Alliance Acute Care F, Lunke S, Eggers S, Wilson M, Patel C, Barnett CP, et al. Feasibility of ultra-rapid exome sequencing in critically ill infants and children with suspected monogenic conditions in the Australian Public Health Care System. JAMA. 2020;323:2503–11.
Bowling KM, Thompson ML, Finnila CR, Hiatt SM, Latner DR, Amaral MD, et al. Genome sequencing as a first-line diagnostic test for hospitalized infants. Genet Med. 2022;24:851–61.
Dimmock D, Caylor S, Waldman B, Benson W, Ashburner C, Carmichael JL, et al. Project Baby Bear: Rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am J Hum Genet. 2021;108:1231–8.
Dimmock DP, Clark MM, Gaughran M, Cakici JA, Caylor SA, Clarke C, et al. An RCT of rapid genomic sequencing among seriously Ill infants results in high clinical utility, changes in management, and low perceived harm. Am J Hum Genet. 2020;107:942–52.
French CE, Delon I, Dolling H, Sanchis-Juan A, Shamardina O, Megy K, et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. 2019;45:627–36.
NICUSeq Study Group, Krantz ID, Medne L, Weatherly JM, Wild KT, Biswas S, et al. Effect of whole-genome sequencing on the clinical management of acutely Ill infants with suspected genetic disease: a randomized clinical trial. JAMA Pediatr. 2021;175:1218–26.
Gubbels CS, VanNoy GE, Madden JA, Copenheaver D, Yang S, Wojcik MH, et al. Prospective, phenotype-driven selection of critically ill neonates for rapid exome sequencing is associated with high diagnostic yield. Genet Med. 2020;22:736–44.
Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in Ill infants. Am J Hum Genet. 2019;105:719–33.
Maron JL, Kingsmore SF, Wigby K, Chowdhury S, Dimmock D, Poindexter B, et al. Novel variant findings and challenges associated with the clinical integration of genomic testing: an interim report of the genomic medicine for Ill neonates and infants (GEMINI) study. JAMA Pediatr. 2021;175:e205906.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438.
Petrikin JE, Cakici JA, Clark MM, Willig LK, Sweeney NM, Farrow EG, et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med. 2018;3:6.
Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3:377–87.
Manickam K, McClain MR, Demmer LA, Biswas S, Kearney HM, Malinowski J, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:2029–37.
Wojcik MH, D’Gama AM, Agrawal PB. A model to implement genomic medicine in the neonatal intensive care unit. J Perinatol. 2023;43:248–52.
Owen MJ, Niemi AK, Dimmock DP, Speziale M, Nespeca M, Chau KK, et al. Rapid sequencing-based diagnosis of thiamine metabolism dysfunction syndrome. N Engl J Med. 2021;384:2159–61.
Diamonstein C, Stevens B, Shahrukh Hashmi S, Refuerzo J, Sullivan C, Hoskovec J. Physicians’ awareness and utilization of genetic services in Texas. J Genet Couns. 2018;27:968–77.
Haga SB, Kim E, Myers RA, Ginsburg GS. Primary care physicians’ knowledge, attitudes, and experience with personal genetic testing. J Pers Med. 2019;9:29.
Hawkins AK, Hayden MR. A grand challenge: providing benefits of clinical genetics to those in need. Genet Med. 2011;13:197–200.
Jenkins BD, Fischer CG, Polito CA, Maiese DR, Keehn AS, Lyon M, et al. The 2019 US medical genetics workforce: a focus on clinical genetics. Genet Med. 2021;23:1458–64.
Lee G, Yu L, Suarez CJ, Stevenson DA, Ling A, Killer L. Factors associated with the time to complete clinical exome sequencing in a pediatric patient population. Genet Med. 2022;24:2028–33.
Suther S, Kiros GE. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11:655–62.
DeBerardinis RJ, Keshari KR. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell. 2022;185:2678–89.
