Abstract
None of the spironolactone trials in heart failure (HF) assessed the blood pressure (BP) responses to exercise, while conflicting results were reported for exercise capacity. In the HOMAGE trial, 527 patients at increased HF risk were randomized to usual treatment with or without spironolactone (25–50 mg/day). The current substudy included 113 controls and 114 patients assigned spironolactone, who all completed the incremental shuttle walk test at baseline and months 1 and 9. Quality of life (QoL) was assessed by EQ5D questionnaire. Between-group differences (spironolactone minus control [Δs]) were analyzed by repeated measures ANOVA with adjustment for baseline and, if appropriate, additionally for sex, age and body mass index. Δs in the pre-exercise systolic/diastolic BP were −8.00 mm Hg (95% CI, −11.6 to −4.43)/−0.85 mm Hg (−2.96 to 1.26) at month 1 and −9.58 mm Hg (−14.0 to −5.19)/−3.84 mm Hg (−6.22 to −1.47) at month 9. Δs in the post-exercise systolic/diastolic BP were −8.08 mm Hg (−14.2 to −2.01)/−2.07 mm Hg (−5.79 to 1.65) and −13.3 mm Hg (−19.9 to −6.75)/−4.62 mm Hg (−8.07 to −1.17), respectively. For completed shuttles, Δs at months 1 and 9 were 2.15 (−0.10 to 4.40) and 2.49 (−0.79 to 5.67), respectively. Δs in QoL were not significant. The correlations between the exercise-induced BP increases and the number of completed shuttles were similar in both groups. In conclusion, in patients at increased risk of developing HF, spironolactone reduced the pre- and post-exercise BP, but did not improve exercise capacity or QoL.

Similar content being viewed by others
Introduction
Exercise capacity provides important diagnostic and prognostic information in patients with chronic cardiac [1,2,3] and pulmonary conditions [4]. Exercise tests are widely used to evaluate the efficacy of new therapies and to assess changes in heart rate and blood pressure (BP) in response to exercise [5,6,7]. Incremental cardiopulmonary exercise testing with metabolic gas exchange measurement (CPET) is the gold standard method to assess maximal aerobic capacity [8]. However, routine CPET is costly and challenging. The incremental shuttle walk test (ISWT) is a symptom-limited maximal test and can track changes in the functional capacity of patients with chronic heart failure (HF) [9,10,11,12]. Exercise capacity as measured by the ISWT correlates closely with the results of CPET [13]. The correlations between distance covered during the ISWT and peak oxygen consumption range between 0.67 and 0.95 [13]. Trials have shown that mineralocorticoid receptor inhibition with spironolactone [14,15,16,17,18], eplerenone [19], finerenone [20], or spironolactone on top sodium-glucose co-transporter 2 inhibitors [21] improves outcomes in HF. However, few trials [15,16,17,18] have assessed the functional effect of spironolactone and none has reported on the BP responses to exercise.
We analyzed data from the HOMAGE (Heart OMics in Aging) trial, a prospective randomized trial in patients at increased risk of developing HF [22]. HOMAGE evaluated the effect of spironolactone on biomarkers of fibrosis and cardiac remodeling [23]. In this prespecified secondary analysis, we investigated the influence of spironolactone on the BP responses to exercise and exercise capacity. We also investigated the correlation between the changes from baseline to the last follow-up visit in the pre- and post-exercise BP and the corresponding changes in circulating biomarkers, echocardiographic measurements, and quality of life (QoL).
