Abstract
Graves’ orbitopathy (GO) affects 25–50% of patients with Graves’ disease. It progresses through phases, from active inflammation to fibrosis. Thyrotropin-related antibodies (TRAb) and Insulin-like growth factor (IGF-1) contribute to GO’s pathogenesis. Conventional treatments like glucocorticoids are often effective, but refractory cases require alternatives like rituximab (RTX), tocilizumab (TCZ), and teprotumumab (TPM). These monoclonal antibodies show promise but carry significant risks. This review aims to assess their efficacy and safety. We retrieved relevant articles up to July 2024 from five databases. Data were extracted from eligible studies by two independent reviewers, including clinical activity scores 7 and 10 (CAS), proptosis, antibody levels, and diplopia. All analyses were conducted using RevMan v5.4. In this review, we included 77 articles. Of these, 58 provided enough data for analysis. TPM, RTX, and TCZ all significantly reduced CAS-7 scores, with TCZ showing the most significant reduction (3.51 points, 95%CI: −4.25, −2.78), followed by TPM (3.1 points, 95%CI: −3.71, −2.49) and RTX. Similarly, for CAS-10, TCZ led with a 5.12-point reduction, significantly outperforming RTX (P = 0.0006). Proptosis decreased significantly with each drug, with TPM leading (2.95 mm), followed by TCZ (1.99 mm) and RTX (0.79 mm). TRAb Levels: TCZ reduced TRAb levels by 8.29 U/L (95%CI: −10.48, −6.09), significantly more than RTX (P = 0.03). Complications varied, with TPM linked to hyperglycemia and ototoxicity, TCZ to hematologic and metabolic issues, and RTX to infusion-related reactions. In conclusion, TCZ and TPM outperform RTX in treating GO, but TPM has higher complications, and RTX, though safer, shows more treatment failures.
Similar content being viewed by others
Introduction
Graves orbitopathy (GO), also known as thyroid eye disease, is a condition closely linked to hyperthyroidism, though it can also occur in euthyroid and hypothyroid patients [1, 2]. It is the most common extrathyroidal manifestation of autoimmune hyperthyroidism and affects ~25–50% of patients with Graves’ disease, with severe cases comprising 5–6% [3]. The incidence varies across populations, with women experiencing around 16 cases per 100,000 and men about 2.9 cases per 100,000 [4]. In Europe, the prevalence is noted at 10 cases per 10,000 individuals, with unilateral cases occurring at a rate of 0.50–1.50 per 10,000 [5]. GO progresses through distinct phases, as designated by the European Group on Graves’ Orbitopathy (EUGOGO) guidelines: the active inflammatory phase, marked by inflammation, pain, redness, and increased proptosis; the plateau phase, where disease activity stabilizes but some symptoms may persist; and the fibrotic or inactive phase, characterized by fibrosis and scarring of orbital tissues, leading to symptoms like diplopia and eyelid retraction [6].
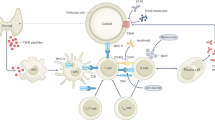
Thyrotropin Receptor Antibodies (TRAb) play a significant role in the development of GO. These antibodies can either act on the thyrotropin receptor (TSHR) as a stimulator (TSAb) or a blocker (TBAb) [7, 8]. Activating these receptors leads to the release of thyroid hormones, contributing to hyperthyroidism and the proliferation of thyrocytes [9]. TSAb stimulates orbital and periorbital tissues, leading to tissue remodeling and inflammation associated with GO [8, 10]. In addition, TRAb and insulin-like growth factor-1 (IGF-1) influence GO development by targeting orbital fibroblasts, causing tissue expansion, optic nerve compression, and exophthalmos [1, 11]. The interaction between TRAb and IGF1 receptors in fibroblasts further suggests a complex cross-talk that maintains the autoimmune process in GO [12, 13]. IGF-1 also upregulates proinflammatory cytokines and hyaluronan production, maintaining the inflammatory environment in GO [14]. IL-6, another proinflammatory cytokine, plays a critical role in the inflammatory process by promoting tissue remodeling and accumulation of adipose tissue, which are hallmarks of the disease [15, 16].
Conventional treatment options for GO primarily involve high-dose intravenous glucocorticoids, recommended as the first-line treatment for moderate-to-severe and active GO according to the EUGOGO guidelines 2016 [5]. Similarly, in cases involving optic neuropathy or corneal ulceration, intravenous glucocorticoids remain the recommended first-line treatment [17]. Despite their effectiveness, with success rates ranging from 60% to 85%, up to 20% of patients may be unresponsive, another 10–20% may experience relapses following steroid withdrawal, and about 5% progress to dysthyroid optic neuropathy despite treatment [18, 19]. Surgical intervention becomes crucial when conservative treatments such as glucocorticoids or orbital radiotherapy prove ineffective. Surgeries like orbital decompression aim to alleviate optic nerve pressure, reduce proptosis, and improve visual function in severe cases [20]. Furthermore, procedures targeting extraocular muscles help correct strabismus and restore proper eye alignment and are generally recommended during the inactive phase to minimize complications and optimize outcomes [21].
Multiple new therapies have emerged to address cases that do not respond well to glucocorticoids and potentially minimize residual deformities of GO. Rituximab (RTX), a monoclonal antibody targeting the CD20 antigen on B cells, has been suggested as a therapeutic option for GO in cases resistant to corticosteroids or where steroid therapy is contraindicated [22, 23]. Recent guidelines now recommend RTX as a second-line treatment for GO [24]. Another promising treatment, tocilizumab (TCZ), an anti-IL-6 receptor therapy, has been shown to reduce clinical activity, TRAb titers, proptosis, eyelid retraction, and diplopia and improve bulbar motility in patients with GO [1]. Recent studies have affirmed the efficacy and safety of TCZ, especially in refractory cases [25, 26]. Conversely, teprotumumab (TPM), a human monoclonal antibody that inhibits the IGF-1 receptor, has demonstrated efficacy in reducing proptosis, diplopia, and clinical activity while improving the quality of life for patients with GO. Multiple studies have confirmed TPM as a valuable second-line treatment for active moderate-to-severe GO [27].
Novel monoclonal antibodies are promising in treating recalcitrant cases of GO; however, their use comes with some considerable adverse effects. For instance, TPM treatment has been associated with inflammatory bowel disease, hyperglycemia, and hearing loss [28]. Similarly, TCZ use was associated with reports of bronchospasm and anaphylactic shock [29]. The current literature does not include reviews comparing the efficacy and safety of these drugs, as mentioned earlier in GO, except for a small review comprising six studies [30]. In our current review, we seek to examine the efficacy of these agents in GO and safety concerns associated with their use. Moreover, we aim to comprehensively review potential use cases and multiple challenges that arise when using these agents.
Methods
The current review was conducted adhering to the tenants of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews of Interventions [31, 32].
Eligibility criteria
Inclusion criteria
-
Articles with cases diagnosed with Graves’ Orbitopathy (GO).
-
Observational or interventional studies where the treatment is rituximab, tocilizumab, or teprotumumab.
-
Articles written in English.
-
Articles could be retrieved in full text.
Exclusion criteria
-
Abstracts, letters to editors, comments, opinions, case reports, and reviews.
-
Articles are written in languages other than English.
Information sources and search strategy
Record retrieval involved several steps. First, we conducted a broad search using generic terms across databases such as PubMed, Scopus, and Embase to identify relevant articles. Following this initial search, we developed a comprehensive strategy that included all relevant terms and their MeSH equivalents. In the subsequent stage, we refined our search by using these terms in PubMed, Scopus, Cochrane Library, and Web of Science. The specific search terms related to GO were as follows: (“Graves’ ophthalmopathy” OR “Graves ophthalmopathy” OR “Graves’ orbitopathy” OR “Graves orbitopathy” OR “thyroid eye” OR “thyroid eyes” OR “thyroid-associated ophthalmopathy” OR “thyroid ophthalmopathy” OR “Graves’ eye”) AND (rituximab OR tocilizumab OR teprotumumab OR Tepezza OR Actemra OR RoActemra OR Tofidence OR MabThera OR Riabni OR Rituxan OR Ruxience OR Truxima). Our final search strategy was conducted on July 2024, using the detailed query. Finally, we manually searched through the references and citations of the retrieved records to find additional relevant studies.
