Abstract
Several clinical subtypes of uveitis exist yet specific immunopathogenic mechanisms involved remain unclear. Ex vivo studies are limited by lack of fresh retinal biopsies and studies have relied on aqueous humour or peripheral blood, which may not directly reflect disease. The aim of this review is to compare the various in vivo models and review their contributions to our understanding of disease processes. These models, although unable to reflect all clinical signs, have provided insight into the contribution of genes and molecules, characterisation of effector T-cells, cell trafficking into retinal tissues, the contribution of tissue-resident myeloid cells and the mechanism(s) of action of several anti-inflammatory compounds. In vivo uveitis models have provided an excellent resource with which to study the molecular and cellular processes involved. Recent refinements in models, improved imaging, and the application of omics have greatly increased the number of readouts and translational opportunities. Future approaches with in vitro models will also be discussed.
Similar content being viewed by others
Introduction
Uveitis, including both infectious and non-infectious forms, describes a range of inflammatory conditions affecting the uveal tissues. In this review we have focussed on non-infectious uveitis (NIU) which affects people of working age, for whom currently available treatments are inadequate. NIU describes more than 30 distinct clinical phenotypes, which can affect either the anterior or posterior segment, or both (pan uveitis). Disease can occur alone or as part of a systemic disease. A significant proportion of patients with anterior uveitis have an associated ankylosing spondylitis and are HLA-B27-positive [1] whereas many patients with posterior uveitis also suffer from sarcoidosis or Behçet’s disease [2]. Due to the complexities of disease classification, management and treatment stratification, several international study groups were created to reach a consensus on clinical guidelines and to facilitate multicentre studies. Hence the agreed definition of NIU is that it can be bilateral, inflammation involves the uveal tract (iris, ciliary body, choroid), and adjacent areas (vitreous humour, retina, optic nerve) are also affected. The pathology of disease determines treatment options, with immunosuppressives such as corticosteroids and/or cyclosporin A often recommended for severe, sight-threatening disease [3]. However, NIU is extremely heterogenous in its clinical severity and duration, and it is difficult to predict who will respond to immunosuppressive treatment.
Investigating immune pathways in NIU
Due to the associated risks, obtaining fresh biopsies from untreated NIU patients with active disease is not feasible. End-stage, fixed ocular specimens can be studied to confirm expression of specific molecules, but results might not reflect ongoing disease pathways. Furthermore, donors will have likely received many different forms of anti-inflammatory treatments over several years, making it difficult to relate specific findings to disease activity.
For patient studies, peripheral blood is often used to investigate immune cells. Ideally this approach should include clinically well-defined, treatment-naïve patients to provide baseline levels. Small volumes of intraocular fluid specimens – aqueous and vitreous – have also been collected, often during cataract surgery or vitrectomy, for use in protein- and cell-based studies. However, whilst such data can be informative, it represents only a single timepoint in the course of disease.
Experimental animal models have therefore been an invaluable tool for studying intraocular inflammation and immune mechanisms at different stages of disease. They enable the identification of intraocular and peripheral cell populations, and responses to treatment. Many animal species have been demonstrated to be inducible for experimental autoimmune uveitis (EAU), although uveitis does not occur spontaneously in the wild. Most preclinical studies have involved rats and mice, where there are similarities with man in respect of ocular anatomy, immune mechanisms and in the role of the blood-retinal barrier in controlling entry of cells and proteins from the periphery into the retina.
In vivo models of anterior uveitis
Anterior uveitis can be a clinically severe, sight-threatening form of disease. Inflammation affects the anterior segment and disease can vary in duration. In vivo rodent models have usually involved immunisation with lipopolysaccharide (endotoxin), often given intravitreally, which results in an acute inflammation affecting the iris and ciliary body which lasts 24–48 h, termed endotoxin-induced uveitis (EIU). Within 16–24 h’ post immunisation a wave of granulocytes, predominantly neutrophils, infiltrate the ocular tissues in response to signalling pathway activation via the endotoxin receptor, TLR-4. This model offers an extremely narrow timeframe with which to investigate disease mechanisms or to administer therapies, and most studies involve pre-treating rodents with potential anti-inflammatory agents prior to inducing EIU [4, 5].
