Abstract
Purpose
To estimate the prevalence and determine predictors of lens opacities (LO) among South Asian Indians aged 41–44 years.
Methods
This cross-sectional study included 1080 participants from the Vellore Birth Cohort, Vellore, South India. All underwent anthropometric measurements, detailed ophthalmic examination including assessment of LO by LOCS III classification and biochemical metabolic measurements. ‘Any cataract’ was defined as any opacity type with a score of >2 or evidence of cataract surgery in either eye. Data collected included information on ocular history, life-style factors, socio-economic and educational status, cooking fuel and sunlight exposure. Multivariable logistic regression analysis was used to examine the association between risk predictors and LO.
Results
The mean age (SD) of participants was 41.8 (1.0) years; 53.8% were male and 50% were rural residents. The overall prevalence of ‘any cataract’ was 13.8% (148/1075, 95% confidence interval (CI) 11.8,16.0). The types of cataract were nuclear 59.1%, cortical 16.9%, posterior subcapsular 4.1%, mixed cataracts 18.9% and pseudophakia 0.7%. Increased risk for LO was observed with a history of asthma (OR 4.51; 95% CI 2.1, 9.7), HbA1C of ≥6.5% (OR 2.29; 95% CI 1.4, 3.7), hypertension (OR 1.73; 95% CI 1.1, 2.7) and, in a subgroup (n = 372), lower 25(OH) vitamin D levels (≤20 ng/dL)(OR 5.56; 95% CI 2.3, 13.2).
Conclusion
The high prevalence of LO at a relatively young age in South Asian Indians suggests earlier onset of ageing. History of asthma, higher HbA1C, hypertension and lower 25(OH) vitamin D levels were associated with LO.
Similar content being viewed by others
Introduction
The global increase in population and life expectancy have led to an increase in the number of people who are blind, from 36 to 43.3 million, despite a reduction in the global prevalence [1, 2]. Age related cataract (lens opacity, LO), accounts for almost 40% of all blindness and 28% of all moderate and severe visual impairment (VI) among those aged 50 years and above, with higher proportions in Asia and lower proportions in Africa [2, 3]. Recognized risk factors for LOs include advancing age, specific ethnic groups such as South Asians [4, 5] and modifiable risk factors such as low socio-economic status, smoking, ultraviolet light exposure, obesity, asthma, hypertension, underlying metabolic disorders (diabetes) and exposure to drugs such as steroids [6]. In Asian Indians, the onset of LOs is earlier than in high income countries [7, 8], which may reflect earlier onset of ageing [9] due to greater exposure to risk factors [10, 11]. Lens opacities have a multifactorial aetiology, with genetic and environmental factors interacting to increase oxidative stress in the lens. Higher levels of hydrogen peroxide, superoxide (O2-) and hydroxyl (OH) free radicals in the lens and aqueous humour are associated with LO [12]. Cells in the lens proliferate throughout life and LOs reflect a lifetime of insults, including oxidative stress [5]. However, randomized clinical trials of antioxidant vitamin supplements (i.e., A, C and E) have not shown any beneficial effects on the incidence or progression of LOs [13] including one from India [14]. There is less evidence of whether vitamin-D deficiency/insufficiency is associated with LOs.
Birth cohort studies have the potential to provide unique insights as exposure to risk factors can be explored across the life course. In this paper we report the prevalence of LO and exposure to risk factors in adults who were recruited to the Vellore Birth Cohort 41–45 years earlier.
Methods
Participants for the current study were traceable members of a subset of the Vellore Birth Cohort (VBC) in which all infants born to women in defined areas of Vellore town and three adjoining rural villages in Tamil Nadu, India, between 1969 and 1973 were included. Subsequently 10,670 singleton live births were followed up during infancy, adolescence and adulthood [15].
Study population
This was a cross-sectional study of adults aged 41–44 years from the Phase-6 follow-up (2013-14) of VBC recruited from one urban and three rural areas nearby. The cohort is described in detail elsewhere [16]. The study adhered to the guidelines of the declaration of Helsinki and was approved by the ethics committees of Christian Medical College, Vellore (IRB min no.7765 dt 22/2/2012), and Public Health Foundation of India.