Methods
Study participants
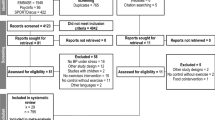
HOMAGE is a multicenter open-label trial with blinded endpoint evaluation (registration number: NCT02556450) [23], conducted in nine centers in the United Kingdom, France, Italy, Ireland, Germany, and the Netherlands. Each center had its own recruitment strategies. The protocol was approved by the Greater Manchester Central Research Ethics Committee (reference, 16/NW/0012; EudraCT number: 2015-000413-48) as well as by each center’s local Ethics Committee. Patients of either sex, aged ≥60 years were eligible, provided that they were at increased risk of developing HF because they already had or were likely to develop coronary heart disease. Additionally, eligible patients had to have a serum N-terminal pro B-type natriuretic peptide (NT-proBNP) of 125–1000 ng/L or a serum BNP of 35–280 ng/L. These ranges excluded patients at low HF risk as well as those with advanced disease requiring further investigation and treatment. Of 877 screened patients (Fig. 1), 527 were randomized to spironolactone 25–50 mg per day (n = 265) on top of usual treatment or usual treatment alone (n = 262) [23]. Of all patients randomized and followed-up in the HOMAGE trial, 450/527 (85.4%), 324/516 (62.8%), and 400/506 (79.1%) completed the ISWT at baseline and at months 1 and 9. The current analyses includes 227 patients who completed the ISWTs at each of these time points, the justification being that evaluating the same patients at each time point increases the comparability of the data over time. Of the 227 patients, 113 were randomized to control and 114 to spironolactone.
Consort diagram showing patient disposition, including screening, randomization and follow-up, and selection of patients for the current analysis. Analyses include 227 patients who completed the ISWT at months 0, 1, and 9. LVEF left ventricular ejection fraction; MRA mineralocorticoid receptor antagonism
Measurements
Exercise capacity was measured by ISWT [24], as described in detail in the online Supplementary Information. Skilled personnel conducted the ISWT around two cones, 9 m apart [22]. Patients walk in a circle around the cones (“the shuttle”), which adds 1 meter to the distance covered. The walking speed is determined by bleeps played from a compact disc. After every minute, walking speed increased. There are up to 12 levels of speed, and potentially 102 shuttles. BP was measured at rest, immediately after the ISWT, and 1, 2, 3, and 5 min later. The ISWT was performed at baseline and at the 1- and 9-month visits. Trained observers recorded BP by conventional sphygmomanometry by auscultation of the Korotkoff sounds. Heart rate and the Borg dyspnea score were recorded pre-exercise and immediately post-exercise. Serum was analyzed for circulating fibrosis markers (Supplementary Fig. 1), high-sensitivity troponin T (hsTnT), and NT-proBNP, as described elsewhere [23, 25]. Commercial radio-immunoassays (Orion Diagnostica, Espoo, Finland) were used to measure serum collagen type-1 C-terminal telopeptide (CITP). Serum procollagen type I C-terminal propeptide (PICP), a marker of collagen type I synthesis was measured using the METRA EIA kit (Quidel Corporation, San Diego, CA). The lower limit of detection was 0.6 ng/mL for CITP and 0.2 ng/mL for PICP. All inter- and intra-assay coefficients of variation were <10%. Glomerular filtration rate was estimated (eGFR) from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [26]. QoL was assessed using the EQ5D visual analog score (https://euroqol.org/eq-5d-instruments). Echocardiography was performed according to the guidelines of the American Society of Echocardiography [27]. The echocardiographic traits of interest include the ratio of the peak early diastolic transmitral flow to the peak of early diastolic mitral annular movement (E/e’) as index of the left ventricular (LV) filling pressure, left atrial volume (LAVI), and left ventricular mass (LVMI), both indexed to body surface area. Patients completed questionnaires to report side effects at month1 and 9.
