Abstract
Background/objectives
Some eyes with neovascular age-related macular degeneration (nAMD) and centre-involving diabetic macular oedema (DMO) fail to respond sufficiently or lose response over time to standard of care intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy. This paper explores clinical scenarios for switching to dual action angiopoietin-2 (Ang-2)/VEGF-A inhibitor faricimab (Vabysmo, Roche Products Limited) in previously anti-VEGF-treated patients.
Methods
A national steering group meeting of UK retina specialists was held in London on 27 October 2023. Clinician practice and experience were reviewed together with pivotal clinical trial data and early findings from real-world settings. Roche Products Limited facilitated and funded the meeting.
Results
While there is no standardised protocol for identifying suboptimal response, the authors review relevant clinical biomarkers of disease activity used in routine clinical practice to determine patient response and guide treatment decisions. Common reasons identified for considering a change of treatment were lack of efficacy demonstrated by suboptimal anatomic or visual improvement and insufficient durability of response. The panel outline strategies for switching to faricimab among eligible patients with a prior anti-VEGF treatment history, with initial monthly loading doses or maintaining the previous treatment interval before attempting to extend, that may be integrated into current treat-and-extend (T&E) clinical pathways for treating patients with nAMD and DMO. General considerations for switching between treatments are also reviewed.
Conclusion
Clinicians may consider a treatment switch to faricimab in nAMD and DMO patients who have suboptimal disease control or insufficient durability of response on initial anti-VEGF therapy.
Similar content being viewed by others
Introduction
Standard of care for the treatment of neovascular age-related macular degeneration (nAMD) and visual impairment due to centre-involving diabetic macular oedema (DMO) is intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy [1, 2]. Anti-VEGF treatment offers significant visual and anatomic benefits in most patients with nAMD and DMO [1,2,3]. However, frequent anti-VEGF injections are often needed to maintain efficacy and some eyes fail to respond, only partially respond, or lose response over time and require review to assess alternative or additional treatment [4,5,6].
Faricimab (Vabysmo, Roche Products Limited) was approved in the United Kingdom (UK) in 2022 for the treatment of nAMD and DMO [7, 8], providing an additional effective treatment option—beyond VEGF inhibition alone—for both treatment-naïve and previously anti-VEGF-treated patients. Faricimab is a bispecific antibody that acts through inhibition of two distinct pathways by neutralisation of both angiopoietin-2 (Ang-2) and VEGF-A [7]. Through dual pathway inhibition, faricimab is designed to reduce vascular permeability and inflammation, inhibit pathological angiogenesis and restore vascular stability [9, 10].
Ang-2 and VEGF-A are considered key drivers of vascular leakage, neovascularisation and inflammation in retinal vascular diseases [11]. Ang-2 competitively inhibits binding of Ang-1 to Tie2 tyrosine kinase receptor, counteracting vascular homoeostasis maintained through Ang-1–dependent Tie2 activation [9]. Ang-2 inhibition in combination with VEGF inhibition may promote vascular stability beyond anti-VEGF therapy alone [9,10,11,12]. Levels of Ang-2 have been shown to be increased in nAMD and DMO and dual Ang-2 and VEGF-A inhibition blockade may provide added clinical benefit over anti-VEGF monotherapy [9, 12,13,14].
Contemporary guidance on switching strategies among anti-VEGF-experienced patients with nAMD and DMO is limited. The aim of this paper is to provide clinician-led practical guidance for switching to faricimab for suboptimal response to previous intravitreal anti-VEGF treatment for nAMD and DMO. The guidance is primarily intended to inform clinicians as well as allied healthcare professionals involved in treating and monitoring patients with nAMD and DMO in UK clinical practice. Application of panel recommendations is at the discretion of the treating clinician.
Methods
A national steering group meeting of 11 UK medical retina specialists and two medical affairs partners from Roche Products Limited was held in London on 27 October 2023, focused primarily on treatment strategies for nAMD and DMO patients who have suboptimal response to previous anti-VEGF therapy. Roche Products Limited facilitated and funded the steering group meeting, and participants from Roche Products Limited provided presentations on faricimab clinical trial designs and outcomes. Following a review of pivotal faricimab clinical trial data and evidence from post hoc exploratory analyses, the panel considered current clinical practice, experience and opinion with respect to:
-
clinical biomarkers and effectiveness outcomes relevant to assessment of patient response to anti-VEGF treatment; and
-
strategies for switching to faricimab as an additional option for patients with suboptimal improvement on initial anti-VEGF treatment.
The meeting was followed by author reviews of a draft summary report; all authors contributed to subsequent development and finalisation of the approved manuscript.
Pivotal clinical trial data and exploratory analyses
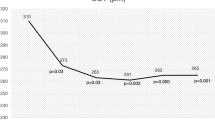
The clinical efficacy, durability of response (frequency of injection) and safety of intravitreal faricimab in nAMD and DMO have been demonstrated in the global phase III TENAYA/LUCERNE (NCT03823287/NCT03823300) and YOSEMITE/RHINE (NCT03622580/NCT03622593) trials, respectively [7, 15,16,17,18]. Across the four trials, ~80% of patients treated with faricimab treat-and-extend (T&E) (previously referred to as personalised treatment interval) achieved 12-week or 16-week dosing intervals at 2 years [7, 17, 18] (Fig. 1). Treatment with faricimab was well tolerated through study ends, with a safety profile comparable to aflibercept (Eylea, Bayer) [7, 17, 18] (Table 1).