Study participants
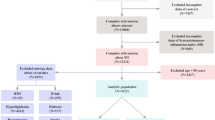
One thousand and eighty of the 2218 traced cohort members who took part in the Phase 5 of the VBC study (1998–2002) were included (Fig. 1) [16].
After obtaining written informed consent trained health workers collected data on socio- demographic status, life-style characteristics (smoking, alcohol consumption) and daily hours of sunlight exposure in participants’ homes using questionnaires. Socioeconomic status, educational status and the physical activity score were determined as described earlier [16,17,18,19]. Smoking status was defined as current smokers of cigarettes or ‘beedis’. Current alcohol consumption was defined as consumption of any local or imported spirits, beer or wine. The main type of fuel used for cooking in their household was noted.
Ophthalmic history and examination
Participants attended the Department of Ophthalmology, Christian Medical College (CMC), Vellore for examination. Following a brief ocular history, distance visual acuity (VA) (uncorrected, presenting and best corrected after retinoscopy and subjective refraction), was measured using a self-illuminated logMAR visual acuity chart at 4 meters, and near vision was tested using a logMAR chart for near vision at 40 cm. Presenting VA in the better eye for distance was categorized according to World Health Organization (WHO) as ’good’ (≥6/12), ‘mild visual impairment’ (VI) (<6/12–6/18), ‘moderate VI’ (<6/18–6/60), ‘severe VI (<6/60–3/60) or blind (<3/60) after conversion to Snellen equivalents. Good near vision was defined as a corrected acuity of N8 equivalent (logMAR 1.0 M) or more in the better eye. A comprehensive ophthalmic examination was performed by a trained ophthalmologist using a Haag Streit slit lamp which included: the intraocular pressure (IOP) measurement using Goldman Applanation tonometry; dilated fundus examination using a 90 D (Volk) lens, and grading LOs using a Lens Opacities Classification System III (LOCS) standard plate [20]. Axial length was measured using ultrasound biometry (Ocuscan, ALCON).
‘Any cataract’ was defined as significant LO or evidence of cataract surgery as an adult in one or both eyes. Using LOCS III classification significant LO was defined as a score of >2 for each type of opacity i.e., for nuclear opalescence (NO), nuclear colour (NC), cortical opacity (CO) or posterior sub-capsular opacity (PSCO); NO or NC of >2 was reported as NC. Those with more than one type were classified as ‘mixed LO’. Inter-observer agreements for 60 participants were : kappa 0.93 (95% CI 0.8–1.0) for right eyes and 0.87 (95% CI 0.68–1.0) for left eyes. If a participant was pseudophakic/aphakic in one eye, the LOCS III grading in the other eye was used. Pseudophakia/aphakia was used if both eyes had undergone cataract surgery, or if the unoperated eye had a LO score of ≤2. If one eye had a condition precluding assessment of LOCS III or evidence of unilateral injury then scores from the other eye were used.
Clinical parameters and biochemical evaluation
Anthropometry included measurements of height, weight, waist circumference (WC), hip circumference (HC) and blood pressure (BP) using standard protocols. Body mass index (BMI) was calculated as the ratio of weight (kg) to height2 (m2). We used WHO definitions for underweight, normal, overweight and obesity [16]. The average of three measurements was used in the analysis. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and diastolic blood pressure (DBP) ≥ 90 mmHg and/or being on medication for hypertension [21]. Blood samples were assayed for fasting plasma glucose by hexokinase method, lipids by colorimetry using Roche Chemistry analysers and glycosylated haemoglobin (HbA1C) by HPLC using Biorad Variant II. As many people with diabetes in India are not diagnosed diabetes was defined as HB A1C of ≥6.5% [22]. Serum 25(OH) vitamin-D levels were measured by electrochemiluminescence assay using Cobas e170 in a subset of 372 participants on whom values were available as they were taking part in another study at the same time [23]. Serum levels ≤20 ng/dL were categorized as deficient [24]. Participants with undiagnosed diabetes or with ocular morbidity requiring treatment were referred to respective clinics at the hospital.