Statistical analysis
For database management and statistical analysis, SAS software, version 9.4 (SAS Institute Inc., Cary, NC) was used. Deviation from the normal distribution was assessed by the Shapiro-Wilk statistic. For comparison of means, we used a t-test or Wilcoxon-Mann-Whitney test depending on the distribution. For comparison of proportions, we applied the χ2-statistic. Pearson correlation coefficients between variables of interest were computed and compared between groups by the Fisher z-transform [28]. Significance was a 2-sided α-level of ≤0.05. Prior to analysis, PICP, CITP, and NT-proBNP were logarithmically transformed (base 10) to approximate the normal distribution. The between-group differences in continuous measurements at months 1 and 9 were calculated by subtracting the mean changes from baseline in the control group from the corresponding changes in the spironolactone group always with adjustment for baseline and additionally, if so mentioned, also for sex, age, and body mass index. For logarithmically transformed variables, within-group changes from baseline to the 1- and 9-month visits and between-group difference were expressed as ratios by multiplying the antilog of the difference between two logarithmically transformed values by 100 to convert results to the percentage scale. Significance of the between-group differences in the serial BP values during the ISWT was analyzed by repeated measures ANOVA without and with adjustment for sex, age, and body mass index. If the baseline QoL data were missing, they were replaced with the 1-month scores, because there were no differences between baseline and the 1-month QoL scores in the 124 patients for whom data were available at both time points. In sensitivity analyses, the data were stratified for sex and the medians of age, LV ejection fraction (LVEF), and eGFR.
Results
Patient characteristics
Descriptive data for the 227 patients are shown in Table 1. The patients were intensively treated with antihypertensive agents (n = 163; 71.8%) and lipid-lowering drugs (n = 203; 89.4%), mainly statins (n = 197; 86.8%), antiplatelet drugs (n = 168; 74.0%), and antidiabetic agents (n = 81; 35.7%). There were no significant differences between the patients randomized to placebo or spironolactone. At baseline (Table 2), exercise capacity measured by the number of completed shuttles and systolic and diastolic BP both before and after exercise were similar in both groups (P ≥ 0.11). The 227 patients included in the substudy had broadly similar characteristics compared to the 300 HOMAGE patients not included (Supplementary Table 1). However, the patients reported here were younger, had a higher eGFR, were less likely to smoke (5.73% versus 10.3%; P = 0.040), and had a higher prevalence of ischemic heart disease (79.3% versus 66.3%; P = 0.001).
Pre-exercise BP
Although systolic BP fell between the baseline and the 1-month visit in the placebo group, the fall was much greater in the spironolactone group (Fig. 2). Compared with the control group, the 9-month pre-exercise systolic BP remained significantly reduced in the spironolactone arm (−6.34 versus −13.5 mm Hg; 95% CI, −10.4 to −2.25 versus −16.7 to −10.2 mm Hg; P-value for the between-group difference: 0.007). These results were unaffected by sex (Supplementary Fig. 2), age (Supplementary Fig. 3), LVEF (Supplementary Fig. 4), or eGFR (Supplementary Fig. 5). In analyses, adjusted for sex, age, body mass index, and the baseline systolic BP level (Table 2), the resting pre-exercise systolic BP at 1 and 9 months decreased 8.00 mm Hg and 9.58 mm Hg more in the spironolactone than in the control group. For diastolic BP, with similar adjustments applied (Table 2), the between-group difference was not significant at 1 month (spironolactone minus control group: −0.85 mm Hg; P = 0.43), but was significant at 9 months (−3.84 mm Hg; P = 0.002).
Post-exercise BP
Figure 2 shows the systolic and diastolic BP responses to ISWT. In unadjusted analyses, at 1 month, systolic/diastolic BP, measured immediately post-exercise, did not change in the control group (−4.27/0.04 mm Hg; 95% CI, −8.90 to 0.35/−2.68 to 2.75 mm Hg; P ≥ 0.070), whereas in the spironolactone group at month 1 the post-exercise systolic BP (−11.9 mm Hg; 95%CI, −16.9 to −6.90 mm Hg; P < 0.001), but not post-exercise diastolic BP (−2.45 mm Hg; 95%CI, −5.37 to 0.47 mm Hg; P = 0.10) decreased. The 9-month post-exercise BP did not change compared to baseline in the control group (−4.15/−0.77 mm Hg; 95% CI, −9.49 to 1.19/−3.30 to 1.76 mm Hg; P ≥ 0.13), but remained reduced on spironolactone (−17.1/−6.13 mm Hg; 95% CI, −22.4 to −11.8/−9.56 to −2.70 mm Hg; P < 0.001). In analyses adjusted for sex, age, BMI, and the baseline BP level (Table 2), the post-exercise systolic BP at months 1 and 9 decreased 8.08 mm Hg and 13.3 mm Hg more in the spironolactone than in the control group. For diastolic BP with similar adjustments applied (Table 2), the between-group difference in the post-exercise diastolic BP was not significant at 1 month (spironolactone minus control group: −2.07 mm Hg; P = 0.27), but was significant at 9 months (−4.62 mm Hg; P = 0.009). Compared to placebo, spironolactone did not affect the increase in BP in response to ISWT (Table 2), pre- and post-exercise heart rate, or Borg score (Table 3).