Faricimab T&E dosing intervals achieved at 2 years in TENAYA/LUCERNE pooled (A) and YOSEMITE/RHINE pooled (B). aPercentages are based on the number of patients randomised to the pooled faricimab arms on Q8W, Q12W or Q16W dosing at week 112, among those who had not discontinued the study at that visit. Treatment interval at a given visit is defined as the treatment interval decision followed at that visit. Interval at week 112 is calculated using data recorded at week 108. bPercentages are based on the number of patients in the pooled faricimab T&E arms on Q4W, Q8W, Q12W or Q16W dosing at week 96, among those who had not discontinued the study at the week 96 visit. Dark blue lines indicate the proportion of faricimab-treated patients on 12-week or 16-week dosing intervals. Q4W every 4 weeks, Q8W every 8 weeks, Q12W every 12 weeks, Q16W every 16 weeks, T&E treat-and-extend. Adapted from Khanani et al. [17].
In post hoc exploratory evaluations of pivotal faricimab clinical trials in nAMD and DMO, findings favour faricimab over aflibercept across multiple anatomic biomarkers for disease control, including absence of retinal fluid, absence of DMO, resolution of macular leakage, secondary epiretinal membrane (ERM) formation and resolution of retinal hyperreflective foci (HRF) [17, 19,20,21,22,23,24,25] (Table 2).
Switching guidance for suboptimal anti-VEGF response in nAMD
Clinical biomarkers
Significant optical coherence tomography (OCT) imaging biomarkers associated with nAMD activity and progression include intraretinal fluid (IRF), subretinal fluid (SRF), pigment epithelial detachment (PED), subretinal hyperreflective material (SHRM), new macular haemorrhage and HRF [26]. Intraretinal fluid is considered the most relevant in terms of impact (progression or improvement) on visual outcome [26]. In eyes treated with anti-VEGF for nAMD, persistent IRF is associated with worse long-term visual acuity compared with resolved IRF, while early resolution of SRF is associated with a greater improvement in visual acuity [26,27,28].
Signs of nAMD disease activity that guide retreatment decisions include: new or increased retinal fluid (IRF, SRF or subretinal pigment epithelium fluid) on OCT; increased PED size; new or persistent macular haemorrhage; new or increased SHRM; or decreased visual acuity of ≥5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters attributable to choroidal neovascularisation activity [29, 30]. Fluorescein leakage or increase in lesion size on FFA may also indicate active disease [31]. Guidance from the National Institute for Health and Care Excellence (NICE) underscores the importance of monitoring of both eyes when one eye only is being treated for nAMD [32].
Clinicians mostly treat nAMD using a flexible T&E dosing regimen after an initial monthly treatment phase (i.e. loading) [7, 29, 33]. Treatment interval extension is considered appropriate when there is no retinal fluid or other OCT biomarkers of active nAMD, with stable or better visual acuity or if visual acuity loss is not considered due to nAMD disease activity [33]. Visual acuity measurements within high throughput real-world services may not provide an accurate assessment of disease activity status.
Determining treatment response
A patient whose visual acuity declines due to persistent exudative disease activity despite an optimally delivered treatment regimen is considered a non-responder [34]. The original diagnosis should be re-evaluated, and the treating clinician should assess protocol adherence to rule out undertreatment [34]. A suboptimal response to anti-VEGF treatment may be considered as presence of macular fluid after the initiation phase of consecutive monthly anti-VEGF injections or persistent/residual macular fluid at any time post the initial loading phase [35]. Mettu et al. defined incomplete response to anti-VEGF therapy in nAMD as persistent disease activity (characterised by persistent fluid exudation, unresolved or new haemorrhage and progressive lesion fibrosis) and/or suboptimal visual recovery (failure to achieve visual acuity of ≥70 ETDRS letters or ≥20/40 Snellen equivalent) despite sustained treatment [36]. Refractory nAMD is generally considered based on the presence of persistent or recurrent IRF or SRF on OCT despite maximal (monthly) or prolonged anti-VEGF dosing for ~12 months or longer after treatment initiation [37].
Options for switching from initial anti-VEGF to faricimab in nAMD
Current practice in treating nAMD is to actively consider switch alternatives for non-response and suboptimal response to previous anti-VEGF therapy at any time after the initial treatment loading phase and for high-need patients being treated regularly every 4–7 weeks (Q4W–Q7W, ~7–12 injections each year). A change may also be considered for treatment-resistant or recalcitrant nAMD, e.g. worsening visual outcomes, or persistent or recurrent retinal fluid on OCT, despite continued timely anti-VEGF injections over a 12-month or longer period [38]. Patients maintained on every-8-week (Q8W) anti-VEGF treatment (~6 injections each year), who cannot be extended further and patients who are not able to maintain intervals longer than 10 or 12 weeks (~4–5 injections per year), may be offered or request the option of a switch to potentially longer-acting treatment, if suitable.
Switching decisions may either be disease activity driven (anatomic control supplemented by visual acuity response) or based on insufficient durability of response per clinician and patient decision. Local service models, disease characteristics and patient-related factors including likely visit adherence may impact clinician decision-making on alternative effective treatment options.
For patients with suboptimal improvement after initial monthly loading and for patients with persistent retinal fluid or other signs of active disease on continued Q4W–Q7W dosing intervals, clinicians should consider initiating the treatment switch to faricimab with a loading phase of four consecutive monthly injections, if possible. Among patients previously treated with anti-VEGF at maximum every-4-week (Q4W) intervals, initial loading with monthly faricimab injections may continue to improve anatomic response.
