Abstract
Due to the covert onset of thymoma, nearly 30% of patients are diagnosed at stage III or IV, losing the opportunity for surgical treatment. We have initiated the application of prednisone in treating refractory thymoma and explored biomarkers to identify potential cases that might benefit from prednisone treatment. In a study involving 96 patients with thymoma, we confirmed a significant tumor shrinkage with prednisone acetate treatment. A reduced diameter ratio indicated that type B1 and B2 thymomas exhibited the most obvious response to prednisone acetate, especially type B2 thymoma. However, the reduced diameter ratio was < 30% in type A, AB, and B3 thymomas. Immunofluorescence and flow cytometry of tumor tissues indicated that TEMRA (T Effector Memory-Expressing CD45RA) cells primarily exist in type B thymoma. However, the percentage of interleukin-8 + TEMRA cells decreased only in B1 and B2 thymoma tissues after prednisone acetate treatment. These findings are particularly significant for patients with type B thymoma with stage III or IV.
Similar content being viewed by others
Introduction
Thymoma is the predominant type of neoplasms in the anterior mediastinum and is frequently associated with various autoimmune diseases [1]. The most common autoimmune disease associated with thymoma is myasthenia gravis (MG), characterized by muscle weakness and fatigue [2]. Studies have revealed that complete surgical resection (R0 resection) is the most important prognostic factor for patients with thymoma [3, 4]. Due to its covert onset, nearly 30% of patients with thymoma are initially diagnosed at stage III or IV and lose the opportunity for surgical treatment [5]. Unfortunately, thymomas are not sensitive to chemotherapy [6,7,8]. Due to the lack of effective targeted therapeutic drugs, we face an embarrassing predicament in treating nonresectable and metastatic thymomas [9, 10].
Interestingly, when we treated patients with severe MG and locally advanced thymoma with prednisone, some cases indicated the remission of MG and the shrinking of the thymoma. Since 2020, we have started to apply prednisone in treating refractory thymoma and tried exploring a biomarker to identify the potential cases that might benefit from prednisone treatment.
Materials and methods
Clinical data were retrospectively collected from the Department of Thoracic Surgery at Beijing Tongren Hospital between 2020 and 2023. The study included patients who underwent needle biopsy or open biopsy for various conditions. Tumor tissues were collected from a total of 96 patients, none of whom had received chemotherapy, radiotherapy, or targeted therapy before the study.
The pathological diagnosis was based on biopsy results before prednisone acetate treatment. The prednisone acetate was administered for at least one month with an oral prednisone dosage of 0.6 mg/kg. The collected specimens were immediately frozen in liquid nitrogen and stored at –80 °C until further analysis. The study protocol and sample collection procedures were approved by the Institutional Review Board of Beijing Tongren Hospital, China. Informed written consent was obtained from each patient before sample collection. All study procedures were conducted following the approved protocol.
Clinical evaluation of the response to the therapy
We assessed the maximum tumor diameter before and one month after taking prednisone acetate using computed tomography (CT) scans. The complete response rate (CRR) was the percentage of all target lesions that disappeared with no new lesions appearing. The partial response rate (PRR) was defined as the percentage of the sum of the maximum diameters of all target lesions that was reduced by ≥ 30%. The objective response rate was equal to CRR + PRR.
Antibodies
Fluorescein isothiocyanate (FITC), phycoerythrin (PE), or biotin-conjugated anti-CCR7 (7D6), anti-interleukin IL-6 (1936), anti-IL-8 (1-77), monoclonal antibodies (mAbs), and isotype controls, immunoglobulin (Ig)G1-FITC, IgG1-PE, biotinylated IgG2a, IgG2b, and IgG1 kappa, were purchased from Thermo Fisher Scientific (81 Wyman Street, Waltham, MA, USA). Peridinin chlorophyll protein conjugated anti-CD45RA (MEM-56) and IgG2b were also acquired from Thermo Fisher Scientific. The allophycocyanin-conjugated anti-CD45 (HI30) and PE-Cyanine7-conjugated anti-CD3 (7D6) were obtained from Thermo Fisher Scientific.
