Abstract
Non-self recognition is a fundamental aspect of life, serving as a crucial mechanism for mitigating proliferation of molecular parasites within fungal populations. However, studies investigating the potential interference of plants with fungal non-self recognition mechanisms are limited. Here, we demonstrate a pronounced increase in the efficiency of horizontal mycovirus transmission between vegetatively incompatible Sclerotinia sclerotiorum strains in planta as compared to in vitro. This increased efficiency is associated with elevated proline concentration in plants following S. sclerotiorum infection. This surge in proline levels attenuates the non-self recognition reaction among fungi by inhibition of cell death, thereby facilitating mycovirus transmission. Furthermore, our field experiments reveal that the combined deployment of hypovirulent S. sclerotiorum strains harboring hypovirulence-associated mycoviruses (HAVs) together with exogenous proline confers substantial protection to oilseed rape plants against virulent S. sclerotiorum. This unprecedented discovery illuminates a novel pathway by which plants can counteract S. sclerotiorum infection, leveraging the weakening of fungal non-self recognition and promotion of HAVs spread. These promising insights provide an avenue to explore for developing innovative biological control strategies aimed at mitigating fungal diseases in plants by enhancing the efficacy of horizontal HAV transmission.
Similar content being viewed by others
Introduction
The ability to discriminate between self and non-self is an ubiquitous phenomenon in all domains of life. In filamentous fungi, this process is typically termed vegetative (or heterokaryon) incompatibility (VIC)1,2, wherein contact between genetically incompatible fungal strains triggers a non-self recognition reaction preventing any subsequent hyphal fusion via numerous pathways including programmed cell death (PCD)1,2. Studies exploring the molecular mechanisms underpinning self/non-self recognition in filamentous fungi have primarily focused on ascomycetes such as Neurospora crassa, Podospora anserina, Cryphonectria parasitica, and Botrytis cinerea3,4,5,6,7,8,9,10,11,12. The genetic loci determining self/non-self recognition are traditionally termed het (heterokaryon) or vic (vegetative incompatibility). Within a fungal species, strains possessing identical alleles at these loci can engage in hyphal anastomosis and are categorized within the same vegetative compatibility group (VCG). Conversely, strains harboring different alleles are incompatible and belong to different VCGs1,9,13. Genes involved in the self/non-self recognition reaction encode heterotrimeric guanine nucleotide-binding proteins (G proteins), proteins involved in generation and scavenging of reactive oxygen species (ROS), and proteins containing HET domains, nucleotide oligomerization ___domain-like receptors, and cell wall remodeling checkpoint regulators5,11,12,14,15. Fundamentally, the self/non-self reaction functions akin to a rudimentary innate immune system, restricting the transmission of deleterious factors, including mycoviruses, between fungal strains, and thereby enhancing organismal fitness in natural environments11.
Mycoviruses are obligate parasites of fungi. Unlike viruses infecting plant and animals, mycoviruses lack an extracellular phase in their replication cycle, with only rare exceptions16. Mycoviruses are transmitted horizontally between fungal strains and vertically from parent to offspring, through respectively hyphal fusion or sporulation. While the majority of mycoviruses do not induce discernible phenotypic alterations in their host fungi and their infections often remain latent, few mycoviruses can modulate fungal traits such as virulence17,18,19. Moreover, mycoviruses seemingly associated with cryptic infections may exert roles that are not readily apparent through conventional experimental approaches, such as the mycovirus-mediated thermal tolerance observed in fungi and plants18. Therefore, mycoviruses potentially play critical ecological roles in natural ecosystems. Of particular interest are mycoviruses that confer hypovirulence to phytopathogenic fungi, garnering considerable attention for their potential application as biological control agents against fungal diseases afflicting trees and crops. For instance, Cryphonectria hypovirus 1 (CHV1) has been successfully utilized in Europe to control the phytopathogenic fungus, Cryphonectria parasitica, responsible for chestnut blight20. However, success has been inconsistent in other geographical regions, particularly in North America. It has been postulated that the intricate structure of C. parasitica VCGs might be one of the factors contributing to the failure of CHV1-mediated control of chestnut blight20,21. Hypovirulence-associated mycoviruses (HAVs) are especially noteworthy due to their ability to spread within populations of phytopathogenic fungi via fungal anastomosis. Therefore, the rapid spread of HAVs in natural host populations is crucial for successfully controlling plant diseases. However, successful dissemination of HAVs within natural host populations is contingent upon uninterrupted horizontal transmission facilitated by hyphal fusion. This process can be hindered by factors such as VIC9,22,23, potentially limiting the efficacy of mycovirus-mediated biological control strategies20.
Despite the prevalence of VIC within fungal species and the intricate structure of VCGs, mycoviruses are ubiquitous in major fungal groups, with individual fungal isolates often harboring diverse mycoviruses17,24,25,26,27. Studies conducted with C. parasitica and Rosellinia necatrix have demonstrated that the rates of horizontal mycovirus transmission are higher in planta than in vitro28,29. Furthermore, highly similar mycoviruses are sometimes found to infect different fungal species or genera in nature26,30. These observations suggest that VIC can be weakened, allowing horizontal mycovirus transmission even between distinct fungal genera. However, the underlying mechanisms driving this phenomenon remain unexplored.
Proline is a pivotal multi-functional amino acid known for its diverse biological functions across plants, mammals, and fungi. Constituting approximately 5% of the total pool of free amino acids in plants under normal conditions, proline proportion can surge to as much as 80% of the amino acid pool following stress31. Interestingly, proline accumulation is a hallmark plant response during the early stages of infection by phytopathogenic fungi32,33,34. In mammals, proline plays a protective role against H2O2-induced cell death and apoptosis, but it has also been shown to promote apoptosis in certain human colon cancer cell lines35. In fungi, proline serves as a potent antioxidant, effectively scavenging ROS and inhibiting apoptosis-like PCD36. Despite the established association between PCD and fungal non-self recognition, the involvement of proline in inhibiting non-self recognition between VIC groups has not been studied previously.
