Abstract
Patients with adrenal aldosterone-producing adenomas (APA) face elevated cardiovascular risks, especially when cortisol is co-secreted, yet the impact on muscle health remains unclear. Myosteatosis, characterized by fatty infiltration into muscles, is linked to cardiometabolic diseases and decreased survival. We aimed to investigate the association between autonomous cortisol secretion (ACS) in APA and muscle quantity and quality. In this study, we analyzed data from 228 APA patients undergoing laparoscopic adrenalectomy between 2009 and 2024, assessing muscle composition via computed tomography. Intermuscular adipose tissue (IMAT), skeletal muscle area and density, visceral and subcutaneous adipose tissue area at L3 were measured. Comparisons were made between ACS and non-ACS groups. We found that among 228 patients, 76 (33.3%) had ACS. Those with ACS exhibited significantly higher IMAT area (P = 0.042) and lower skeletal muscle area (P = 0.002) and density (P < 0.001). Multivariable regression confirmed ACS positively associated with IMAT area and negatively associated with skeletal muscle area and density. At 1-year follow-up, ACS patients (n = 15) experienced decreased IMAT area (P = 0.001) and increased skeletal muscle area (P = 0.031) post-adrenalectomy, while those without ACS (n = 29) showed no IMAT change but increased visceral (P < 0.001) and subcutaneous (P = 0.008) adipose tissue area. In summary, myosteatosis and sarcopenia are linked to ACS in APA patients, and these parameters improve following adrenalectomy.

Similar content being viewed by others
Introduction
The prevalence of metabolic syndrome and obesity in patients with primary aldosteronism (PA) is high. Excess aldosterone results in cardiac remodeling and metabolic sequelae, including visceral obesity, insulin resistance, and impaired glucose homeostasis [1]. Concurrent autonomous cortisol secretion (ACS) is often underdiagnosed in PA due to incomplete screening. ACS not only leads to a higher prevalence of obesity and diabetes [2] but also independently increases cardiovascular events among patients with PA [3, 4]. Studies have indicated that in patients with ACS but without PA, increased cortisol secretion is linked to higher visceral adipose tissue (VAT) and reduced muscle quantity [5, 6]. However, little is known about ACS-induced changes in body composition among patients with PA. One study found no difference in total, visceral, and subcutaneous fat volumes between ACS and non-ACS groups in patient with PA [7]. Conversely, another study revealed that among patients with aldosterone-producing adenoma (APA), a subtype of PA, those with ACS exhibited significantly lower skeletal muscle area compared to those without ACS [8].
Myosteatosis, described as excess fat deposition in the skeletal muscle, is currently gaining attention because it provides a distinct perspective in sarcopenia research [9]. Multiple studies have demonstrated that myosteatosis is a negative prognostic factor in cancer and is associated with a higher complication rate in various diseases such as cardiovascular disease and hepatic steatosis [10]. While Cushing’s syndrome is widely recognized for its association with visceral obesity and muscle wasting, research on ACS cohorts is limited, with no prior investigations into the impact of PA [6, 11]. In addition, the relationship between myosteatosis and ACS has not been investigated. The aim of our study was to clarify the relationship between muscle fat content, especially myosteatosis, and ACS in patients with PA.
Materials and methods
Data sources
The multicenter Taiwan Primary Aldosteronism Investigators (TAIPAI) database maintains a standardized database of PA and prospectively collects biochemical, imaging, and clinicopathological data. The study population and criteria used for the diagnosis of PA were described in a previous publication [12]. The study protocol was approved by the Ethics Committee of the National Taiwan University Hospital. Informed consent was obtained from all participants prior to inclusion in the study.
Study population
We conducted a retrospective analysis of 228 adult patients with APA who underwent unilateral adrenalectomy between January 2009 and April 2024. Both men and women aged >18 years were eligible for this study. Of these, 44 recruited patients underwent a follow-up abdominal CT-scan 1-year after post-adrenalectomy.
Diagnosis criteria
-
1.
Primary aldosteronism
The diagnosis of PA was established in hypertensive patients by fulfillment of the following criteria : (1) autonomous excess aldosterone production evidenced by an aldosterone-renin ratio > 35 ng/mL/h; (2) plasma aldosterone concentration (PAC) > 16 ng/dL in a seated saline infusion test, or PAC/ plasma renin activity > 35 ng/dL shown in a captopril/losartan challenge test [12].