Wojcik MH, Reimers R, Poorvu T, Agrawal PB. Genetic diagnosis in the fetus. J Perinatol. 2020;40:997–1006.
Adams S, Llorin H, Dobson LJ, Studwell C, Wilkins-Haug L, Guseh S, et al. Postnatal genetic testing on cord blood for prenatally identified high-probability cases. Prenat Diagn. 2023;43:1120–31.
Almannai M, Marom R, Sutton VR. Newborn screening: a review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr Opin Pediatr. 2016;28:694–9.
Wilson J, Jungner G, WHO. Principles and practice of screening for disease. World Health Organization, 1968.
Advisory Committee on Heritable Disorders in Newborns and Children. Recommended Uniform Screening Panel. 2023. https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp.
Baker MW, Groose M, Hoffman G, Rock M, Levy H, Farrell PM. Optimal DNA tier for the IRT/DNA algorithm determined by CFTR mutation results over 14 years of newborn screening. J Cyst Fibros. 2011;10:278–81.
Ding Y, Owen M, Le J, Batalov S, Chau K, Kwon YH, et al. Scalable, high quality, whole genome sequencing from archived, newborn, dried blood spots. NPJ Genom Med. 2023;8:5.
Downie L, Halliday J, Lewis S, Amor DJ. Principles of genomic newborn screening programs: a systematic review. JAMA Netw Open. 2021;4:e2114336.
Holm IA, Agrawal PB, Ceyhan-Birsoy O, Christensen KD, Fayer S, Frankel LA, et al. The BabySeq project: implementing genomic sequencing in newborns. BMC Pediatr. 2018;18:225.
Genetti CA, Schwartz TS, Robinson JO, VanNoy GE, Petersen D, Pereira S, et al. Parental interest in genomic sequencing of newborns: enrollment experience from the BabySeq Project. Genet Med. 2019;21:622–30.
Ceyhan-Birsoy O, Murry JB, Machini K, Lebo MS, Yu TW, Fayer S, et al. Interpretation of genomic sequencing results in healthy and Ill newborns: results from the BabySeq project. Am J Hum Genet. 2019;104:76–93.
Pereira S, Smith HS, Frankel LA, Christensen KD, Islam R, Robinson JO, et al. Psychosocial effect of newborn genomic sequencing on families in the babyseq project: a randomized clinical trial. JAMA Pediatr. 2021;175:1132–41.
The GUARDIAN Study. What is the GUARDIAN study? 2021. https://guardian-study.org/.
Genomics England. Newborn genomes programme. 2023. https://www.genomicsengland.co.uk/initiatives/newborns.
Knowles JK, Helbig I, Metcalf CS, Lubbers LS, Isom LL, Demarest S, et al. Precision medicine for genetic epilepsy on the horizon: Recent advances, present challenges, and suggestions for continued progress. Epilepsia. 2022;63:2461–75.
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–32.
Prakash V. Spinraza-a rare disease success story. Gene Ther. 2017;24:497.
Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381:1644–52.
Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16:630–43.
Elliott AM, du Souich C, Lehman A, Guella I, Evans DM, Candido T, et al. RAPIDOMICS: rapid genome-wide sequencing in a neonatal intensive care unit-successes and challenges. Eur J Pediatr. 2019;178:1207–18.
Freed AS, Clowes Candadai SV, Sikes MC, Thies J, Byers HM, Dines JN, et al. The impact of rapid exome sequencing on medical management of critically Ill children. J Pediatr. 2020;226:202–12.
Smith HS, Swint JM, Lalani SR, de Oliveira Otto MC, Yamal JM, Russell HV, et al. Exome sequencing compared with standard genetic tests for critically ill infants with suspected genetic conditions. Genet Med. 2020;22:1303–10.
Hayeems RZ, Luca S, Assamad D, Bhatt A, Ungar WJ. Utility of genetic testing from the perspective of parents/caregivers: a scoping review. Children. 2021;8:259.