A model of acute anterior uveitis was developed in Fischer 344 rats immunised with bovine ocular melanin (EMIU). This was found to be CD4+T cell mediated as it was inhibited by anti-CD4 antibody. The disease entered remission at 4 weeks’ post induction and was associated with subsequent spontaneous relapses [6]. EMIU therefore holds greater potential as a preclinical model for anterior uveitis.
Evidence for autoimmune pathways in posterior uveitis
Initially the evidence for posterior uveitis having an autoimmune aetiology was due to it sharing many features with more established autoimmune diseases, including multiple sclerosis, type 2 diabetes, and rheumatoid arthritis. The observed infiltration of CD4+T cells into retinal tissues, an upregulation of HLA-DR expression by tissue-resident cells, and the clinical efficacy of the T-cell inhibitor cyclosporin A all suggested posterior uveitis to be an autoimmune disease. Further evidence arose from the development of the in vivo model EAU in which purified retinal antigens emulsified in adjuvant were administered to naïve animals, resulting in development of a localised intraocular inflammation [7]. The specific autoantigen(s) involved in posterior uveitis remain unclear despite considerable research in this area, and therefore posterior uveitis is usually referred to as being an immune-mediated retinal inflammatory disease.
In vivo models of posterior uveitis
Models of EAU induced by uveitogenic retinal proteins or peptides in mice and rats share similar immunological mechanisms and pathological characteristics, although the progression and severity of the disease varies, based on species-, strain- and dose-dependent differences [8]. Purified retinal antigens used for inducing EAU include S-Ag (arrestin), interphotoreceptor binding protein (IRBP), rhodopsin/opsin, phosducin, and recoverin. For inducing EAU in inbred mice IRBP is the antigen commonly used and, by comparing sequential IRBP peptide sequences, C57BL/6 mice mainly responded to peptide 1–20, whilst other mouse strains developed EAU in response to different sequences [9], highlighting that genes involved in antigen presentation to CD4+T cells (MHC Class 2) determine responsiveness to specific retinal antigens. The time course and severity of EAU development also varies; B10RIII mice develop an acute and severe form of EAU 7–10 days post immunisation and inflammation persists for 14–20 days, whereas C57BL/6 J mice exhibit a milder form of EAU, detectable by 14–17 days which peaks on day 20–23 and then declines, but with low levels of retinal inflammation still detectable on day 75. The C57BL/6 model can therefore be used for more translational studies as the inflammation is reversible and there is an extended timeframe with which to introduce any therapy post EAU induction.
Another method for inducing EAU involves adoptive transfer (AT), where retinal antigen-specific, activated CD4+T cells are administered to naïve mice. AT has several advantages as it avoids the need for adjuvants or pertussis in the immunisation protocol. However, it often takes longer to develop, is monophasic, and clinically less severe. When originally developed, AT provided the definitive evidence that CD4+T cells induce EAU, whereas CD8+T cells, B cells or serum could not [10]. More recently AT has been used to demonstrate the differential capacity of CD4+T cell subsets to induce EAU (described below).
There are also spontaneous models of EAU using transgenic mice. Several studies have involved the IRBP-specific T cell receptor-transgenic mouse (R161H-/-) in B10RIII mice [11]. Another spontaneous model uses the Autoimmune Regulator (AIRE)(-/-) genetically deficient mice [12]. These models are more reflective of human disease in terms of their spontaneousness, and each model exhibited a different clinical progression with persistent cellular infiltrates in the R161H model, suggesting a more translational model for human disease. A spontaneous relapsing/remitting model (R14) has also been described in rats immunised with an IRBP peptide in which oral tolerance of the same peptide was demonstrated to effectively downregulate the disease [13].
A chronic model of EAU has recently been described in which peptides 1–20 and 161–180 were co-administered, resulting in disease being detected at 16 weeks post immunisation. Furthermore, long-lived memory T cells (CD44hi, IL-7R+, IL-15R+, CD4+) were detected in retinal tissues 12–16 weeks post-EAU induction which, upon isolation, were still able to adoptively transfer disease to naïve mice [14]. These long-lived cells are thought to be responsible for the persistent low grade intraocular inflammation observed after disease resolution.