Among patients considered unable to extend beyond Q8W treatment intervals, a treatment switch to faricimab may be initiated by matching the previous interval, or with initial monthly injections per clinician discretion, followed thereafter by flexible dosing using a T&E regimen. Patients who are interval matched but who fail to respond sufficiently on first clinic review post switch may benefit from a new initiation phase of consecutive monthly injections. Figure 2 provides an overview of guidance for switching to faricimab T&E in previously treated nAMD.
aSuboptimal response defined as intraretinal fluid or subretinal fluid on OCT, other anatomic features of active or worsening disease (e.g. new haemorrhage OR new SHRM), or unchanged (≤5-letter improvement)/reduced VA due to nAMD, after three consecutive monthly intravitreal injections. bTreatment-resistant nAMD generally defined as persistent retinal fluid on OCT despite continued intravitreal anti-VEGF injections over a 12-month period. cPrimarily maintained at this treatment interval or later shortened. dIn patients previously anti-VEGF-treated at maximum Q4W intervals, initial loading with monthly injections may continue to improve anatomic features. nAMD neovascular age-related macular degeneration, OCT optical coherence tomography, Q4W–Q7W every 4–7 weeks, Q8W every 8 weeks, Q10W–Q12W every 10–12 weeks, Q16W every 16 weeks, SHRM subretinal hyperreflective material, T&E treat-and-extend, VA visual acuity.
Switching guidance for suboptimal anti-VEGF response in DMO
Clinical biomarkers
Early and sustained control of central subfield thickness (CST) and fluid resolution are both important for optimising vision outcomes in DMO [39, 40]. Additional biomarkers associated with treatment response in DMO include intraretinal cysts, large outer nuclear layer cyst, disorganisation of retinal inner layers (DRIL), ellipsoid zone disruption and amount and ___location of HRF [41].
Retreatment decisions in the management of DMO are based mainly on comparison of OCT features and visual acuity over recent visits. Change in OCT CST/central retinal thickness (CRT) should be assessed against the lowest CST/CRT achieved and visual acuity change against the best-achieved score in response to treatment, which usually occur following the monthly treatment phase but may be following further intensive treatment [42]. Extension of treatment interval using a personalised T&E regimen is considered appropriate once CST/CRT on OCT and visual acuity are stable [42].
Determining treatment response
Non-response to treatment for DMO may be considered where there is worsening visual outcomes, or unchanged/increased CST/CRT, despite an optimally delivered treatment regimen. As for nAMD, diagnosis should be re-evaluated for eyes that are non-responsive to initial intravitreal treatment. A suboptimal clinical response at ≥12 weeks from treatment initiation may be considered where there is <20% reduction from reference OCT CST/CRT (machine matched for comparisons) and unchanged (≤5-letter improvement) or reduced visual acuity due to DMO with residual disease activity. The panel suggested a practical threshold for defining insufficient anatomic response as a reduction of less than 20% in reference CST/CRT (documented for example at time of diagnosis) and/or retinal thickness above an indicative absolute CST/CRT value or threshold as determined by the treating clinician (e.g. 390 µm if NICE guidance recommendation of CRT criterion of ≥400 µm was used to initiate anti-VEGF treatment) [43]. Treatment-resistant or refractory DMO is generally considered as persistent DMO, increased retinal thickness, or reduced visual acuity attributable to DMO, despite continued anti-VEGF treatment over a 12-month or longer period [44].
Options for switching from initial anti-VEGF to faricimab in DMO
Clinicians may consider switching to faricimab in patients with DMO who are either non-responsive or considered suboptimal responders after monthly loading with anti-VEGF monotherapy, and for patients with persistent active disease following regular anti-VEGF treatment post loading. Other options for these patients include intravitreal corticosteroid implant treatment or supplemental focal laser.
In DMO, clinical management is focused predominantly on achieving effective disease control by continuing to treat until resolution of macular oedema, with an early switch considered for a poor or incomplete response with persistent DMO. High retreatment burden, patient-related factors including adherence restrictions or disease-related characteristics (e.g. unresolved maculopathy) may impact clinical decisions on appropriate switch options and timings. Patients who are maintained stable with controlled diabetic maculopathy on longer dosing intervals of every 10 or 12 weeks are thought most likely to transition to a pro re nata (PRN, as needed) treatment plan with continued unchanged therapy. The Diabetic Retinopathy Clinical Research Network Protocol I study showed that the need for intravitreal anti-VEGF injections in DMO decreases annually over 5 years [45].
Ideally all suitable patients with previously treated DMO who are switched to faricimab would receive an initial monthly loading phase where possible, followed thereafter by a personalised T&E approach. Extension increments of 4 weeks are considered suitable, but there is flexibility for shorter adjustment increments at the discretion of the treating clinician.
Adjunctive macular laser or change to intravitreal corticosteroid treatment may be appropriate in eyes with suboptimal response to anti-VEGF agents.
In a landmark comparative effectiveness study of different anti-VEGF treatments for DMO, supplemental laser was administered if DMO persisted and was not improving starting at month 6; over 2 years, 41–65% of eyes received laser therapy at least once [46]. Macular laser can be effective for people who have visual impairment due to centre-involving DMO and have thinner retinas (CRT < 400 µm) and may delay the need for anti-VEGF treatment [47]. Either observation or macular laser may be considered for people with DMO and good vision (i.e. ≥79 letters) [47].
Vasoactive growth factors and inflammatory cytokines other than VEGF and Ang-2 contribute to the breakdown of the blood-retinal barrier and the onset of DMO [48]. Intravitreal corticosteroid implants dexamethasone (Ozurdex, AbbVie) and fluocinolone acetonide (Iluvien, Alimera Sciences) suppress inflammation and inhibit VEGF expression and are effective in reducing CRT and improving visual acuity in DMO; however, they are associated with a high risk of intraocular pressure elevation and the development or progression of cataract in phakic patients [49,50,51,52].