BP during ISWT
In unadjusted analyses, the correlations between the exercise-induced BP increases and the number of completed shuttles were similar in both groups (Supplementary Table 2). Furthermore, the correlations between baseline BMI and the resting systolic and diastolic BP and the exercise-induced changes in systolic/diastolic BP at months 1 and 9 ranged from −0.16 to 0.15 without any between-group difference (P ≥ 0.052).
As shown in Fig. 3 for systolic and diastolic BP, repeated measures ANOVA did not reveal a significant between-group difference at the baseline visit in the pre-exercise BP and the post-exercise BP up to 5 min after completion of the ISWT (P ≥ 0.051). However, at months 1 and 9, BP throughout the ISWT was significantly lower in the spironolactone group compared to control (P < 0.001; Fig. 3). Adjustment for sex, age, and body mass index produced confirmatory results.
Analysis of accessory variables
Table 3 in the Supplementary Information summarizes the effects of spironolactone versus control on circulating biomarkers, serum electrolytes, echocardiographic functional measurements, and QoL. There were no differences between control and spironolactone in the QoL changes at 1 and 9 months (Supplementary Table 3). To assess the effects of spironolactone versus control in more detail, correlations were computed between the changes from baseline to the 9-month visit in the pre- and post-exercise systolic BP and in the accessory variables (Supplementary Table 4). The between-group differences in these correlations were not significant with the exception for the change in serum potassium in relation to the change in the post-exercise systolic BP at 9 months (r = 0.068 versus r = −0.254; P = 0.015). The side-effects reported by patients were all mild not necessitating withdrawal of patients from the trial. In the active-treatment group, three men experienced loss of libido, gynecomastia, or tenderness of the breast; in the control group no patient reported such complaints.
Discussion
To the best of our knowledge, HOMAGE is the first randomized clinical trial allowing to study the effects of usual therapy compared to spironolactone on top of usual therapy on the BP response to ISWT and exercise capacity as captured by the number completed shuttles. Systolic BP measured immediately post-exercise at 1 and 9 months and the diastolic BP at 9 months significantly decreased in the intervention group, irrespective of adjustment (Fig. 2 and Table 2). At months 1 and 9, systolic and diastolic BP throughout the ISWT were lower on spironolactone than in control patients (Fig. 3). The BP-lowering effects of spironolactone on the ISWT-induced BP rise were broadly consistent in analyses dichotomized by sex or the medians of age, LVEF or eGFR (Supplementary Figs. 2–5). Spironolactone did not affect the increase in systolic BP in response to ISWT. In unadjusted analyses, the number of completed shuttles was higher on spironolactone. Adjusted analyses showed a similar trend, albeit that formal significance was lost (Table 2). Spironolactone treatment did not affect QoL. In exploratory analyses, spironolactone did not affect the correlations between the exercise-induced BP changes and the number of completed shuttles (Supplementary Table 2) or the correlations between the changes in the pre- and post-exercise BP at 9 months and the corresponding changes in the accessory measurements (Supplementary Table 4).