Isolation of tumor tissue
Tumor tissues were dissected, minced into small pieces, and digested for 1 h at 37 °C in Roswell Park Memorial Institute (RPMI) medium containing 2% fetal bovine serum supplemented with 300 U/mL type IV collagenase (Sigma–Aldrich) and 100 U/mL DNase I (Sigma–Aldrich). The pieces and fluid were passed through a sieve. The cells were suspended by vigorous pipetting, washed twice in RPMI medium, and collected by centrifugation.
Flow cytometry analysis
For immunofluorescence staining, 106 cells were incubated with biotinylated mAbs for 20 min at 4 °C. After washing with HBSS/5% FCS, the biotin-labeled cells were incubated with streptavidin-coupled PE for 20 min at 4 °C, washed twice with peripheral blood smear/0.5% body surface area, and analyzed on FACS Calibur using cell quest software (Becton Dickinson). To assess the frequency of the CD45RA + CCR7-T cells, data were collected on 2 × 105 total thymocytes. For the analysis of IL-8 expression, the cells were stained for CD45RA and CCR7 surface expression, fixed and permeabilized using IntraPrep Permeabilization reagents (Immunotech), and labeled with biotinylated anti-IL-8 and streptavidin-coupled PE.
Immunofluorescence
The cells were fixed on slides using poly-L-lysine, and the Duolink (Duolink II; Olink Biosciences) assay was performed according to the manufacturer’s instructions. The cells were fixed with 2% formaldehyde for 15 min, permeabilized with 1% Triton X-100 for 10 min, and incubated with CD45RA, CCR7 and Mki67 antibodies for 1 h at room temperature. Images were collected on a confocal microscope (LSM 700; Carl Zeiss) with Zen 2011 software using 63× objectives at room temperature. The images were then processed using ImageJ (National Institutes of Health).
Statistical analysis
The expression level of cytokines was determined using the Statistical Package for the Social Sciences software (version 23.0; IBM, Armonk, NY, USA), and statistical analyses were conducted using Graphpad Prism software (version 8.0; Graphpad Software Inc., San Diego, CA, USA). Measurement data with normal distribution are expressed as mean ± standard deviation, and groups are compared using a t-test, while for comparing TEMRA cell levels across different subtypes before treatment, an analysis of variance (ANOVA) was employed. Furthermore, Dunnett’s test was used for post-hoc pairwise comparisons, which has corrected for the issues related to multiple comparisons. For measurement data that did not conform to the normal distribution, the rank sum test was used for comparison between groups. Differences were considered statistically significant at a p < 0.05.
Results
Patient characteristics
Patient characteristics are listed in Table 1. All cases were classified according to the World Health Organization (WHO) and Masaoka’s clinical staging. All samples were obtained during surgical procedures at Beijing Tongren Hospital and were reviewed by senior pathologists. The study included 96 patients from Masaoka’s stage III (58.3%) to stage IV (41.7%). Among all the patients, 58 (60.4%) were male and 38 (39.6%) were female, with a median age of 43 years (range 21–65). Primary thymomas were classified into subtypes: type A, AB, B1, B2, and B3 according to the WHO criteria. Thymic cyst tissue was used as the control group. Fifty-six (58.3%) patients were in stage III, and 40 (41.7%) were in stage IV, according to Masaoka. Forty-five (46.9%) of them exhibited autoimmune diseases such as MG, dermatomyositis, and Good’s syndrome.
Response
We assessed the maximum tumor diameter before and one month after taking prednisone acetate using CT scans. A significant decrease in the maximum tumor diameter was observed after prednisone acetate treatment (Fig. 1). A reduced diameter ratio of at least 30% was obtained in 58 patients, resulting in an overall response rate of 60.4%. This included all patients with type B1 and B2. Patients with type B1 and B2 thymomas exhibited the most obvious response to prednisone acetate, especially type B2 thymoma. In type B1, 18 patients’ reduced diameter ratio reached 30–60%, and 10 patients reached 60–90%. In type B2, 13 patients’ reduced diameter ratio reached 30–60%, and 16 patients reached 60–90%; even 1 patient’s tumor disappeared in a CT scan. However, there was no significant difference in type A, AB, and B3 thymomas after prednisone acetate treatment. Only one patient with type A, 4 with type AB, and 5 with type B3 achieved a reduced diameter ratio of 30%–60%, while the others indicated no change after taking prednisone acetate (Table 2, Fig. 2). All patients subsequently underwent tumor resection after prednisone acetate treatment, and the tumor tissue was sent to further study.