Readouts of EAU
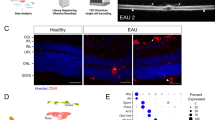
Rodent models of EAU have the crucial advantage of enabling a plethora of readouts that characterise the pathological features of the disease. Fundus photography can allow the visualisation of many EAU-induced features in C57BL6 mice. This includes optic disc inflammation, vasculitis and retinal tissue damage where significant changes can be seen from around 14–21 days post immunisation (DPI) in the IRBP 1–20 (Fig. 1) and IRBP 651-670 induced models [15,16,17,18,19,20,21,22]. Fluorescein angiography (FA) allows visualisation of leakage of fluorescein to mark features of increased vascular leakage, a feature of EAU [15, 19, 22, 23]. Optical coherence tomography (OCT) enables tracking of the changes in the organisation of retinal components and the thicknesses of retinal layers, where features including increased infiltration of inflammatory cells, as illustrated (Fig. 1), and retinal disorganisation and detachment can be observed in EAU mice [15, 18, 20, 21, 23, 24]. Electroretinography (ERG) identifies functional deterioration of the retina where scotopic (dark-adapted) and photopic (light-adapted) a- and b-wave amplitudes can be significantly reduced in the EAU mice [16, 18, 20, 25]. Despite the range of possible observations throughout the progression of EAU, investigations focus on in vivo readouts that answer research questions whilst considering the safety of animals with time spent under anaesthesia.
The possibilities of ex vivo observations in these models of EAU have also majorly expanded. Haematoxylin & eosin (H&E) retinal staining has been used to assess the severity of EAU; it can help to identify features like vasculitis, infiltrating immune cells (seen in parallel to OCT) and retinal layer disorganisation [15, 20, 21, 23, 25]. Immunostaining in the EAU retina has allowed more specific visualisation of a reduced amount of rod, cone, bipolar, amacrine, horizontal and retinal ganglion cells, and an increase of CD45+leucocytes and CD11b+myeloid cells [8, 15, 16, 23]. Immunohistochemical observations offer the advantage of visualising the organisation and morphology of cell types in different layers of the retinae through cross-sections or flat-mounted samples. However, these can often be more qualitative assessments with a limited number of markers at one time on a selected number of planes or layers.
Readouts assessed with flow cytometry (FC) offer a high-throughput analysis of quantitative data including cell size, granularity, and immunophenotyping with multiple antibodies on millions of cells. More recently, single-cell RNA sequencing (scRNA-seq) has provided high-resolution transcriptomic data, revealing cell heterogeneity and therefore rarer subsets in disease-associated populations.
Pathogenic cells in EAU
Myeloid cells have an essential role in the retina, clearing debris/pathogens and in homoeostasis of retinal tissues. In conjunction with immunohistology, FC results from retinal cell populations have demonstrated increased levels of CD4+T cells, CD45+ leucocytes, CD11b+macrophages and MHC class 2+microglia [26,27,28,29].
A certain subset of these myeloid cells are macrophages which are tissue-resident (microglia) or derived from peripheral blood (monocyte-derived). Microglia are distinct in that they are an embryonic yolk sac-derived self-renewing population [30], predominantly inhabiting the inner retinal segment, migrating to the outer layer in response to inflammation and changing from ramified to amoeboid morphology in the process [31].
While monocytes share a common myeloid progenitor with microglia, monocytes are generated by bone marrow haematopoiesis during adulthood [32]. These monocytes can extravasate via the choroid into the outer retinal layer where they differentiate into macrophages, which show highly plastic morphology [33]. Monocyte-derived macrophages are typically classified into pro-inflammatory (M1) or homoeostatic (M2) roles, depending on their microenvironment and subsequent antibody and cytokine expression [34].