Selection process
Two independent reviewers screened the titles and abstracts of all available records, having removed identifying data such as author names and affiliations to minimize bias. They followed a checklist of eligibility criteria to guide their screening process. A third reviewer was on hand to resolve any conflicts. Following the initial screening, the same two reviewers assessed the full texts of the selected articles for eligibility, comparing their findings to resolve any remaining disagreements.
Data collection
Two independent reviewers extracted data from full-text articles into a shared Google spreadsheet, following a two-part process using a predetermined set of variables. In the first part, they gathered baseline information, including the author’s last name, study design, study arms, country of origin, follow-up duration, and outcomes. They also collected details such as the number of patients, their age, gender distribution, body mass index (BMI), severity of GO, treatment regimen, and response to previous steroid therapy. In the second part, for analysis, they extracted data on clinical activity score (CAS), exophthalmometric measurements, antibody levels, Gorman diplopia score (GDS), and intraocular pressure (IOP).
Outcome measures
All variables were expressed as Mean ± Standard deviation (SD). Change scores were calculated as the difference between the last follow-up and initial values at baseline. In studies where data were expressed in forms other than Mean ± SD, we used the available formula from the Cochrane Handbook to convert them back into means and standard deviations.
Clinical activity score (CAS)
CAS is an important tool in the management of GO; it is used to classify the disease into active (CAS ≥ 3) or inactive (CAS < 3) according to EUGOGO guidelines [33]. Some studies have used the original scoring system of 10 items, where baseline evaluation is conducted using the first seven items and later assessments use the last three items for follow-up [34]. Other studies adhered to a seven-item score throughout follow-up assessments. Thus, we analyzed each method separately for consistency, labeling the outcomes as either CAS-7 or CAS-10.
Proptosis
Proptosis measurements were obtained using Hertel’s exophthalmometer. Whenever studies provided separate measurements for both eyes, we averaged the readings, given the minor differences in both eyes, and to allow for combination with other studies that provided a single composite value.
Thyrotropin receptor antibodies (TRAb)
Since the TRAb assay does not differentiate between stimulating and blocking antibodies, we decided not to separate it from the thyrotropin receptor-stimulating antibody (TSAb) assay. Although TSAb is the modern and more accurate alternative, most studies adhered to the more conventional TRAb assay.
Gorman diplopia score (GDS)
GDS is a subjective grading system used to assess the severity of diplopia in patients with GO. This scoring system typically ranges from 0 to 3, with different levels indicating varying degrees of diplopia. For instance, a score of 0 represents no diplopia, 1 for intermittent diplopia, 2 for inconstant diplopia (e.g., gaze-evoked), and 3 for constant diplopia in the primary gaze or while reading [35]. Similar to proptosis, we combined the values for both eyes to make the outcome compatible with studies providing a single average value.
Intraocular pressure (IOP)
IOP monitoring can help early detection of compressive optic neuropathy and correlates well with the severity of the underlying GO, aiding in choosing appropriate lines of treatment [36].
Risk of bias
Two different reviewers assessed the quality of all full-text articles, and conflicts were resolved by a third reviewer. The reviewers evaluated the methodical quality of all cohort studies using the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort, Case-Control, and Case Series Studies [37, 38]. Similarly, they used the Risk of Bias 2 (RoB2) provided by Cochrane to evaluate all Randomized control trials (RCTs) included in the review [39]. As for Non-randomized trials (NRCTs), the Risk of Bias in Non-randomized Studies- of Interventions (ROBINS-I) tool was used to assess the quality [40].
Statistical analysis
All analyses were carried out using RevMan v5.4.1 software to determine the overall change scores of CAS-7 and CAS-10, proptosis, antibody levels, IOP, and GDS. All outcomes were compared among RTX, TCZ, and TPM, where eligible. The analysis was conducted by pooling the estimates for each intervention and representing them as subgroups in RevMan software. This allows comparing results from all interventions in a single forest plot for each parameter. When more than two subgroups were present in an outcome, we checked for a significant subgroup difference, and if so, a pairwise analysis was undertaken to examine the differences. Combined means and corresponding 95% confidence intervals (CIs) were reported. Statistical significance was determined with a P-value of less than 0.05. Heterogeneity among included studies was assessed using the I2 statistic, and the random effects model was used to account for differences between populations and inclusion criteria. The results were displayed on a forest plot, providing information about individual studies and the heterogeneity of the effect measure. Funnel plots were generated and manually inspected to check for asymmetry representing publication bias.
Results
Study selection
After searching four databases, we collected 1152 records. Removing 635 duplicates left us with 917 unique records. We then screened the titles and abstracts, excluding 815 entries. We retrieved the full text of the remaining 102 records and evaluated them against our eligibility criteria. During this evaluation, we excluded three review articles, five non-English articles, 11 articles that were reviews, and nine articles that did not meet the specified population criteria. Ultimately, 77 articles were included in the review, with 58 providing sufficient data for analysis. The flow diagram for study selection is shown in Fig. 1.
Characteristics of the included studies
This review comprised 77 studies: 39 cohort studies, 23 case series, 10 randomized clinical trials, three case-control studies, and 2 non-randomized trials [26, 41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116]. The total number of subjects was 6356, distributed as 434 subjects treated with RTX, 212 treated with TCZ, and 5710 cases treated with TMP. The cases treated with TPM are further divided into 1381 cases from primary studies and 4329 from postmarketing pharmacovigilance studies.
Thirty-eight articles investigated TPM [41,42,43, 48, 49, 52, 54,55,56, 63,64,65,66,67, 69,70,71, 73, 80,81,82, 90, 92, 94,95,96, 100,101,102,103,104,105,106,107, 110, 112, 114, 115]; the mean age was 53.94 years, and 72.15% of cases were female patients. The mean follow-up was 9.9 months (range: 2–20.4), and the prevalent treatment regimen prescribed was a single dose of 10 mg/kg of TPM followed by seven doses of 20 mg/kg, with an interim gap of three weeks between individual doses. The patients had active disease in 19, inactive in five, and mixed in 11 studies. Most studies did not explicitly state glucocorticoid resistance in their inclusion criteria.
As for RTX, 27 articles examined its use in GO [44, 46, 51, 57,58,59,60, 62, 68, 72, 74,75,76,77,78, 85,86,87,88,89, 91, 97, 98, 108, 109, 111, 113, 116]; the mean age was 51.12 years and females constituted 68.8% of cases. The follow-up period averaged 17.42 months (range: 3–67). Patients had active disease in 25 articles, inactive disease in one, and mixed activity in a single article. TCZ was studied in 14 studies [26, 44, 45, 47, 50, 53, 61, 75, 79, 83, 84, 93, 99, 103]; the patients were 68.8% females with a mean age of 51.89 years. The follow-up period was 14.48 months (range: 4–24), and the disease was reported as active in 13 articles and to have mixed activity in another. All articles exclusively included patients resistant to glucocorticoid treatment. A complete depiction of baseline characteristics is presented in Table 1.
Risk of bias
The reviewers categorized cohort studies as “good,” “fair,” or “poor” based on NIH criteria. A “good” study has the lowest risk of bias and produces valid results, a “fair” study may have some bias but not enough to invalidate findings, and a “poor” rating indicates significant bias, leading to exclusion from the evidence body. Four of the 39 cohort studies were deemed to have fair quality, and 35 had good quality. There were 23 case series in the review: 19 with good and another four with fair quality. Using the RoB2 tool for RCTs, assessors labeled studies as having “low risk”, “some concern”, or “high risk” of bias. Five trials had a low risk, one had some concern, and four had a high risk of bias. As for NRCTs, there were two studies; one had a moderate, and another had a low risk of bias. Supplementary Tables 1–3 and Supplementary Figs. 1 and 2.