a The maximum tumor diameter was measured before prednisone acetate treatment (left) and one month after prednisone acetate treatment (right) using a CT scan in patients with thymoma. b Type B1 and B2 were sensitive to prednisone acetate treatment. Statistical differences were determined using the t-test. Adjusted p-values were considered *** (adjusted p < 0.05).
Toxicity
Eleven patients experienced electrolyte disturbances, such as hypokalemia and hypocalcemia. A total of 53 patients exhibited insomnia, 40 patients experienced loss of appetite, and 25 patients developed obesity. None of the patients exhibited wound infection or delayed wound healing.
Discovery study of TEMRA cells’ proportion for thymoma
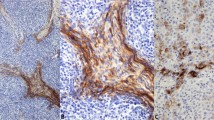
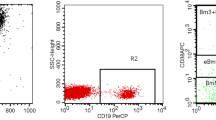
Glucocorticoids significantly impacted T lymphocytes [11], and the thymus was a crucial immune organ that impacted T-cell homeostasis and function. Thymic tumors such as thymomas were associated with various autoimmune diseases, including MG, Good’s syndrome, dermatomyositis, Addison’s disease, and rheumatoid arthritis [12]. These associations might be related to abnormalities in T-cell function. TEMRA cells, characterized by the re-expression of CD45RA, were implicated in immunosenescence and autoimmune diseases. Aging is a dynamic process that progresses with concomitant organ function deterioration, leading to loss of quality of life and declining immunity. This was highlighted by the increased incidence of malignancy and loss of immunity to previously encountered pathogens [13]. Several studies and reports have confirmed that cytomegalovirus (CMV) infection was closely associated with thymoma and autoimmune diseases. It was widely recognized that the activation by persistent viruses such as CMV in vivo could decrease the proportion of naive T cells during aging and increase the proportion of senescent cells, including TEMRA cells [14,15,16,17]. Before prednisone acetate treatment, immunofluorescence analysis of tumor tissues revealed the presence of TEMRA cells (CD45RA + CCR7-) predominantly in type B thymoma (Fig. 3). We further validated this result using flow cytometry analysis, which yielded consistent findings (Fig. 4). The results proved that the proportion of TEMRA cells increased in type B1, B2 and B3 thymomas tissue, while the proportion of TEMRA cells in type A and AB thymomas tissue was similar to that in the control group.
The proportions of TEMRA cells (CD45 + CD3 + CD45RA + CCR7-) in tissues from patients with thymoma with various pathological patterns (A, n = 14; AB, n = 8; B1, n = 10; B2, n = 16; and B3, n = 14) and age-matched controls (thymic cysts, n = 10) were analyzed by flow cytometry. a Representative flow cytometry plots of TEMRA cells in tissues from patients (Q1 panel). b Type B1, B2, and B3 thymoma tissues had more TEMRA cells than other types. The statistical differences were determined using the t-test. Adjusted p-values were considered *** (adjusted p < 0.05).
To exclude the effect of prednisone on cancer cells, we further counted the positive levels of Mki67 before and after treatment, and found that the levels of Mki67 in different types of thymoma tissues did not respond significantly to hormone therapy (Fig. 5).
Tumor tissues were marked by Mki67 antibodies in various pathological types of tissues by immunofluorescence. After treatment, the Mki67 level increased in one patient each for types A, AB, and B3, while it decreased in 1, 1, 3, and 2 patients respectively for types A, AB, B1, and B2. However, the changes in Mki67 in any of the groups were not statistically significant (P > 0.05).
