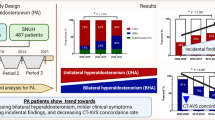
Abstract
Primary aldosteronism (PA) is associated with a higher prevalence of abdominal aortic calcification (AAC). Unilateral and bilateral PA are the most common subtypes of PA. However, no studies have addressed the difference in the prevalence of AAC between the two subtypes. In addition to aldosterone, parathyroid hormone (PTH), an important regulator of calcium metabolism, was also reported to be elevated in individuals with unilateral PA. Therefore, we hypothesized that the prevalence of AAC may be higher in individuals with unilateral PA, which may be related to the plasma aldosterone concentration (PAC) and PTH levels. We included 156 PA patients who underwent adrenal venous sampling and 156 with essential hypertension (EH) matched by age and sex. Of the former, 76 were diagnosed with unilateral PA, and 80 were diagnosed with bilateral PA. The aortic calcification index (ACI) presented the severity of AAC and was measured by adrenal computed tomography scan. Our results showed that compared with the EH group, the prevalence and severity of AAC were higher in PA patients (32.7 vs. 19.6%; 4.32 ± 3.61% vs. 2.53 ± 2.42%, respectively). In the PA subgroup analysis, unilateral PA was associated with a higher and more severe AAC than bilateral PA (40.7 vs. 25.0%; 5.12 ± 4.07% vs. 3.08 ± 2.34%, respectively). Moreover, PAC and PTH levels were higher in individuals with unilateral PA than in those with bilateral PA (P < 0.05). After risk adjustment, multivariate regression analysis revealed that PAC and PTH were positively-associated with AAC in patients with PA (P < 0.05). In conclusion, unilateral PA patients exhibited a higher prevalence of AAC and more severe AAC due to elevated PAC and PTH levels.
Similar content being viewed by others
Introduction
Primary aldosteronism (PA), characterized by autonomous aldosterone secretion and suppressed plasma renin activity, affects 5–13% of patients with hypertension [1, 2]. Increasing evidence has shown that patients with PA are at higher risk for cardiovascular morbidity and mortality [3,4,5], among which vascular calcification (VC) is an important indicator of cardiovascular mortality. A recent meta-analysis of 30 prospective cohort studies demonstrated that the presence of VC was associated with a 3- to 4-fold higher risk for cardiovascular morbidity and mortality [6]. In addition, findings from the latest study showed that compared with EH matched controls, the abdominal aorta calcification (AAC) prevalence was almost twofold higher in patients with PA (39.1 vs. 20.3%) [7]. The well-recognized cause is that absolute aldosterone excess exhibits a series of procalcification effects, such as promoting oxidative stress, inflammation, and apoptosis [8]. These results highlight that aldosterone is one of the major regulators of VC.
Bilateral PA (largely represented by bilateral adrenal hyperplasia) and unilateral PA (largely represented by aldosterone-producing adenoma (APA)) are the two major subtypes of PA, which together account for over 90% of cases [9, 10]. Unilateral PA patients differ from bilateral PA patients due to higher aldosterone levels, lower potassium, and typical adenoma [11]. Interestingly, in recent years, several studies have confirmed that parathyroid hormone (PTH) levels are higher in unilateral PA patients than in bilateral PA patients and are useful for the clinical diagnosis of APA [12,13,14]. Increasing evidence has also shown that PTH levels are elevated and positively correlated with plasma aldosterone concentration (PAC) levels in patients with PA [15, 16]. Notably, the interaction between PAC and PTH modified the risk of cardiovascular mortality: higher PAC increased the effect of PTH on cardiovascular death and vice versa [17]. Moreover, PTH, as an important regulator of calcium metabolism, was strongly linked with the development and progression of VC in patients with chronic kidney disease [18, 19].
Based on the above observations, we hypothesized that the prevalence of AAC may be higher in patients with unilateral PA, which may be related to high PAC and PTH levels.