Statistical analysis
Participants’ characteristics are presented as means with standard deviation (SD) for normally distributed variables; median (inter-quartile range; IQR) for skewed variables, and proportions for categorical variables. Baseline characteristics are summarized using two sample t-tests and chi-square tests stratified by gender and place of residence. Risk factors for LO were chosen based on clinical importance. Univariate and multivariable logistic regression analyses were used to study predictors of LO and the results are presented as odds ratios (OR) and 95% confidence intervals (CI). All variables were entered simultaneously in the multivariate model which included age, gender, education and current smoking, alcohol consumption, household possession score, hours outdoor, cooking fuel used, history of asthma, HbA1c, hypertension, body mass index, axial length and physical activity score. A subgroup analysis (n = 374) was undertaken to explore the association between serum 25(OH) vitamin D levels and LO in individuals on whom 25(OH) vitamin D was measured [23]. All statistical analyses were performed using Stata/IC version 16 (StataCorp. 2019. College Station, TX: LLC).
Results
A total of 1080 traced cohort members who agreed to participate were examined; five were excluded (two had history of bilateral eye injuries and three had missing LOCS III data) leaving 1075 for analysis (Fig. 1). The mean age (SD) of participants at examination was 41.8 (1.0) (range 41–44) years and 53.8% (n = 578) were male (Table 1). The mean BMI (SD) was 25.4 (4.8) kg/m2 (in the overweight range). Only men reported smoking (32.5%) and consuming alcohol (45%).
Women had higher physical activity scores than men and urban and rural women spent longer outside the home than their male counterparts. Most participants (80%) used liquid petroleum gas (LPG) as a cooking fuel. Approximately 3% had a history of asthma, 12.5% had an HbA1c of ≥6.5% and 21.7% had hypertension.
General ocular findings
Family history of spectacle use / holding things close to see was reported in one or both parents by 26.1%, in sibling alone in 4.5%, children alone in 2.5% and a combination of more than one first degree relative in 16.8% of participants. Reported spectacle use for near alone, distance alone and for both was 7.7%, 5.5% and 2.2% respectively. Self-reported ocular history included trauma in one eye [33, 3.1%], night blindness [6, 0.6% (mostly women)], surgery in either eye for cataract, glaucoma, or trauma [8, 0.7%] and current use of eye drops [8, 0.7%].
Among the 1065 participants with VA data, 95% had ‘good’ VA, 2% had ‘mild VI’ and 3% had ‘moderate VI’ ; 99.7% had normal near vision with correction. The mean (SD) IOP was 13.7 (2.8) mm Hg.
Lens opacities
The overall prevalence of ‘any cataract’ was 13.8% (95% CI 11.8, 16.0) [men: 15.1% (95% CI 12.2,18.2); women: 12.3% (95% CI 9.5,15.5)]. Combining gender and place of residence the prevalence was as follows: rural men, 17.3% (50/289), rural women 10.4% (26/250), urban men 13.2% (38/288) and urban women 14.2% (35/247). There was no significant difference by sex (P = 0.16) or place of residence (P = 0.83). Nuclear cataract was the commonest type of LO (59.1%) followed by cortical (16.9%), posterior subcapsular (4.1) and mixed opacities (18.9%). Only 0.7% were pseudophakic in both eyes.
In unadjusted logistic regression analysis, higher household asset scores and higher educational status were significantly associated with LO, but were not significant in the multivariable model (Table 2). The following remained statistically significant in the multivariable model: higher HbA1C (OR 2.29; 95% CI 1.4, 3.7), hypertension (OR 1.73; 95% CI 1.1, 2.7) and a history of asthma (OR 4.51; 95% CI 2.1, 9.7). Positive history of rheumatoid arthritis (P = 0.31) and use of systemic glucocorticoids (P = 0.92) were not associated with LO.
In the subgroup analysis of 372 individuals, LOs were significantly associated with low 25(OH) vitamin D levels (OR 5.56; 95% CI 2.3, 13.2) (P < 0.001) (Table 3). The proportion of people in the population with LO from exposure to vitamin D deficiency which could be prevented by correcting vitamin D deficiency was 56% (i.e., the population attributable risk) (Supplementary Table 1). A sensitivity analysis comparing the sub-group with vitamin D data (n = 372) with those without (n = 703) showed no significant differences in most characteristics except education and type of LO. Those without vitamin D levels were better educated (high school and above; 48.4% versus 48.1%, P = 0.01) and had a higher prevalence of any LO (15.5% versus 10.5%, P = 0.02).