Discovery study of IL-8’s level in TEMRA cells for thymoma
Several researchers have proposed IL-8 as a potential biomarker for thymoma identification, as its levels in naive T cells in peripheral blood were significantly elevated in patients with thymoma compared to those with other thymic tumors [18]. However, we found that the proportion of TEMRA cells increased in type B thymoma tissues except naive T cells. Consequently, we employed flow cytometry to investigate whether IL-8 was also secreted by TEMRA cells before prednisone acetate treatment. Our results indicated that IL-8 was detected in TEMRA cells, but the level of IL-8 + TEMRA cell in type B3 was lower than B1 and B2 thymomas tissue (Fig. 6). This might explain why prednisone acetate exhibited an obvious effect on shrinking B1 and B2 thymomas than B3 thymoma.
The proportions of IL-8 + TEMRA cells in patients with various types of thymoma tissues (B1, B2, and B3) were measured. a Numbers adjacent to the outlined areas of flow cytometry plots indicate the percentages of IL-8 + TEMRA cells. b IL-8 + TEMRA cells can be detected in B1, B2, and B3 thymoma tissues, but the level of IL-8 + TEMRA cells in type B3 was lower than B1 and B2 thymoma tissues. The statistical differences were determined using the t-test. Adjusted p-values were considered *** (adjusted p < 0.05).
Therefore, we chose all of the type B2 tumor tissue and six of type B1 tumor tissue, which exhibited a significant response to prednisone acetate to detect the IL-8+ level TEMRA cell after prednisone acetate treatment. The result demonstrated decreased IL-8+ level TEMRA cells after prednisone acetate treatment (Fig. 7).
The proportions of IL-8 + TEMRA cells in patients with various types of thymoma tissues (B1 and B2) were measured after prednisone acetate treatment. a Numbers adjacent to the outlined areas of flow cytometry plots indicate the percentages of IL-8 + TEMRA cells. b The level of IL-8 + TEMRA cells decreased after prednisone acetate treatment. The statistical differences were determined using the t-test. Adjusted p-values were considered *** (adjusted p < 0.05).
Discussion
In this study, we confirmed a significant tumor shrinkage effect of prednisone acetate treatment in 24 patients with thymoma, including 16 patients with type B2 and 8 with type B1. In reviewing past studies, we found that the study focused on the effect of hormones on CD4 + CD8+ double-positive immature thymocytes. Double-positive thymocytes revealed the highest glucocorticoid receptor expression and were sensitive to glucocorticoid-induced apoptosis. Type B1 thymoma was the best responder to the steroid pulse therapy [19,20,21,22]. However, our study found that type B2 was a better responder than type B1. We tried finding another way to explain this phenomenon.
Our study observed that the percentage of IL-8 + TEMRA cells decreased in B1 and B2 thymoma tissues after prednisone acetate treatment. Age-related changes in human T-cell populations, particularly terminally differentiated CD8+ effector memory CD45RA + TEMRA cells, contribute to immunosenescence and are increased in age-related chronic inflammatory diseases [23], such as Alzheimer’s disease [24] and myocarditis. Glucocorticoid treatment has progressed TEMRA CD8+ cells into an exhausted phenotype, with increased expression levels of proinflammatory chemokines. These cells interact with innate immune cells and lack key anti-inflammatory signals [25].
Since 2015, we have also noticed an investigation into IL-8’s role in thymoma progression and relapse [26]. Recently, many researchers have evaluated IL-8 as a biomarker, mainly for inflammatory diseases [27, 28]. IL-8 is known for its significance in many cancers [28, 29]. The impact of IL-8 on Melanoma pathogenesis, prognosis, and therapy has also been investigated in some studies [30]. In type B thymoma, our study revealed that TEMRA cells can also secrete IL-8, with higher proportions of IL-8 + TEMRA cells in type B1 and B2 compared to type B3.