Methods
Study design and patients
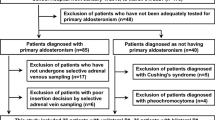
This was a single-center, cross-sectional study conducted in the Hypertension Center of People’s Hospital of Xinjiang Uygur Autonomous Region. From January 2017 to January 2018, a total of 156 patients diagnosed with PA who underwent AVS were included in our study. AVS was performed in 156 PA patients to differentiate between bilateral and unilateral PA. The process was as follows: successful catheterization was judged by an adrenal/peripheral vein cortisol ratio >3:1. PACs were cortisol corrected by dividing the right and left adrenal vein PACs by their respective cortisol concentrations; a cortisol-corrected aldosterone lateralization ratio (high to low side) of more than 2:1 indicated unilateral PA. AVS results showed that there were 76 unilateral PA and 80 bilateral PA patients in our study. The study also included 156 patients with EH matched by age (±3 years) and sex admitted to our center in the same period. All patients underwent adrenal computed tomography (CT) scanning with thin slices (3.0 mm).
The study was performed in accordance with the principles of the Declaration of Helsinki and its amendments and approved by the Ethics Committee of The People’s Hospital of Xinjiang Uygur Autonomous Region. All participants provided signed informed consent.
Diagnostic criteria
The initially positive screening test for PA was based on ARR ⩾ 20 (ng/dl)/(ng/ml/h) in addition to PAC exceeding 12 ng/dl. The diagnosis of PA was based on saline infusion test (SIT) criteria in accordance with the Endocrine Society Guidelines [2]. In the process of the SIT, patients remained in a seated position for 30 min before and during the infusion of 2 L of saline over 4 h. Subjects with a postinfusion PAC > 10 ng/dl were considered to be positive for ‘determined PA’, and patients with a postinfusion PAC between 5 and 10 ng/dl were considered to have ‘undetermined PA’ that was diagnosed or rejected based on clinical manifestations such as drug-resistant hypertension, spontaneous or diuretic-induced hypokalemia, and adrenal incidentaloma or hyperplasia found by thin layer CT scan [20], and those with a postinfusion PAC < 5 ng/dl were considered to be negative for PA. All patients were recruited and requested to discontinue angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, dihydropyridine calcium antagonists, and β-receptor blockers for at least 4 weeks or diuretics and mineralocorticoid antagonists for at least 6 weeks or were not taking any antihypertensive medications at least 2 weeks before the ARR measurement. To standardize the treatment and to eliminate the interference of drugs, all patients participating in this study did not take therapeutic doses of calcium or vitamin D within 4–6 weeks. Antihypertensive drugs were also switched to alpha-blockers (doxazosin), slow-releasing calcium channel blockers (verapamil), or a combination of the two.
Patients with clinical or laboratory evidence of associated conditions were excluded from this study, such as history of hyperpaparathyroidism, chronic kidney disease, Cushing syndrome, malignancy including adrenocortical carcinoma, cardiovascular complications (myocardial infarction, angina, stroke, or heart failure), severe incorrigible hypokalemia, or a previous diagnosis of secondary hypertension.
The diagnosis of EH was based on the following criteria: a known history of hypertension with antihypertensive drug treatment or three documented office systolic blood pressure measurements ≥140 mmHg or diastolic blood pressure measurements ≥90 mmHg on different days; secondary forms of hypertension were excluded by reviewing records for medical history and performing physical examinations and appropriate biochemical tests and imaging studies [21].
Measurement of the abdominal aortic calcification index
AAC can be quantitatively measured on adrenal CT using the ACI, which was measured using techniques described in Naoki Fujita’s study [22]. This necessitates measurement of intra-aortic calcification lesions in several cross-sections using abdominal CT imaging (Fig. 1). All patients underwent an adrenal CT scan to evaluate abdominal aortic plaques. The plaque area (an area ≥1 mm2 with density of >130 HU) was classified as a calcified plaque. All imaging procedures were performed on the same equipment using the same parameters, and the ACI of every patient was measured by the same physician who was blinded to the patient’s clinical characteristics. The process was as follows: adrenal CT images focusing around the left renal artery were scanned ten times at 3 mm intervals, as previously described. For each slice, the cross-section of the aorta was evenly divided into 12 sectors, and the number of calcified sectors as the calcification score of each slice was counted. The ACI was calculated using the following formula: ACI (%) 100*(total calcification score on all slices)/(12*10). ACI > 0 indicated the occurrence of AAC.
