Abstract
Although high blood pressure (BP) and BP variability have been reported to be associated with cognitive impairment, few studies have investigated the association between home BP (HBP) and cognitive function in the oldest-old. The aim of this study was to evaluate whether the value of and the day-to-day variability in HBP was associated with cognitive function in a Japanese community-dwelling oldest-old population. Among 111 participants aged 85–87 years, cognitive function was assessed using the Japanese version of the Montreal Cognitive Assessment (MoCA-J). HBP was measured two times every morning for a median of 30 days. The value of and variability in HBP were calculated as the average and coefficient of variation (CV) of the measurements, respectively. The associations of HBP variability with MoCA-J were examined using multiple linear regression models. Of 111 participants, 47.7% were men, and 64.0% were taking medications for hypertension. The mean HBP was 141.9 ± 14.8/72.2 ± 8.4 mmHg, and the mean CV of HBP was 6.7 ± 1.9/6.8 ± 2.4. The mean total MoCA-J score was 22.9 ± 3.5. The MoCA-J score was significantly lower with increasing CVs of both systolic BP (b = −0.36, p = 0.034) and diastolic BP (b = −0.26, p = 0.046) after adjustment for possible confounding factors. The value of HBP was not associated with MoCA-J. In the community-dwelling oldest-old population, higher day-to-day HBP variability, but not the value of HBP, was associated with cognitive impairment. When measuring HBP, attention should be paid not only to the values but also to their variations.
Similar content being viewed by others
Introduction
With the increase in the aging population, the number of people living with cognitive impairment has been increasing worldwide [1]. The prevalence of cognitive impairment increases exponentially with age, with the most likely population to be affected being the oldest-old, or people aged 85 years or older [1, 2]. Regarding dementia, the prevalence was estimated to be approximately 1.5% at age 65 years, and the prevalence doubles every 4 years to reach approximately 30% at 80 years [3]. In Japan, with the oldest population in the world, cognitive impairment in the oldest-old has become a great concern for health and social care.
Hypertension, also a common disorder in the oldest-old, is a well-known risk factor for vascular dementia [4]. It has also been reported to be a risk factor for cognitive decline [5], mild cognitive impairment (MCI) [6], and Alzheimer’s disease [7]. In recent years, in addition to blood pressure (BP), BP variability has been increasingly recognized as being associated with cognitive impairment [8, 9]. However, the majority of previous studies exploring BP, BP variability, and cognitive function focused on BP values and visit-to-visit variability based on casual office BP measurements. Home BP (HBP) measurements are more reproducible, have no white-coat effect and have more prognostic significance than casual office BP measurements [10]. The utility of HBP measurement is marked in older adults who demonstrate higher white-coat hypertension prevalence and BP variability than younger adults [10, 11]. To date, few studies have investigated the association between the value and variability of HBP and cognitive impairment [12,13,14,15]. Furthermore, only one epidemiological study has targeted the oldest-old [15]. As the influence of BP on cognitive function has been suggested to vary in different age groups [5, 16] and the oldest-old show distinct sociodemographic characteristics compared with those of younger adults, studies on this specific age group are considered important. BP variation increases with age [11]. Based on the current literature, we hypothesized that the influence of BP variability on cognitive function might be prominent in the oldest-old population.
The aim of this study was to evaluate whether HBP and the day-to-day HBP variability were associated with cognitive function precisely evaluated by the Japanese version of the Montreal Cognitive Assessment (MoCA-J) in a Japanese community-dwelling oldest-old population to obtain a better understanding of the association between BP and cognitive function in the oldest-old.