In the double-blind placebo-controlled RALES trial of spironolactone (25 mg/day) [15], based on the NYHA classification, three categories were used to assess changes in the symptoms of HF: improvement, no change, and worsening or death. In the placebo group, the condition of 33% of the patients improved, but it did not change in 18%, and it worsened in 48%; in the spironolactone group, the condition improved in 41%, did not change in 21% and worsened in 38%. The between-group difference was significant (P < 0.001) [15]. In three double-blind trials with a single-center [18] or multicenter [16, 17] design, 80 [18], 150 [17], or 422 [16] HF patients with preserved LVEF (HFpEF) were randomized to placebo or spironolactone (25 mg/day) and followed up for 6 [17], 9 [18] or 12 [16] months. The number of analyzed patients amounted to 71 [18], 131 [17], and 422 [16], respectively. In the single-center trial (80% women; mean age 71 years) [18], the baseline-adjusted peak VO2, was 13.9 mL/min on placebo and 13.5 mL/min on spironolactone, resulting in a between-group difference −0.4 mL/kg/min (95% CI, −1.1 to 0.4 mL/kg/min; P = 0.38). There was no improvement in the echocardiographic measurements [18]. In the Polish trial (84% women; mean age: 67 years) [17], spironolactone compared to placebo increased peak VO2 (2.9 versus 0.3 mL/min/kg) in relation to a reduction in the exercise-induced E/e’ ratio (−3.0 versus +0.5). In ALDO-DHF (52% women; mean age, 67 years) [16] spironolactone improved LV diastolic function but did not affect VO2 or QoL. The adjusted mean between-group difference amounted to −1.5 (95% CI, −2.0 to −0.9; P < 0.001) for the E/e’ ratio and −0.1 mL/min/kg (95%CI: −0.6 to −0.8 mL/min/kg; P = 0.81) for VO2.
Among the aforementioned trials, QoL was either not reported [17] or did not change [16, 18]. However, in the EPHESUS study (39% women; mean age, 64 years), 6632 patients with reduced LVEF (HFrEF) following myocardial infarction were randomized to placebo or eplerenone (25–50 mg/day) and followed up for 16 months on average [19]. Using the Swiss insurance system as reference, eplerenone was cost-effective in reducing mortality and increasing QoL [29]. Compared to eplerenone, the affinity of spironolactone for androgen, glucocorticoid, and progesterone receptors is 102–103 higher [30], potentially explaining why eplerenone increased QoL in EPHESUS [19], but not in the current study or other trials of spironolactone [16, 18]. In a trial set up to investigated whether treatment with spironolactone might be meaningful in the prevention of HF, community-dwelling subjects aged ≥65 years old, with at least one non-ischemic HF risk factor (hypertension, type-2 diabetes, or obesity) were randomized to echocardiography-guided therapy or usual care. LV dysfunction resolved more frequently with spironolactone than in untreated patients [31]. However, the study was underpowered to determine whether screening-guided spironolactone therapy reduced incident HF because spironolactone was frequently discontinued due to the too strict renal function criteria [31].
The current analyses highlighted the signature of mineralocorticoid antagonism (MRA) by spironolactone on serum electrolytes, eGFR, and the circulating fibrosis biomarkers. Neither the increase in serum potassium nor the decrease in eGFR limited the beneficial effects of spironolactone on the pre- and post-exercise BP, the decrease in serum NT-proBNP or the shift in PICP and the PICP/CITP ratio indicative of an antifibrotic effect (Supplementary Fig. 1) [32]. BP lowering is probably a major contributor to the beneficial effects of MRA in HF patients [14,15,16,17,18,19] or the HOMAGE patients at risk of HF because of coronary heart disease. In HFpEF patients, greater time within the target systolic BP range is associated with a decreased risk of adverse cardiovascular outcomes and mortality, especially among younger patients [33]. In HFpEF patients, abnormal sodium burden, assessed from the serum sodium concentration [34], is an independent predictor of long-term all-cause mortality and HF hospitalization. However, as shown by the PATHWAY-2 trial [35] and in keeping with the lowering of serum sodium, spironolactone is effective in mitigating the adverse effects of the increased sodium load. In this 3-month double-bind trial, 25% of 126 patients enrolled in an endocrinal substudy (n = 31) had an inappropriately elevated aldosterone as assessed by ratio of plasma aldosterone to plasma renin and a plasma aldosterone exceeding the mean value in all 126 patients (250 pmol/L [9 ng/dL]). In these 31 patients, spironolactone 25 mg/d, compared with placebo, reduced systolic BP by 18.3 mm Hg (95%CI, 16.2–20.5 mm Hg) and the thoracic fluid content by 6.8% (95% CI, 4.0–8.8%) [35]. In TOPCAT-Americas [36] compared to placebo, spironolactone increased worsening of renal function (doubling of serum creatinine), but this feature did not preclude substantial beneficial effects in terms of lowering the risk of cardiovascular and all-cause mortality. In the current study, the BP lowering effects of spironolactone were similar if dichotomized by median eGFR (Supplementary Fig. 5).