Laboratory measurements
Nonfasting blood samples were drawn between 08:00 am and 11:00 am after patients had been ambulant for at least 2 h and seated for 15 min. Plasma aldosterone was measured by radioimmunoassay using a commercially available kit (Beckman Coulter, Brea, CA, USA) with intra- and interassay coefficients of variation of 5.6% and 8.5%, respectively, and the reference range was 7.64–1600 pg/ml. PRA was measured by an iodine angiotensin I radioimmunoassay kit (Northern Biotechnology Institutes, Beijing, China) with intra- and interassay coefficients of variation below 10% and 15%, respectively, and the reference range was 0.2–12 ng/ml per hour. Serum PTH levels were measured by electrochemiluminescence immunoassay (Roche Inc, Swiss). The intra- and interassay coefficients of variation were 4.2–5.7% and 6.3–8.8%, respectively, and the reference range was 2–210 pg/ml. Measurement of 25-hydroxyvitamin D [25(OH)D] was performed by a Roche COBAS e601 fully automatic electrochemiluminescence immunoanalyzer. The intra- and interassay coefficients of variation were <5% and <10%, respectively, and the reference range was 1.5–160 ng/ml. Serum calcium and phosphorus (Pi) were measured on a C16000 automated biochemistry analyzer (Abbott Laboratories, Abbott Park, IL, USA). The intra- and interassay coefficients of variation were also <5%, and the reference ranges were 2.1–2.9 mmol/l and 0.97–1.62 mmol/l, respectively. Hyperparathyroidism was considered when serum PTH levels were higher than the upper limit of the normal range of 65 pg/ml. Hypovitaminosis D was defined as 25-(OH)D levels below 20 ng/ml.
Statistical analysis
Statistical analyses of the clinical data were carried out using SPSS version 19.0. Continuous variables with a normal distribution are reported as the mean (±SD); skewed data are reported as the median (interquartile range). Comparisons of baseline characteristics of different types of PA and EH patients were compared using independent sample t-tests, chi-square tests, or Fisher’s exact tests according to the types of variables. The prevalence of AAC between the two groups was compared with the chi-square test. Multivariate logistic analysis was used to adjust for confounding factors and isolate the relationship with AAC, where age, duration of hypertension, and PAC were included in the model as confounding factors. The risk of AAC was expressed as an odds ratio (OR) with a 95% confidence interval (95% CI). A two-sided P value < 0.05 denoted the presence of a statistically significant difference.
Results
Baseline characteristics of the study population
The clinical and biochemical characteristics of PA and EH patients matched with respect to age and sex are shown in Table 1. Patients with PA had higher PAC, PTH, and 24 h urinary calcium levels than EH patients, whereas serum potassium and TC levels were lower than those in the EH group (all p < 0.05). The prevalence of AAC was significantly different between patients with PA and EH (32.7 vs. 19.6%; P = 0.013). Moreover, the degree of ACI was more severe in PA patients than in EH patients (4.32 ± 3.61% vs. 2.53 ± 2.42%, P = 0.028).
Comparison of patients with different types of PA
A comparison of the clinical and biochemical parameters of patients with different types of PA is shown in Table 2. Compared with bilateral PA patients, unilateral PA patients had higher PAC, ARR, and PTH levels, whereas the duration of hypertension, BMI, AC, and serum potassium were lower than those in the bilateral PA group (all P < 0.05). The prevalence and severity of AAC were significantly higher in the unilateral PA group than in the bilateral PA group (40.7 vs. 25.0%, P = 0.036; 5.12 ± 4.07% vs. 3.08 ± 2.34%, P = 0.048, respectively).