The precise role of yolk-sac derived microglia during retinal inflammation was investigated in EIU using single-eye mRNA-Seq and Cx3cr1 Cre mice to track microglia transcriptional changes over 2 weeks. It was thus demonstrated that these microglia resumed their homeostatic state 2 weeks after acute retinal inflammation [35]. Evidence for the role of retinal microglia in EAU has come from studies using scRNA-seq. Whilst it has been known that CC chemokines are heavily involved in the EAU pathogenesis, recent investigations have shown that blockage of CCR5/CCL5 interaction can have therapeutic potential by reducing infiltrating T-cells and microglial activation [8, 16].
The AT model of EAU had provided evidence that CD4+T cells initiate the EAU disease process and further studies have compared populations of retinal antigen-specific CD4+T cells [36]. Whilst both Th1 and Th17 cell subsets are uveitogenic, the course of disease varies, suggesting that each cell subset utilises different immune pathways. Further studies have also identified a significant increase of a unique subset of T cells - Th17/Th1 - in the retina, which expressed both IFNγ and IL-17, were significantly upregulated at peak disease and were resistant to the inhibitory effects of dexamethasone [37].
Analysis of retinal cell infiltrates identified multiple subsets of regulatory T cells (Treg) which modulate disease [38]. In a recent review, several EAU models, induced with different peptides and in different strains, have recently been compared to help select the most appropriate model to use for each research study [39].
Applying EAU to understand role of human Treg
Whilst many studies of EAU have provided insights into human disease, some findings have been initially demonstrated in uveitis patients which have subsequently been confirmed in EAU. One example of this was the finding of increased levels of a subset of CD4+CD25+ FoxP3+ Tbet+ TIGIT+ Treg in peripheral bloods from NIU patients in clinical remission in comparison with active disease [40]. This Treg subset was subsequently investigated in a mouse model of EAU and it was reported that TIGIT stimulation, administered from day 20 onwards (active EAU) significantly suppressed disease scores and inhibited Th17 cell infiltration [41]. These findings suggested that certain subsets of Treg cells could be regulating the disease and might offer a potential therapy in man. In a previous clinical trial (UVEREEG; NCT024944929) [42], intravitreal administration of polyclonal Treg was performed in patients with bilateral severe forms of NIU. Whilst results were inconclusive, further understanding of the different Treg subsets could offer a future alternative immunosuppression for these patients.
Organoids as models of uveitis
Organoids are multicellular, three-dimensional tissue cultures that recapitulate many aspects of the complex structure and function of the corresponding in vivo tissue [43]. These tissues are cellularly heterogeneous, in contrast to feeder cell based cultures, yet are more simplified than animal models [44].
Organoids therefore enable the investigation of the interaction of immune cells with epithelial cells in a reductionist manner [44]. They may be co-cultured with T cells to evoke immune-mediated epithelial cell differentiation [45, 46]. For example, the triple co-culture of epithelial cells, cytotoxic T cells and bacteria identified the H. pylori-induced expression of the checkpoint inhibitor programmed cell death ligand-1 [47].
In terms of ocular diseases, labs have grown optic cups and retinal organoids with emergent electrophysiological properties [48,49,50], as well as corneal organoids [51]. This technology has been successfully employed to model genetic diseases including Leber congenital amaurosis [52] and retinitis pigmentosa [53]. In the future, it is possible that these technologies could be applied to further elucidate the pathogenesis of uveitis.
Conclusion
The in vivo models of uveitis and imaging methods have been extensively refined over the last 30 years, allowing investigators to clinically score disease temporally and identify several relevant effector cells and molecules. These models have therefore become extremely informative in preclinical studies. However, given their heterogeneity, it is crucial to select the model which best fits the research question. In addition, while many potential therapies have been demonstrated to be effective for reversing EAU, the results do not always translate to patients. Hence, effective and safe treatments for the various clinical subtypes of uveitis remain elusive.