It is recommended that clinicians consider intravitreal corticosteroid treatment if suitable based on inadequate response after 12 months or after 2 years based on burden of injections at this stage [47, 53]. Intravitreal corticosteroid treatment may also be considered in DMO eyes with contraindications to anti-VEGF [47]. Vitrectomy should be considered for DMO patients who do not respond to anti-VEGF treatment with evidence of vitreomacular traction or presumed ERM-related oedema [47]. Figure 3 provides an overview of guidance for switching to faricimab T&E in previously treated DMO.
aTreatment-resistant DMO generally defined as persistent DMO, increased retinal thickness, or unchanged (≤5-letter improvement)/reduced VA due to DMO with residual disease activity, despite continued intravitreal anti-VEGF treatment over a 12-month period. b[47]. cMacular laser may be considered as adjunct therapy [47]. anti-VEGF anti-vascular endothelial growth factor, CRT central retinal thickness, CST central subfield thickness, DMO diabetic macular oedema, ERM epiretinal membrane, OCT optical coherence tomography, ≤Q8W every 4–8 weeks, Q16W every 16 weeks, T&E treat-and-extend, VA visual acuity.
Different treatment plans for each eye are feasible for patients with bilateral DMO (e.g. intravitreal corticosteroid treatment in one eye and anti-VEGF for the other).
The panel recommended non-ophthalmic physician review for all patients, especially those with uncontrolled diabetes and for patients with progressive retinopathy or resistant maculopathy, to address all treatable systemic risk factors. Routine access to support from diabetologists or general practitioners is recommended.
General considerations when switching to faricimab among previously anti-VEGF-treated nAMD and DMO
Bilateral involvement
For bilateral involvement, clinicians ideally use the same intravitreal anti-VEGF agent for both eyes; clinicians should therefore consider switching both eyes at the same time if possible when switching between these agents. If bilateral injections are planned during the same visit, clinicians should use a separate batch of medication for each eye or reschedule another appointment if separate lots are not available [54]. The safety and efficacy of faricimab administered in both eyes concurrently have not been studied [7].
Patient counselling and consent
-
Patients should be informed of potentially serious risks of intravitreal injection procedures as well as risks associated with intravitreal VEGF inhibitors [54]. Evidence suggests that treatment with faricimab has similar adverse events to aflibercept and ranibizumab (Lucentis, Novartis) [15,16,17,18, 55,56,57].
-
Intraocular inflammation rates in phase III clinical trials with faricimab were low and comparable to aflibercept [19] (Table 1). Post marketing, rare cases of retinal vasculitis and/or retinal occlusive vasculitis have been reported with the use of faricimab and aflibercept [7, 58, 59]. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency, according to product label updates [7, 58, 59].
-
When initiating intravitreal VEGF inhibitor treatment, caution is advised in nAMD patients with a large and/or high PED who may be at increased risk of retinal pigment epithelial (RPE) tear development [7, 60, 61]. Several reports suggest that eyes with a baseline PED height ≥600 µm have an increased risk of RPE tear following intravitreal anti-VEGF therapy [62, 63]. The frequency of RPE tear development through 2 years in the TENAYA and LUCERNE nAMD trials (pooled) was 2.9% with faricimab and 1.5% with aflibercept; most of these adverse events were reported as mild to moderate, without impact to vision and occurred during the loading phase [7].
-
Documented patient consent obtained prior to first intravitreal injection procedure typically applies for a course of repeated anti-VEGF treatments. Professional guidance recommends that repeat written consent should be obtained if there is a change to the treatment plan or drug used, change in the clinical condition and/or change in perceived benefit-risk to the patient [34, 35].
-
Some patients may need a period of continued monthly faricimab dosing after a loading phase to maximise treatment response.
Post switch
-
Patients who experience hypersensitivity or have worsening anatomic response following a treatment switch to faricimab could either be switched back to previous anti-VEGF or given a different anti-VEGF agent.
Modelling of treatment regimens
-
Clinical modelling of alternative treatments and regimens should ideally capture a range of related healthcare costs and benefits for the patient, the practice and the healthcare system overall, including: net acquisition, administration and monitoring cost of therapy; healthcare resource use and costs (e.g. OCT procedures, non-consultant led outpatient visits, nurse or technician-led monitoring, consultant-led reviews, post injection management and overall episode costs); capacity improvements over time, supported by modelling assumptions of injection frequencies through years 3–5; and impact on patients and their carers [64, 65].
Discussion
This panel review provides practice considerations and recommendations for assessing response to intravitreal anti-VEGF therapy for nAMD and DMO and outlines potential options and approaches for switching to faricimab in eyes with suboptimal improvement or limited durability of response. Panel discussions on biomarkers of disease activity have concentrated on OCT-guided findings that can be routinely assessed in clinical practice. Lack of efficacy demonstrated by suboptimal anatomic or visual improvement and high treatment burden are the most common reasons for considering a change of treatment in patients with nAMD and DMO. Switch strategies guided by disease control and durability of response, and incorporating monthly loading doses or interval-matched initiation, may be integrated into current faricimab clinical pathways for treating patients with nAMD and DMO [33, 42].
Professional guidance recommends a treatment switch in patients with nAMD and DMO experiencing allergy or presumed tachyphylaxis and for practical reasons [34, 47]. Draft guidance from NICE on treating DMO recommends that clinicians should consider using macular laser as rescue treatment or changing anti-VEGF treatment if initial treatment alone does not stabilise (i.e. within 5 letters of visual acuity from baseline) or improve the patient’s vision after the loading phase [47]. A further review of treatment response should take place after 12 months and clinicians should consider intravitreal corticosteroid treatment if the response is suboptimal [47].