Factors associated with AAC in patients with PA
We next determined which factors were associated with the prevalence of AAC in patients with PA (Table 3). In a univariate analysis, we found that age, duration of hypertension, abdominal circumference, PAC, PRA, ARR, serum K+, PTH, Pi, and unilateral subtype were all factors significantly associated with AAC (all P < 0.05). Therefore, we selected age, duration of hypertension, abdominal circumference, and Pi as AAC risk factors in our logistic regression analysis.
After adjusting for AAC risk factors, including age, duration of hypertension, abdominal circumference, and Pi, we performed multivariate logistic regression analysis in patients with PA to elucidate the factors contributing to AAC (Table 4). PAC and PTH levels were positively-associated with AAC in patients with PA (OR 1.235, 95% CI 1.100–1.373, P < 0.001; OR 1.038, 95% CI 1.013–1.064, P = 0.002, respectively). Overall, the results supported the significance of PAC and PTH levels in terms of the prediction of AAC in patients with PA.
Discussion
To our knowledge, this is the first study to explore AAC in patients with different types of PA and its association with PTH levels. The major findings from our study are that (1) in patients with PA, the prevalence and severity of AAC was higher than those in EH patients matched by age and sex. In the PA subgroup analysis, unilateral PA patients exhibited a higher prevalence and more severe AAC than bilateral PA patients. (2) Importantly, in addition to traditional risk factors such as age, duration of hypertension, abdominal circumference, and Pi, PAC and PTH levels were independent risk factors for AAC in patients with PA, especially in patients with unilateral PA.
Our data are in accordance with the findings from Pinming Liu et al. [7], who demonstrated that compared with EH matched controls, the AAC prevalence was higher in patients with PA. Given the evidence of a high prevalence of AAC in PA patients, studies have described the interaction between aldosterone and VC. Growing in vitro and in vivo studies have shown that high levels of aldosterone have direct inflammatory and fibrotic effects on myocardial and vascular endothelial cells, including tumor necrosis factor-α, interleukin-1β, interleukin-6, and monocyte chemoattractant protein-1 [23]. Moreover, aldosterone was shown to increase the activity of NADPH oxidase. Previous studies have revealed that H2O2 generated from NADPH oxidase promoted a phenotypic switch of vascular smooth muscle cells (VSMCs) from a contractile to an osteogenic phenotype by upregulating the expression of Runx2, causing VC [24]. Furthermore, the pro-apoptotic role of aldosterone in VC has also been gradually revealed. Apoptotic bodies derived from VSMCs were shown to act as nucleating structures for calcium crystal formation. Conversely, inhibition of VSMC apoptosis can prevent VC, suggesting that VSMC apoptosis is another important mechanism for VC [25]. These results highlight that downregulation of aldosterone may be a potential therapy for attenuating VC.
In the present study, PTH levels were elevated in unilateral PA patients. The underlying mechanisms are as follows: (1) PTH could directly act on adrenocortical cells through its receptor, PTH1R, which was more abundant in aldosterone-producing adrenocortical cells than other types of cells [26,27,28]. After adrenalectomy, a marked decrease in aldosterone and PTH levels was observed, providing evidence for a direct causal relation between the tumors-associated with unilateral PA and the increase in PTH [29]; (2) aldosterone excess in the setting of PA may decrease serum calcium levels by increased urinary calcium loss, and may thereby contribute to secondary hyperparathyroidism. It is likely that the additional mechanism involves an increased sensitivity of the parathyroid cells to ionized calcium lowering, as reported recently [12, 30]; (3) expanded extravascular fluid volume with decreased proximal reabsorption of sodium and calcium and increased distal delivery with a distal reabsorption of sodium but not of calcium might explain secondary hyperparathyroidism [31]; and (4) unilateral PA patients had lower 25-(OH) D levels than bilateral PA patients in this study. Several investigations have shown an inverse association between PTH and 25-(OH)D [32]. Due to the inverse correlation between 25-(OH)D and PTH concentrations, patients with deficient vitamin D status would experience increased PTH secretion from the parathyroid gland.