Research indicates that thorough surgical removal (R0 resection) is a critical prognostic indicator for patients with thymoma. Approximately 30% of individuals diagnosed with thymoma exhibit disease progression to stages III or IV due to the tumor’s subtle onset. Regrettably, the majority of patients presenting with stage III or IV thymoma do not achieve R0 resection status. Accordingly, neoadjuvant therapy is imperative for patients diagnosed with stage III or IV thymoma to enhance their treatment outcomes. Nevertheless, the development of neoadjuvant therapy for thymoma has been stagnant over recent decades, partly attributed to the limitations and challenges associated with immunotherapy and targeted therapy interventions. Our study and some past case reports indicated that prednisone acetate can significantly shrink thymoma [31,32,33,34]. Consequently, the suitable timing and scene for using glucocorticoids in treating thymoma is crucial. We proposed confirming the pathological type of thymoma before operation and measuring the percentage of IL-8 + TEMRA cells in needle biopsy samples by flow cytometry, which were necessary before prednisone acetate treatment. Our results indicate the level of IL-8 + TEMRA cell in type B3 was lower than B1 and B2 thymomas tissue, and prednisone acetate decreases the level of IL-8 + TEMRA cell in type B1 and B2. So, we think the high level of IL-8 + TEMRA cell is a biomarker indicating that glucocorticoid treatment can be effective for patients with type B1 and B2 thymomas. In this way, we can evaluate whether the patient is suitable for predisone acetate. It can break through the limitations of current chemotherapy, immunotherapy and targeted therapy to a certain extent, and provide surgical opportunities for patients with inoperable thymoma. It’s extremely meaningful in prolonging the life of thymoma patients.
Conclusion
Our study confirms an increase in TEMRA cells in type B thymoma. This phenomenon indicates that type B thymoma may promote cell senescence, accumulating TEMRA cells and inflammatory cytokines. Furthermore, the high level of IL-8 + TEMRA cells may serve as a biomarker, indicating that glucocorticoid treatment can be effective for patients with type B1 and B2 thymomas. This discovery is significant for patients with type B thymoma in stage III or IV, as it can enhance their survival time and quality of life, offering important value for overcoming thymoma.
Data availability
All data supporting the findings of this study are available in the article, supplementary information, or from the corresponding authors upon reasonable request.
References
Falkson CB, Vella ET, Ellis PM, Maziak DE, Ung YC, Yu E. Surgical, radiation, and systemic treatments of patients with thymic epithelial tumors: a systematic review. J Thorac Oncol. 2023;18:299–312.
Richman DP. The Future of Research in Myasthenia. JAMA Neurol. 2015;72:812–4.
Girard N, Mornex F, Van Houtte P, Cordier JF, van Schil P. Thymoma: a focus on current therapeutic management[J]. J Thorac Oncol. 2009;4:119–26.
Du X, Cui J, Yu XT, Yu L. Risk factor analysis of thymoma resection and its value in guiding clinical treatment[J]. Cancer Med. 2023;12:13408–14.
Girard N, Lal R, Wakelee H, Riely GJ, Loehrer PJ. Chemotherapy definitions and policies for thymic malignancies[J]. J Thorac Oncol. 2011;6:S1749–55.
Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan[J]. Ann Thorac Surg. 2003;76:878–84.
Hassan M, Seoud DE. Multimodality treatments in locally advanced stage thymomas[J]. Hematol Oncol Stem Cell Ther. 2009;2:340–4.
Riely GJ, Huang J. Induction therapy for locally advanced thymoma[J]. J Thorac Oncol. 2010;5:S323–6.
Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II Trial[J]. J Clin Oncol. 2019;37:2162–70.
Strobel P, Hohenberger P, Marx A. Thymoma and thymic carcinoma: molecular pathology and targeted therapy[J]. J Thorac Oncol. 2010;5:S286–90.
Kalfeist L, Galland L, Ledys F, Ghiringhelli F, Limagne E, Ladoire S. Impact of glucocorticoid use in oncology in the immunotherapy era. Cells. 2022;11:770.
Yu XT, Yu L, Du X, Yu Z, Yang XG, Jiang YX. Clinical and genetic characteristics of thymoma patients with autoimmune hepatitis and myocarditis. Front Oncol. 2021;11:746304.
Laphanuwat P, Gomes D, Akbar AN. Senescent T cells: Beneficial and detrimental roles. Immunol Rev. 2023;316:160–75.
Pereira BI, De Maeyer RPH, Covre LP, Belaid DN, Lanna A, Ward S, et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat Immunol. 2020;21:684–94.