S. sclerotiorum, a cosmopolitan phytopathogenic fungus, exhibit a broad host range, infecting over 700 plant species and causing significant economic losses annually37. S. sclerotiorum harbors a diverse array of mycoviruses, some of which recognized as HAVs with potential as biological control agents due to their substantial impact on virulence25,38,39,40,41,42,43,44,45. Given that S. sclerotiorum does not produce typical asexual spores, RNA mycoviruses in S. sclerotiorum primarily rely on hyphal anastomosis for horizontal transmission. However, the VCGs of S. sclerotiorum exhibit notable complexity. For instance, a study of 30 individual S. sclerotiorum strains isolated from a single rapeseed field illustrated that they are associated with 27 different VCGs46. Therefore, the complex S. sclerotiorum VCGs pose a potential impediment to horizontal mycovirus transmission and consequently challenge the efficacy of HAV-based biocontrol applications. Nevertheless, the fact that S. sclerotiorum strains isolated from their natural habitat harbor diverse mycoviruses indicates the existence of mechanisms facilitating mycovirus transmission in nature and suggests that even fungal populations with a high degree of VIC are amenable to mycovirus-based control strategies25,27,38.
In this study, our primary objective was to investigate how mycoviruses overcome limitations in effective horizontal transmission imposed by the fungal non-self recognition system in natural environments. Our findings reveal that the transmission frequency of mycoviruses between S. sclerotiorum VIC strains is markedly higher in planta as compared to in vitro, concomitant with an increase in plant proline levels upon S. sclerotiorum infection. We present compelling evidence that plant proline serves to attenuate non-self recognition reactions in S. sclerotiorum, thus facilitating horizontal mycovirus transmission. By elucidating the mechanism underlying elevated horizontal mycovirus transmission levels in planta, our study offers valuable insights and proposes a straightforward yet innovative strategy for enhancing the application of HAVs in plant disease management in agricultural settings.
Results
Mycovirus transmission between S. sclerotiorum strains is enhanced in plants
S. sclerotiorum strain Ep-1PN, co-infected with Sclerotinia sclerotiorum debilitation-associated RNA virus (SsDRV) and Sclerotinia sclerotiorum RNA virus L (SsRV-L), exhibited several phenotypic traits associated with hypovirulence, including abnormal colony morphology, reduced growth rate, and decreased virulence on oilseed rape (Brassica napus) plants (Fig. 1A, B)43,47. Strain Ep-1PNA367 is a virulent HAVs-free strain, obtained from HAVs-infected Ep-1PN through a single ascospore isolation and therefore vegetatively compatible with Ep-1PN. Co-culturing of Ep-1PN and Ep-1PNA367 on Potato Dextrose Agar (PDA) resulted in the unrestricted horizontal transmission of mycoviruses from Ep-1PN (donor strain) to Ep-1PNA367 (recipient strain), converting Ep-1PNA367 into a hypovirulent strain as evidenced by its abnormal colony morphology and reduced growth rate (Fig. 1A)43. Conversely, mycoviruses were rarely transmitted on PDA from Ep-1PN to incompatible strains 1980m, SG9, and SCH941A1, as illustrated by the lack of traits associated with hypovirulence in the recipient strains (Fig. 1A). On oilseed rape plants, the HAVs-infected strain Ep-1PN caused significantly smaller lesions as compared to the four virulent strains. Notably, co-inoculation of all four virulent strains with Ep-1PN resulted in significantly smaller lesions as compared to the virulent strains alone (Fig. 1B–D). These observations suggested that, in planta, mycoviruses were horizontally transmitted from Ep-1PN to the virulent strains despite VIC, thereby conferring hypovirulence.
A Colony morphology of S. sclerotiorum strains Ep-1PN (EP), Ep-1PNA367 (A367), 1980m, SG9, and SCH941A1 (941A1) at 7 dpi (upper panel), and co-cultures of the HAVs-infected hypovirulent strain Ep-1PN as donor and the other four HAVs-free virulent strains as recipients for 5 dpi (lower panel) on PDA at 20 °C. Ep-1PN is compatible with its single-ascospore derivative Ep-1PNA367, and incompatible with the other three strains. B Virulence assay of Ep-1PNA367, 1980m, SG9, and SCH941A1 (upper panel), and their co-inoculations with Ep-1PN (lower panel) on living oilseed rape plants (72 hpi, 20 °C). The white bar represents 1 cm. C Diameters of lesions caused by Ep-1PN, Ep-1PNA367, 1980m, SG9, and SCH941A1 alone, and following co-inoculation of those strains with Ep-1PN on living oilseed rape plants (72 hpi, 20 °C), n = 76 biologically independent samples. D Analysis of diameters of lesions caused by co-inoculation of Ep-1PN with either Ep-1PNA367 or 1980m using the Bland-Altman method, illustrating that only 6.8% (5/74) of lesion size values were outside the 95% consistency limit (95% limits of agreement) of the maximum error range. E Horizontal transmission efficiency of two mycoviruses, SsDRV and SsRVL, from Ep-1PN to 1980m on PDA, living oilseed rape plants, and oilseed rape leaves previously stored in −80 °C (n = 3 and N = 40; n = 3 indicates that we conducted three independent biological repeats and N = 40 indicates that we performed 40 co-cultures for each biological repeat, with each recipient strain from the 40 co-cultures sub-cultured on plates containing hygromycin or neomycin and used to measure mycovirus transmission efficiency). Data are presented as mean ± SD, two-tailed Student’s t test. ****p < 0.0001. Source data are provided as a Source Data file.
To test this hypothesis, we re-isolated incompatible strain 1980m, which is labeled with a hygromycin resistance marker, from plant lesions through three rounds of sub-culturing on PDA with hygromycin (Supplementary Fig. 1). Subsequent reverse transcription followed by polymerase chain reaction (RT-PCR) confirmed the presence of mycoviruses SsDRV and SsRVL in 1980m, with the transmission frequency being significantly higher in planta (79% for SsDRV and 25% for SsRVL) than on PDA (25% for SsDRV and 6% for SsRVL) (Fig. 1E). This increased transmission rate was only observed when experiments were conducted on fresh leaves and not leaves previously stored in −80 °C (Fig. 1E and Supplementary Fig. 1), indicating that the leaves must be metabolically active to influence transmissibility. Therefore, we concluded that oilseed rape plants can enhance horizontal transmission of mycoviruses between incompatible strains.