In our study, AAC depended more on PAC and PTH levels, not PA subtypes. AAC would be higher in patients with the unilateral subtype due to the high PAC and PTH levels. Recently, the results from the latest study indicated that the PTH-PTH1R pathway could stimulate aldosterone biosynthesis and calcium metabolic abnormalities in PA patients. Although the specific role of PTH in VC in PA patients is unknown, the relationship between PTH and VC has been verified in patients with renal failure and type 2 diabetes [33, 34]. PTH exerts its effects through the PTH 1 receptor, which is expressed in cardiomyocytes, VSMCs, and endothelial cells. Elevation of serum PTH disrupts energetic metabolism in cardiomyocytes, leading to calcium overload and inducing calcium metabolism disorders and vascular inflammation, causing oxidative stress and endothelial dysfunction by activating protein kinase A or C, which can result in damage to vessels, thereby contributing to vascular fibrosis and calcification [33, 34]. In addition, PTH promotes osteoblastic differentiation of endothelial cells by increasing the expression of bone morphogenetic protein (BMP) 2 and BMP4 via the Erk1/2 and NF-κB signaling pathways, which suggests a potential role of PTH in the promotion of VC [35]. In recent years, studies have suggested that surgical resection of the parathyroid might reduce VC-promoting factors by reducing the levels of Pi, calcium-Pi product concentration, PTH levels, etc., thus controlling the progression of VC [36]. These results highlight the important role of PTH in VC. Moreover, observational studies described that elevated PTH levels were closely related to higher aldosterone in patients with primary hyperparathyroidism [37]. Accordingly, patients with PA also exhibit associated-elevations in aldosterone with higher PTH levels [38, 39]. These small observational studies have suggested an important relationship between PTH and aldosterone under pathophysiologic conditions. Excessive levels of PAC and PTH cause endothelial dysfunction in a reactive oxygen species-dependent manner, which leads to the impairment of selected signal transduction pathways, alteration of NO production and impairment of endothelial phenotypes, resulting in vessel stiffness by direct action on vascular smooth muscle and further development of atherosclerosis and VC [40, 41]. These observations confirmed our hypothesis that high PAC and PTH levels were independently associated with AAC.
This study has several limitations that should be highlighted. First, the number of PA patients enrolled in this study was relatively small. Therefore, further studies with larger sample sizes are needed to verify our findings. Second, studies on the roles of PTH in promoting AAC in PA patients are limited, and the underlying mechanisms need to be explored in detail. Despite these limitations, the present study was the first to assess AAC in different types of PA and its association with PTH levels.
In conclusion, we provide clinical evidence that patients with unilateral PA exhibited a higher prevalence of AAC and more severe AAC than bilateral PA patients. High PAC and PTH levels are probably important predictors of AAC in patients with PA, especially in patients with unilateral PA.
Data availability
There are no linked data sets for this submission. The following reason is given: data will be available on request.
References
Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline[J]. J Clin Endocrinol Metab. 2008;93:3266–81.
Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. The Task Force Committee on Primary Aldosteronism, the Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism-the Japan Endocrine Society 2009[J]. Endocr J. 2011;58:711–21.
Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis[J]. Lancet Diabetes Endocrinol. 2018;6:41–50.
Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study[J]. Hypertension. 2013;62:331–6.
Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A. et al. Long-term cardio-and cerebrovascular events in patients with primary aldosteronism[J]. J Clin Endocrinol Metab. 2013;98:4826–33.
Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis[J]. Vasc Health Risk Manag. 2009;5:185–97.
Liu P, Zhang S, Gao J, Lin Y, Shi G, He W, et al. Downregulated serum 14, 15-epoxyeicosatrienoic acid is associated with abdominal aortic calcification in patients with primary aldosteronism[J]. Hypertension. 2018;71:592–8.
Gao J, Zhang K, Chen J, Wang MH, Wang J, Liu P, et al. Roles of aldosterone in vascular calcification: an update[J]. Eur J Pharm. 2016;786:186–93.
Fabrizio B, Silvia M, Tracy W, Denis R, Jacopo B, Martina T, et al. Subtype diagnosis of primary aldosteronism: is adrenal vein sampling always necessary?[J]. Int J Mol Sci. 2017;18:848.