-
2.
Aldosterone-producing adenoma
The diagnosis of APA was based on (1) the evidence of PA at the screening tests mentioned above; (2) lateralization of aldosterone secretion with adrenal vein sampling confirmed; (3) histopathological evidence of adenoma after adrenalectomy; and (4) PA being corrected by adrenalectomy.
-
3.
Patients with autonomous cortisol secretion
Patients were categorized as having ACS based on an overnight 1 mg dexamethasone suppression test (DST). According to the guideline proposed by European Endocrinology Society, a cutoff point of 1.8 μg/dl is applied to exclude ACS [13]. ACS diagnosis required serum cortisol ≥5 μg/dL after a 1 mg DST. For levels between 1.8 and 4.9 μg/dL, at least one additional criteria such as ACTH < 10 pg/mL, nocturnal cortisol ≥5 μg/dL, low DHEA-S, or UFC > 70 μg/24-h were needed.
-
4.
Post-adrenalectomy outcome
We used the Primary Aldosteronism Surgery Outcome (PASO) consensus criteria to classify the clinical and biochemical outcomes of adrenalectomy [14]. These criteria provide standardized definitions for complete, partial, and absent success based on blood pressure control, use of antihypertensive medications, and levels of plasma potassium, aldosterone, and renin. The outcomes were categorized accordingly as complete, partial, or absent success
Biochemical measurements
Serum cortisol was measured using a chemiluminescent immunoassay (Architect, Abbott, VA, USA), and PAC and plasma renin activity were measured using commercial radioimmunoassay kits (Biochem Immunosystems, Bologna, Italy, and Stillwater, MN, USA, respectively). Anti-hypertensive medication and medication that may interfere with DST were discontinued prior to the study [12].
Body composition analysis by computed tomography (CT)
Various CT scanners were used for brain CT in this study, with detector ranges from 64 to 320. These scanners included LightSpeed VCT by GE Healthcare (Milwaukee, Wisconsin, USA), Brilliance 64 and Brilliance iCT 256 by Philips Healthcare (Best, The Netherlands), SOMATOM Sensation 64, SOMATOM Definition AS by Siemens Healthineers (Erlangen, Germany), and Aquilion ONE by Toshiba Medical Systems (Tokyo, Japan). All CT examinations were performed using the following parameters: 120 kVp and automated dose modulation. The imaging slice thickness was 5 mm, and the scan range was from the upper edge of the diaphragm to the lower pole of both kidneys. A blinded trained researcher with the guidance of two radiologists (B-CL and C-CC) analyzed the CT images using sliceOmatic image analysis software (version 5.0, Tomovision, Montreal, QC, Canada). For body composition analysis, using predefined Hounsfield units (HU) ranges, the total areas of skeletal muscle (SMA, −29 to 150 HU), visceral adipose tissue (VAT, −150 to −50 HU), subcutaneous adipose tissue (SAT, −190 to −30 HU), and intermuscular adipose tissue (IMAT, −190 to −30 HU) were measured at the mid L3 vertebral level of selected axial CT slices (Fig. 1); afterward, the SMA, VAT, SAT, and IMAT were manually tagged and quantified. Single-slice abdominal images at the L3 vertebra correlate strongly with whole-body volumes of skeletal muscles and adipose tissues, as reported in previous studies [15]. Skeletal muscle density (SMD) and IMAT density is defined by mean HU of the SMA and IMAT, respectively.
Schematic of abdominal compartments segmentation using semi-automatic software. The A cross-sectional computerized tomography image at the mid L3 vertebral level is quantified based on different densities of B subcutaneous adipose tissue (red), visceral adipose tissue (green), skeletal muscle (yellow), and intermuscular adipose tissue (light blue)
Statistical analysis
All data are presented as the mean ± standard deviation unless otherwise specified. Baseline characteristics were compared using a two-sample t-test for continuous variables and a chi-square test for categorical variables. Multivariable analyses were performed using a multivariable linear regression model after adjustment for confounding factors. All parameters were systematically checked for collinearity. The linear relationship between two continuous variables was assessed using Pearson’s correlation. Comparisons of body composition parameters pre- and post-adrenalectomy were conducted using the Wilcoxon signed-rank test. All statistical analyses for significance and resulting P values were two-sided with P < 0.05. We utilized SPSS Statistics software version 25 (IBM, Armonk, NY, USA) for all analyses. GraphPad Prism 9 software was used for creating all figures.