To assess the representativeness of participants in our study from cohort members examined in 1998–2002 (n = 2218) [19] the age, gender, place of residence, educational status and SES among those examined in the current study (2013/2014) were compared with those who were not. There were significant differences only in place of birth (rural/urban; (P < 0.001) and SES (P < 0.001) and not in age, sex and educational status (Supplementary Table 2).
Discussion
Lens opacities increase over the age of 50 years and only a few studies have reported the prevalence of LO among individuals aged less than 50 years (Table 4) [25,26,27,28,29,30,31,32,33,34,35,36]. Our prevalence estimate (13.8%) is comparable to the south Indian Aravind Comprehensive Eye Study (AECS), which used similar methods (age range 40–49 years, 15.7%) [35], but was higher than in a Chinese study of 45–49 year olds (5.9%, 95% CI 4.9–7.0) [29]. The prevalence of LOs using the LOCS II grading of 2 or more in the Barbados Eye studies (age range 40–49 years) were between 3.0 and 4.7% [25, 28, 37]. Comparing prevalence estimates between studies needs caution, due to methodological differences in the definitions and classification systems used for LOs (Table 4). However, the prevalence does seem to be lower in high-income countries than in middle-income countries, which may be explained by lower exposure to modifiable risk factors, such as lifestyle factors, and better control of blood glucose amongst people with diabetes.
In our study, nuclear LOs were the commonest type (8.1%), which is similar to other Indian studies such as AECS (8.2%) [35], but higher than in the Andhra Pradesh Eye Disease Survey (APEDS)(3.5%) [38]. In high-income countries cortical LO are commoner in both younger and older populations [28]. Different LO types may be associated with specific risk factors, the most commonly reported being cortical LO and high UVB exposure [39]. However, in our study the number of participants with LOs were too few for analysis by type of LO.
Men had a slightly higher prevalence of LO than women, but this was not statistically significant. This differs from other studies where women generally have a higher prevalence, particularly earlier studies [28, 40, 41]. Reasons for the gender difference are not fully understood, but may be due to a fall in oestrogen-mediated anti-ageing effects on the lens in women [42]. Less pronounced gender differences in LO in younger populations were also reported from the Swedish national cataract register [43].
The Beaver Dam Eye Study showed a U-shaped relationship between SES and cataracts, with higher frequencies at extremes of SES [44], reflecting different exposure to risk factors amongst those very poor and very affluent. Our study did not show any significant relationship with SES, despite a detailed SES assessment using multiple indicators such as household assets and education. This is in contrast to APEDS, where the prevalence of LOs was higher among those with a lower SES based on monthly income [38]. The different indicators used to calculate SES may explain the differences.
There is clear evidence from observational studies that smoking increases the risk of cataract [45], which is mediated by oxidative damage from smoke constituents [46]. In our study there was no association with between LO and smoking as has been reported from East Asia [40, 47] but other studies in India have shown an association [38, 41]. There was also no association between biomass cooking fuels and LO, unlike other studies from India [48]. Possible explanations for both of these findings are the relatively young age of our study participants who would have had fewer packs-years of smoking exposure and fewer cumulative years of exposure to biomass cooking fuels due to the use of gas for cooking.
The increased risk of LO among individuals with asthma has been reported previously [49, 50], which may reflect steroid use. In a large general practice study in the United Kingdom, (n = 201,816; age 3–90 years), corticosteroid use was associated with increased cataract risk (relative risk 1.3) but this was not evident in those under the age of 40 years [51]. Our study lacked information on duration of steroid use.
Previous studies show variable associations between obesity and LOs [40, 52, 53]. Pooled estimates from a meta-analysis of 17 studies, including one from Asia, demonstrated a 2% increase in age-related cataracts with every 1 kg/m2 increase in BMI for PSC only, but the pooled effect showed a weak association [54]. In our study, there was no significant difference in cataract prevalence between individuals who were underweight, overweight and obese (Table 2). Our finding that individuals with higher HbA1C are at greater risk of LO aligns with many other studies [40, 41, 52, 55]. Lens damage is attributed to osmotic and oxidative stress and non-enzymatic glycation of lens proteins [56].