Study limitations
This report on a subset of patients from the HOMAGE trial has several limitations. First, we excluded patients, who did not complete three ISWTs, i.e., at months 0, 1, and 9. However, not including the same patients at each time point would have precluded a direct comparison of the sort- and long-term results. Second, the between-group differences in the number of completed shuttles (Table 2) or the changes in QoL (Supplementary Table 3) did not reach significance, probably because of a lack of statistical power, given that only 227 of the 527 [23] (43.0%) HOMAGE patients were analyzed with a follow-up not longer than 9 months. Spironolactone-specific complaints include loss of libido, gynecomastia, or tenderness of the breast [30], but occurred only in three men and did not explain the absence of an effect of active treatment on QoL. The number of shuttles completed and the self-assessed QoL were accessory variables. The trial’s primary endpoint was the interaction between PIINP (Supplementary Fig. 1) and galectin-3 [23]. Although the analyses of exercise capacity and QoL were prespecified according to the HOMAGE protocol [22], the power to detect a significant difference, assuming an effect size of 20% with a 2-sided α-level of 0.05, was low, respectively, 0.05 for the number of completed shuttles and 0.20 for QoL. These HOMAGE result should therefore be considered as exploratory because they do not exclude a type I error, albeit that these outcomes are in line with other trials of spironolactone [16, 18]. Third, women were underrepresented in our study, to some extent limiting its generalizability. Fourth, BP was measured in the clinics during the trial visits, which could have biased the BP values compared to out-of-office BP levels. However, due to consistency of the BP effects in subgroups and the replication of the BP results in other HF cohorts [37], these findings might be regarded as robust. Fifth, the clinical centers were free to organize the ISWT according to the local working routines irrespective of the time interval between the intake of medications, including spironolactone in the active-treatment group and the test. However, spironolactone undergoes rapid extensive metabolism to three active metabolites with prolonged half-lives (13.8–16.5 h) [30], so that this feature of conducting the trial is unlikely to have biased the results. Finally, given the relatively small sample size and the short follow-up, we could not relate the ISWT results to outcome. However, there are multiple long-term studies in patients [10, 38, 39] showing that exercise capacity as assessed by the ISWT predicts mortality [38, 39] and cardiovascular [39] or cardiac [10] endpoints.
Conclusions
In patients at increased risk of developing HF, MRA by spironolactone reduced the pre- and post-exercise BP but did not result in significant increases in exercise capacity or QoL. Guidelines for the management of symptomatic HF (stages III-IV) [40, 41] unanimously recommend the use of MRAs giving the overwhelming evidence from randomized clinical trials [14,15,16,17,18,19,20,21]. Given the current results, use of spironolactone 25–50 mg/day may also be considered in the management of patients at increased risk of developing HF, because of coronary heart disease; its use is not limited by hyperkalemia or an excessive reduction in eGFR.
References
Martens P, Augusto SN, Finet JE, Tang WHW. Distinct impact of noncardiac comorbidities on exercise capacity and functional status in chronic heart failure. JACC: Heart Fail. 2023;11:1365–76.
Fagard R, Pardaens K, Vanhaecke J. Prognostic significance of exercise versus resting blood pressure in patients with chronic heart failure. J Hypertens. 1999;17:1977–81.
von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail. 2021;23:92–113.