El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism[J]. Front Endocrinol. 2016;7:72.
Zhang Y, Niu W, Zheng F, Zhang H, Zhu L. Identifying unilateral disease in Chinese patients with primary aldosteronism by using a modified prediction score[J]. J Hypertens. 2017;35:2486.
Rossi GP, Ragazzo F, Seccia TM, Maniero C, Barisa M, Calo LA, et al. Hyperparathyroidism can be useful in the identification of primary aldosteronism due to aldosterone-producing adenoma[J]. Hypertension. 2012;60:431–6.
Zhang LX, Gu WJ, Li YJ, Wang Y, Mu YM. PTH is a promising auxiliary index for the clinical diagnosis of aldosterone-producing adenoma[J]. Am J Hypertens. 2015;29:575–81.
Fortina F, Bellosta S. Hyperparathyroidism secondary to hyperaldosteronism[J]. High Blood Press Cardiovasc Prev. 2010;17:27–30.
Brunaud L, Germain A, Zarnegar R, Rancier M, Alrasheedi S, Caillard C, et al. Serum aldosterone is correlated positively to parathyroid hormone (PTH) levels in patients with primary hyperparathyroidism[J]. Surgery. 2009;146:1035–41.
Chau K, Holmes D, Melck A, Clifford Chan-Yan. Secondary hypertension due to concomitant aldosterone-producing adenoma and parathyroid adenoma[J]. Am J Hypertens. 2014;28:280–2.
Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease[J]. Metabolism. 2014;63:20–31.
Malluche HH, Blomquist G, Monier-Faugere MC, Cantor TL, Davenport DL. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis[J]. J Am Soc Nephrol. 2015;26:2534–44.
Di LL, Gorini A, Bellasi A, Morrone LF, Rivera R, Russo L, et al. Fibroblast growth factor 23 and parathyroid hormone predict extent of aortic valve calcifications in patients with mild to moderate chronic kidney disease[J]. Clin Kidney J. 2015;8:732–6.
Luo Q, Li NF, Yao XG, Zhang DL, Abulikemu SFY, Chang GJ, et al. Potential effects of age on screening for primary aldosteronism[J]. J Hum Hypertens. 2016;30:53.
Whitworth JA. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension[J]. J Hypertens. 2003;21:1983–92.
Fujita N, Hatakeyama S, Yamamoto H, Tobisawa Y, Yoneyama T, Yoneyama T, et al. Implication of aortic calcification on persistent hypertension after laparoscopic adrenalectomy in patients with primary aldosteronism[J]. Int J Urol. 2016;23:412–7.
Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis[J]. Nat Rev Nephrol. 2013;9:459.
Mayyas F, Alzoubi KH, Van Wagoner DR. Impact of aldosterone antagonists on the substrate for atrial fibrillation: aldosterone promotes oxidative stress and atrial structural/electrical remodeling[J]. Int J Cardiol. 2013;168:5135–42.
Mathew JT, Patni H, Chaudhary AN, Liang W, Gupta A, Chander PN, et al. Aldosterone induces mesangial cell apoptosis both in vivo and in vitro[J]. Am j physiol Ren physiol. 2008;295:73–81.
Mazzocchi G, Aragona F, Malendowicz LK, Nussdorfer GG. PTH and PTH-related peptide enhance steroid secretion from human adrenocortical cells[J]. Am J Physiol Endocrino Metab. 2001;280:E209–E213.
Kawashima M, Takahashi T, Yanai H, Ogawa H, Yasuoka T. Direct action of parathyroid hormone-related peptide to enhance corticosterone production stimulated by adrenocorticotropic hormone in adrenocortical cells of hens[J]. Poult Sci. 2005;84:1463–9.
Rizk-Rabin M, Assie G, Rene-Corail F, Perlemoine K, Hamzaoui H, Tissier F, et al. Differential expression of parathyroid hormone–related protein in adrenocortical tumors: autocrine/paracrine effects on the growth and signaling pathways in H295R Cells[J]. Cancer Epidemiol Biomark Prev. 2008;17:2275–85.