The high prevalence of overweight and obesity, and diabetes compared with other countries was also reported in the Phase 5 VBC study [16]. The prevalence of diabetes in our cohort was also higher than other NCD-RisC estimates for India, with little difference between rural and urban populations [16]. In India, the number of people with diabetes is predicted to increase to more than 130 million by 2045 [57], which is likely to further increase the burden of diabetic retinopathy and cataract. The age of onset of diabetes is also generally lower in India than in other populations, which may in part be explained by the ‘thin-fat’ Indian phenotype [58]. India is also undergoing rapid urbanization, with easy access to unhealthy food and reduced levels of physical activity [59]. Considering the inadequate resources for diabetes care and eye care, India faces a huge public eye health problem.
A meta-analysis reported hypertension to be a risk factor for LO, particularly posterior subcapsular opacities [15, 60] but findings were not consistent across studies. Inflammation has been postulated as a likely mechanism. Our estimates (OR 1.73, 95% CI 1.1, 2.7) are comparable to the findings of a meta-analysis of cohort studies (RR 1.08; 95% CI: 1.05–1.12) and case-control or cross-sectional studies (OR 1.28; 95% CI: 1.12–1.45) [60]. Hypertension is also an important risk factor for diabetic retinopathy and could exacerbate the increase in avoidable blindness from cataract and diabetic retinopathy [57].
Despite other studies of sunlight exposure and cataract showing a modest association, including in India [61], sunlight exposure was not significantly associated with LO in our study. This may reflect underestimation of sunlight exposure which was questionnaire based and prone to recall bias.
In the subgroup analysis, vitamin-D deficiency gave a 5-fold higher odds of LO, than those with normal values, which is a stronger association than in other studies [62,63,64,65], in South Korean men [65] and in younger women in the USA, for example [64]. Vitamin D deficiency is more frequent in individuals with pigmented skin, lower midday sunlight exposure and those who live at higher latitudes [65, 66]. Photoxidation of lens proteins [67] and altered calcium signalling are implicated in cataractogenesis [68]. LOs in vitamin-D deficiency may be mediated through reduced antioxidant activity [65], and alteration in calcium homeostasis [69]. Lower levels of vitamin D have also been detected in aqueous and vitreous humour in patients with cataract than those with retinal diseases [70]. To our knowledge, this is the first study to show such a strong association between vitamin D deficiency and any cataract in young adults and further studies are warranted.
This is the first observational study of a birth cohort, which provides insights into early ageing manifested by early onset of LOs. The relatively large sample size with rich phenotype and risk factor data adds strength to the study and allows for further follow-up studies of eye conditions. Our study may be limited by selection bias, which is inherent in longitudinal cohort studies where participants can be hard to trace.
Rapid socioeconomic development in India, with changing lifestyles, leading to more obesity, hypertension and diabetes, is likely to increase the burden of cataract blindness in younger adults with alarming public health implications. Modifiable risk factors need to be addressed through eye health promotion, which needs to be integrated into policies and programs for the control of NCDs.
In conclusion, the prevalence of LOs in this birth cohort was higher than in many other studies, but similar to another study in south India. Nuclear cataracts were the commonest form of cataract. A history of bronchial asthma, hypertension and hyperglycaemia were significantly associated with LOs. The strong association with lower serum vitamin D levels needs further investigation in India, as it is a potentially modifiable risk factor.
Summary
What was known before
-
In Asian Indians, the onset of LOs is earlier than in high income countries, which may reflect earlier onset of ageing.
-
Bronchial asthma, hypertension and diabetes are associated with lenticular opacities.
What this study adds
-
A high prevalence of lens opacities in this young population compared with most other studies.
-
Further studies are required to explore the role of low serum vitamin D levels which is a potentially modifiable factor.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–e97.
Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, et al. The Lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 2021;9:e489–e551.
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e34.
Murthy G, Gupta SK, John N, Vashist P. Current status of cataract blindness and Vision 2020: the right to sight initiative in India. Indian J Ophthalmol. 2008;56:489–94.
West SK, Munoz B, Istre J, Rubin GS, Friedman SM, Fried LP, et al. Mixed lens opacities and subsequent mortality. Arch Ophthalmol. 2000;118:393–7.
West SK, Valmadrid CT. Epidemiology of risk factors for age-related cataract. Surv Ophthalmol. 1995;39:323–34.