Results
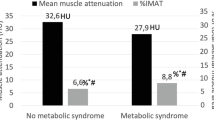
Table 1 shows the demographic data and body composition parameters of each group of patients. The mean age of the individuals was 52.6 ± 10.6 years, and half (49.6%) of them were male. Of 228 patients with APA, 76 (33.3%) had ACS. APA patients with ACS were older (55.7 ± 10.4 vs. 51.0 ± 10.3, P = 0.002), with higher serum potassium (3.8 ± 0.6 mmol/L vs. 3.6 ± 0.5 mmol/L, P = 0.012), higher post-DST serum cortisol (5.2 ± 3.8 μg/dL vs. 0.9 ± 0.5, P < 0.001), less baseline adrenocorticotropic hormone (8.4 ± 7.2 pg/mL vs. 21.1 ± 12.2 pg/mL, P < 0.001), less dehydroepiandrosterone sulfate (2.7 ± 2.2 μmol/L vs. 4.2 ± 2.7 μmol/L, P < 0.001) compared to those without ACS. Other clinical characteristics, hypertensive medications, lipid profile and comorbidities related to metabolic and cardiovascular diseases showed no significant differences between both groups.
Patients with APA/ACS showed myosteatosis and sarcopenia
We compared muscle quantity and quality between patients with ACS and those without ACS. The increased IMAT area reflects intermuscular fatty deposition, while decreased SMD indicates higher intramyocellular lipids (IMCL). In Table 2, the ACS group exhibited significantly higher IMAT area (9.6 ± 4.9 cm2 vs. 8.3 ± 4.4 cm2, P = 0.042), lower IMAT density (−29.3 ± 15.8 HU vs. −23.8 ± 13.5 HU, P = 0.006) and lower SMD (32.2 ± 10.6 HU vs. 37.3 ± 8.4 HU, P < 0.001) compared to non-ACS patients. Additionally, the ACS group showed significantly lower SMA (119.4 ± 39.1 cm2 vs. 135.3 ± 33.7 cm2, P = 0.002) compared to non-ACS group, indicating reduced muscle quantity. Other body composition parameters, including VAT area, VAT density, SAT area and SAT density, did not differ between the groups.
ACS was independently associated with IMAT area, SMD and SMA
We further examined the correlation between IMAT area, SMD, and SMA with post-DST serum cortisol levels and ACS status. In Fig. 2, post-DST serum cortisol levels positively correlated with IMAT area (R2 = 0.027, P = 0.012) and negatively correlated with SMA (R2 = 0.060, P < 0.001) and SMD (R2 = 0.123, P < 0.001). We then assessed if the association between muscle fat content, muscle quantity, and ACS was independent of metabolic factors. Bivariate analyses showed a significant association of ACS with SMA, SMD, IMAT area and IMAT density. After adjusting for confounders (age, sex, BMI, systolic BP, diabetes status, triglycerides, and cholesterol levels) in multivariate analyses, the relationship between ACS and SMA, SMD, IMAT area and IMAT density remained significant (all P < 0.05) (Table 3).
Associations of the body composition parameters with post-DST serum cortisol levels in patients with APA. The scatter plots illustrate the relationships between IMAT A, SMA B and SMD C and post-DST serum cortisol levels. APA aldosterone-producing adenoma, DST dexamethasone suppression test, IMAT intermuscular adipose tissue, SMA skeletal muscle area, SMD skeletal muscle density
Improvement of IMAT area and SMA in patients with APA/ACS post-adrenalectomy
A total of 44 patients underwent a follow-up abdominal CT scan one year after adrenalectomy. The rate of complete or partial clinical success is 73.3% (11/15) for the ACS group and 93.1% (27/29) for the non-ACS group; while the rate of complete or partial biochemical success is 80.0% (12/15) for the ACS group and 86.2% (25/29) for the non-ACS group, all without significant difference. Table 4 and Supplemental Fig. 1 illustrate that the ACS group (n = 15) exhibited a significant decrease in IMAT area (9.7 ± 5.7 cm2 to 7.3 ± 4.7 cm2, P = 0.001) and an increase in SMA (111.4 ± 36.1 cm2 to 117.0 ± 37.1 cm2, P = 0.031), with no change in IMAT density, SMD, VAT area, VAT density, SAT area and SAT density. Conversely, patients without ACS (n = 29) showed no alterations in IMAT area, IMAT density, VAT density, SAT density, SMA, or SMD but had significant increases in VAT area (132.9 ± 67.8 cm2 to 169.4 ± 82.6 cm2, P < 0.001) and SAT area (165.6 ± 67.4 cm2 to 191.5 ± 99.3 cm2, P = 0.008).