Methods
Participants
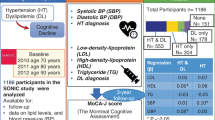
This cross-sectional study was part of the SONIC study, an ongoing prospective cohort study targeting community-dwelling older people in their 70, 80, 90s, or over 100 years old in two regions (eastern and western regions) of Japan. A detailed description of the study design and protocol has been published elsewhere [16,17,18]. We used a narrow age-range cohort design, with each cohort including individuals whose ages fell within a 3-year range. In 2017, a second follow-up survey of those in their 80s was conducted, and 273 individuals aged 85–87 years participated in the survey in the western region. After excluding subjects with clear cognitive and/or physical function decline, we recruited 133 participants to measure BP at their home. After the exclusion of 15 participants who did not consent to participate in the study, 3 who had a history of stroke, and 4 who had missing data, 111 participants were included in the present analysis.
The study protocol was approved by the ethical committee of Osaka University Hospital (approval number: 266). Written informed consent was obtained from all participants.
BP measurement
Physicians or nurses instructed the participants on how to perform HBP measurements using an explanation form. After the explanation, participants measured their BP with an upper-arm electronic oscillometric device (HEM-7080 IC; Omron Healthcare Co., Ltd, Kyoto, Japan) and wrote the value as a sample on the recording sheet at the survey site with physicians or nurses present. The HEM-7080 IC was successfully validated in the oldest-old population [19]. The HEM-7080 IC automatically records up to 350 BP measurements and can extract these data for analysis. The participants were asked to measure their BP 2 times every morning within 1 h after waking up, after urination, before breakfast and taking medication, after a minimum of 1–2 min of rest, in a sitting position for 30 days using the HEM-7080 IC. The mean of two measurements per morning was used as the value for each day. The value and variability in HBP were calculated using all the obtained measurements as the average and within-participant coefficient of variation (CV) of the measurements, respectively. The CV was calculated with the formula: SD of the daily HBP/mean daily HBP × 100.
Office BP was measured twice consecutively by a trained physician or nurse with the participant in a sitting position after at least a few minutes of rest on the left arm using a mercury sphygmomanometer. The mean of the two readings was defined as the office BP.
Assessment of cognitive function
Cognitive function was assessed using the MoCA-J by trained geriatricians and/or psychologists. The MoCA-J is a brief cognitive screening tool for detecting MCI in elderly people and assesses the following domains of cognition: visuoexecutive, naming, attention, language, abstraction, recall, and orientation [20]. Years of education were determined from a self-administered questionnaire, and 1 point was added to the total MoCA-J score if an individual had 12 years or fewer of education. The total MoCA-J score ranges from 0 to 30, with higher scores reflecting a higher cognitive function. The MoCA-J has demonstrated good reliability and validity for predicting early cognitive decline compared with the performance of conventional cognitive tests [20, 21].
Other factors
The data collection procedure included an interview, a self-administered questionnaire, physical examinations, and laboratory tests. Information on medications was obtained from a prescription record that the participants brought. Medical history was assessed during an interview with reference to the prescription record. Diabetes was defined as a casual plasma glucose level ≥ 200 mg/dL (11.1 mmol/L), HbA1c ≥ 6.5%, a self-reported diagnosis of diabetes, or the use of antidiabetic agents based on the Japan Diabetes Society criteria [22]. Psychological well-being was evaluated using the WHO 5-item well-being index (WHO-5). The WHO-5 is a sensitive and specific screening tool for depression, and its applicability across study fields is very high [23]. Gait speed was measured over a 2.44-m (8-ft) walking course. Participants were instructed to walk at their normal, comfortable pace. Measurements were taken twice, and the mean speed of the two walking trials was used for the analysis.
Statistical analysis
Descriptive data are presented as n (%) or mean ± SD. Differences in the clinical characteristics and BP profiles associated with the CV quartiles of HBP were assessed by analysis of variance and the chi-squared test. Scatter plots with linear regression were used to show the associations and ensure linear relationships between BP, CV of HBP, and MoCA-J for both systolic BP (SBP) and diastolic BP (DBP). The associations of CV of HBP with MoCA-J were examined using multiple linear regression models [16, 18, 24] including sex, the corresponding mean HBP, antihypertensive medication, diabetes mellitus, history of arrhythmia, WHO-5, and gait speed. Analysis with same multiple linear regression models but including home pulse pressure instead of mean HBP was also performed. The education level was not included in the model since a 1-point correction for education was adopted to calculate the MoCA-J score; further analysis was performed with the model adjusted for years of education. The association between CV of HBP and MoCA-J subdomain scores was tested with quartiles of CV of HBP using the Jonckheere–Terpstra test because the subdomain scores were small. All statistical analyses were performed with SPSS Statistics ver. 24.0 for Windows (IBM Japan, Tokyo, Japan). A two-tailed value of P < 0.05 was considered significant.