Thompson JR. The demand incidence of cataract in Asian immigrants to Britain and their descendants. Br J Ophthalmol. 1989;73:950–4.
Mohan M, Sperduto RD, Angra SK, Milton RC, Mathur RL, Underwood BA, et al. India-US case-control study of age-related cataracts. India-US Case-Control Study Group. Arch Ophthalmol. 1989;107:670–6.
Brian G, Taylor H. Cataract blindness-challenges for the 21st century. Bull World Health Organ. 2001;79:249–56.
Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, Kazmi K, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA. 2007;297:286–94.
Gujral UP, Mohan V, Pradeepa R, Deepa M, Anjana RM, Narayan KM. Ethnic differences in the prevalence of diabetes in underweight and normal weight individuals: The CARRS and NHANES studies. Diabetes Res Clin Pr. 2018;146:34–40.
Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–82.
Mathew MC, Ervin AM, Tao J, Davis RM. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;2012:CD004567.
Gritz DC, Srinivasan M, Smith SD, Kim U, Lietman TM, Wilkins JH, et al. The antioxidants in prevention of cataracts study: effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol. 2006;90:847–51.
Antonisamy B, Raghupathy P, Christopher S, Richard J, Rao PS, Barker DJ, et al. Cohort Profile: the 1969-73 Vellore birth cohort study in South India. Int J Epidemiol. 2009;38:663–9.
Vasan SK, Antonisamy B, Gowri M, Selliah HY, Geethanjali FS, Jebasingh FS, et al. Prevalence, incidence and predictors of cardiovascular risk factors: longitudinal data from rural and urban South India and comparison with global data. BMJ Open Diabetes Res Care. 2020;8:e001782.
Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–68.
Samuel P, Antonisamy B, Raghupathy P, Richard J, Fall CH. Socio-economic status and cardiovascular risk factors in rural and urban areas of Vellore, Tamilnadu, South India. Int J Epidemiol. 2012;41:1315–27.
Raghupathy P, Antonisamy B, Fall CH, Geethanjali FS, Leary SD, Saperia J, et al. High prevalence of glucose intolerance even among young adults in south India. Diabetes Res Clin Pr. 2007;77:269–79.
Chylack LT Jr., Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–6.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57. 2020.
International Expert C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34.
Karuppusami R, Antonisami B, Vasan SK, Gowri M, Selliah HY, Arulappan G, et al. Association of serum 25-Hydroxy vitamin D with total and regional adiposity and cardiometabolic traits. PLoS One. 2020;15:e0243850.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Varma R, Torres M. Prevalence of lens opacities in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1449–56.
Giuffre G, Giammanco R, Di Pace F, Ponte F. Casteldaccia eye study: prevalence of cataract in the adult and elderly population of a Mediterranean town. Int Ophthalmol. 1994;18:363–71.
Congdon N, West SK, Buhrmann RR, Kouzis A, Munoz B, Mkocha H. Prevalence of the different types of age-related cataract in an African population. Invest Ophthalmol Vis Sci. 2001;42:2478–82.
Leske MC, Connell AM, Wu SY, Hyman L, Schachat A. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol. 1997;115:105–11.
Tang Y, Wang X, Wang J, Huang W, Gao Y, Luo Y, et al. Prevalence of age-related cataract and cataract surgery in a Chinese adult population: the Taizhou Eye Study. Invest Ophthalmol Vis Sci. 2016;57:1193–200.
Athanasiov PA, Casson RJ, Sullivan T, Newland HS, Shein WK, Muecke JS, et al. Cataract in rural Myanmar: prevalence and risk factors from the Meiktila Eye Study. Br J Ophthalmol. 2008;92:1169–74.
Rosman M, Zheng Y, Lamoureux E, Saw SM, Aung T, Tay WT, et al. Review of key findings from the Singapore Malay Eye Study (SiMES-1). Singapore Med J. 2012;53:82–7.
Seah SK, Wong TY, Foster PJ, Ng TP, Johnson GJ. Prevalence of lens opacity in Chinese residents of Singapore: the tanjong pagar survey. Ophthalmology. 2002;109:2058–64.