Fungal VIC-associated genes are downregulated in planta
To unravel the potential mechanism underlying enhanced mycovirus transmissibility in planta, strain 1980m was co-cultured separately with HAVs-infected strain Ep-1PN and HAVs-free strain Ep-1PNA367 on PDA and plants. Subsequently, genes related to the self/non-self recognition reaction and the G protein signaling pathway were identified in the S. sclerotiorum genome and their in vitro and in planta expression levels were compared (Fig. 2 and Supplementary Fig. 2). In total, forty genes encoding proteins with a conserved HET ___domain were identified, the majority of which were downregulated in planta as compared to in vitro (Fig. 2C and Supplementary Fig. 2). Additionally, six genes encoding G proteins, including three Gα (sscle_11g084840, sscle_05g042960, and sscle_16g109000), two Gβ (sscle_07g056270 and sscle_03g023580), and one Gγ (sscle_06g053750), were identified. All except Gβ2 were downregulated in planta as compared to in vitro (Fig. 2A, B and Supplementary Fig. 2A). Notably, the presence of HAVs in EP-1PN did not appear to have a significant effect on the expression levels of the aforementioned genes (Supplementary Fig. 2). Therefore, the attenuation of the VIC reaction is attributable to plant-fungus interactions, rather than mycovirus infection. We further compared the expression levels of genes encoding G proteins in other phytopathogenic fungi during growth in vitro and infection in planta by utilizing publicly available transcriptome data (Supplementary Fig. 3). Our analysis revealed that the majority of these genes were downregulated in planta as compared to their in vitro expression, suggesting that their suppression during plant-fungus interactions may be universal.
A Expression cluster analysis of S. sclerotiorum genes encoding G protein subunits in 1980 and Ep-1PN co-cultures in vitro and in planta. B Expression levels of genes encoding G protein subunits in 1980 alone, and 1980 and Ep-1PN co-culture in vitro and in planta. Data are presented as mean ± SD, two-tailed Student’s t test. n = 3 biologically repetitions. C Expression cluster analysis of S. sclerotiorum genes encoding proteins containing HET conserved domains in 1980 and Ep-1PN co-cultures in vitro and in planta. The relative expression of selected genes was plotted based on the threshold of RPKM value in RNA-seq data (Bioproject Accession: PRJNA908905). Red and blue indicate respectively high and low expression levels. Source data are provided as a Source Data file.
Plant proline accumulation induced by fungal infection enhances mycovirus transmission
Proline levels are partly influenced by pyrroline-5-carboxylate (P5C) levels, its precursor molecule synthesized enzymatically by pyrroline-5-carboxylate synthase (P5CS)48,49. Proline levels increased respectively 4-fold and 10-fold in response to infection of oilseed rape with HAVs-infected hypovirulent strain Ep-1PN and HAVs-free virulent strain Ep-1PNA367 (Fig. 3A). It appears that this increase in proline levels is a response to fungal infection and is therefore more pronounced in the presence of the virulent strain that induces more severe infection as compared to the isogenic hypovirulent strain. A similar proline increase was also observed in Arabidopsis thaliana Col-0 plants during S. sclerotiorum infection (Fig. 3B). Plants normally possess two p5cs genes, and p5cs2 but not p5cs1 expression levels were upregulated in both oilseed rape and A. thaliana Col-0 plants infected with Ep-1PNA367 (Supplementary Fig. 4A), indicating that increased proline stems at least partially from upregulation of the enzyme responsible for synthesizing its precursor molecule.
A Proline content of oilseed rape plants inoculated by HAVs-free virulent strain Ep-1PNA367 (A367) and HAVs-infected hypovirulent strain Ep-1PN (EP) at 24 hpi, and of Ep-1PNA367 and Ep-1PN mycelia (n = 15). B Proline content of A. thaliana Col-0 wildtype and p5cs2 mutant inoculated by Ep-1PNA367 at 24 hpi (n = 11). C Virulence assay of strains 1980m, Ep-1PN, and Ep-1PN and 1980m co-inoculation (Ep & 1980m) on A. thaliana Col-0 wildtype and p5cs2 mutant, and virulence assay of Ep & 1980m co-inoculation on the p5cs2 mutant following spraying with exogenous proline (36 hpi, 20 °C) (n = 14). The lesions indicated by white arrows in the upper panel was enlarged in the lower panel. D Diameters of lesions caused by Ep-1PN and 1980m co-culture on A. thaliana Col-0 wildtype, p5cs2 mutant, and p5cs2 mutant plus proline (36 hpi, 20 °C) (n = 11). E Diameters of lesions caused by 1980m with or without proline on oilseed rape (72 hpi, 20 °C), illustrating that proline addition does not induce plant resistance to S. sclerotiorum infection (n = 57). F Transmission efficiency of two mycoviruses, SsDRV and SsRVL, from Ep-1PN to 1980m on A. thaliana Col-0 wildtype and p5cs2 mutant (n = 3 for independent experiments, N = 8 for co-cultures). G Transmission efficiency of two mycoviruses, SsDRV and SsRVL, from Ep-1PN to 1980m on PDA containing proline at concentrations ranging from 3.2 µM to 320 mM (n = 3 for independent experiments, N = 12 for co-cultures). H Co-culture of Ep-1PN with 1980m on PDA (left) and PDA + P (right) (n = 16). I Upper panel, colony morphology of virus-free virulent SG9 (recipient) and six virus-infected hypovirulent strains SZ-150, SX276, Ep-1PNA367T1, WF-1, and AH98 (donors) on PDA or PDA + P for 7 days at 20 °C and corresponding mycovirus transmission efficiency (n = 24). The hypovirulent strains were infected by diverse mycoviruses, including +ssRNA mycoviruses (SsDRV, SsRVL, SsEV1, and SsHV1), dsRNA mycoviruses (SsPV1 and SsMYRV4), and −ssRNA mycoviruses (SsNSRV1), as indicated below each fungal colony. Lower panel, co-culture model diagram of SG9 and mycovirus-infected (VI) hypovirulent strains on PDA (left). Strain SG9 was co-cultured with six hypovirulent strains on PDA or PDA + P, and the associated mycovirus transmission efficiency is shown below for each fungal strain (n = 24). The transmission efficiency of all mycoviruses was increased on PDA + P. Data are presented as mean values ± SD, two-tailed Student’s t test, n represents biologically independent samples, *p < 0.05; ***p < 0.001; ****p < 0.0001; ns not significant. Source data are provided as a Source Data file.