Discussion
Myosteatosis and sarcopenia is associated with ACS
Disruption of muscular metabolism is associated with metabolic impairment in hypercortisolism. Nevertheless, research on myosteatosis in ACS has not been conducted to date [16]. In this study, we used the IMAT area and SMD as indicators for evaluating myosteatosis. We demonstrated a previously unrecognized association between ACS and myosteatosis in APA patients, distinct from sarcopenia, providing independent prognostic information in various diseases [10]. Furthermore, this association remained significant after adjusting for multiple metabolic confounders. Importantly, the detrimental change in body composition parameter showed improvement after adrenalectomy, highlighting the efficacy of targeted therapy for APA patients. On the other hand, we found that visceral fat area increased after adrenalectomy in non-ACS patients with APA, which seems consistent with the findings of a prior study [17].
Although most studies only used SMD to diagnose myosteatosis [10], IMAT and SMD could have different physiological implications and clinical relevance in patients. They represent different fat content and distribution patterns. IMAT is an extramyocellular lipid deposition within muscles’ fascia and can be directly measured via CT segmentation [10]. IMCL refers to microscopic fat infiltration within myocytes, reflected by lower SMD on CT scans. This is because muscle radiodensity decreases as intramuscular fat deposition increases [18]. Our result showed that IMAT and IMCL mostly have the same tendency but are not always congruent, such as among patient after adrenalectomy, which suggests that different physiological mechanisms may cause the accumulation or resolution of IMAT and IMCL. The precise mechanisms of glucocorticoid-mediated IMAT and IMCL deposition have not been fully established. IMAT is responsible for mediating insulin resistance [19] and is involved in proinflammatory pathways in muscle, and further studies are needed to explore its prognostic implication in PA patients.
Moreover, we observed that the ACS group exhibited lower skeletal muscle quantity compared to the non-ACS groups among patients with APA. This aligns with findings from a prior study comparing ACS status in APA patients, which similarly reported significantly lower skeletal muscle area in those with ACS [8]. Interestingly, other studies involving general PA patients or nonfunctioning adrenal incidentaloma did not demonstrate such an association between muscle mass and ACS status [7, 20].
Post-adrenalectomy changes of body composition
Another notable aspect of our study was the post-adrenalectomy improvement in IMAT and SMA. We observed a decrease in IMAT and an increase in SMA among the ACS group, contrasting with the non-ACS group where no significant changes were noted. This trend was not observed in SMD. Previous research has indicated that metabolic factors such as hypertension, BMI, and metabolic syndrome improve in ACS patients post-adrenalectomy, while cardiometabolic parameters remain unchanged or worsen in nonfunctioning adrenal incidentaloma patients [4]. Our findings suggest that IMAT may hold more clinical relevance than SMD after adrenalectomy in ACS patients. However, given the limited sample size, further validation in larger cohorts is warranted.
Additionally, we observed an increase in VAT and SAT after adrenalectomy in the non-ACS group, consistent with findings from other studies. One study reported significant weight gain in APA patient post-adrenalectomy [21], while another noted increased abdominal adipose tissue after adrenalectomy, particularly in those with KCNJ5 mutations [22]. This may be due to aldosterone-induced adipose tissue shrinkage and fibrosis in APA patients, as indicated by elevated fibronectin and collagen I protein levels [23, 24]. Moreover, APA patients experience increased sympathetic activation, which could lead to higher energy expenditure and reduced adipose tissue size [25]. Post-adrenalectomy, these effects are reversed, resulting in an increase in SAT and VAT.