References
Wakefield D, Clarke D, McCluskey P. Recent developments in HLA B27 anterior uveitis. Front Immunol. 2021;11:6–8134. https://doi.org/10.3389/fimmu.2020.608134
Guan Y, Li F, Li N, Yang P. Decoding Behcet’s uveitis: an in-depth review of pathogenesis and therapeutic advances. J Neuroinflamm. 2024;21:133. https://doi.org/10.1186/s12974-024-03123-6
Abdulla D, Ali Y, Menezo V, Taylor SRJ. The use of sustained release intravitreal steroid implants in non-infectious uveitis affecting the posterior segment of the eye. Ophthalmic Ther. 2022;11:479–87.
Merida S, Sancho-Tello M, Almansa I, Desco C, Peris C, Moreno ML, et al. Bevacizumab diminishes inflammation in an acute endotoxin-induced uveitis model. Front Pharm. 2018;9:649. https://doi.org/10.3389/fphar.2018.00649
Shi X, Zhu S, Jin H, Fang J, Xing X, Wang Y, et al. The anti-inflammatory effects of KS23, a novel peptide derived from globular adiponectin, on endotoxin-induced uveitis in rats. Front Pharm. 2021;11:585446. https://doi.org/10.3389/fphar.2020.585446
Smith JR, Rosenbaum JT, Williams KA. Experimental melanin-induced uveitis: experimental model of human acute anterior uveitis. Ophthalmic Res. 2008;40:136–40.
Caspi RR, Chan CC, Fujino Y, Najafian F, Grover S, Hansen CT, et al. Recruitment of antigen-nonspecific cells plays a pivotal role in the pathogenesis of a T cell-mediated organ-specific autoimmune disease, experimental autoimmune uveoretinitis. J Neuroimmunol. 1993;47:177–88.
Agarwal RK, Silver PB, & Caspi RR. Rodent Models of Experimental Autoimmune Uveitis. Methods Mol Biol. 2021;900. https://doi.org/10.1007/978-1-60761-720-422
Cortes LM, Mattapallil MJ, Silver PB, Donoso LA, Liou GI, Zhu W, et al. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Invest Ophthalmol Vis Sci. 2008;49:1946–56.
Uchio E, Kijima M, Ishioka M, Tanaka S, Ohno S. Suppression of actively induced EAU by CD4+T cells. Graefes Arch Clin Exp Ophthalmol. 1997;235:97–102.
Chen J, Qian H, Horai R, Chan CC, Caspi RR. Mouse models of EAU: comparative analysis of adjuvant-induced vs spontaneous models of uveitis. Curr Mol Med. 2015;15:550–7.
Yin M, Smith JA, Chou M, Chan J, Jittayasothorn Y, Gould DB, et al. Tracing the role of Aire in immune tolerance to the eye with a TCR transgenic mouse model. Proc Natl Acad Sci USA. 2024;121:e2311487121. https://doi.org/10.1073/pnas.2311487121
Huber A, Diedrichs-Möhring M, Wildner G. Spontaneously relapsing-remitting experimental autoimmune uveitis in rats allows successful therapeutic oral tolerance induction in ongoing disease. Mol Immunol. 2015;63:215–26.
Fan N-W, Qiurong Z, Wang S, Ortiz G, Huckfeldt RM, Chen Y. Long-lived autoreactive memory CD4+T cells mediate the sustained retinopathy in chronic autoimmune uveitis. FASEB J. 2023;37. https://doi.org/10.1096/fj.202202164R
Yuan F, Zhang R, Li J, Lei Q, Wang S, Jiang F, et al. CCR5-overexpressing mesenchymal stem cells protect against experimental autoimmune uveitis: insights from single-cell transcriptome analysis. J Neuroinflamm. 2024;21:1–24.
Liu J, Liao X, Li N, Xu Z, Yang W, Zhou H, et al. Single-cell RNA sequencing reveals inflammatory retinal microglia in experimental autoimmune uveitis. MedComm (Beijing). 2024;5:e534. https://doi.org/10.1002/mco2.534
Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008;87:319–26.
Fan NW, Li J, Mittal SK, Foulsham W, Elbasiony E, Huckfeldt RM, et al. Characterization of Clinical and Immune Responses in an Experimental Chronic Autoimmune Uveitis Model. Am J Pathol. 2021;191:425–37.