Maniero C, Fassina A, Seccia TM, Toniato A, Rossi GP. Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism[J]. J Hypertens. 2012;30:390–5.
Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study[J]. J Clin Endocrinol Metab. 2012;97:E75–E79.
Rossi E, Perazzoli F, Negro A, Sani C, Davoli S, Dotti C, et al. Acute effects of intravenous sodium chloride load on calcium metabolism and on parathyroid function in patients with primary aldosteronism compared with subjects with essential hypertension[J]. Am J Hypertens. 1998;11(1 Pt 1):8.
Kota S, Jammula S, Kota S, Meher L, Modi K. Correlation of vitamin D, bone mineral density and parathyroid hormone levels in adults with low bone density[J]. Indian J Orthop. 2013;47:402–7.
Neves KR, Graciolli FG, Dos Reis LM, Graciolli RG, Neves CL, Magalhães AO, et al. Vascular calcification: contribution of parathyroid hormone in renal failure[J]. Kidney Int. 2007;71:1262–70.
Mary A, Hartemann A, Brazier M, Aubert CE, Kemel S, Salem JE, et al. Higher parathyroid hormone levels are associated with increased below-the-knee arterial calcification in type 2 diabetes[J]. Diabetes Metab. 2018;44:305–8.
Cheng Z, Ye T, Ling Q, Wu T, Wu G, Zong G. Parathyroid hormone promotes osteoblastic differentiation of endothelial cells via the extracellular signal-regulated protein kinase 1/2 and nuclear factor-κB signaling pathways[J]. Exp Ther Med. 2018;15:1754–60
Williams Rachael. Parathyroidectomy might reduce cardiovascular calcification in dialysis patients[J]. Nat Clin Pract Nephrol. 2005;1:64–5.
Krysiak R, Kobielusz-Gembala I, Okopień B. Primary aldosteronism in a patient after surgical treatment of primary hyperparathyroidism[J]. Przegl Lek. 2011;68:388–90.
Concistré A, Petramala L, Zinnamosca L, Settevendemmie A, Corpaci F, Marinelli C, et al. Primary aldosteronism with concurrent primary hyperparathyroidism: clinical case load in a single centre.[J]. Eur Rev Med Pharm Sci. 2015;19:971–6.
Asbach E, Bekeran M, König A, Katharina L, Gregor H, Marcus T, et al. Primary and secondary hyperparathyroidism in patients with primary aldosteronism–findings from the german conn’s registry[J]. Exp Clin Endocrinol Diab. 2020;128:246–54.
Gao X, Yamazaki Y, Onodera Y, Ogata H, Omata K, Morimoto R. et al. The crosstalk between aldosterone and calcium metabolism in primary aldosteronism~A possible calcium metabolism-associated aberrant “neoplastic” steroidogenesis in adrenals~[J]. J Steroid Biochem Mol Biol. 2019;21:1–37.
Deska M, Romuk E, Segiet OA, Truchanowski W, Stolecka D, Birkner E, et al. Oxidative stress and angiogenesis in primary hyperparathyroidism[J]. Eur Surg. 2017;49:118–26.
Funding
This study was supported by the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences [2019PT330003].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was conducted in accordance with the principles of the Declaration of Helsinki and its amendments and was approved by the Ethics Committee of People’s Hospital of Xinjiang Uygur Autonomous Region.
Informed consent
All participants provided signed informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tuersun, T., Luo, Q., Zhang, Z. et al. Abdominal aortic calcification is more severe in unilateral primary aldosteronism patients and is associated with elevated aldosterone and parathyroid hormone levels. Hypertens Res 43, 1413–1420 (2020). https://doi.org/10.1038/s41440-020-0529-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0529-7
Keywords
This article is cited by
-
Radiofrequency Echographic Multispectrometry (REMS) can Overcome the Effects of Structural Internal Artifacts and Evaluate Bone Fragility Accurately
Calcified Tissue International (2023)
-
Long-term impact of spironolactone compliance on microalbuminuria in patients with primary aldosteronism
Hypertension Research (2021)