Results
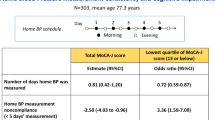
The characteristics of the participants are shown in Table 1. Of 111 participants aged 85–87 years old, 47.7% were men and 64.0% were taking medication for hypertension. The participants’ profiles of HBP and office BP are shown in Table 2. HBP was measured for a median of 30 days (range, 6–30 days). The mean HBP was 141.9 ± 14.8/72.2 ± 8.4 mmHg, and the mean CV of HBP was 6.7 ± 1.9/6.8 ± 2.4. There were no significant differences in the clinical characteristics or BP profiles based on the CV quartiles of HBP (Supplementary Table 1, Supplementary Table 2).
The mean total MoCA-J score was 22.9 ± 3.5 (range: 14–30). Scatter plots of the associations between HBP variables (mean HBP and CV of HBP) and MoCA-J are presented in Fig. 1, suggesting that the MoCA-J score decreased with an increase in HBP variability. Neither HBP nor office BP (r = 0.04, p = 0.704 for SBP; r = 0.06, p = 0.507 for DBP) was associated with cognitive function. The regression coefficients for the MoCA-J score are shown in Table 3. In the multiple linear models, the MoCA-J was significantly lower with increasing CVs of both SBP and DBP after adjustment for possible confounding factors. The significant associations between CV and the MoCA-J score were substantially unchanged after adjustment for pulse pressure instead of mean HBP (b = −0.33, p = 0.051 for SBP; b = −0.27, p = 0.040 for DBP). Further adjustment for education level did not change the significance of the association between CV and the MoCA-J score (b = −0.34, p = 0.042 for SBP; b = −0.28, p = 0.032 for DBP). In addition to BP variability, gait speed was associated with the MoCA-J score. Concerning the association between the CV quartiles of HBP and the subdomain score of MoCA-J, the subdomain score of visuoexecutive decreased significantly with higher CV levels of SBP, and the score of attention decreased with higher CV levels of DBP (Supplementary Table 3).
Discussion
Among community-dwelling oldest-old people aged 85–87 years, a negative association between BP variability assessed by CV of HBP and cognitive function assessed by the MoCA-J score was found. However, the value of HBP was not associated with cognitive function.
There are growing reports that BP variability affects cognitive impairment [8, 9]. Although BP variability was defined mainly as visit-to-visit BP variability based on office BP in most studies [8], some studies have shown an association between HBP variability and cognitive decline or dementia [12,13,14,15]. The Ohasama study demonstrated that day-to-day BP variability in systolic HBP and home SBP values were associated with cognitive decline [13]. The Hisayama study investigated significant associations between higher day-to-day HBP variability and dementia [14]. These studies included subjects with average ages of 63 and 71 years, respectively. A study in China targeting people aged 80 years or older demonstrated that a higher CV of systolic HBP was associated with a lower MMSE score [15]. Consistent with these findings, our analyses indicated that increased HBP variability is an independent contributor to the MoCA-J score in Japanese oldest-old individuals.