Results of syntheses
CAS-7
The seven-item clinical activity score was reported in 26 studies: 10 studies investigating TPM, 10 studies on RTX, and six studies evaluating TCZ. All three drugs were able to significantly reduce CAS-7 after completion of respective treatment regimens. TCZ reduced CAS-7 by 3.51 points (95%CI: −4.25, −2.78); TPM decreased it by a mean 3.1 points (95%CI: −3.71, -2.49); and RTX was able to diminish it by a mean 2.81 points (95%CI: −3.57, −2.06). Despite TCZ and TPM achieving greater reductions in CAS-7 than RTX, the differences between all three drugs were not statistically significant Fig. 2.
CAS-10
The ten-item clinical activity score was reported in 19 studies, and one study compared both TCZ and RTX: 10 studies investigated RTX, six studies on TCZ, and three studies evaluated TCZ. All three monoclonals were able to significantly reduce CAS-10 after completion of the respective treatment course. TCZ reduced CAS-10 by 5.12 points (95% CI: −6.26, −3.98); TPM decreased it by a mean 4.08 points (95% CI: −5.77, −2.39); and RTX was able to diminish it by a mean 3.02 points (95% CI: −3.58, −2.46). TCZ achieved more reduction in CAS-10 than TPM; however, the difference was not statistically significant, and conversely, TPM and RTX were not significantly different. On the other hand, TCZ achieved significantly more reduction than RTX (P = 0.0006). Reductions achieved by TPM and RTX using CAS-7 did not differ significantly from those measured via CAS-10. Nevertheless, the reductions achieved by TCZ differed significantly between CAS-7 and CAS-10 (P = 0.0119). Assimilating all data from both CAS-7 and CAS-10, the findings match those using CAS-10 alone, with TCZ performing significantly better than RTX (P = 0.0025) Fig. 3.
Proptosis
Proptosis was reported in a form suitable for analysis in 40 studies and one article comparing TCZ and RTX: 21 studies using TPM reported proptosis, as well as 11 RTX studies and eight TCZ studies included proptosis measurements. The three monoclonal treatments each resulted in a significant reduction of proptosis following their respective courses. TCZ led to a reduction of 1.99 mm (95% CI: −2.52, −1.45); TPM showed a decrease of 2.95 mm (95% CI: −3.21, −2.68); and RTX was associated with a reduction of 0.79 mm (95% CI: −1.20, −0.38). The differences between all three drugs were statistically significant: TCZ vs. RTX (P = 0.0010), TPM vs. TCZ (P = 0.0019), and TPM vs. RTX (P < 0.0001) Fig. 4.
TRAb
The level of total antibodies was explored in 18 studies, and one study compared TCZ to RTX: 15 articles on RTX and 4 on TCZ. Both drugs reduced TRAb levels significantly; TCZ reduced them by 8.29 U/L (95% CI: −10.48, −6.09) and RTX by 5.22 U/L (95% CI: −6.83, −3.61), and TCZ produced a significantly greater reduction in TRAb levels than RTX (P = 0.03) Fig. 5.
GDS and IOP
TPM reduced Diplopia scores significantly (MD: −0.96 points; 95% CI: −1.50, −0.43). However, the RTX effect was not significant (P = 0.30). Moreover, all three drugs had comparable reductions in IOP Figs. 6 and 7.
Complications
An exhaustive analysis of complication severity according to the common terminology criteria of adverse events (CTCAE) is beyond the scope of this review; however, we provide a comprehensive summary of the adverse events that arose during the use and follow-up of each drug.
Rituximab (RTX)
Adverse effects associated with RTX use occurred mainly during infusions and were self-limited. Mild (Grade 1) events included throat itching, nose stuffiness, mild hypotension, and rhinorrhea [88,46], minor infusion-related rashes (4, [68]), headache (2, [78]), mild temperature elevation [89], mild urticaria [109], fatigue (1, [113]), and minor infections during follow-up (2, [59]). Moderate (Grade 2) adverse events included hypotension (4, [59]), nausea (4, [59, 89, 58]), chills (2, 59, 78), tachycardia (1, [59]), heart palpitations (1, [44]), hypertension exacerbation [74], and serum sickness-like reaction (2, [59]). Severe (Grade 3) reactions included Cytokine release syndrome with vision decline and chemosis controlled with hydrocortisone (7, [51, 85, 88, 113]) and arthritis exacerbation (1, [58]).
Tocilizumab (TCZ)
Adverse effects associated with TCZ were mainly hematologic, metabolic, and infection-related. Mild (Grade 1) events included minor neutropenia (1, [44]), mild neutropenia (3, [61]), mild liver enzyme increases (1, [44]), deranged lipid profiles (2, [61]), cutaneous rash (1, [44]), hypercholesterolemia (16, [45, 84]), pruritus/urticaria (3, [84]), weight increase (4, [45]), and severe but self-limiting back pain (1, [61]). Moderate (Grade 2) adverse events included neutropenia (7, [84, 79]), hypertransaminasemia (2, [84]), upper respiratory tract infection (1, [83]), respiratory tract infection (1, [79]), otitis (2, [26]), and asthenia (6, [84]). Severe (Grade 3) reactions included thrombocytopenia (2, [84]), elevated transaminases (2, [83]), cellulitis (3, [84]), autoimmune hepatitis (1, [83]), and delayed injection-site reactions (1, [79]). Life-threatening (Grade 4) events were observed in cases of acute pyelonephritis (1, [83]) and anaphylactic shock with bronchospasm (1, [84]). There was one Grade 5 (death) event reported due to metastatic cancer [79].
Teprotumumab (TPM)
The most prominent adverse events arising during TPM therapy were hyperglycemia and ototoxicity. The complications were graded according to CTCAE as follows: Mild (Grade 1) events included autophony and patulous eustachian tube [55], dry skin (5, [66]), nail changes (3, [66]), paresthesia (3, [96]), alopecia (3, [96]), hearing impairment (2, [56]), upper abdominal pain (1, [73]), and mild nausea (9, [66]). Moderate (Grade 2) adverse events included hypoacusis, hearing impairment (5, [55]; 5, [64]; 5, [73]; 5, [66]), ototoxicity (5, [73]), hyperglycemia (2, [55]; 5, [96]; 10, [64]), muscle spasms (8, [96]; 1, [73]), fatigue (10, [66]), diarrhea (6, [96]; 10, [66]), hypertension (2, [66]), menstrual abnormalities (9, [101]). Severe (Grade 3) reactions included muscle cramps (20, [64]; 31, [66]), new hearing symptoms (8, [73]), sensorineural hearing changes (5, [66]), and dysgeusia (7, [66]). Life-threatening (Grade 4) events were present, including intracerebral hemorrhage (1, [55]), serious pneumothorax and infusion reactions (2, [56]), serious diarrhea (1, [96]), inflammatory bowel disease (1, [96]), Escherichia sepsis (1, [96]), Hashimoto’s encephalopathy (1, [96]), and urinary retention (1, [96]).
Publication bias
Only CAS-7, CAS-10, and proptosis had enough studies to justify inspection for publication bias. Manual inspection of the funnel plots did not reveal asymmetry in any of the subgroups in the three eligible outcomes (Supplementary Figs. 3–5).
Discussion
Graves’ orbitopathy (GO) is the most common extrathyroidal manifestation of Graves’ disease. GO leads to a multitude of presentations that affect vision, appearance, and quality of life [3]. Around a fifth of active patients fail to respond to conventional glucocorticoid therapy [18, 19]. This review aims to analyse the efficacy of rituximab (RTX), tocilizumab (TCZ), and teprotumumab in improving the various clinical parameters related to GO. We compared the performance of all three drugs in reducing the clinical activity score (CAS), proptosis measurements, thyrotropin receptor antibodies (TRAb), intraocular pressure (IOP), and Gorman diplopia scale (GDS).