Non-self recognition during hyphal fusion often coincides with accumulation of reactive oxygen species (ROS) and initiation of programmed cell death (PCD)1,5. Proline is known to act as a ROS scavenger and inhibit apoptosis-like PCD36,50,51,52. Therefore, we hypothesized that the enhanced mycovirus transmission between incompatible fungal strains observed in planta is facilitated by proline produced by the plant. To test this hypothesis, an A. thaliana Col-0 mutant with a T-DNA insertion in p5cs2 leading to impaired proline production during S. sclerotiorum infection (Fig. 3B) was used in phenotypic comparisons with wildtype. Co-inoculation of wildtype and p5cs2 mutant plants with Ep-1PN and 1980m revealed significantly larger lesions in the p5cs2 mutant as compared to wildtype, while exogenous proline application mitigated the increased susceptibility of p5cs2 mutant plants (Fig. 3C, D). Subsequently, 1980m was re-isolated from lesions of wildtype and p5cs2 mutant plants and HAVs infection was confirmed with the transmission frequency being significantly lower in the p5cs2 mutant as compared to wildtype (Fig. 3F). Importantly, reduction in disease severity was observed only in the presence of both proline and the HAVs-infected strain (Fig. 3E), supporting the notion that proline enhances mycovirus transmission rather than inducing plant resistance against the pathogenic fungus.
Proline provided exogenously enhances mycovirus transmission
To provide further evidence that proline enhances mycovirus transmission and to explore potential mechanisms in a tractable in vitro system, we established fungal cultures on PDA supplemented with proline in concentrations ranging from 3.2 μM to 320 mM, where Ep-1PN and 1980m exhibited robust growth. In this in vitro system, the highest transmission frequency of SsDRV and SsRVL from Ep-1PN to 1980m was observed at 3.2 mM proline (Fig. 3G), a concentration subsequently used for all further experiments and designated as PDA + P. Moreover, increased hyphal branching of 1980m was noted at its margin within the contact area (Fig. 3H). Together, these results suggest that proline, whether produced by plants or applied exogenously, can directly facilitate mycovirus transmission between incompatible S. sclerotiorum strains.
To evaluate whether this phenomenon is a widespread occurrence in S. sclerotiorum, we measured the horizontal transmission rates of HAVs in six previously reported hypovirulent strains, namely SZ-150, SX276, Ep-1PN, WF-1, AH98, and SCH733 (Supplementary Table 1 and Fig. 3I), to the incompatible strains SG9 and 1980m. Each HAV-infected donor strain was co-cultured with the each virus-free recipient strain on PDA and PDA + P. Across all combinations, the addition of proline resulted in increased HAVs transmission from donors to recipients, irrespective of whether the mycoviruses comprised dsRNA or ssRNA genomes (Fig. 3I and Supplementary Fig. 5). This finding suggests that the proline-mediated facilitation of mycovirus transmission is a broad-spectrum phenomenon in S. sclerotiorum.
Proline promotes hyphal fusion, inhibits expression of VIC-associated genes, and reduces ROS accumulation in fungi
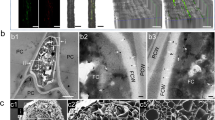
To investigate the cellular, biochemical and molecular mechanisms underpinning the proline-mediated enhancement of horizontal mycovirus transmission, we first examined the impact of proline on hyphal fusion between incompatible strains, particularly the induction of PCD associated with VIC reactions1,9,53. Following Evans blue assay and scanning electron microscopy (SEM) visualization, a typical necrotic zone was clearly observed at the interface between 1980m and incompatible strains Ep-1PNA367 or SCH733 on PDA but was significantly attenuated on PDA + P (Fig. 4A and Supplementary Figs. 5A and 6). Importantly, this necrotic zone was not at all evident between compatible strains Ep-1PN and Ep-1PNA367 (Fig. 1A). This observation, together with the fact that the mycovirus transmission efficiency should theoretically be 100% between two compatible strains on PDA41 and therefore higher than that of two incompatible strains on PDA + P (Fig. 3G), suggest that proline weakens but do not completely eliminates the PCD response between incompatible strains. Examination of the interface between 1980m and its incompatible strains by transmission electron microscopy (TEM) revealed organelle degradation and extensive cytoplasmic vacuole formation on PDA, whereas cytoplasmic vacuole formation was reduced, and hyphal fusion was enhanced on PDA + P (Fig. 4B). Propidium iodide (PI) staining, used for detecting dead cells, further confirmed cell death during co-culture of 1980G and Ep-1PNA367G on PDA, whereas no visible PI staining was evident on PDA + P, indicating the absence of cell death (Fig. 4C). Confocal fluorescence microscopy allowed visualization of hyphal fusion between 1980m and Ep-1PNA367G, evident on PDA + P but not on PDA (Fig. 4D).