Excessive BP variability may lead to lower cognitive performance due to cerebral functional impairment and structural vascular changes [8, 25, 26]. Hemodynamic instability associated with exaggerated BP variability could increase stress on the vessel wall and lead to microvascular damage and vascular endothelial dysfunction [8, 25]. Excessive BP variability may cause decreased perfusion and oxygenation as BP decreases, especially in individuals without hypertension [26]. In older people, higher BP could be a compensatory mechanism to maintain organ perfusion. Even in individuals with hypertension, excessive BP variability might cause decreased perfusion in the oldest-old. Cerebral microvascular damage and disturbed perfusion lead to functional impairment and structural vascular changes of the brain via neuronal cell injury and ischemia, which may lead to cognitive impairment or dementia. As BP variation increases with age [11], the influence of BP variability on microvascular damage, vascular endothelial dysfunction, and reduced perfusion might be prominent in the oldest-old population. In addition, increased BP variability may reflect arterial stiffness or autonomic neuropathy [27, 28], which may partly mediate the association between BP variability and cognitive impairment, although home pulse pressure was not associated with cognitive function.
Not only SBP variability but also DBP variability was associated with cognitive function in the present study. The association between DBP variability and cognitive impairment was less consistent in previous studies [13, 14, 29, 30]. This inconsistency may have arisen from different characteristics of the study populations, different definitions of BP variability or cognitive function or other unknown causes; age could also partly explain the inconsistency. DBP is the main determinant of the mean pressure, which is an indicator of organ blood flow. Excessive DBP variability may cause cerebral ischemia. This possibility might be greater in older people, as DBP tends to spontaneously decrease with aging as a result of stiffening of the arterial wall and decreasing vessel wall compliance [31].
In the present study, neither HBP nor office BP was associated with cognitive function. Many epidemiological studies have identified hypertension or high BP in mid-life as a risk factor for later-life cognitive decline or the development of dementia [5]. On the other hand, a conflicting association between late-life BP and cognitive functioning, with no associations or a U- or J-shaped association, has been demonstrated [5]. In some long-duration follow-up studies, trajectories of BP differed depending on the dementia status and antihypertensive medication [32]. The association between BP and cognitive impairment is complex and involves various factors, such as age, duration of hypertension, hypertensive medication, and comorbid health status, especially in older people [5, 33]. In this age group, it might be difficult to discuss the association with cognitive function using only once-measured BP levels, whether based on casual office BP measurements or HBP measurements.
In the present analysis, gait speed was significantly associated with cognitive function. Several previous studies indicated that a slow gait speed was a predictor of cognitive decline or dementia [34]. Our study also showed that physical and cognitive functions are related in oldest-old people. It was shown that physical function may need to be considered when examining the cognitive function of the elderly.
To the best of our knowledge, the present study provides the first evidence that increased day-to-day HBP variability is associated with poorer cognitive function assessed by MoCA-J in a community-dwelling oldest-old population. The underlying mechanism and clinical significance might differ between the types of BP variability [35]. The high availability, feasibility, utility, and tolerability of HBPM have been acknowledged. Measurement of HBP is possible for both hypertensive and nonhypertensive individuals. The utility of HBP measurement is marked in older adults who demonstrate a higher hypertension prevalence, white-coat hypertension prevalence, and BP variability than younger adults [10, 11]. It is clinically valuable for the association between HBP variability and cognitive function to be clarified. However, another BP variability index, for example, orthostatic hypotension, which is supposed to indicate major BP variability in older people, has also been reported to be associated with cognitive impairment [36]. Further investigations are required to elucidate the relationship between each BP variability index and to clarify the influence of the variability indices on cognitive impairment in the oldest-old population. In addition, MoCA-J, exhibiting a comparatively normal distribution, has been reported to show higher sensitivity to a subtle cognitive change than conventional tools such as the mini-mental state examination [20, 21]. MoCA-J is a useful tool for detecting MCI, a transitional stage between natural aging and dementia. In addition, different global cognitive tests incorporate different cognitive domains. In the present study, increased HBP variability was associated with the visuoexecutive and attention domains. Zhao JH et al. reported that patients with cerebral hypoperfusion after cerebral angiostenosis or occlusion had lower scores in the visuoexecutive, attention, abstraction, and recall domains [37]. Working memory and attention inhibition are closely related to executive functions [38]. These cognitive processes rely on the integrity of frontal and subcortical brain structures [5, 38]. The exact mechanism responsible for the effect of BP variability on specific domains of cognitive impairment is unclear, and hypoperfusion caused by exaggerated BP variability might lead to ischemia in the cerebral area. Using different cognitive tests could contribute to a further exploration of the relationship between HBP and cognitive function.