In our analysis of the clinical efficacy of TPM, TCZ, and RTX, all three drugs significantly reduced both the seven-item (CAS-7) and ten-item (CAS-10) in patients after treatment. TCZ achieved the most substantial reduction in CAS-7 and CAS-10, although the differences compared to TPM were not statistically significant. However, TCZ did perform significantly better than RTX in reducing CAS-10 (P = 0.0006), and this superiority was confirmed when data from both CAS-7 and CAS-10 were combined (P = 0.0025). Proptosis also decreased significantly with all three treatments, with TPM showing the greatest reduction, followed by TCZ, and RTX showing the least; the differences between all three were statistically significant. Moreover, TCZ also outperformed RTX in reducing TRAb levels (P = 0.03), and in terms of diplopia, TPM significantly reduced scores, while RTX did not have a significant effect. All three drugs demonstrated comparable efficacy in lowering intraocular pressure (IOP).
Contrasting our results with the previous meta-analysis conducted on monoclonal therapy for GO showed agreement in the main parameters of disease. Fatani et al. reported that monoclonal antibodies, including TPM and RTX, showed significant improvements in CAS, proptosis, and diplopia compared to glucocorticoids or placebo [30]. Similarly, our analysis found that all three drugs significantly reduced CAS and proptosis, with TCZ significantly outperforming TPM and RTX in regards to CAS, and both TCZ and TPM performing better than RTX in regards to proptosis. In contrast to Shen et al., who found a non-significant improvement in proptosis for RTX, our results showed a significant reduction in proptosis for all three drugs, however, TPM had the greatest impact in comparison with the other two agents [23]. Conversely, Li et al. reported a modest effect of TPM but not RTX on CAS and proptosis, our analysis showed that TCZ and TPM outperformed RTX significantly in these measures, nonetheless, RTX still had a smaller but significant effect, Thus, although RTX underperforms in its effect on CAS and proptosis in comparison to other agents, it is still an effective and considerable agent [117].
Categorizing the complications reported in the results of the current review showed that TPM exhibited the highest number of adverse events across several categories. TPM had 48 mild (Grade 1) events, 62 moderate (Grade 2) events, and 50 severe (Grade 3) events, along with 11 life-threatening (Grade 4) events. In contrast, TCZ reported the highest number of mild (Grade 1) events at 54 but had fewer moderate (31) and severe (12) events, with only 2 life-threatening events. RTX had the fewest total adverse events overall, with 34 mild, 22 moderate, and 14 severe events, and notably, no life-threatening (Grade 4) or fatal (Grade 5) events. TPM, therefore, stands out for its higher incidence of more severe and life-threatening complications, while RTX caused the least number of complications across all grades.
Notably, our results match the findings of the postmarketing pharmacovigilance study by Huang et al. [65]. The authors found that the most commonly reported adverse effects of TPM were hyperglycemia, muscle spasms, fatigue, and hearing disorders. The effect of TPM on blood glucose levels was investigated by Amarikwa et al. [43]. The authors noted that 40.9% were already on antihyperglycemic medications at baseline. Management strategies included adding new medications (36.4% of cases) or adjusting existing ones (31.8%), with insulin modifications in 18.2% of cases. At the last follow-up, ~50 weeks after the first infusion, 36.4% of patients with hyperglycemia had returned to their baseline glycaemic status, while 63.6% still had persistent hyperglycemia above baseline. These results show the high rate of complications during the use of TPM, some of which can sometimes arise due to pre-existing conditions; thorough investigations are required to identify baseline conditions that may increase the risk of developing complications.
The safety profile of TCZ was less severe than TPM; however, the reports by Moi et al. that three cases of cancer developed during the follow-up were quite concerning [79]. Multiple studies on rheumatoid arthritis patients treated with TCZ were available to counsel. Rubbert-Roth et al. found that the overall malignancy risk in tocilizumab-treated patients was comparable to that of rheumatoid arthritis (RA) patients treated with anti-TNF agents [118]. This finding was corroborated by Wadström et al., who reported no increased overall cancer risk with tocilizumab compared to other biologic disease-modifying antirheumatic drugs (bDMARDs) like abatacept and rituximab [119]. Furthermore, Cho et al. noted that postmarketing surveillance and large studies have not shown a significant increase in malignancy risk for tocilizumab-treated RA patients [120]. Collectively, these studies suggest that tocilizumab does not significantly elevate cancer risk, and the cases reported by Moi may have occurred sporadically. However, this does not preclude the need for more trials with longer follow-up durations to settle such concerns further.
RTX had the lowest incidence of complications in the current review, and the incidence could be further reduced by lowering the dose of RTX. Studies examining different dosing regimens of RTX reveal a complex balance between efficacy and adverse effects. Lower doses of RTX (100 mg) generally demonstrated fewer adverse effects, with Bennedjaï et al., Insull et al., and Karasek et al. reporting only minor issues such as heart palpitations and infusion-related rashes [44, 68, 72]. However, these lower doses were associated with higher treatment failure rates, as seen in [44] (5 out of 14 patients) and Karasek (3 out of 10 patients). Many of the failures were due to the development of optic neuropathy, which required urgent decompression surgery. Conversely, higher doses (2 × 1000 mg/2 week) showed improved efficacy but more frequent adverse effects. Salvi et al. reported 13/15 infusion reactions, including two cases of cytokine release syndrome, while Stan et al. noted 11/13 adverse events in the RTX group, with 5 considered moderate or severe [88, 97]. Careful selection of patients who are unlikely to proceed to optic neuropathy for low RTX regimens might improve the safety profile while maintaining efficacy.
This study was the first to incorporate all available evidence regarding the efficacy of monoclonal therapy for GO using RTX, TPM, and TCZ. In addition, we compared the safety profiles of each drug after categorizing the adverse events according to the Common Terminology of Adverse Events. Conversely, we highlighted some of the key problems that arise during treatment with different monoclonals and provided suggestions on how to address them. Nonetheless, the underlying evidence was heterogeneous in regard to thyroid status, disease activity, and resistance to glucocorticoid therapy. Only two double-armed studies directly compared more than one monoclonal. Furthermore, we could not assess whether complications arose due to the effect of monoclonal therapy or were the mere result of underlying conditions or faulty procedures. Future controlled trials might investigate the efficacy and safety of RTX, TCZ, and TPM to confirm the findings from our research.
Conclusion
In conclusion, this review provides a comprehensive analysis of the efficacy and safety of rituximab (RTX), tocilizumab (TCZ), and teprotumumab (TPM) in the treatment of Graves’ orbitopathy (GO). Our findings demonstrate that all three drugs effectively reduce clinical activity scores, proptosis, and thyrotropin receptor antibodies; however, TCZ and TPM generally outperform RTX in these parameters. RTX was associated with the fewest adverse events overall, especially when administered at lower doses. This came with the price of higher treatment failure rates, particularly in cases of optic neuropathy. TPM, while highly effective, exhibited the highest incidence of severe and life-threatening complications, necessitating careful patient selection and monitoring. The safety profile of TCZ was more favorable than that of TPM, but concerns about malignancy risk, although questionable and not definitively established, warrant further investigation. Future controlled trials are essential to validate these findings, further analyse adverse effects, and refine the therapeutic approaches for GO.
Data availability
The datasets were generated from open-source databases. All data are available within the manuscript and its supplemental files.
References
Klet S, Marković B, Janić T, Stojković M, Ćirić J, Beleslin BN, et al. Steroid-resistant Graves’orbitopathy–therapeutic options. Med Glas Spec Boln za Boles štitaste žlezde Boles Metab. 2023;28:78–90.
Ventura M, Melo M, Carrilho F. Selenium and thyroid disease: from pathophysiology to treatment. Int J Endocrinol. 2017;2017:1297658. https://doi.org/10.1155/2017/1297658.
Lu C, Lai CL, Yang CM, Liao KC, Kao CS, Chang TC, et al. The relationship between obesity-related factors and Graves’ orbitopathy: a pilot study. Medicine. 2022;58:1748 https://doi.org/10.3390/medicina58121748.
Gupta P, Kaur N, Goyal A, Aggarwal A. Atypical asymmetric presentation of severe Graves’ orbitopathy. Cureus. 2023;15:e45907. https://doi.org/10.7759/cureus.45907.