A Evans blue staining of two incompatible strains 1980m and Ep-1PNA367G co-cultured on PDA and PDA + P, for the detection of the necrotic zone and PCD response at their interface. The interface region indicated by the white frame in the upper panel was enlarged in the lower panel. Scale bar, 3 mm. B TEM visualization of cell fusion between Ep-1PNA367G and 1980m on PDA and PDA + P. The fusion pore is indicated by white arrows. The vacuole formation and organelle degradation are indicated by asterisks, and cell wall thickening is indicated by black arrows. Scale bar, 1 µm. C PI staining and visualization using confocal microscopy for detection of the cell death at the interface between 1980m and Ep-1PNA367G on PDA and PDA + P. The white box indicates regions where apoptosis occurs in the two incompatible strains stained by PI, while the addition of proline significantly reduces the extent of apoptosis in these regions. Scale bar, 50 µm. D Ep-1PNA367G and 1980m interface on PDA and PDA + P visualized under confocal microscopy; the orientation of the arrows indicates that the hyphae originally labeled with one fluorescent group showed a signal of another fluorescent group, suggesting the fusion of hyphae carrying different fluorescent labels. Scale bar, 50 µm. E Volcano plot showing the number of DEGs in co-cultured VIC strains on PDA and PDA + P. F GO enrichment analysis of down-regulated DEGs from (E). The pathways marked by magenta are closely related to PCD or ROS. G Heatmap showing the expression levels of vic-related genes when two VIC strains interface on PDA, PDA + P, and oilseed rape plants. H Expression levels of genes related to G proteins and ROS in strain 1980 alone, and co-cultured with Ep-1PNA367 on PDA and PDA + P, as shown by RT-qPCR. Data are mean ± SD of n = 3 independent experiments. I NBT staining of 1980m treated with supernatant from Ep-1PNA367G. Scale bar, 0.5 cm. J Transmission efficiency of SsDRV and SsRVL from Ep-1PN to 1980m or deletion transformants (n = 24 for co-cultures). Source data are provided as a Source Data file.
Subsequently, we conducted transcriptome profiling of two incompatible S. sclerotiorum strains on PDA, PDA + P and in planta. In total, 1285 differentially expressed genes (DEGs; >2-fold level), of which 1595 and 690 were respectively downregulated and upregulated, were noted on PDA + P as compared to PDA (Fig. 4E). Gene Ontology (GO) “biological process” and “cellular component” terms found to be enriched in the upregulated genes included cell wall organization or biogenesis, vacuole, cell wall, membrane, and extracellular region (Supplementary Fig. 8). Conversely, GO “biological process” terms found to be enriched in the downregulated genes included transport (transmembrane, lipid, ion, etc.), response to environmental factors (oxidative stress, heat, chemical, and DNA damage stimulus, etc.), and cell wall remodeling (Fig. 4F). Consistent with observations in planta (Fig. 2), expression levels of genes encoding HET and G proteins were predominantly downregulated on PDA + P as compared to PDA (Fig. 4G). However, no significant change in the expression levels of these genes was noted between PDA and PDA + P when co-culturing two compatible S. sclerotiorum strains (Supplementary Fig. 7), suggesting that no PCD reaction occurred in this case as expected based on our observations at the microscopic level. The expression levels of two ROS-related genes were induced upon contact of incompatible strains on PDA, but this induction was inhibited on PDA + P (Fig. 4H). Additionally, nitroblue tetrazolium (NBT) staining showed that proline inhibits ROS accumulation in 1980m exposed to Ep-1PNA367 supernatant following lysis (Fig. 4I).
Fungal VIC-associated genes downregulated by proline impede mycovirus transmission
To examine whether any of the genes whose expression is downregulated by proline in vitro and/or in planta play a role in horizontal mycovirus transmission, we generated deletion mutants for a subset of them in the genetic background of strain 1980. This deletion mutant analysis encompassed genes Ssvic1 to Ssvic6, Ssvib1, and Sscwr1 to Sscwr3, which encode proteins containing HET/NT80 or LMPO conserved domains (Supplementary Figs. 9 and 10). Ssvic2 deletion resulted in reduced growth, while Ssvib1 deletion affected sclerotia development, including their morphology and numbers. The remaining deletions exhibited colony morphology and growth rates similar to their parent strain (Fig. 4J). Subsequently, Ep-1PN (donor) was co-cultured with 1980m or individual deletion mutants (recipients) on PDA (Fig. 4J). The hyphae at the margin of most deletion mutants serving as recipient strains displayed excessive branching and reduced growth, particularly in the case of ∆Ssvic3. The recipient strains were then subcultured on neomycin-containing PDA for three generations to fully eliminate the donor and the transmission rate of mycoviruses was assessed using RT-PCR. Transmission rates were significantly increased in ∆Ssvic1, ∆Ssvic3, ∆Ssvic4, ∆Ssvic6, ∆Sscwr1, and ∆Sscwr2 as compared to their parent strain (Fig. 4J). Remarkably, approximately 75% of the ∆Ssvic3 sub-isolates tested exhibited phenotypic traits associated with hypovirulence, consistent with the SsDRV transmission rate (Fig. 4J).
Exogenous proline enhances the biocontrol efficiency of HAVs and increases oilseed rape yield
To ascertain the applicability of laboratory findings for enhanced control of Sclerotinia disease using HAVs in conjugation with exogenous proline, we conducted field experiments on oilseed rape for a period of over 2 years, from 2021 to 2022. In 2021, the disease incidence decreased by 41.9% and yield increased by 11.2% following combined Ep-1PN and proline treatment, while disease incidence decreased by 26.9% and yield increased by 4.8% following treatment with Ep-1PN alone as compared to non-treated controls (Fig. 5). Similar results were observed in the field experiments conducted in 2022.
A Symptoms of Sclerotinia stem rot on oilseed rape after spraying with hyphal fragment suspensions of Ep-1PN with and without proline. In the upper panel, grayish-yellow colors signify diseased plants; in the lower panel, grayish-yellow discoloration of stems is evident and caused by S. sclerotiorum. B Application of Ep-1PN with and without proline improved yield and suppressed stem rot in field experiments as compared to controls (proline and water). C The putative model for plant-derived proline-mediated inhibition of fungal non-self recognition reaction to promote mycovirus transmission. The interaction between plants and phytopathogenic fungi results in increasing proline accumulation, which promotes mycovirus transmission between incompatible fungi via inhibition of fungal non-self recognition system. Donor strain Ep-1PN is incompatible with recipient strain 1980. Source data are provided as a Source Data file.