Clinical trial and early real-world data suggest that switching to faricimab may provide rapid anatomic improvement with stable or improved vision and with potential for extended treatment intervals in some patients with nAMD and DMO [66,67,68,69,70]. Across 21 National Health Service sites in the UK, early treatment patterns of faricimab among patients with nAMD and DMO previously treated with anti-VEGF confirm treatment interval extensions after the first 4 months with maintenance of vision [71, 72]. In a small retrospective cases series from a single UK tertiary care centre, faricimab was shown to be a safe and effective option for patients with treatment-resistant nAMD on aflibercept Q4W–Q6W, with clinical improvement on OCT observed in 80% of patients (n = 68) [73]. The authors noted that there was no evidence that switching with either 2 or 3 monthly loading injections had an independent effect on clinical outcomes [73].
This article focuses on anti-VEGF-experienced patients and treatment switch to dual Ang-2/VEGF pathway inhibition with faricimab as an option for suboptimal response or presumed acquired resistance to previous therapy. However, changing to a different anti-VEGF drug may also be a suitable option among patients who have an incomplete response or diminished response over time with previous anti-VEGF therapy [74, 75]. Moreover, reported outcomes of faricimab in previously anti-VEGF-treated patients are mostly limited to observational, non-controlled studies involving early data captured in routine practice, with no standardised measurements of visual acuity and variable clinician dosing approaches. An early positive anatomic and/or visual response following treatment switch may not necessarily translate to long-term patient response and more intensive treatment may provide additional clinical benefit in some eyes. Clinicians will continue to face heterogeneity in clinical response, including challenging or complex cases that do not respond satisfactorily to anti-VEGF treatments. Intravitreal corticosteroid implant treatment is a potentially effective option for patients with DMO who have an inadequate response to continued anti-VEGF therapy [47, 76, 77].
Closing comments
To conclude, there was strong emphasis from panel members that disease control and frequency of injection should be at the forefront of clinician thinking when assessing treatment response and switch strategies in the management of nAMD and DMO. Emerging data suggest that dual Ang-2 and VEGF-A pathway inhibition with faricimab may offer potential for greater disease control with less frequent dosing over time compared with targeting the VEGF pathway alone [78]. Among patients who respond satisfactorily to faricimab, the treatment interval may potentially be extended, reducing burden and risks associated with frequent injections while maintaining or improving visual outcomes. Fewer injection and clinic monitoring visits provide meaningful benefits for patients and carers [79, 80]. Additionally, decreased injection frequency across a subset of patients may help to improve clinic capacity, thereby preventing treatment delays and potentially facilitating implementation of new or additional services [81].
Current clinician guidance on switch strategies may be further adapted as additional longer-term data and evidence emerge from multiple, ongoing real-world studies [71, 72, 78]. Future research will also add to current understanding of treatment patterns and patient outcomes utilising different switch strategies, including responses over time with and without a monthly initiation phase.
Summary
What was known before
-
Some eyes fail to respond or only partially respond to multiple anti-VEGF treatments and frequent intravitreal injections are often needed to maintain efficacy.
-
There is a need for standardised protocols defining treatment response and practical criteria for considering a treatment switch for patients who respond insufficiently to previous anti-VEGF therapy.
What this study adds
-
Clinician-led practice considerations and recommendations for assessing patient response to intravitreal anti-VEGF and potential options and approaches for switching to faricimab in eyes with suboptimal improvement or limited durability of response on previous anti-VEGF therapy.
Data availability
Consensus recommendations were developed based on objective expert opinion and clinical experience. Correspondence and requests for further information should be addressed to the corresponding author.
References
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98:1144–67.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185–222.
Virgili G, Curran K, Lucenteforte E, Peto T, Parravano M. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2023;2023:CD007419.
Wallsh JO, Gallemore RP. Anti-VEGF-resistant retinal diseases: a review of the latest treatment options. Cells. 2021;10:1049.
Khachigian LM, Liew G, Teo KYC, Wong TY, Mitchell P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J Transl Med. 2023;21:133.
Sharma D, Zachary I, Jia H. Mechanisms of acquired resistance to anti-VEGF therapy for neovascular eye diseases. Investig Ophthalmol Vis Sci. 2023;64:28.
Vabysmo 120 mg/mL solution for injection [Summary of Product Characteristics]; Roche Products Limited, UK, January 2024.
Vabysmo 120 mg/mL solution for injection [Summary of Product Characteristics]; Roche Registration GmbH, Germany, June 2023.
Khanani AM, Russell MW, Aziz AA, Danzig CJ, Weng CY, Eichenbaum DA, et al. Angiopoietins as potential targets in management of retinal disease. Clin Ophthalmol. 2021;15:3747–55.
Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8:1265–88.
Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye. 2021;35:1305–16.
Heier JS, Singh RP, Wykoff CC, Csaky KG, Lai TYY, Loewenstein A, et al. The angiopoietin/tie pathway in retinal vascular diseases: a review. Retina. 2021;41:1–19.
Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree IA. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89:480–3.
Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16:635–61.
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al; TENAYA and LUCERNE Investigators. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40.
Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al; YOSEMITE and RHINE Investigators. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741–55.
Khanani AM, Haskova Z, Kotecha A, Margaron P, Ives J, Tan Y, et al. Faricimab in nAMD and DME: latest updates. Paper presented at Angiogenesis, Exudation, and Degeneration 2023 virtual meeting; 10–11 February 2023.
Wong TY, Haskova Z, Asik K, Baumal CR, Csaky KG, Eter N, et al. Faricimab treat-and-extend for diabetic macular edema: two-year results from the randomized phase 3 YOSEMITE and RHINE trials. Ophthalmology. 2024;131:708–23.
Gale R, London N, Kotecha A, Willis JR, Souverain A, Shildkrot Y, et al. Faricimab rapidly improves fluid parameters in patients with nAMD. Paper presented at 23rd European Society of Retina Specialists Congress; Amsterdam, The Netherlands: 5–8 October 2023.