Visceral fat is not associated with ACS
In theory, glucocorticoids bind to adipocyte glucocorticoid receptors and induce adipogenesis, especially in the VAT. Intriguingly, our results showed no significant difference in VAT between the ACS and non-ACS groups, consistent with prior studies [8, 11, 20]. There are several possible explanations for this. First, glucocorticoid signaling is polymorphic and differs among people. Hence, using an absolute cut-off value of serum cortisol level to diagnose ACS may not suit everyone because the same cortisol level did not reflect the same effect. Second, the effect of aldosterone on VAT should also be considered. Recent research indicates lower VAT in APA patients compared to those with essential hypertension, suggesting a potential counterbalancing effect between cortisol and aldosterone in APA/ACS [17]. These data further support that both cortisol and aldosterone play complex roles in adipogenesis.
Limitations
This retrospective study had several limitations. Firstly, the limited number of participants with follow-up abdominal CT scans reduced statistical power. Secondly, ACS can be clinically latent, making it challenging to ascertain the duration of cortisol excess prior to imaging, which may impact body composition redistribution. Additionally, the lack of standardized cutoff values and complementary diagnostic criteria in ACS protocols may introduce bias [12]. Thirdly, there is no standardized diagnostic consensus for myosteatosis. While most studies utilize abdominal CT and analyze body composition at the L3 level due to its correlation with whole-body muscle mass, we adhered to this methodology.
Conclusions
We found that high muscle fat content and low muscle mass were independently associated with APA/ACS after adjusting for metabolic factors. These adverse changes improved following adrenalectomy, underscoring the efficacy of targeted therapy for PA patients. Given the accessibility of routine abdominal CT scans for APA patients, utilizing IMAT area and SMD as myosteatosis indicators to predict cardiovascular risk in ACS/APA could be beneficial. Assessing muscle quality at baseline and longitudinally may offer valuable insights. Further research, including ‘muscular fat mapping,’ is needed to elucidate skeletal muscle quality’s role in preventing cardiovascular and metabolic sequelae.
References
Chang YY, Tsai CH, Peng SY, Chen ZW, Chang CC, Lee BC, et al. KCNJ5 somatic mutations in aldosterone-producing adenoma are associated with a worse baseline status and better recovery of left ventricular remodeling and diastolic function. Hypertension. 2021;77:114–25.
Bleier J, Shlomai G, Fishman B, Dotan Z, Rosenzweig B, Tirosh A. The quantitative relationship between autonomous cortisol secretion, dysglycemia and the metabolic syndrome. Endocr Pract. 2020;26:974–82.
Nakajima Y, Yamada M, Taguchi R, Satoh T, Hashimoto K, Ozawa A, et al. Cardiovascular complications of patients with aldosteronism associated with autonomous cortisol secretion. J Clin Endocrinol Metab. 2011;96:2512–8.
Khan U. Nonfunctioning and subclinical cortisol secreting adrenal incidentalomas and their association with metabolic syndrome: a systematic review. Indian J Endocrinol Metab. 2019;23:332–46.
Debono M, Prema A, Hughes TJ, Bull M, Ross RJ, Newell-Price J. Visceral fat accumulation and postdexamethasone serum cortisol levels in patients with adrenal incidentaloma. J Clin Endocrinol Metab. 2013;98:2383–91.
Kim JH, Kwak MK, Ahn SH, Kim H, Cho YY, Suh S, et al. Alteration in skeletal muscle mass in women with subclinical hypercortisolism. Endocrine. 2018;61:134–43.
Mansour N, Bruedgam D, Dischinger U, Kurzinger L, Adolf C, Walter R, et al. Effect of mild cortisol cosecretion on body composition and metabolic parameters in patients with primary hyperaldosteronism. Clin Endocrinol. 2024;100:212–20.
Park SS, Ahn CH, Kim SW, Yoon JW, Kim JH. Subtype-specific Body Composition and Metabolic Risk in Patients With Primary Aldosteronism. J Clin Endocrinol Metab. 2024;109:e788–98.
Dolly A, Dumas JF, Servais S. Cancer cachexia and skeletal muscle atrophy in clinical studies: what do we really know? J Cachexia Sarcopenia Muscle. 2020;11:1413–28.