Discussion
Fungal non-self recognition mechanisms are commonly regarded as the primary barrier to horizontal mycovirus transmission. However, our understanding of how mycoviruses are horizontally transmitted between fungi largely comes from in vitro experiments, with limited insights into whether plants possess mechanisms enhancing mycovirus transmission between pathogenic fungi, thus decreasing disease severity. Moreover, in vitro experiments do not fully capture the natural process of horizontal mycovirus transmission in plant-associated fungi, as they overlook the important factor of “plant”. Therefore, we investigated the impact of host plants (oilseed rape and Arabidopsis) on mycovirus transmission between incompatible strains of a phytopathogenic fungus (S. sclerotiorum). Our findings revealed a significant increase in the transmission rate of mycoviruses in planta as compared to in vitro. This phenomenon mirrors observations in other mycovirus-fungus-plant interaction systems. For example, the transmission rate of the prototypic CHV1 between incompatible strains of C. parasitica on trees consistently surpasses that observed in in vitro experiments40,41, and mycovirus transmission can occur between incompatible R. necatrix strains on apple branches42,43. Plants appear to attenuate VIC response in phytopathogenic fungi, having significant implications for mycovirus ecology and holding practical importance for their application in biological control. Therefore, there is considerable interest in elucidating the mechanisms that underpin the plant-mediated weakening of VIC barriers.
In this study, we have observed an increase in plant-derived proline concentration in response to infections by phytopathogenic fungi. Elevated proline levels have been found to decrease fungal non-self recognition reactions, thereby facilitating enhanced mycovirus transmission between otherwise incompatible fungal strains and explaining the widespread mycovirus distribution in fungi. To the best of our knowledge, this data represents the first example of a plant-associated factor shown to promote horizontal mycovirus transmission and points to a novel plant defense mechanism against phytopathogenic fungi. Previous studies have indicated that higher plants exhibit elevated proline content in response to various environmental stresses54. Proline, acting as a signaling molecule, modulates mitochondrial activities, influences cell proliferation and death, and regulates gene expression, essential for the plant’s recovery from stress52. Our data have shown that the addition of proline alone did not confer a protective effect against S. sclerotiorum infection neither in laboratory nor field experiments, suggesting that proline primarily decreases disease severity by enhancing HAVs transmission through suppression of fungal non-self recognition reactions. Therefore, the plant’s ability to diminish VIC reactions through proline production presents a novel potential strategy and mechanism against fungal infection in agriculture. However, the effect induced by plant proline in response to fungal infection is likely localized and sub-optimal, as the application of exogenous proline together with a HAVs-infected strain provides further protection to susceptible crops against phytopathogenic fungi. This treatment not only decreases disease severity, but more importantly, increases crop yields.
We have provided compelling evidence to support the notion that proline alleviates non-self recognition reaction in S. sclerotiorum. Firstly, proline treatment promotes hyphal fusion at the cellular level and reduces fungal ROS levels at the biochemical level. Secondly, proline inhibits the expression of genes encoding HET ___domain-containing proteins, and disruptions of these genes (Supplementary Figs. 9 and 10), including Ssvic, Ssvib1, and Sscwr, significantly promote mycovirus transmission. Previous studies have shown that homologs of S. sclerotiorum genes downregulated by proline are essential for cell fusion and non-self recognition in C. parasitica, N. crassa, B. cinerea, and P. anserina9,11,22,23,55. Additionally, deletions of VIC-related genes (vic1a-2, vic3b-1,and pix6-2) in C. parasitica significantly promote CHV1 transmission between incompatible strains23,56. It would be interesting to further investigate how proline affects the expression of genes that regulate hyphal fusion in S. sclerotiorum.
Horizontal mycovirus transmission potentially occurs among fungi sharing the same ecological niche and is influenced by interactions between fungi and plants. Multiple mycoviruses have been observed to infect individual fungal strains at a high rate in nature57. In our study, we have discovered a significant increase in proline concentration in plants (oilseed rape, Arabidopsis, etc.) upon infection with a phytopathogenic fungus (S. sclerotiorum). This increase assists mycoviruses to circumvent the fungal non-self recognition reaction, potentially resulting in the mycovirus co-infections of individual fungal strains observed in nature. Furthermore, certain mycoviruses, such as Botrytis virus F or Sclerotinia sclerotiorum botybirnavirus 1, have been identified to infect fungi24,25,30,58, including B. cinerea, S. sclerotiorum, and Leptosphaeria biglobosa, that occupy similar ecological niches in oilseed rape plants. Interestingly, a number of mycoviruses are found in different fungal genera, or even kingdoms30,59,60. However, whether proline can also promote hyphal fusion between different fungal species or genera remains to be determined in the future.
HAVs have potential as biological control agents but questions regarding their ability to spread in the natural populations of the pathogen and their ecological fitness under field conditions have to be addressed to expand their application in disease management strategies. Despite hindering fungal growth and rendering their hosts less ecologically fit than their virus-free counterparts, HAVs continue to spread in nature. For instance, CHV1 clearly impedes growth and sporulation, yet is naturally disseminated in European populations of C. parasitica20,21. Furthermore, some mycoviruses exhibit poor vertical transmission rates to conidia coupled with restrictions imposed by fungal non-self-recognition systems43,61,62, but they endure in the natural fungal populations over time. Recent research has shown that HAVs can induce a change in the host fungus life style. For instance, SsHADV1 and Pestalotiopsis theae chrysovirus-1 can convert phytopathogenic fungi into beneficial endophytic fungi, promoting plant health63,64. Curvularia thermal tolerance virus infects an endophytic fungus and confers thermal tolerance to the plant18, benefiting the survival of all three organisms under high temperatures (up to 65 °C) that would not be able to tolerate on their own. Here, we propose an additional explanation for the ecological stability of mycoviruses: plant proline promotes horizontal transmission of HAVs, continuously leading to new infections and compensating for the lower ecological fitness of the host. This tri-interaction system involving plants, phytopathogenic fungi, and HAVs, benefits plant health, survival of hypovirulent fungi, and mycovirus transmission. From this perspective, mycoviruses emerge as key players in the evolution and balance of local plant ecosystems, functioning similarly to viruses in marine ecosystems.