Buldygina YV, Terekhova GM, Strafun LS, Savosko II, Lysova ZG, Shlyakhtych SL. Assessing the efficacy of various treatment regimens for patients with endocrine ophthalmopathy associated with Graves’ disease. J Ophthalmol. 2022;96:311–28.
Brammen L, Riss P, Lukas J, Gessl A, Dunkler D, Li S, et al. Total thyroidectomy (Tx) versus thionamides (antithyroid drugs) in patients with moderate-to-severe Graves’ ophthalmopathy - a 1-year follow-up: study protocol for a randomized controlled trial. Trials. 2018;19:495. https://doi.org/10.1186/s13063-018-2876-0.
Kumata K, Nagata K, Matsushita M, Kuwamoto S, Kato M, Murakami I, et al. Thyrotropin Receptor Antibody (TRAb)-IgM levels are markedly higher than TRAb-IgG levels in Graves’ disease patients and controls, and TRAb-IgM production is related to Epstein-Barr virus reactivation. Viral Immunol. 2016;29:459–63. https://doi.org/10.1089/vim.2016.0043.
Kwon H, Kim WG, Jang EK, Kim M, Park S, Jeon MJ, et al. Usefulness of measuring thyroid stimulating antibody at the time of antithyroid drug withdrawal for predicting relapse of Graves disease. Endocrinol Metab. 2016;31:300–10. https://doi.org/10.3803/EnM.2016.31.2.300.
Menon LP, Naqvi S, Maradana J, Edem D. Recurrent Graves’ disease following near-total thyroidectomy: a case report and literature review. Cureus. 2024;16:e52260. https://doi.org/10.7759/cureus.52260.
Park J, Kim J, Kim SS, Choi HY. Prognostic significance of thyroid-stimulating hormone receptor antibodies in moderate-to-severe Graves’ orbitopathy. Front Endocrinol. 2023;14:1153312. https://doi.org/10.3389/fendo.2023.1153312.
Ujianto M, Sari F, Rakhman MF. Unveiling modern strategies for diagnosing and treating grave orbitopathy. J Klin dan Ris Kesehat. 2024;3:89–106.
Taylor PN, Zhang L, Lee RWJ, Muller I, Ezra DG, Dayan CM, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020;16:104–16. https://doi.org/10.1038/s41574-019-0305-4.
Walasik-Szemplińska D, Kamiński G, Sudoł-Szopińska I. Impact of radioiodine treatment on doppler flow parameters in the central retinal artery and ophthalmic artery in patients with hyperthyroidism. J Ultrasound Med. 2021;40:305–18. https://doi.org/10.1002/jum.15401.
Byeon HJ, Chae MK, Ko J, Lee EJ, Kikkawa DO, Jang SY, et al. The role of adipsin, complement factor D, in the pathogenesis of Graves’ orbitopathy. Invest Ophthalmol Vis Sci. 2023;64:13 https://doi.org/10.1167/iovs.64.11.13.
Nowak M, Siemińska L, Karpe J, Marek B, Kos-Kudła B, Kajdaniuk D. Serum concentrations of HGF and IL-8 in patients with active Graves’ orbitopathy before and after methylprednisolone therapy. J Endocrinol Investig. 2016;39:63–72. https://doi.org/10.1007/s40618-015-0322-7.
Soler ZM, Platt MP, Leung MK, Mong S, Metson R. Sinonasal abnormalities in patients with Graves’ orbitopathy. Laryngoscope. 2011;121:656–60. https://doi.org/10.1002/lary.21392.
Ponto KA, Pitz S, Mann WJ, Weber MM, Pfeiffer N, Kahaly GJ. Vorgehen bei endokriner Orbitopathie. Evidenzbasierte Empfehlungen [Management of Graves’ orbitopathy: evidence-based recommendations]. Dtsch Med Wochenschr. 2009;134:2521–4. https://doi.org/10.1055/s-0029-1243057.
Devarbhavi P, Maiti A, Sinha A, Basu A, Chakraborty S, Dey L, et al. Rituximab therapy in steroid resistant thyroid associated ophthalmopathy. Case Rep Int. 2017;6:1–4. https://doi.org/10.5348/crint-2017-36-CR-5.
Sisti E, Menconi F, Leo M, Profilo MA, Mautone T, Mazzi B, et al. Long-term outcome of Graves’ orbitopathy following high-dose intravenous glucocorticoids and orbital radiotherapy. J Endocrinol Investig. 2015;38:661–8. https://doi.org/10.1007/s40618-015-0241-7.
Eckstein A, Schittkowski M, Esser J. Surgical treatment of Graves’ ophthalmopathy. Best Pr Res Clin Endocrinol Metab. 2012;26:339–58. https://doi.org/10.1016/j.beem.2011.11.002.
Eing F, Cruz AA. Surgical treatment of globe subluxation in the active phase of the myogenic type of Graves orbitopathy: case reports. Arq Bras Oftalmol. 2012;75:131–3. https://doi.org/10.1590/s0004-27492012000200012.
Salvi M, Campi I. Medical Treatment of Graves’ Orbitopathy. Horm Metab Res. 2015;47:779–88. https://doi.org/10.1055/s-0035-1554721.
Shen WC, Lee CH, Loh EW, Hsieh AT, Chen L, Tam KW. Efficacy and safety of rituximab for the treatment of Graves’ orbitopathy: a meta-analysis of randomized controlled trials. Pharmacotherapy. 2018;38:503–10. https://doi.org/10.1002/phar.2111.
Oakman G, Ong C. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis with overlap IgA nephropathy: a case report. Case Rep Nephrol Dial. 2024;14:36–41. https://doi.org/10.1159/000536618.
Duarte AF, Xavier NF, Sales Sanz M, Cruz AAV. Efficiency and safety of tocilizumab for the treatment of thyroid eye disease: a systematic review. Ophthalmic Plast Reconstr Surg. 2024;40:367–73. https://doi.org/10.1097/IOP.0000000000002573.
Sánchez-Bilbao L, Martínez-López D, Revenga M, López-Vázquez Á, Valls-Pascual E, Atienza-Mateo B, et al. Anti-IL-6 Receptor tocilizumab in refractory Graves’ orbitopathy: national multicenter observational study of 48 patients. J Clin Med. 2020;9:2816 https://doi.org/10.3390/jcm9092816.
Bartalena L. Role of teprotumumab in the treatment of active moderate-to-severe Graves’ orbitopathy. Eur Thyroid J. 2022;11:e220185 https://doi.org/10.1530/ETJ-22-0185.
Lin F, Yao Q, Yu B, Deng Z, Qiu J, He R. The efficacy and safety of teprotumumab in thyroid eye disease: evidence from randomized controlled trials. Int J Clin Pr. 2023;2023:6638089. https://doi.org/10.1155/2023/6638089.
Xavier NF, Lucena DT, Cruz AAV. Monoclonal antibodies for the treatment of graves orbitopathy: precision medicine?. Ophthalmic Plast Reconstr Surg. 2023;39:307–15. https://doi.org/10.1097/IOP.0000000000002315.
Fatani WA, Hamdan DM, Taher NO, Alsharef JF, Aldubi RM, Alwagdani AM, et al. Monoclonal antibodies for the treatment of Graves’ ophthalmopathy: a systematic review and meta-analysis. Saudi J Ophthalmol. 2023;37:137–48. https://doi.org/10.4103/sjopt.sjopt_176_22.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71 https://doi.org/10.1136/bmj.n71.
Higgins JP: Cochrane handbook for systematic reviews of interventions version 5.0.1. The Cochrane Collaboration. http://www.cochrane-handbook.org 2008.
Limone PP, Bianco L, Mellano M, Garino F, Giannoccaro F, Rossi A, et al. Is concomitant treatment with steroids and radiotherapy more favorable than sequential treatment in moderate-to-severe graves orbitopathy?. Radio Med. 2021;126:334–42. https://doi.org/10.1007/s11547-020-01244-5.
Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol. 1997;47:9–14. https://doi.org/10.1046/j.1365-2265.1997.2331047.x.