There were several limitations of this study. First, due to the nature of the cross-sectional study design, our findings do not clarify the causal relationship between HBP variability and cognitive function. A reverse association may be present as well. Irregularity or errors in self-measurement and poor medication compliance could lead to greater BP variability. Second, our sample size was small. However, as we used a narrow age-range design, age, which largely affects cognitive function and BP variability, was controlled for. Third, the study participants were recruited from a pool of volunteers who could participate in the survey on site. Most of the participants live independently and had a relatively higher level of cognitive function. A previous study involving Japanese community-dwelling older people reported that the mean MoCA-J was 21.4 for individuals 75–85 years old and 20.5 for individuals ≥80 years [39]. Thus, the participants were not a representative sample of oldest-old individuals in the general population. The results cannot be applied to all older people.
Among community-dwelling oldest-old people, increased day-to-day BP variability assessed by HBP measurements was significantly associated with poorer cognitive function assessed by MoCA-J. When measuring HBP, attention should be paid not only to the values but also to their variations. Even for the oldest-old who can live independently in the community, cognitive function tests might be recommended if BP variability is relatively high. It is desirable to clarify the causal relationship between HBP variability and cognitive decline in the oldest-old population with a prospective study.
References
Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer report 2015—the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International (ADI);2015.
Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29:1595–608.
Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–66.
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713.
Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24.
Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506.
Meng XF, Yu JT, Wang HF, Tan MS, Wang C, Tan CC, et al. Midlife vascular risk factors and the risk of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42:1295–310.
Nagai M, Hoshide S, Dote K, Kario K. Visit-to-visit blood pressure variability and dementia. Geriatr Gerontol Int. 2015;15:26–33.
Jung HW, Kim KI. Blood pressure variability and cognitive function in the elderly. Pulse. 2013;1:29–34.
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29.
Kamide K, Kabayama M. Implications of blood pressure variations in older populations. Hypertens Res. 2019;42:19–25.
Yeung SE, Loken Thornton W. "Do it-yourself": home blood pressure as a predictor of traditional and everyday cognition in older adults. PLoS ONE. 2017;12:e0177424.
Matsumoto A, Satoh M, Kikuya M, Ohkubo T, Hirano M, Inoue R, et al. Day-to-day variability in home blood pressure is associated with cognitive decline: the Ohasama study. Hypertension. 2014;63:1333–8.
Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, et al. Day-to-day blood pressure variability and risk of dementia in a general Japanese elderly population: The Hisayama Study. Circulation. 2017;136:516–25.
Liu Z, Zhao Y, Zhang H, Chai Q, Cui Y, Diao Y, et al. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res. 2016;39:245–53.
Ryuno H, Kamide K, Gondo Y, Nakama C, Oguro R, Kabayama M, et al. Differences in the association between high blood pressure and cognitive functioning among the general Japanese population aged 70 and 80 years: The SONIC study. Hypertens Res. 2016;39:557–63.
Gondo Y, Masui Y, Kamide K, Ikebe K, Arai Y, Ishizaki T. SONIC Study: A longitudinal cohort study of the older people as part of a Centenarian study. In NA Pachana, editor. Encyclopedia of geropsychology. Singapore: Springer Science+Business Media; 2016.
Ikebe K, Gondo Y, Kamide K, Masui Y, Ishizaki T, Arai Y, et al. Occlusal force is correlated with cognitive function directly as well as indirectly via food intake in community-dwelling older Japanese: From the SONIC study. PLoS ONE. 2018;13:e0190741 https://doi.org/10.1371/journal.pone.0190741.
Godai K, Kabayama M, Saito K, Asayama K, Yamamoto K, Sugimoto K, et al. Validation of an automated home blood pressure measurement device in oldest-old populations. Hypertens Res. 2019. https://doi.org/10.1038/s41440-019-0330-7.