Kohler JN, Turbitt E, Biesecker BB. Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet. 2017;25:662–8.
Callahan KP, Mueller R, Flibotte J, Largent EA, Feudtner C. Measures of utility among studies of genomic medicine for critically Ill infants: a systematic review. JAMA Netw Open. 2022;5:e2225980.
Smith HS. Genomic medicine’s critical outcome measure-utility. JAMA Netw Open. 2022;5:e2225988.
Diaby V, Babcock A, Huang Y, Moussa RK, Espinal PS, Janvier M, et al. Real-world economic evaluation of prospective rapid whole-genome sequencing compared to a matched retrospective cohort of critically ill pediatric patients in the United States. Pharmacogenomics J. 2022;22:223–9.
D’Gama AM, Agrawal PB. Role of genomic medicine and implementing equitable access for critically ill infants in neonatal intensive care units. J Perinatol. 2023;43:963–7.
Wojcik MH, Del Rosario MC, Agrawal PB. Perspectives of United States neonatologists on genetic testing practices. Genet Med. 2022;24:1372–7.
Wojcik MH, Callahan KP, Antoniou A, Del Rosario MC, Brunelli L, ElHassan NO, et al. Provision and availability of genomic medicine services in Level IV neonatal intensive care units. Genet Med. 2023;25:100926.
Douglas MP, Parker SL, Trosman JR, Slavotinek AM, Phillips KA. Private payer coverage policies for exome sequencing (ES) in pediatric patients: trends over time and analysis of evidence cited. Genet Med. 2019;21:152–60.
Trosman JR, Weldon CB, Slavotinek A, Norton ME, Douglas MP, Phillips KA. Perspectives of US private payers on insurance coverage for pediatric and prenatal exome sequencing: Results of a study from the Program in Prenatal and Pediatric Genomic Sequencing (P3EGS). Genet Med. 2020;22:283–91.
Best S, Brown H, Lunke S, Patel C, Pinner J, Barnett CP, et al. Learning from scaling up ultra-rapid genomic testing for critically ill children to a national level. NPJ Genom Med. 2021;6:5.
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20:1554–63.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Franck LS, Kriz RM, Rego S, Garman K, Hobbs C, Dimmock D. Implementing rapid whole-genome sequencing in critical care: a qualitative study of facilitators and barriers to new technology adoption. J Pediatr. 2021;237:237–43.e2.
Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017;19:e367.
Bupp CP, Ames EG, Arenchild MK, Caylor S, Dimmock DP, Fakhoury JD, et al. Breaking barriers to rapid whole genome sequencing in pediatrics: Michigan’s Project Baby Deer. Children. 2023;10:106.
Lewis C, Buchannan J, Clarke A, Clement E, Friedrich B, Hastings-Ward J, et al. Mixed-methods evaluation of the NHS Genomic Medicine Service for paediatric rare diseases: study protocol. NIHR Open Res. 2021;1:23.
Funding
AMD was supported by NICHD/NIH (T32 HD 098061). PBA is supported by NHGRI/NIH (R01HG011798) and “Because of Bella” foundation.
Author information
Authors and Affiliations
Contributions
AMD and PBA conceived the manuscript, AMD drafted the manuscript, and AMD and PBA critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
D’Gama, A.M., Agrawal, P.B. Genomic medicine in neonatal care: progress and challenges. Eur J Hum Genet 31, 1357–1363 (2023). https://doi.org/10.1038/s41431-023-01464-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01464-z
This article is cited by
-
Regulatory Frameworks for Approval of Orphan Drugs: A Comparative Overview Highlighting Need for Global Harmonization and Cohesive Guidelines
Current Pharmacology Reports (2025)
-
Advancements in pharmacological interventions for atopic dermatitis current strategies and future directions
Inflammopharmacology (2025)
-
Ambivalence and regret in genome sequencing
European Journal of Human Genetics (2023)