Quah Qin Xian N, Alnahrawy A, Akshikar R, Lee V. Real-world efficacy and safety of mycophenolate mofetil in active moderate-to-sight-threatening thyroid eye disease. Clin Ophthalmol. 2021;15:1921–32. https://doi.org/10.2147/OPTH.S305717.
Fan SX, Zeng P, Li ZJ, Wang J, Liang JQ, Liao YR, et al. The clinical features of Graves’ orbitopathy with elevated intraocular pressure. J Ophthalmol. 2021;2021:9879503. https://doi.org/10.1155/2021/9879503.
Quality assessment tool for observational cohort and cross-sectional studies [https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools].
Health NIo: Quality assessment tool for case series studies. NHLBI NIH.gov Web site[Accessed September 24, 2020] 2017.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 https://doi.org/10.1136/bmj.d5928.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919 https://doi.org/10.1136/bmj.i4919.
Adetunji MO, Nguyen BJ, McGeehan B, Tamhankar MA, Briceño CA. Effect of teprotumumab on intraocular pressure in thyroid-associated ophthalmopathy. Taiwan J Ophthalmol. 2022;12:325–9. https://doi.org/10.4103/tjo.tjo_30_22.
Al-Sharif EM, Zhou J, Shoji MK, Acuff K, Liu CY, Korn BS, et al. Effects of teprotumumab on eyelid retraction in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2025;41:22–27. https://doi.org/10.1097/IOP.0000000000002707.
Amarikwa L, Mohamed A, Kim SH, Kossler AL, Dosiou C. Teprotumumab-related hyperglycemia. J Clin Endocrinol Metab. 2023;108:858–64. https://doi.org/10.1210/clinem/dgac627.
Bennedjaï A, Bouheraoua N, Gatfossé M, Dupasquier-Fediaevsky L, Errera MH, Tazartes M, et al. Tocilizumab versus rituximab in patients with moderate to severe steroid-resistant Graves’ orbitopathy. Ocul Immunol Inflamm. 2022;30:500–5. https://doi.org/10.1080/09273948.2020.1808688.
Boutzios G, Chatzi S, Goules AV, Mina A, Charonis GC, Vlachoyiannopoulos PG, et al. Tocilizumab improves clinical outcome in patients with active corticosteroid-resistant moderate-to-severe Graves’ orbitopathy: an observational study. Front Endocrinol. 2023;14:1186105. https://doi.org/10.3389/fendo.2023.1186105.
Campi I, Vannucchi G, Muller I, Lazzaroni E, Currò N, Dainese M, et al. Therapy with different dose regimens of rituximab in patients with active moderate-to-severe Graves’ orbitopathy. Front Endocrinol. 2022;12:790246. https://doi.org/10.3389/fendo.2021.790246.
Ceballos-Macías José J, Rivera-Moscoso R, Flores-Real Jorge A, Vargas-Sánchez J, Ortega-Gutiérrez G, Madriz-Prado R, et al. Tocilizumab in glucocorticoid-resistant Graves orbitopathy. A case series report of a Mexican population. Ann Endocrinol. 2020;81:78–82. https://doi.org/10.1016/j.ando.2020.01.003.
Dallalzadeh LO, Villatoro GA, Chen L, Sim MS, Movaghar M, Robbins SL, et al. Teprotumumab for thyroid eye disease-related strabismus. Ophthalmic Plast Reconstr Surg. 2024;40:434–9. https://doi.org/10.1097/IOP.0000000000002611.
Davis JB, Mudalegundi S, Henderson AD, Carey AR. Stability of ocular alignment after teprotumumab therapy in a cohort of patients with thyroid eye disease and baseline diplopia. J Neuroophthalmol. 2024;44:527–32. https://doi.org/10.1097/WNO.0000000000002066.
de-Pablo-Gómez-de-Liaño L, Fernández-Vigo JI, Troyano-Rivas J, Niño-Rueda C, Romo-López Á, Gómez-de-Liaño R. Response to tocilizumab treatment in Graves’ ophthalmopathy by measuring rectus muscle thickness and chemosis using optical coherence tomography. Arch Soc Esp Oftalmol. 2018;93:386–91. https://doi.org/10.1016/j.oftal.2018.04.011.
Deltour JB, d’Assigny Flamen M, Ladsous M, Giovansili L, Cariou B, Caron P, et al. Efficacy of rituximab in patients with Graves’ orbitopathy: a retrospective multicenter nationwide study. Graefes Arch Clin Exp Ophthalmol. 2020;258:2013–21. https://doi.org/10.1007/s00417-020-04651-6.
Diniz SB, Cohen LM, Roelofs KA, Rootman DB. Early experience with the clinical use of teprotumumab in a heterogenous thyroid eye disease population. Ophthalmic Plast Reconstr Surg. 2021;37:583–91. https://doi.org/10.1097/IOP.0000000000001959.
Dorado Cortez O, Grivet D, Perrillat N, Gain P, Thuret G. Treatment of corticosteroid-resistant Graves’ orbitopathy with tocilizumab: a single-centre prospective study. Orbit. 2023;42:411–7. https://doi.org/10.1080/01676830.2022.2119262.
Douglas RS, Couch S, Wester ST, Fowler BT, Liu CY, Subramanian ps, et al. Efficacy and safety of teprotumumab in patients with thyroid eye disease of long duration and low disease activity. J Clin Endocrinol Metab. 2023;109:25–35. https://doi.org/10.1210/clinem/dgad637.
Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020;382:341–52. https://doi.org/10.1056/NEJMoa1910434.
Douglas RS, Kahaly GJ, Ugradar S, Elflein H, Ponto KA, Fowler BT, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129:438–49. https://doi.org/10.1016/j.ophtha.2021.10.017.
Du Pasquier-Fediaevsky L, Andrei S, Berche M, Leenhardt L, Héron E, Rivière S. Low-dose rituximab for active moderate to severe graves’ orbitopathy resistant to conventional treatment. Ocul Immunol Inflamm. 2019;27:844–50. https://doi.org/10.1080/09273948.2018.1453078.
Eid L, Coste-Verdier V, Longueville E, Ribeiro E, Nicolescu-Catargi B, Korobelnik JF. The effects of Rituximab on Graves’orbitopathy: a retrospective study of 14 patients. Eur J Ophthalmol. 2020;30:1008–13. https://doi.org/10.1177/1120672119845224.
El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedüs L. B lymphocyte depletion with the monoclonal antibody rituximab in Graves’ disease: a controlled pilot study. J Clin Endocrinol Metab. 2007;92:1769–72. https://doi.org/10.1210/jc.2006-2388.
Erdei A, Paragh G, Kovacs P, Karanyi Z, Berenyi E, Galuska L, et al. Rapid response to and long-term effectiveness of anti-CD20 antibody in conventional therapy resistant Graves’ orbitopathy: a five-year follow-up study. Autoimmunity. 2014;47:548–55. https://doi.org/10.3109/08916934.2014.939266.
Habroosh FA, Albrashdi SS, Alsaadi AH, Eatamadi H. Tocilizumab use for optic nerve compression in thyroid eye disease: a prospective longitudinal cohort. Int Ophthalmol. 2024;44:222 https://doi.org/10.1007/s10792-024-03143-4.
Heemstra KA, Toes RE, Sepers J, Pereira AM, Corssmit EP, Huizinga TW, et al. Rituximab in relapsing Graves’ disease, a phase II study. Eur J Endocrinol. 2008;159:609–15. https://doi.org/10.1530/EJE-08-0084.
Hilliard G, Pruett J, Donahue SP, Velez FG, Peragallo JH, Ditta LC, et al. Outcomes of strabismus surgery following teprotumumab therapy. Am J Ophthalmol. 2024;262:186–91. https://doi.org/10.1016/j.ajo.2024.01.005.
Ho TC, Maamari RN, Kossler AL, Sears CM, Freitag SK, Reshef ER, et al. Outcomes of patients with thyroid eye disease partially treated with teprotumumab. Ophthalmic Plast Reconstr Surg. 2023;39:150–5. https://doi.org/10.1097/IOP.0000000000002267.