Ahn H, Kim DW, Ko Y, Ha J, Shin YB, Lee J, et al. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev. 2021;70:101398.
Yener S, Baris M, Peker A, Demir O, Ozgen B, Secil M. Autonomous cortisol secretion in adrenal incidentalomas and increased visceral fat accumulation during follow-up. Clin Endocrinol. 2017;87:425–32.
Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, et al. Case detection and diagnosis of primary aldosteronism - The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005.
Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175:G1–34.
Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–99.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8.
Drey M, Berr CM, Reincke M, Fazel J, Seissler J, Schopohl J, et al. Cushing’s syndrome: a model for sarcopenic obesity. Endocrine. 2017;57:481–5.
Er LK, Lin MC, Tsai YC, Hsiao JK, Yang CY, Chang CC, et al. Association of visceral adiposity and clinical outcome among patients with aldosterone producing adenoma. BMJ Open Diabetes Res Care. 2020;8:e001153.
Correa-de-Araujo R, Addison O, Miljkovic I, Goodpaster BH, Bergman BC, Clark RV, et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol. 2020;11:963.
Kovalik JP, Slentz D, Stevens RD, Kraus WE, Houmard JA, Nicoll JB, et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes. 2011;60:1882–93.
Delivanis DA, Iniguez-Ariza NM, Zeb MH, Moynagh MR, Takahashi N, McKenzie TJ, et al. Impact of hypercortisolism on skeletal muscle mass and adipose tissue mass in patients with adrenal adenomas. Clin Endocrinol. 2018;88:209–16.
Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25:177–86.
Chen KM, Chang YL, Wu TH, Lee BC, Chen PT, Liu KL, et al. Aldosterone-producing adenoma-harbouring KCNJ5 mutations is associated with lower prevalence of metabolic disorders and abdominal obesity. J Hypertens. 2021;39:2353–60.
Wu C, Zhang H, Zhang J, Xie C, Fan C, Zhang H, et al. Inflammation and fibrosis in perirenal adipose tissue of patients with aldosterone-producing adenoma. Endocrinology. 2018;159:227–37.
Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25.
Kontak AC, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Nesbitt SD, et al. Reversible sympathetic overactivity in hypertensive patients with primary aldosteronism. J Clin Endocrinol Metab. 2010;95:4756–61.
Acknowledgements
We thank the staff of the Second Core Lab of the Department of Medical Research in National Taiwan University Hospital for technical support. We also extend our gratitude to Ms. Ying-Ting Hung and Ms. Pin-Chen Chen for their technical support throughout the study.
The TAIPAI Study Group
Vin-Cent Wu4, Tai-Shuan Lai4, Shih-Chieh Jeff Chueh4, Shao-Yu Yang4, Kao-Lang Liu4, Chin-Chen Chang4, Bo-Ching Lee4, Shuo-Meng Wang4, Kuo-How Huang4, Po-Chih Lin4, Yen-Hung Lin4, Chi-Sheng Hung4, Lian-Yu Lin4, Shih-Cheng Liao4, Ching-Chu Lu4, Chieh-Kai Chan4, Leay-Kiaw Er5, Ya-Hui Hu5, Che-Hsiung Wu5, Yao-Chou Tsai5, Zheng-Wei Chen6, Chien-Ting Pan6, Che-Wei Liao7, Cheng-Hsuan Tsai8, Yi-Yao Chang9, Chen-Hsun Ho10, Wei-Chieh Huang11, Ying-Ying Chen12
Funding
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST-111-2628-B-002-025-MY3).
Author information
Authors and Affiliations
Consortia
Contributions
Study concept and design: B-CL, C-CC. Acquisition of data: B-CL, P-TC, and K-LL. Analysis and interpretation of data: B-CL, Y-LC and C-CC. Statistical analysis: B-CL and C-CC. Writing—original draft preparation: B-CL, Y-LC. Writing—review and editing: B-CL, P-TC, and C-CC. Critical revision of the manuscript for important intellectual content: V-CW, Y-HL, and C-CC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, BC., Chang, YL., Chen, PT. et al. Myosteatosis and sarcopenia are linked to autonomous cortisol secretion in patients with aldosterone-producing adenomas. Hypertens Res 48, 519–528 (2025). https://doi.org/10.1038/s41440-024-01933-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01933-y