Athanasiov PA, Edussuriya K, Senaratne T, Sennanayake S, Sullivan T, Selva D, et al. Cataract in central Sri Lanka: prevalence and risk factors from the Kandy Eye Study. Ophthalmic Epidemiol. 2010;17:34–40.
Shah SP, Dineen B, Jadoon Z, Bourne R, Khan MA, Johnson GJ, et al. Lens opacities in adults in Pakistan: prevalence and risk factors. Ophthalmic Epidemiol. 2007;14:381–9.
Nirmalan PK, Krishnadas R, Ramakrishnan R, Thulasiraj RD, Katz J, Tielsch JM, et al. Lens opacities in a rural population of southern India: the Aravind Comprehensive Eye Study. Invest Ophthalmol Vis Sci. 2003;44:4639–43.
Husain R, Tong L, Fong A, Cheng JF, How A, Chua WH, et al. Prevalence of cataract in rural Indonesia. Ophthalmology. 2005;112:1255–62.
Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–9.
Krishnaiah S, Vilas K, Shamanna BR, Rao GN, Thomas R, Balasubramanian D. Smoking and its association with cataract: results of the Andhra Pradesh eye disease study from India. Invest Ophthalmol Vis Sci. 2005;46:58–65.
Richter GM, Torres M, Choudhury F, Azen SP, Varma R. Risk factors for cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino Eye Study. Ophthalmology 2012;119:547–54.
Tang Y, Wang X, Wang J, Jin L, Huang W, Luo Y, et al. Risk factors of age-related cataract in a Chinese adult population: the Taizhou Eye Study. Clin Exp Ophthalmol. 2018;46:371–9.
Nirmalan PK, Robin AL, Katz J, Tielsch JM, Thulasiraj RD, Krishnadas R, et al. Risk factors for age related cataract in a rural population of southern India: the Aravind Comprehensive Eye Study. Br J Ophthalmol. 2004;88:989–94.
Zetterberg M, Celojevic D. Gender and cataract-the role of estrogen. Curr Eye Res. 2015;40:176–90.
Lundstrom M, Stenevi U, Thorburn W. Gender and cataract surgery in Sweden 1992-1997. A retrospective observational study based on the Swedish National Cataract Register. Acta Ophthalmol Scand. 1999;77:204–8.
Klein R, Klein BE, Jensen SC, Moss SE, Cruickshanks KJ. The relation of socioeconomic factors to age-related cataract, maculopathy, and impaired vision. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1969–79.
Ye J, He J, Wang C, Wu H, Shi X, Zhang H, et al. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. 2012;53:3885–95.
Shalini VK, Luthra M, Srinivas L, Rao SH, Basti S, Reddy M, et al. Oxidative damage to the eye lens caused by cigarette smoke and fuel smoke condensates. Indian J Biochem Biophys. 1994;31:261–6.
Xu L, Cui T, Zhang S, Sun B, Zheng Y, Hu A, et al. Prevalence and risk factors of lens opacities in urban and rural Chinese in Beijing. Ophthalmology. 2006;113:747–55.
Ravilla TD, Gupta S, Ravindran RD, Vashist P, Krishnan T, Maraini G, et al. Use of cooking fuels and cataract in a population-based study: the india eye disease study. Environ Health Perspect. 2016;124:1857–62.
Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337:8–14.
Mitchell P, Cumming RG, Attebo K, Panchapakesan J. Prevalence of cataract in Australia: the Blue Mountains eye study. Ophthalmology. 1997;104:581–8.
Jick SS, Vasilakis-Scaramozza C, Maier WC. The risk of cataract among users of inhaled steroids. Epidemiology. 2001;12:229–34.
Kuang TM, Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P. Body mass index and age-related cataract: the Shihpai Eye Study. Arch Ophthalmol. 2005;123:1109–14.
Sabanayagam C, Wang JJ, Mitchell P, Tan AG, Tai ES, Aung T, et al. Metabolic syndrome components and age-related cataract: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2011;52:2397–404.
Ye J, Lou LX, He JJ, Xu YF. Body mass index and risk of age-related cataract: a meta-analysis of prospective cohort studies. PLoS One. 2014;9:e89923.
Pan CW, Boey PY, Cheng CY, Saw SM, Tay WT, Wang JJ, et al. Myopia, axial length, and age-related cataract: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2013;54:4498–502.
Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6:92–108.
Indian Institute of Public Health, Hyderabad, 2019. Guidelines for the Prevention and Management of Diabetic Retinopathy and Diabetic Eye Disease in India. Hyderabad, India: Indian Institute of Public Health. Available from: https://phfi.org/wp-content/uploads/2019/09/2019-Guidelines-for-the-Prevention-and-Management-of-Diabetic-Retinopathy.pdf
Kapoor N. The tropical phenotype of obesity. Thin fat obesity: the tropical phenotype of obesity. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Ahmed SF, Chrousos G et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.;2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK568563/.
Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, et al. Prevalence of generalized & abdominal obesity in urban & rural India-the ICMR-INDIAB Study (Phase-I) [ICMR- NDIAB-3]. Indian J Med Res. 2015;142:139–50.
Yu X, Lyu D, Dong X, He J, Yao K. Hypertension and risk of cataract: a meta-analysis. PLoS One. 2005;9:e114012.
Vashist P, Tandon R, Murthy GVS, Barua CK, Deka D, Singh S, et al. Association of cataract and sun exposure in geographically diverse populations of India: The CASE study. First Report of the ICMR-EYE SEE Study Group. PLoS One. 2020;15:e0227868.
Park S, Choi NK. Serum 25-hydroxyvitamin D and Age-Related Cataract. Ophthalmic Epidemiol. 2017;24:281–6.
Brown CJ, Akaichi F. Vitamin D deficiency and posterior subcapsular cataract. Clin Ophthalmol. 2015;9:1093–8.
Rao P, Millen AE, Meyers KJ, Liu Z, Voland R, Sondel S, et al. The relationship between serum 25-hydroxyvitamin D levels and nuclear cataract in the carotenoid age-related eye study (CAREDS), an ancillary study of the women’s health initiative. Invest Ophthalmol Vis Sci. 2015;56:4221–30.
Jee D, Kim EC. Association between serum 25-hydroxyvitamin D levels and age-related cataracts. J Cataract Refract Surg. 2015;41:1705–15.
Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91:115–24.
Fan X, Zhang J, Theves M, Strauch C, Nemet I, Liu X, et al. Mechanism of lysine oxidation in human lens crystallins during aging and in diabetes. J Biol Chem. 2009;284:34618–27.
Berridge MJ. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150434.
Gosak M, Markovic R, Fajmut A, Marhl M, Hawlina M, Andjelic S. The analysis of intracellular and intercellular calcium signaling in human anterior lens capsule epithelial cells with regard to different types and stages of the cataract. PLoS One. 2015;10:e0143781.
Rullo J, Pennimpede T, Mehraban Far P, Strube YN, Irrcher I, Urton T, et al. Intraocular calcidiol: Uncovering a role for vitamin D in the eye. J Steroid Biochem Mol Biol. 2020;197:105536.
Funding
The study was funded by internal research grants from the Christian Medical College, Vellore (IRB 7765/02-2012), the Indian Institute of Public Health, Hyderabad (WTP project grant/09- 2012) and London school of Hygiene and & Tropical Medicine, UK (ITCRBH76 project grant/06-2012).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research; BA, CG, GVSM, NJ, PP, AB, TK, RI, LMA, AA, NT, TP, GA were involved in the design of the study; PP, BA, NM, JPC, FJ, HYC were responsible for data collection; PP, BA, PS, HYC, MG, SKV, CG were responsible for data analysis and drafted the manuscript. All authors reviewed the manuscript and the interpretations and approved of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41433_2025_3836_MOESM1_ESM.docx
Calculation of Attributable risk between vitamin D deficiency (Yes/No) and lens opacity(Yes/No) (sub-group analysis, n=372)
41433_2025_3836_MOESM2_ESM.docx
Comparison of cohort characteristics at 26-32 years (examined in 1998/2002) (n=2218) with whether they were included (n=1075) or excluded (1143) from the lens opacity study at 41-44 years (2013/2014)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paul, P., Antonisamy, B., John, N. et al. Prevalence and risk factors of pre-senile lens opacities in the 1969-73 Vellore Birth Cohort. Eye (2025). https://doi.org/10.1038/s41433-025-03836-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-025-03836-9