Huang J, Su A, Yang J, Zhuang W, Li Z Sr. Postmarketing safety concerns of teprotumumab: a real-world pharmacovigilance assessment. J Clin Endocrinol Metab. 2024;110:159–65. https://doi.org/10.1210/clinem/dgae417.
Hubschman S, Sojitra B, Ghiam S, Sears C, Hwangbo N, Goldberg RA, et al. Teprotumumab and orbital decompression for the management of proptosis in patients with thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2024;40:270–5. https://doi.org/10.1097/IOP.0000000000002563.
Hwang CJ, Rebollo NP, Mechels KB, Perry JD. Reactivation after teprotumumab treatment for active thyroid eye disease. Am J Ophthalmol. 2024;263:152–9. https://doi.org/10.1016/j.ajo.2023.12.001.
Insull EA, Sipkova Z, David J, Turner HE, Norris JH. Early low-dose rituximab for active thyroid eye disease: an effective and well-tolerated treatment. Clin Endocrinol. 2019;91:179–86. https://doi.org/10.1111/cen.13970.
Jain AP, Gellada N, Ugradar S, Kumar A, Kahaly G, Douglas R. Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease. Br J Ophthalmol. 2022;106:165–71. https://doi.org/10.1136/bjophthalmol-2020-317806.
Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diab Endocrinol. 2021;9:360–72. https://doi.org/10.1016/S2213-8587(21)00056-5.
Kahaly GJ, Subramanian PS, Conrad E, Holt RJ, Smith TJ. Long-term efficacy of teprotumumab in thyroid eye disease: follow-up outcomes in three clinical trials. Thyroid. 2024;34:880–9. https://doi.org/10.1089/thy.2023.0656.
Karasek D, Cibickova L, Karhanova M, Kalitova J, Schovanek J, Frysak Z. Clinical and immunological changes in patients with active moderate-to-severe Graves’ orbitopathy treated with very low-dose rituximab. Endokrynol Pol. 2017;68:498–504. https://doi.org/10.5603/EP.a2017.0040.
Keen JA, Correa T, Pham C, Claussen AD, Hansen MR, Carter KD, et al. Frequency and patterns of hearing dysfunction in patients treated with teprotumumab. Ophthalmology. 2024;131:30–36. https://doi.org/10.1016/j.ophtha.2023.08.001.
Khanna D, Chong KK, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010;117:133–139.e2. https://doi.org/10.1016/j.ophtha.2009.05.029.
Lee C, Park JW, Kim YD, Woo KI. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant thyroid eye disease: a prospective study. Int Ophthalmol. 2024;44:179 https://doi.org/10.1007/s10792-024-03117-6.
Li J, Xiao Z, Hu X, Li Y, Zhang X, Zhang S, et al. The Efficacy of Rituximab combined with 131I for ophthalmic outcomes of Graves’ ophthalmopathy patients. Pharmacology. 2017;99:144–52. https://doi.org/10.1159/000453618.
McCoy AN, Kim DS, Gillespie EF, Atkins SJ, Smith TJ, Douglas RS. Rituximab (Rituxan) therapy for severe thyroid-associated ophthalmopathy diminishes IGF-1R(+) T cells. J Clin Endocrinol Metab. 2014;99:E1294–9. https://doi.org/10.1210/jc.2013-3207.
Mitchell AL, Gan EH, Morris M, Johnson K, Neoh C, Dickinson AJ, et al. The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory Graves’ orbitopathy. Clin Endocrinol. 2013;79:437–42. https://doi.org/10.1111/cen.12141.
Moi L, Hamedani M, Ribi C. Long-term outcomes in corticosteroid-refractory Graves’ orbitopathy treated with tocilizumab. Clin Endocrinol. 2022;97:363–70. https://doi.org/10.1111/cen.14655.
Mukit FA, Manley A, Patel AB, Hashemi M, Laplant JF, Fleming JC, et al. Side effects and adverse events after treatment with teprotumumab for thyroid eye disease: a retrospective observational case series. Cureus. 2024;16:e58585. https://doi.org/10.7759/cureus.58585.
Ozzello DJ, Dallalzadeh LO, Liu CY. Teprotumumab for chronic thyroid eye disease. Orbit. 2022;41:539–46. https://doi.org/10.1080/01676830.2021.1933081.
Parunakian E, Ugradar S, Tolentino J, Malkhasyan E, Raika P, Ghaly J, et al. Teprotumumab improves light sensitivity in patients with thyroid eye disease. Graefes Arch Clin Exp Ophthalmol. 2024;262:2999–3006. https://doi.org/10.1007/s00417-024-06491-0.
Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, et al. Tocilizumab in graves orbitopathy study group. efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol. 2018;195:181–90. https://doi.org/10.1016/j.ajo.2018.07.038.
Pérez-Moreiras JV, Varela-Agra M, Prada-Sánchez MC, Prada-Ramallal G. Steroid-resistant Graves’ orbitopathy treated with tocilizumab in real-world clinical practice: a 9-year single-center experience. J Clin Med. 2021;10:706 https://doi.org/10.3390/jcm10040706.
Précausta F, Arsène S, Renoult-Pierre P, Laure B, Crinière L, Pisella PJ. Treatment by rituximab on six Grave’s ophthalmopathies resistant to corticosteroids. Ann Endocrinol. 2017;78:20–26. https://doi.org/10.1016/j.ando.2016.12.002.
Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33–40. https://doi.org/10.1530/eje.1.02325.
Salvi M, Vannucchi G, Campi I, Currò N, Simonetta S, Covelli D, et al. Rituximab treatment in a patient with severe thyroid-associated ophthalmopathy: effects on orbital lymphocytic infiltrates. Clin Immunol. 2009;131:360–5. https://doi.org/10.1016/j.clim.2008.12.005.
Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–31. https://doi.org/10.1210/jc.2014-3014.
Savino G, Mandarà E, Gari M, Battendieri R, Corsello SM, Pontecorvi A. Intraorbital injection of rituximab versus high dose of systemic glucocorticoids in the treatment of thyroid-associated orbitopathy. Endocrine. 2015;48:241–7. https://doi.org/10.1007/s12020-014-0283-1.
Sears CM, Wang Y, Bailey LA, Turbin R, Subramanian PS, Douglas R, et al. Early efficacy of teprotumumab for the treatment of dysthyroid optic neuropathy: a multicenter study. Am J Ophthalmol Case Rep. 2021;23:101111. https://doi.org/10.1016/j.ajoc.2021.101111.
Silkiss RZ, Reier A, Coleman M, Lauer SA. Rituximab for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2010;26:310–4. https://doi.org/10.1097/IOP.0b013e3181c4dfde.
Simmons BA, Tran C, Pham CM, Shriver EM. The effect of teprotumumab on eyelid position in patients with thyroid eye disease. Plast Reconstr Surg Glob Open. 2022;10:e4287 https://doi.org/10.1097/GOX.0000000000004287.
Smith LD, Moscato EE, Seiff SR. Tocilizumab for the management of thyroid-associated orbitopathy. Ophthalmic Plast Reconstr Surg. 2022;38:188–92. https://doi.org/10.1097/IOP.0000000000002027.
Smith TJ, Cavida D, Hsu K, Kim S, Fu Q, Barbesino G, et al. Glycemic trends in patients with thyroid eye disease treated with teprotumumab in 3 clinical trials. Ophthalmology. 2024;131:815–26. https://doi.org/10.1016/j.ophtha.2024.01.023.
Smith TJ, Cockerham K, Barretto N, Hirst A, Oliver L, Enstone A, et al. Bridging and validation of the specific graves ophthalmopathy quality of life questionnaire with health state utility values. Endocr Pr. 2024;30:470–5. https://doi.org/10.1016/j.eprac.2024.02.002.
Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61. https://doi.org/10.1056/NEJMoa1614949.
Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100:432–41. https://doi.org/10.1210/jc.2014-2572.
Stan MN, Salvi M. MAnagement of endocrine disease: rituximab therapy for Graves’ orbitopathy - lessons from randomized control trials. Eur J Endocrinol. 2017;176:R101–R109. https://doi.org/10.1530/EJE-16-0552.