Akbar AN, Soares MV, Plunkett FJ, Salmon M. Differential regulation of CD8+ T cell senescence in mice and men. Mech Ageing Dev. 2000;121:69–76.
Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2014;54:1–5.
Chang R, Duan S, Li S, Zhang P. Viral infection in thymoma and thymic tumors with autoimmune diseases. Thorac Cancer. 2021;12:2971–80.
Gao S, Jiang JH, Jin C, Gao J, Xiong D, Yang PJ, et al. Interleukin-8 as a candidate for thymoma identification and recurrence surveillance. Nat Commun. 2020;11:4881.
Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–6.
Cohen AH, Bluestein HG, Redelman D. Dexyadenosine modulates human suppressor T cell function and B cell differentiation stimulated by Staphylococcus aureus protein A. J Immunol. 1984;132:1761–6.
Berki T, Palinkas L, Boldizsar F, Nemeth P. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int Immunol. 2002;14:463–9.
Wiegers GJ, Knoflach M, Bock G, Niederegger H, Dietrich H, Falus A, et al. CD4(+)CD8(+) TCR(low) thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis. Eur J Immunol. 2001;31:2293–301.
Salumets A, Tserel L, Rumm AP, Turk L, Kingo K, Saks K, et al. Epigenetic quantification of immunosenescent CD8(+) TEMRA cells in human blood. Aging Cell. 2022;21:e13607.
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577:399–404.
Zhu H, Galdos FX, Lee D, Waliany S, Huang YV, Ryan J, et al. Identification of pathogenic immune cell subsets associated with checkpoint inhibitor-induced myocarditis. Circulation. 2022;146:316–35.
Wu Y, Das A, Hayday A, Lal R, Gibbons D. Does IL8 (CXCL8) have a role in thymoma progression and as a marker for relapse?. J Thorac Dis. 2015;7:AB109.
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–92.
Filimon A, Preda IA, Boloca AF, Negroiu G. Interleukin-8 in melanoma pathogenesis, prognosis and therapy-an integrated view into other neoplasms and chemokine networks. Cells. 2021;11:120.
Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7:1543–88.
Srivastava SK, Bhardwaj A, Arora S, Tyagi N, Singh AP, Carter JE, et al. Interleukin-8 is a key mediator of FKBP51-induced melanoma growth, angiogenesis and metastasis. Br J Cancer. 2015;112:1772–81.
Green JD, Forman WH. Response of thymoma to steroids[J]. Chest. 1974;65:114–6.
Posner JB, Howieson J, Cvitkovic E. Disappearing” spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases[J]. Ann Neurol. 1977;2:409–13.
Zouvelou V, Vamvakaris I, Tentolouris-Piperas V, Potaris K, Velonakis G. The effect of glucocorticoids on radiology and histology of thymoma in myasthenia gravis[J]. Acta Neurol Belg. 2022;122:1073–5.
Tateyama H, Takahashi E, Saito Y, Fukai I, Fujii Y, Niwa H, et al. Histopathologic changes of thymoma preoperatively treated with corticosteroids[J]. Virchows Arch. 2001;438:238–47.
Acknowledgements
We are grateful to all current and former members of our laboratories and departments for their excellent contributions to the research projects cited in this article. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
This study was supported by the Clinical Technology Innovation Project of Beijing Hospital Authority (XMLX201839) and the National Natural Science Foundation of China (Grant Number: 81550004).
Author information
Authors and Affiliations
Contributions
Xintao Yu and Lei Yu: Conceptualization, methodology, and software. Xintao Yu: Data curation and writing-original draft preparation. Jian Cui: Visualization, investigation, and supervision. Xiang Gao: Software and validation. Xingguo Yang: Writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics
Informed consent was obtained from all participants who signed consent forms for this study. The study was approved by the Human Research Ethics Board of Beijing Tongren Hospital, Capital Medical University, and all experiments were performed following relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, Xt., Cui, J., Yang, Xg. et al. Novel modulation of T effector memory cells-expressing CD45RA by prednisone in inoperable advanced type B thymoma patients. Genes Immun 26, 222–228 (2025). https://doi.org/10.1038/s41435-025-00329-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-025-00329-3