Singh R, Aggarwal D, Dutta K, Jaggi S, Sodhi MK, Saini V. Assessment of the feasibility of 1-min sit-to-stand test in evaluating functional exercise capacity in interstitial lung disease patients. J Exerc Rehabil. 2023;19:363–9.
Hecht I, Arad M, Freimark D, Klempfner R. Blood pressure dynamics during exercise rehabilitation in heart failure patients. Eur J Prev Cardiol. 2017;24:818–24.
Nishiyama Y, Morita H, Harada H, Katoh A, Adachi H, Koga A, et al. Systolic blood pressure to exercise as predictor of mortality in patients with chronic heart failure. Int Heart J. 2010;51:111–5.
Carneiro HA, Song RJ, Lee J, Schwartz B, Vasan RS, Xanthakis V. Association of blood pressure and heart rate responses to submaximal exercise with incident heart failure: the Framingham Heart Study. J Am Heart Assoc. 2021;10:e019460.
Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225.
Keell SD, Chambers JS, Francis DP, Edwards DF, Stables RH. Shuttle-walk test to assess chronic heart failure. Lancet. 1998;352:705.
Morales FJ, Montemayor T, Martinez A. Shuttle versus six-minute walk test in the prediction of outcome in chronic heart failure. Int J Cardiol. 2000;76:101–5.
Francis DP. Low-cost shuttle walk test for assessing exercise capacity in chronic heart failure. Int J Cardiol. 2000;76:105–6.
Pulz C, Diniz RV, Alves ANF, Tebexreni AS, Carvalho AC, de Paola ÂAV, et al. Incremental shuttle and six-minute walking tests in the assessment of the functional capacity in chronic heart failure. Can J Cardiol. 2008;24:131–5.
Parreira VF, Janaudis-Ferreira T, Evans RA, Mathur S, Goldstein RS, Brooks D. Measurement properties of the incremental shuttle walk test: a systematic review. Chest. 2014;145:1357–69.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17.
Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction. The Aldo-HF randomized controlled clinical trial. J Am Med Assoc. 2013;309:781–91.
Kosmala W, Rojek M, Przewlocka-Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:1823–34.
Upadhya B, Hundley WG, Brubaker PH, Morgan TM, Stewart KS, Kitzman DW. The effect of spironolactone on exercise tolerance and arterial function in older patients with HFpEF. J Am Geriatr Soc. 2017;65:2374–82.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–84.
Banerjee M, Maisman I, Pal R, Mukhopadhyay S. Mineralocorticoid receptor antagonists with sodium-glucose co-transporter-2 inhibitors in heart failure: a meta-analysis. Eur Heart J. 2023;44:3686–96.
Pellicori P, Ferreira JP, Mariottoni B, Brunner-La Rocca HP, Ahmed FZ, Verdonschot J, et al. Effects of spironolactone on serum markers of fibrosis in people at high risk of developing heart failure: rationale, design and baseline characteristics of a proof-of-concept, randomised, precision-medicine, prevention trial. The Heart OMics in AGing (HOMAGE) trial. Eur J Heart Fail. 2020;22:1711–23.
Cleland JGF, Ferreira JP, Mariottoni B, Pellicori P, Cuthbert J, Verdonschot JAJ, et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart ‘OMics’ in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 2021;42:684–96.
Singh SJ, Jones PW, Evans R, Morgan MDL. Minimum clinically improvement for the incremental shuttle walking test. Thorax. 2008;63:775–7.
Ravassa S, López B, Ferreira JP, Girerd N, Bozec E, Pellicori P, et al. Biomarker-based assessment of collagen cross-linking identifies patients at risk of heart failure more likely to benefit from spironolactone effects on left atrial remodelling. Insights from the HOMAGE clinical trial. Eur J Heart Fail. 2022;24:321–31.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. A report from the American Society of Echocardiography’s guidelines and standard committee and the task force on echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–119.
Kleinbaum DG, Kupper LL, Nizam A, Muller KE. The correlation coefficient and straight-line regression analysis. In: applied regression analysis and other multivariate methods and over. Hampshire, UK: BOOKS/COLE CENGAGE Learning; 2010. p. 91–106.