Chen X, Kexzik JM, Forrester JV, Goldberg GL, Wicks IP, Bernard CC, et al. In vivo multi-modal imaging of experimental autoimmune uveoretinitis in transgenic reporter mice reveals the dynamic nature of inflammatory changes during disease progression. J Neuroinflamm. 2015;12:1–12.
Yang JM, Yun K, Jeon J, Yang HY, Kim B, Jeong S, et al. Multimodal evaluation of an interphotoreceptor retinoid-binding protein-induced mouse model of experimental autoimmune uveitis. Exp Mol Med. 2022;54:252–62.
Harimoto K, Ito M, Karasawa Y, Sakurai Y, Takeuchi M. Evaluation of mouse experimental autoimmune uveoretinitis by spectral ___domain optical coherence tomography. BJO. 2014;98:808–12.
Bowers CE, Calder VL, Greenwood J, Eskandarpour M. Experimental Autoimmune Uveitis: An Intraocular Inflammatory Mouse Model. J Vis Exp. 2022; Jan 12: https://doi.org/10.3791/61832
Chu CJ, Herrmann P, Carvalho LS, Liyanage SE, Bainbridge JWB, Ali RR, et al. Assessment and in vivo scoring of murine experimental autoimmune uveoretinitis using optical coherence tomography. PLoS One. 2013;8:e63002.
Shome A, Mugisho OO, Niederer RL, Rupenthal ID. Comprehensive grading system for experimental autoimmune uveitis in mice. Biomed. 2023;11:2022 https://doi.org/10.3390/biomedicines11072022
Chen J, Qian H, Horai R, Chan CC, Caspi RR. Use of optical coherence tomography and electroretinography to evaluate retinal pathology in a mouse model of autoimmune uveitis. PLoSOne. 2013;8:e63904. https://doi.org/10.1371/journal.pone.0063904
Copland DA, Wertheim MS, Armitage WJ, Nicholson LB, Raveney BJ, Dick AD. The Clinical Time-Course of Experimental Autoimmune Uveoretinitis Using Topical Endoscopic Fundal Imaging with Histologic and Cellular Infiltrate Correlation. Invest Ophthalmol Vis Sci. 2008;49:5458–65.
Bradley LJ, Ward A, Hsue MCY, Liu J, Copland DA, Dick AD, et al. Quantitative assessment of experimental ocular inflammatory disease. Front Immunol. 2021;12:630022. https://doi.org/10.3389/fimmu.2021.630022
Okunuki Y, Mukai R, Nakao T, Tabor SJ, Butovsky O, Dana R, et al. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proc Natl Acad Sci USA. 2019;116:9989–98.
Shirahama S, Okunuki Y, Lee MY, Karg MM, Refaian N, Krasniqi D, et al. Retinal microglia exacerbate uveitis by functioning as local antigen-presenting cells. bioRxiv; 2024. https://doi.org/10.1101/2024.03.23.586440
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–43.
Guo M, Schwarz TD, Dunaief JL, Cui QN. Myeloid cells in retinal and brain degeneration. FEBS J. 2022;289:2337–61.
Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2007;14:392–404.
Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts’. Cell Commun Sig. 2013;11:29. https://doi.org/10.1186/1478-811X-11-29
Hickman E, Smyth T, Cobos-Uribe C, Immormino R, Rebuli ME, Moran T, et al. Expanded characterization of in vitro polarized M0, M1, and M2 human monocyte-derived macrophages: Bioenergetic and secreted mediator profiles. PLoS ONE. 2022;18:e0279037 https://doi.org/10.1371/journal.pone.0279037
Bell OH, Copland DA,Ward A, Nicholson LB, Lange CAK, Chu CJ, et al. Single eye mRNA-Seq reveals normalisation of the retinal microglial transcriptome following acute inflammation. Front Immunol. 2020;10. https://doi.org/10.3389/fimmu.2019.03033
Horai R, Silver PB, Chen J, Agarwal RK, Chong WP, Jittayasothorn Y, et al. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun. 2013;44:21–33.