Lanzetta P, Rachitskaya A, Camino A, Gibson K, Mar F, Shildkrot Y, et al. Faster time to retinal fluid control with faricimab versus aflibercept in patients with DMO in the phase 3 YOSEMITE/RHINE Trials. Paper presented at 23rd European Society of Retina Specialists Congress; Amsterdam, The Netherlands: 5–8 October 2023.
Nudleman N, Goldberg R, Csaky K, Kolomeyer AM, Gibson K, Mar F, et al. Faricimab reduces macular leakage vs aflibercept in patients with DME. Paper presented at American Society of Retina Specialists annual meeting; Seattle, WA, USA: 28 July–1 August 2023.
Jaffe GJ, Ogura Y, Deák G, Gibson K, Schmidt-Erfurth U, Wang T, et al. Impact of faricimab vs aflibercept on epiretinal membrane formation over 2 years in eyes with DMO in the YOSEMITE/RHINE phase 3 trials. Paper presented at American Society of Retina Specialists annual meeting; Seattle, WA, USA: 28 July–1 August 2023.
Maunz A, von Schulthess E, Patel KM, Chakravarthy U, Bachmeier I, Lloyd Jones I, et al. Automated segmentation of hyperreflective foci in diabetic macular edema shows greater volume reduction by faricimab vs aflibercept in phase 3 YOSEMITE and RHINE. Investig Ophthalmol Vis Sci. 2023;64:PB0039.
Chakravarthy U, Maunz A, von Schulthess E, Patel K, Bachmeier I, Lloyd Jones I, et al. Automated segmentation of hyperreflective foci in DME shows greater volume reduction in eyes treated with faricimab compared to aflibercept in the phase 3 YOSEMITE and RHINE clinical trials. Paper presented at 23rd European Society of Retina Specialists Congress; Amsterdam, The Netherlands: 5–8 October 2023.
Watkins C, Paulo T, Bührer C, Holekamp NM, Bagijn M. Comparative efficacy, durability and safety of faricimab in the treatment of diabetic macular edema: a systematic literature review and network meta-analysis. Adv Ther. 2023;40:5204–21.
Hanson RLW, Airody A, Sivaprasad S, Gale RP. Optical coherence tomography imaging biomarkers associated with neovascular age-related macular degeneration: a systematic review. Eye. 2023;37:2438–53.
Core JQ, Pistilli M, Hua P, Daniel E, Grunwald JE, Toth CA, et al. Comparison of age-related macular degeneration treatments trials (CATT) research group. Predominantly persistent intraretinal fluid in the comparison of age-related macular degeneration treatments trials. Ophthalmol Retin. 2022;6:771–85.
Riedl S, Vogl WD, Waldstein SM, Schmidt-Erfurth U, Bogunović H. Impact of intra- and subretinal fluid on vision based on volume quantification in the HARBOR trial. Ophthalmol Retin. 2022;6:291–7.
Bailey C, Cackett P, Kotagiri A, Mahmood S, Minos E, Narendran N, et al. Practical implementation of a q4-q16 aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration: Updated guidance from a UK expert panel. Eye. 2023;37:1916–21.
Kodjikian L, Parravano M, Clemens A, Dolz-Marco R, Holz FG, Munk MR, et al. Fluid as a critical biomarker in neovascular age-related macular degeneration management: literature review and consensus recommendations. Eye. 2021;35:2119–35.
Arpa C, Khalid H, Chandra S, Wagner S, Fasler K, Faes L, et al. Ten-year survival trends of neovascular age-related macular degeneration at first presentation. Br J Ophthalmol. 2021;105:1688–95.
National Institute for Health and Care Excellence. Serious eye disorders: Quality Standard. Published 12 February 2019. www.nice.org.uk/guidance/qs180.
Kotagiri A, Gale R, Pearce I, Sivaprasad S, Cackett P, Elsherbiny S, et al. Intravitreal faricimab for neovascular age-related macular degeneration: Consensus pathway by an expert panel of UK retina specialists. Poster presented at The Royal College of Ophthalmologists annual congress; Birmingham, UK: 22–25 May 2023.
The Royal College of Ophthalmologists. Commissioning guidance—age-related macular degeneration services. June 2021. https://www.rcophth.ac.uk/resources-listing/commissioning-guidance-age-related-macular-degeneration-services/.
Chorev M, Haderlein J, Chandra S, Menon G, Burton BJL, Pearce I, et al. A multi-modal AI-driven cohort selection tool to predict suboptimal non-responders to aflibercept loading-phase for neovascular age-related macular degeneration: PRECISE study report 1. J Clin Med. 2023;12:3013.
Mettu PS, Allingham MJ, Cousins SW. Incomplete response to Anti-VEGF therapy in neovascular AMD: exploring disease mechanisms and therapeutic opportunities. Prog Retin Eye Res. 2021;82:100906.
Zola M, D’Alessandro E, Sherif M, Nguyen A, De Azevedo D, Haeller C, et al. Refractory neovascular age-related macular degeneration: time-dependent changes of central retinal thickness with anti-VEGF treatment. Graefes Arch Clin Exp Ophthalmol. 2021;259:1477–86.
Rush RB, Rush SW. Intravitreal faricimab for aflibercept-resistant neovascular age-related macular degeneration. Clin Ophthalmol. 2022;16:4041–6.
Khoramnia R, Nguyen QD, Kertes PJ, Ramsay LS, Vujosevic S, Anderesi M, et al. Exploring the role of retinal fluid as a biomarker for the management of diabetic macular oedema. Eye. 2024;38:54–60.
Brown DM, Emanuelli A, Bandello F, Barranco JJE, Figueira J, Souied E, et al. KESTREL and KITE: 52-week results from two phase III pivotal trials of brolucizumab for diabetic macular edema. Am J Ophthalmol. 2022;238:157–72.