In addition to biocontrol applications in agriculture, our research provides a promising avenue for enhancing CHV1-mediated control of chestnut blight through the application of exogenous proline. Despite the potential of CHV1-based biological control, its success has been limited, particularly in North America. A previous explanation for this limited success is the complex structure of VCGs among C. parasitica strains in North America, which restricts CHV1 horizontal transmission. Previous studies have shown that C. parasitica infection increases proline production in chestnuts65, and that CHV1 transmission rates are higher in planta than in vitro21,38,66,67,68. Therefore, we hypothesize that the enhanced transmission of CHV1 in chestnuts is mediated by plant proline, similarly to transmission of HAVs in S. sclerotiorum. In our research, plant proline levels induced by S. sclerotiorum are approximately 10-fold lower than the exogenous proline added in PDA and field experiments. Mycovirus transmission rate is significantly higher on PDA containing 3.2 mM proline than on plant leaves without exogenous proline. Addition of exogenous proline (3.2 mM) along with HAVs-infected Ep-1PN led to better biological control efficiency than Ep-1PN alone in our field experiments. Therefore, increasing the concentration of proline above the level produced by the plant in response to infection, through addition of exogenous proline, leads to a greater enhancement of mycovirus transmission in S. sclerotiorum.
In summary, fungi have evolved intricate non-self recognition mechanisms to prevent the spread of molecular parasites such as mycoviruses. In response to infection by phytopathogenic fungi, plants utilize proline to hinder fungal non-self recognition reactions, thereby reducing fungal resistance to mycoviruses (Fig. 5C). Our research elucidates the mechanism by which mycovirus survival and transmission can be enhanced in natural ecosystems and highlights a straightforward practical enhancement in the application of mycoviruses for fungal disease control under field conditions.
Methods
Strains and culture conditions
Information on S. sclerotiorum strains is summarized in Supplementary Table 1. All strains were routinely cultured on PDA at 20–22 °C to observe morphology and determine growth rate. Mycelial plugs were stored at 4 °C and dried sclerotia at −20 °C. The pathogenicity of all strains was determined by inoculating leaves of oilseed rape and A. thaliana with mycelial plugs.
Plants and growth conditions
A commercial cultivar of oilseed rape (Brassica napus cv. Huashuang No. 4) was grown in pots with garden soil at 20 °C. A. thaliana (L.) Heynh., accession Columbia-0 (Col-0), was used as the wildtype. The T-DNA insertion lines p5cs2-1 (SALK_203144) in the Col-0 background were obtained from Arashare (https://www.arashare.cn/), and confirmed using PCR with three primer pairs (Supplementary Fig. 12 and Supplementary Table 2). After stratification at 4 °C in the dark for 2–4 days, A. thaliana seeds were planted and grown in soil at 20–22 °C, with 50% relative humidity and a 16-h light/8-h dark photoperiod for 4–5 weeks.
Horizontal transmission assays
HAV-infected hypovirulent donor strains (Supplementary Table 1) were co-cultured with HAV-free virulent recipient strains, labeled by a hygromycin (1980R or 1980m) resistance gene, in vitro (PDA or proline-containing PDA) or in planta (oilseed rape or A. thaliana). Following contact of donor and recipient strains for 3–5 days in vitro or 24–48 hin planta, the recipient strain was re-isolated for sub-culturing on PDA containing 50 ng/µl hygromycin or 50 ng/µl cephalosporin. RT-PCR with mycovirus-specific primers was used to establish infection status in recipient strains and quantify horizontal transmission rates (Supplementary Table 2).
RNA extraction, RT-PCR, and RNA-seq analysis
S. sclerotiorum strains were cultured alone or co-cultured with (in)compatible strain on: (1) cellophane membrane overlaying PDA with or without proline; (2) oilseed rape or Arabidopsis plants. Subsequently, mycelia and plant issue were, respectively, collected and total RNA was extracted using a commercial RNA extraction kit (Takara, Code No.: 9108). RNA quantity and quality were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA) and a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA).
Total RNA (about 1 μg) was treated with RNase-free DNase I (Takara, Code No.: 2270A) and used to synthesize complementary (c)DNA with M-MLV reverse transcriptase (Takara, Code No.: 2641A) and oligo (dT) primers. Quantitative (q)PCR was performed using iTaq SYBR Green Supermix in a Bio-Rad CFX384 Real-Time PCR System (Bio-Rad). The actin gene was used as a control. All RT-qPCR primers are listed in Supplementary Table 2.
RNA-seq was performed using the HiSeq 2500 platform with at least three biological replicates (Illumina, USA). Approximately two million reads were obtained for each sample. Raw sequence reads were trimmed and clipped to remove low-quality reads and adapter sequences using Trimmomatic (version 0.9.25). Processed reads were mapped against the S. sclerotiorum reference genome (http://fungi.ensembl.org/Sclerotinia_sclerotiorum_1980_uf_70_gca_001857865/Info/Index) using HISAT2 (version: 2.0)69. The aligned SAM files were sorted and converted into BAM files using SAMtools70. A count table was generated using featurecounts (version: 1.6.0)71. Differential gene expression analysis was performed using the R package DEseq2 and heatmaps were generated using R72,73.
Evans Blue and NBT staining
To detect PCD in S. sclerotiorum, Evans Blue staining was conducted as previously described53. Briefly, incompatible strains 1980m and Ep-1PNA367G were co-cultured on PDA with or without 3.2 mM proline. Upon contact, the strains allowed to interface for 12 h and then stained in a 0.5% (w/v) Evans blue solution for 30 min. The interface was observed under a stereomicroscope after being washed twice with phosphate buffer saline (PBS, pH = 7.4).
To detect ROS levels in S. sclerotiorum, NBT staining was performed as previously described, with minor modifications53. The incompatible strains 1980m and Ep-1PNA367G were cultured separately on PDA for 36 h. The mycelium of Ep-1PNA367G was collected, ground into powder in liquid nitrogen, and re-suspended in PBS. The supernatant was collected by centrifugation at 12,000 × g for 5 min and 3.2 mM proline was added. This mixture was used to treat 1980m on PDA for 30 min. Then, 1980m was washed twice with PBS and stained with 0.5% (w/v) NBT solution for 30 min in the dark. The reaction was terminated with ethanol, and ROS levels were quantified colorimetrically. All biological experiments were repeated at least five times.