Szucs TD, Holm MV, Schwenkglenks M, Zhang Z, Weintraub WS, Burnier M, et al. Cost-effectiveness of eplerenone in patients with left ventricular dysfunction after mycardial infarction—an analysis of the EPHESUS Study from a Swiss perspective. Cardiovasc Drugs Ther. 2006;20:193–204.
Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol. 2008;31:153–8.
Potter E, Stephenson G, Harris J, Wright L, Marwick TH. Screening-guided spironolactone treatment of subclinical left ventricular dysfunction for heart failure prevention in at-risk patients. Eur J Heart Fail. 2022;24:620–30.
López B, González A, Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–54.
Huang R, Lin Y, Liu M, Xiong Z, Zhang S, Zhong X, et al. Time in target range for systolic blood pressure and cardiovascular outcomes in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2022;11:e022765.
Zhen Z, Choy M, Dong B, Dong Y, Liang W, Liu C, et al. Prognostic impact of abnormal sodium burden in heart failure patients with preserved ejection fraction. Eur J Clin Invest. 2023;53:e14115.
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes GT, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6:464–75.
Beldhuis IE, Myhre PL, Bristow M, Claggett B, Damman K, Fang JC, et al. Spironolactone in patients with heart failure, preserved ejection fraction, and worsening renal function. J Am Coll Cardiol. 2021;77:1211–21.
Ferreira JP, Collier T, Clark AL, Mamas MA, Brunner-La Rocca HP, Heymans S, et al. Spironolactone effect on the blood pressure of patients at risk of developing heart failure: an analysis from the HOMAGE trial. Eur Heart J Cardiovasc Pharmacother. 2022;8:149–56.
Billings CG, Hurdman JA, Condliffe R, Elliot CA, Smith IA, Austin M, et al. Incremental shuttle walk test distance and autonomic dysfunction predict survival in pulmonary arterial hypertension. J Heart Lung Transplant. 2017;36:871–9.
Greenwood SA, Castle E, Lindup H, Mayes J, Waite I, Grant D, et al. Mortality and morbidity following exercise-based renal rehabilitation in patients with chronic kidney disease: the effect of programme completion and change in exercise capacity. Nephrol Dial Transplant. 2019;34:618–25.
The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–200.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032.
Acknowledgements
The authors are indebted to the many investigators, who were involved in designing and running the HOMAGE trial. Their names are listed in the Supplementary Material.
the HOMAGE Investigators
Fozia Z. Ahmed10, Kei Asayama2, Erwan Bozec12, Hans P. Brunner La Rocca9, Andrew L. Clark13, Franco Cosmi12, John G. F. Cleland3, Joe Cuthbert13, Tim Collier17, Javier Díez6, Frank Edelmann11, João P. Ferreira4,5, Arantxa González6, Nicolas Girerd12, Stephanie Grojean18, Mark Hazebroek9, Stephane Heymans9, Tine W. Hansen2, Javed Khan3, Begoñia López6, Chen Liu1, Roberto Latini19, Beatrice Mariottoni7, Ken McDonald20, Gladys E. Maestre2, María U. Moreno12, Mamas A. Mamas10,21, Anne Pizard12, Burkert Pieske11, Johannes Petutschnigg11, Pierpaolo Pellicori3, Patrick Rossignol12, Philippe Rouet22, Suzanna Ravassa6, Jan A. Staessen2,8,16, Lutgarde Thijs23, Job A. J. Verdonschot9, Fang-Fei Wei1,2, Faiez Zannad12
Funding
HOMAGE was funded by the European Union Seventh Framework Program. OMRON Healthcare, Co., Ltd., Kyoto, Japan provided a non-binding grant to the Non-Profit Research Association Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, FF., Pellicori, P., Ferreira, J.P. et al. Effects of spironolactone on exercise blood pressure in patients at increased risk of developing heart failure: report from the HOMAGE trial. Hypertens Res 47, 3225–3236 (2024). https://doi.org/10.1038/s41440-024-01843-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01843-z