Stevens SM, Pirakitikulr N, Lee BW. Subcutaneous tocilizumab for active thyroid eye disease refractory to orbital radiation and systemic steroids in tobacco smokers. Taiwan J Ophthalmol. 2022;12:39–43. https://doi.org/10.4103/tjo.tjo_59_21.
Teo HM, Smith TJ, Joseph SS. Efficacy and safety of teprotumumab in thyroid eye disease. Ther Clin Risk Manag. 2021;17:1219–30. https://doi.org/10.2147/TCRM.S303057.
Terrarosa AK, DeMaria LN, North VS, Garcia MD, Kim ET, Belinsky I. Menstrual irregularities and amenorrhea in thyroid eye disease patients treated with teprotumumab. Ophthalmic Plast Reconstr Surg. 2024;40:312–5. https://doi.org/10.1097/IOP.0000000000002569.
Ting MAJ, Topilow NJ, Ediriwickrema LS, Yoon JS, Liu CY, Korn BS, et al. A comparison of proptosis reduction with teprotumumab versus surgical decompression based on fat-to-muscle ratio in thyroid eye disease. Orbit. 2024;43:222–30. https://doi.org/10.1080/01676830.2023.2282509.
Toro-Tobon D, Rachmasari KN, Bradley EA, Wagner LH, Tooley AA, Stokken JK, et al. Medical therapy in patients with moderate to severe, steroid-resistant, thyroid eye disease. Thyroid. 2023;33:1237–44. https://doi.org/10.1089/thy.2023.0167.
Ugradar S, Braun J, Wang Y, Zimmerman E, Douglas RS. Facial and eyelid changes in thyroid eye disease are reversed by teprotumumab. Plast Reconstr Surg Glob Open. 2021;9:e3809 https://doi.org/10.1097/GOX.0000000000003809.
Ugradar S, Kang J, Kossler AL, Zimmerman E, Braun J, Harrison AR, et al. Teprotumumab for the treatment of chronic thyroid eye disease. Eye. 2022;36:1553–9. https://doi.org/10.1038/s41433-021-01593-z.
Ugradar S, Shi L, Wang Y, Mester T, Yang H, Douglas RS. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye. 2021;35:2607–12. https://doi.org/10.1038/s41433-020-01297-w.
Ugradar S, Wang Y, Mester T, Kahaly GJ, Douglas RS. Teprotumumab for thyroid eye disease: early response is not required for benefit. Eye. 2022;36:1403–8. https://doi.org/10.1038/s41433-021-01539-5.
Vannucchi G, Campi I, Bonomi M, Covelli D, Dazzi D, Currò N, et al. Rituximab treatment in patients with active Graves’ orbitopathy: effects on proinflammatory and humoral immune reactions. Clin Exp Immunol. 2010;161:436–43. https://doi.org/10.1111/j.1365-2249.2010.04191.x.
Vannucchi G, Campi I, Covelli D, Currò N, Lazzaroni E, Palomba A, et al. Efficacy profile and safety of very low-dose rituximab in patients with Graves’ orbitopathy. Thyroid. 2021;31:821–8. https://doi.org/10.1089/thy.2020.0269.
Vinson KB, Kirzhner M. Effects of teprotumumab on patients with long-standing, active thyroid eye disease. Am J Ophthalmol Case Rep. 2022;26:101348. https://doi.org/10.1016/j.ajoc.2022.101348.
Wang R, Song D, Zhong Y, Li H. Potential role of IGF-1R in the interaction between orbital fibroblasts and B lymphocytes: an implication for B lymphocyte depletion in the active inflammatory phase of thyroid-associated ophthalmopathy. BMC Immunol. 2024;25:31 https://doi.org/10.1186/s12865-024-00613-3.
Wang XL, Xu SS, Zhou JB, Song ZH. An observational study on the safety of teprotumumab based on FAERS database. Endocrine. 2024;85:313–20. https://doi.org/10.1007/s12020-024-03852-x.
Wang Y, Hu H, Chen L, Zhang H, Yang T, Xu X, et al. Observation study of using a small dose of rituximab treatment for thyroid-associated ophthalmopathy in seven Chinese patients: One pilot study. Front Endocrinol. 2023;13:1079852. https://doi.org/10.3389/fendo.2022.1079852.
Xin Y, Xu F, Gao Y, Bhatt N, Chamberlain J, Sile S, et al. Pharmacokinetics and exposure-response relationship of teprotumumab, an insulin-like growth factor-1 receptor-blocking antibody, in thyroid eye disease. Clin Pharmacokinet. 2021;60:1029–40. https://doi.org/10.1007/s40262-021-01003-3.
Zhang C, Ersan S, Yousef Y, Sandhur B, Desilets J, McGlone C, et al. The effect of teprotumumab infusion on ocular alignment in patients with symptomatic thyroid eye disease. J AAPOS. 2024;28:103959. https://doi.org/10.1016/j.jaapos.2024.103959.
Zhang Z, Feng X, Guo Y, Kang X, Wang D, Zhang J, et al. Efficacy of rituximab in treating steroid-resistant Graves’ orbitopathy in active moderate-to-severe and sight-threatening forms: a retrospective observation from China. Heliyon. 2024;10:e31932. https://doi.org/10.1016/j.heliyon.2024.e31932.
Li H, Yang L, Song Y, Zhao X, Sun C, Zhang L, et al. Comparative effectiveness of different treatment modalities for active, moderate-to-severe Graves’ orbitopathy: a systematic review and network meta-analysis. Acta Ophthalmol. 2022;100:e1189–98. https://doi.org/10.1111/aos.15074.
Rubbert-Roth A, Sebba A, Brockwell L, Kelman A, Porter-Brown B, Pulley J, et al. Malignancy rates in patients with rheumatoid arthritis treated with tocilizumab. RMD Open. 2016;2:e000213 https://doi.org/10.1136/rmdopen-2015-000213.
Westermann R, Cordtz RL, Duch K, Mellemkjaer L, Hetland ML, Magnussen B, et al. Cancer risk with tocilizumab/sarilumab, abatacept and rituximab treatment in patients with rheumatoid arthritis: a Danish cohort study. Rheumatology. 2025;64:1019–28. https://doi.org/10.1093/rheumatology/keae140.
Cho SK, Lee J, Han M, Bae SC, Sung YK. The risk of malignancy and its incidence in early rheumatoid arthritis patients treated with biologic DMARDs. Arthritis Res Ther. 2017;19:277 https://doi.org/10.1186/s13075-017-1482-y.
Davis JB, Mudalegundi S, Henderson AD, Carey AR. Stability of Ocular Alignment After Teprotumumab Therapy in a Cohort of Patients With Thyroid Eye Disease and Baseline Diplopia. J Neuroophthalmol. 2024;44:527–532 https://doi.org/10.1097/WNO.0000000000002066.
José JC, Rivera-Moscoso R, Jorge AF, Vargas-Sánchez J, Ortega-Gutiérrez G, Madriz-Prado R, et al. Tocilizumab in glucocorticoid-resistant graves orbitopathy. A case series report of a mexican population. InAnnales d’Endocrinologie Vol. 81, pp. 78–82. Elsevier Masson, 2020.
Author information
Authors and Affiliations
Contributions
Abdulelah Abumohssin: Writing—review and editing, Writing— original draft, Validation, Resources, Methodology, Conceptualization. Abdulasalam Alqutub: Writing—review and editing, Writing—original draft, Validation, Supervision, Methodology, Data curation. Saja Aljuhani: Writing—review and editing, Writing—original draft, Data curation. Abdulrahman Alqutub: Writing—review and editing, Validation, Supervision. Rayan Alshareef: Writing—review and editing, Writing—original draft, Visualization, Validation, Supervision, Methodology, Data curation, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abumohssin, A.G., Alshareef, R.A., Aljohani, S. et al. Comparative efficacy and safety of rituximab, tocilizumab, and teprotumumab in Graves’ orbitopathy: a systematic review and meta-analysis. Eye 39, 1901–1932 (2025). https://doi.org/10.1038/s41433-025-03845-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-025-03845-8