Chen YH, Eskandarpour M, Gondrand A, Zhang X, Gu R, Galatowicz G, et al. Functionally distinct IFNg+IL-17A+ Th cells in experimental autoimmune uveitis: T-cell heterogeneity, migration and steroid response. Eur J Immunol. 2020;50:1941–51.
Silver PB, Horai R, Chen J, Jittayasothorn Y, Chan CC, Villasmil R, et al. Retina-specific T regulatory cells bring about resolution and maintain remission of autoimmune uveitis. J Immunol. 2015;194:3001–9.
Chen Y-H, Lightman S, Calder VL. CD4+T-cell plasticity in non-infectious retinal inflammatory disease. Int J Mol Sci. 2021;22:9584. https://doi.org/10.3390/ijms22179584
Gilbert RM, Zhang X, Sampson RD, Ehrenstein MR, Nguyen DX, Chaudhry M, et al. Clinical remission of sight-threatening non-infectious uveitis is characterized by an upregulation of peripheral T-regulatory cell polarized towards T-bet and TIGIT. Front Immunol. 2018;9:907. https://doi.org/10.3389/fimmu.2018.00907
Peters K, McDonald T, Muhammad F, Brady A, Dostal J, Lee DJ. TIGIT stimulation suppresses autoimmune uveitis by inhibiting Th17 cell infiltration. J Leuk Biol. 2024;00:1–7. https://doi.org/10.1093/jleuko/qiae116
Gregoire S, Terrada C, Martin GH, Fourcade G, Baeyens A, Marodon G, et al. Treatment of uveitis by in situ administration of ex vivo-activated polyclonal regulatory T cells. J Immunol. 2016;196:2109–18.
Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L, et al. Organoids. Nat Rev Methods Prim. 2022;2:1–21.
Bar-Ephraim YE, Kretzschmar K, Clevers H. Organoids in immunological research. Nat Rev Immunol. 2020;20:279–93.
Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–4.
Schreurs RRCE, Baumdick ME, Sagebiel AF, Kaufmann M, Mokry M, Klarenbeek PL, et al. Human Fetal TNF-α-Cytokine-Producing CD4+ Effector Memory T Cells Promote Intestinal Development and Mediate Inflammation Early in Life. Immunity. 2019;50:462–476.e8.
Holokai L, Chakrabarti J, Broda T, Chang J, Hawkins JA, Sundaram N, et al. Increased Programmed Death-Ligand 1 is an Early Epithelial Cell Response to Helicobacter pylori Infection. PLOS Pathog. 2019;15:e1007468.
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6.
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell. 2012;10:771–85.
Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, et al. Blood-Derived Human iPS Cells Generate Optic Vesicle–Like Structures with the Capacity to Form Retinal Laminae and Develop Synapses. Investig Ophthalmol Vis Sci. 2012;53:2007–19.
Foster JW, Wahlin K, Adams SM, Birk DE, Zack DJ, Chakravarti S. Cornea organoids from human induced pluripotent stem cells. Sci Rep. 2017;7:41286.
Parfitt DA, Lane A, Ramsden CM, Carr AJF, Munro PM, Jovanovic K, et al. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell. 2016;18:769–81.
Deng WL, Gao ML, Lei XL, Lv JN, Zhao H, He KW, et al. Gene correction reverses ciliopathy and photoreceptor loss in ipsc-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Rep. 2018;10:1267–81.
Acknowledgements
I would like to acknowledge several of my colleagues whose work has been cited herein including Professor S. Lightman, and Drs. R. Gilbert, Yi-H. Chen & M. Eskandarpour.
Author information
Authors and Affiliations
Contributions
OSK undertook a literature search and wrote the section on future in vitro models of EAU and provided feedback for the final manuscript proof. SC contributed the images for Fig. 1 and prepared the figure legend and provided feedback for the final manuscript proof. SW did a literature search and wrote the section on retinal myeloid cells. VC did a literature search, wrote the remaining sections and prepared the final proof for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knutson, O.S., Choi, S., Williams, S. et al. Comparative models of uveitis. Eye 39, 1446–1450 (2025). https://doi.org/10.1038/s41433-025-03693-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-025-03693-6