Chang YC, Huang YT, Hsu AY, Meng PP, Lin CJ, Lai CT, et al. Optical coherence tomography biomarkers in predicting treatment outcomes of diabetic macular edema after ranibizumab injections. Medicina. 2023;59:629.
Chakrabarti S, Gale R, Sivaprasad S, Bailey C, Yang Y, Peto T, et al. Intravitreal faricimab for diabetic macular oedema: Consensus pathway by an expert panel of UK retina specialists. Poster presented at The Royal College of Ophthalmologists annual congress; Birmingham, UK: 22–25 May 2023.
National Institute for Health and Care Excellence. Faricimab for treating diabetic macular oedema (TA799). Technology appraisal guidance. June 2022. www.nice.org.uk/guidance/ta799.
Rush RB, Rush SW. Faricimab for treatment-resistant diabetic macular edema. Clin Ophthalmol. 2022;16:2797–801.
Bressler SB, Odia I, Glassman AR, Danis RP, Grover S, Hampton GR, et al. Changes in diabetic retinopathy severity when treating diabetic macular edema with ranibizumab: DRCR.net Protocol I 5-year report. Retina. 2018;38:1896–904.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9.
National Institute for Health and Care Excellence. Guideline. Diabetic retinopathy: management and monitoring. Draft for consultation, August 2023. https://www.nice.org.uk/guidance/gid-ng10256/documents/draft-guideline.
Urias EA, Urias GA, Monickaraj F, McGuire P, Das A. Novel therapeutic targets in diabetic macular edema: beyond VEGF. Vis Res. 2017;139:221–7.
Salvetat ML, Pellegrini F, Spadea L, Salati C, Musa M, Gagliano C, et al. The treatment of diabetic retinal edema with intravitreal steroids: how and when. J Clin Med. 2024;13:1327.
National Institute for Health and Care Excellence. Dexamethasone intravitreal implant for treating diabetic macular oedema (TA824). Technology appraisal guidance. September 2022. https://www.nice.org.uk/guidance/ta824.
National Institute for Health and Care Excellence. Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema (TA953). Technology appraisal guidance. March 2024. https://www.nice.org.uk/guidance/ta953.
Scottish Medicines Consortium. Fluocinolone acetonide 190 micrograms intravitreal implant (Iluvien®) SMC No. (864/13). January 2014. https://www.scottishmedicines.org.uk/media/1737/fluocinolone__iluvien__resubmission_final_january_2014_for_website.pdf.
NHS England. Operational note: Commissioning recommendations following the national procurement for medical retinal vascular medicines. National procurement for Anti-VEGF and Intravitreal Corticosteroids. August 2022. https://www.england.nhs.uk/wp-content/uploads/2022/08/B1720-Commissioning-recommendations-following-national-procurement-medical-retinal-vascular-medicines-August-2.pdf.
The Royal College of Ophthalmologists. Intravitreal injection therapy. August 2018. https://www.rcophth.ac.uk/resources-listing/intravitreal-injection-therapy/.
Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126:1155–70.
Sahni J, Dugel PU, Patel SS, Chittum ME, Berger B, Del Valle Rubido M, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the AVENUE phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:955–63.
Khanani AM, Patel SS, Ferrone PJ, Osborne A, Sahni J, Grzeschik S, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:964–72.
VABYSMO® (faricimab-svoa) injection, for intravitreal use. Highlights of prescribing information; Genentech, Inc., October 2023.
EYLEA® (aflibercept) injection, for intravitreal use. Highlights of prescribing information; Regeneron Pharmaceuticals, Inc., December 2023.
Eylea 40 mg/ml solution for injection in pre-filled syringe [Summary of Product Characteristics]; Bayer plc, UK, November 2023.
Lucentis 10 mg/ml solution for injection [Summary of Product Characteristics]; Novartis Pharmaceuticals UK Limited, July 2023.
Khanani AM, Eichenbaum D, Schlottmann PG, Tuomi L, Sarraf D. Optimal management of pigment epithelial detachments in eyes with neovascular age-related macular degeneration. Retina. 2018;38:2103–17.
Sarraf D, Joseph A, Rahimy E. Retinal pigment epithelial tears in the era of intravitreal pharmacotherapy: risk factors, pathogenesis, prognosis and treatment (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:142–59.
Gale R, Cox O, Keenan C, Chakravarthy U. Health technology assessment of new retinal treatments; the need to capture healthcare capacity issues. Eye. 2022;36:2236–8.
Meer EA, Oh DH, Brodie FL. Time and distance cost of longer acting anti-VEGF therapies for macular degeneration: contributions to drug cost comparisons. Clin Ophthalmol. 2022;16:4273–9.
Panos GD, Lakshmanan A, Dadoukis P, Ripa M, Motta L, Amoaku WM. Faricimab: transforming the future of macular diseases treatment - a comprehensive review of clinical studies. Drug Des Devel Ther. 2023;17:2861–73.
Bakri SJ, Tabano D, Borkar D, Garmo V, Ahmed A, Myers R, et al. Real-world outcomes and treatment patterns with faricimab in AMD FARETINA. Paper presented at American Academy of Ophthalmology 2023 retina subspecialty day; San Francisco, CA, USA: 3–6 November 2023.
Borkar D, Tabano D, Garmo V, Leng T, Shaia J, Kuo BL, et al. Early treatment patterns and outcomes in patients with diabetic macular edema treated with faricimab: the FARETINA-DME Study. Paper presented at American Society of Retina Specialists annual meeting; Seattle, WA, USA: 28 July–1 August 2023.
Khanani AM, Aziz AA, Khan H, Gupta A, Mojumder O, Saulebayeva A, et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study - 6 month results. Eye. 2023;37:3574–81.