Fujiwara Y, Suzuki H, Yasunaga M, Sugiyama M, Ijuin M, Sakuma N, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int. 2010;10:225–32.
Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50:1039–52.
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–28.
Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84:167–76.
Conway KS, Forbang N, Beben T, Criqui MH, Ix JH, Rifkin DE. Relationship between 24-Hour ambulatory blood pressure and cognitive function in community-living older adults: the UCSD Ambulatory Blood Pressure Study. Am J Hypertens. 2015;28:1444–52.
Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43:2526–34.
Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe? a review. Front Physiol. 2014;5:120.
Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Factors affecting the variability of home-measured blood pressure and heart rate: the Finn-home study. J Hypertens. 2010;28:1836–45.
Zhang Y, Agnoletti D, Blacher J, Safar ME. Blood pressure variability in relation to autonomic nervous system dysregulation: the X-CELLENT study. Hypertens Res. 2012;35:399–403.
Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. 2012;30:1556–63.
Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600.
Duprez DA. Systolic hypertension in the elderly: addressing an unmet need. Am J Med. 2008;121:179–84.
Stewart R, Xue QL, Masaki K, Petrovitch H, Ross GW, White LR, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54:233–40.
Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24:886–93.
Quan M, Xun P, Chen C, Wen J, Wang Y, Wang R, et al. Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta-analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72:266–70.
Parati G, Ochoa JE, Lombardi C, Salvi P, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–55.
Peters R, Anstey KJ, Booth A, Beckett N, Warwick J, Antikainen R, et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J. 2018;39:3135–43.
Zhao JH, Tian XJ, Liu YX, Yuan B, Zhai KH, Wang CW, et al. Executive dysfunction in patients with cerebral hypoperfusion after cerebral angiostenosis/occlusion. Neurol Med Chir. 2013;53:141–7.
Saunders NL1, Summers MJ. Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychology. 2011;25:237–48.
Narazaki K, Nofuji Y, Honda T, Matsuo E, Yonemoto K, Kumagai S. Normative data for the montreal cognitive assessment in a Japanese community-dwelling older population. Neuroepidemiology. 2013;40:23–29.
Acknowledgements
We are grateful to all SONIC participants who participated in these studies. We sincerely appreciate all staff involved in the SONIC study, especially Yumiko Aoshima, Tae Matsue, and Yasuyo Takamine, for their secretarial work and support.
SONIC Study Group
Kazuya Taira10, Werayuth Srithumsuk10, Nonglak Klinpudtan10, Naoko Wada10, Atsuko Higuchi10, Serina Yokoyama3, Satomi Maeda3, Motonori Nagasawa3, Taku Fujimoto3, Kennichi Matsuda11, Taiji Ogawa11, Masahiro Kitamura11, Yoshinobu Maeda11
Funding
This study was supported by a grant-in-aid from JSPS KAKENHI (KK: 15K08910, 19K07888, KA: 17H04126).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of SONIC study group are listed below Acknowledgements.
Supplementary information
Rights and permissions
About this article
Cite this article
Godai, K., Kabayama, M., Gondo, Y. et al. Day-to-day blood pressure variability is associated with lower cognitive performance among the Japanese community-dwelling oldest-old population: the SONIC study. Hypertens Res 43, 404–411 (2020). https://doi.org/10.1038/s41440-019-0377-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0377-5
This article is cited by
-
Assessment of blood pressure variability: characteristics and comparison of blood pressure measurement methods
Hypertension Research (2024)
-
Relationship between defecation status and blood pressure level or blood pressure variability
Hypertension Research (2024)
-
Vagus nerve size determined via ultrasonography is associated with white matter lesions in patients with vascular risk factors
Journal of Ultrasound (2024)
-
Short- to long-term blood pressure variability: Current evidence and new evaluations
Hypertension Research (2023)
-
The relationship between day-to-day variability in home blood pressure measurement and multiple organ function
Hypertension Research (2022)