Pandit SA, Momenaei B, Wakabayashi T, Mansour HA, Vemula S, Durrani AF, et al. Real-world outcomes of faricimab in patients with previously treated neovascular age-related macular degeneration. Ophthalmol Retina. 2024;8:360–6.
Talks J, de Salvo G, Patel PJ, de Silva SR, Gale RP, McKibbin M, et al. FARWIDE-nAMD: Early treatment patterns of faricimab among neovascular age-related macular degeneration patients in the UK. Paper presented at 23rd European Society of Retina Specialists Congress; Amsterdam, The Netherlands: 5–8 October 2023.
Bailey C, Peto T, Downey L, Kiire C, Pearce I, Reynolds R, et al. FARWIDE-DMO: Early treatment patterns of faricimab among DMO patients in the UK. Paper presented at 23rd European Society of Retina Specialists Congress; Amsterdam, The Netherlands: 5–8 October 2023.
Raimondi R, Falfeli T, Bogdanova-Bennet A, Varma D, Habib M, Kotagiri A, et al. Outcomes of treatment-resistant neovascular age-related macular degeneration switched from aflibercept to faricimab. Ophthalmol Retina. 2024;8:537–44.
Spooner K, Hong T, Wijeyakumar W, Chang AA. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017;11:161–77.
Sepetis AE, Clarke H, Gupta B. Functional and structural characteristics in patients with diabetic macular oedema after switching from ranibizumab to aflibercept treatment. Three year results in real world settings. Int J Retin Vitreous. 2022;8:23.
Sanders FWB, Dumont Jones R, Jones DR, Phillips SV, Williams GS. Treatment of diabetic macular edema or macular edema following retinal vein occlusion based on repeated injection of the dexamethasone intravitreal implant: a retrospective real-world analysis. Clin Ophthalmol. 2023;17:3177–87.
Busch C, Zur D, Fraser-Bell S, Laíns I, Santos AR, Lupidi M, et al. International Retina Group. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55:789–96.
Penha FM, Masud M, Khanani ZA, Thomas M, Fong RD, Smith K, et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int J Retin Vitreous. 2024;10:5.
Gale RP, Finger RP, Eldem B, Aslam T, Barratt J, Daien V, et al. The management of neovascular age-related macular degeneration: a systematic literature review of patient-reported outcomes, patient mental health and caregiver burden. Acta Ophthalmol. 2023;101:e26–42.
Spooner KL, Guinan G, Koller S, Hong T, Chang AA. Burden of treatment among patients undergoing intravitreal injections for diabetic macular oedema in Australia. Diabetes Metab Syndr Obes 2019;12:1913–21.
Narayan A, Watson S-L. Faricimab: the solution to treatment resistant neovascular age related macular degeneration? A real-world study. Poster presented at The Royal College of Ophthalmologists annual congress 2023; Birmingham, UK: 22–25 May 2023.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Acknowledgements
Third-party medical writing support was provided by Rod McNeil, BA (Hons), MBA, of Rod McNeil Associates and funded by Roche Products Limited, in accordance with Good Publication Practice (GPP3) guidance [82].
Funding
All authors except AP and SK received honoraria from Roche Products Limited for their contributions to the UK national steering group meeting, although the authors were not remunerated for writing this manuscript. The meeting was facilitated and funded by Roche Products Limited in response to a need from academics to convene and discuss this clinically significant subject. Funding for open access was provided by Roche Products Limited.
Author information
Authors and Affiliations
Contributions
All authors contributed to development and finalisation of the manuscript and approved the manuscript for journal submission. Eleven ophthalmology consultants were selected by Roche Products Limited and invited to participate based on their expertise in clinical practice for nAMD and DMO across the different regions of the UK. This was to allow representation across England and the devolved nations. In addition, two members of the medical team at Roche Products Limited were selected by Roche to participate in the steering group meeting based on their knowledge and expertise in the faricimab clinical trial data.
Corresponding author
Ethics declarations
Competing interests
LD: Advisory board attendances for and speaker honoraria from Alimera Sciences, AbbVie, Bayer, Biogen, Novartis and Roche; travel grants from AbbVie, Bayer and Novartis. SS: Financial support from AbbVie, Amgen, Apellis, Bayer, Biogen, Boehringer Ingelheim, Novartis, Eyebiotech, EyePoint Pharmaceuticals, Janssen Pharmaceuticals, Nova Nordisk, Optos, Ocular Therapeutix, Kriya Therapeutics, OcuTerra, Roche, Stealth Biotherapeutics and Sanofi. Is the current Editor in Chief of Eye. RC: Advisory board attendances for and speaker honoraria and travel grants from Roche, Bayer, Novartis and Alimera Sciences. CB: Advisory board attendances for and honoraria and travel support from Alimera Sciences, Apellis, Bayer, Boehringer Ingelheim, Janssen Pharmaceuticals, Novartis and Roche. SC: Honoraria from and consultancy for Roche, AbbVie and Alimera Sciences and travel support from Bayer and Alimera Sciences. SE: Advisory board attendances for and travel support from AbbVie, Alimera Sciences, Bayer, Novartis, Roche and Topcon. JP: Consultancy for and travel support from Bayer and Roche. GS: Advisory board attendances for and honoraria and travel support from Bayer and Roche. S-LW: Advisory board attendances for and honoraria and travel support from Roche, Bayer, and Novartis. GW: Honoraria from Roche. AP: Employee of Roche Products Limited. SK: Employee of Roche Products Limited. AL: Travel support from Roche; equity in EyeBio.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Downey, L., Sivaprasad, S., Chhabra, R. et al. Dual pathway inhibition with faricimab for previously treated neovascular age-related macular degeneration and diabetic macular oedema: guidance from a UK panel of retina specialists. Eye 38, 3077–3086 (2024). https://doi.org/10.1038/s41433-024-03223-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-024-03223-w