Microscopy
The cassettes of green and mCherry fluorescent protein (GFP and mCherry) under the control of two Aspergillus nidulans oliC promoters are shown in Supplementary Fig. 11 (Genbank accession number: PP209073-PP209074). Strains 1980 and Ep-1PNA367 were labeled respectively with mCherry and GFP using a standard polyethylene glycol (PEG)-mediated protoplast transfection protocol as previously described74. The resulting strains were denoted as 1980m and Ep-1PNA367G. These labeled strains were co-cultured on a sterilized glass slide covered by a thin PDA layer, with or without proline, at 20 °C. Upon contact, an Olympus confocal microscope or TEM or SEM was used to observe the interface between the strains. The excitation and emission wavelengths were 552–580 and 395–488 nm for mCherry and GFP, respectively. All biological experiments were repeated at least five times.
Generation of gene deletion mutants
S. sclerotiorum genes (Supplementary Table 2) were knocked out using the split-marker method, as shown in Supplementary Fig. 8. Briefly, segments approximately 1.2 kb in length located upstream and downstream of each gene were amplified from the S. sclerotiorum genome with specific primers (Supplementary Table 2). These segments were then ligated with two segments of NE and EO carrying a 0.6 kb overlapping region. NP and PT were obtained from a neomycin-resistance gene cassette using the ClonExpress® II One Step Cloning Kit (Vazyme Code No.: C112-01). Equimolar amounts of the purified NP and PT fragments were used to transfect S. sclerotiorum protoplasts using a standard PEG-mediated protocol as previously described75. Transfectants were transferred onto PDA containing 100 ng/μl hygromycin or neomycin for continuous cultivation and screening until stable phenotypes were observed. Initially obtained transfectants were typically heterozygous since S. sclerotiorum cells are multinucleate. Therefore, multiple rounds of single-protoplast isolation for each candidate transfectant were conducted following a previously reported protocol with minor modifications75. Fresh hyphae of candidate transfectants were incubated at 28 °C in 1 ml cell wall digestive solution (1% megalyase in 0.7 M NaCl solution) for 10 min under shaking (140 × g), and the mixture was filtered through paper to remove mycelial fragments. The filtrate then was centrifuged at 5000 × g for 5 min, and the precipitate was resuspended in 600 μL STC (1 M sorbitol, 50 mM Tris-HCl pH = 8.0, 50 mM CaCl2) and spread on regenerative medium containing hygromycin or neomycin. Subsequently, each single-protoplast transfectant was confirmed to be homozygous by multiple PCR amplifications as shown in Supplementary Fig. 10.
Liquid chromatography-mass spectrometry (LC-MS)
S. sclerotiorum strains were inoculated on leaves of B. napus (2 months old) or A. thaliana (5–6 weeks old). The leaves were collected at 12 hpi, freeze-dried and crushed using a mixer mill (MM 400, Retsch) with zirconia beads for 1.5 min at 30 Hz. Proline was extracted overnight at 4 °C from 100 mg leaf powder with 1.0 ml 70% (v/v) aqueous methanol containing 0.1 mg/l lidocaine as an internal standard. Following centrifugation at 10,000 × g for 10 min, 0.4 ml of each lipid-soluble extract was mixed and filtered (SCAA-104, 0.22-μm pore size; ANPEL, Shanghai, China, www.anpel.com.cn) before LC-MS analysis. LC-MS/MS was performed by coupling a Dionex Ultimate 3000 ultra-performance liquid chromatography system (Thermo Fisher Scientific) with a TSQ AltisTM triple-quadrupole mass spectrometer (Thermo Fisher Scientific).
Field experiments
Experiments were conducted on oilseed rape fields in Hubei province, in Huanggang for 2 years (2021 and 2022) and in Wuhan for 1 year (2022). Plants were regularly treated with fertilizer, but not fungicide, during the growing season. Twelve plots of approximately 50 m2 (5 m × 10 m width × length) were defined in each field and each treatment was applied to at least three plots. Hyphal fragment suspensions of Ep-1PN were prepared as previously described63. Hyphal fragment suspensions with or without 3.2 mM proline were sprayed on aerial plant parts at the flowering stage (earlier than the epidemic phase of stem rot), and 3.2 mM proline or water instead of hyphal fragment suspension was used as control. 100 plants were randomly chosen from each plot, and the disease severity for each was rated on a scale of 0 to 476. Plants within 20 m2 areas were harvested from each plot, and their seeds were weighed to calculate the disease index. All data were subjected to statistical analysis.
Quantification and statistical analysis
The quantitative data for mycovirus transmission rates, proline content, and fungal virulence assays are presented as mean ± standard deviation (SD). The statistical analyses were performed by Student’s t test analysis of variance, Figures were generated by the GraphPad Prism 8.0 software.
Data availability
The RNA-seq data generated in this study have been deposited in the NCBI database under accession code (Bioproject Accession: PRJNA908905; PRJNA1065649; PRJNA1068540). All of our raw data including full uncropped images in the manuscript are provided in figshare (https://doi.org/10.6084/m9.figshare.25108211) Source data are provided with this paper.
References
Glass, N. L., Jacobson, D. J. & Shiu, P. K. The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu. Rev. Genet. 34, 165–186 (2000).
Saupe, S. J. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 64, 489–502 (2000).
Ament-Velásquez, S. L. et al. Allorecognition genes drive reproductive isolation in Podospora anserina. Nat. Ecol. Evol. 6, 910–923 (2022).
Daskalov, A., Heller, J., Herzog, S., Fleißner, A. & Glass, N. L. Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi. Microbiol. Spectr. 5, https://doi.org/10.1128/microbiolspec.FUNK-0015-2016 (2017).
Glass, N. L. & Dementhon, K. Non-self recognition and programmed cell death in filamentous fungi. Curr. Opin. Microbiol. 9, 553–558 (2006).
Milgroom, M. G., Smith, M. L., Drott, M. T. & Nuss, D. L. Balancing selection at nonself recognition loci in the chestnut blight fungus, Cryphonectria parasitica, demonstrated by trans-species polymorphisms, positive selection, and even allele frequencies. Heredity 121, 511–523 (2018).
Pál, K. et al. Sexual and vegetative compatibility genes in the aspergilli. Stud. Mycol. 59, 19–30 (2007).
Saupe, S. J. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin. Cell Dev. Biol. 22, 460–468 (2011).
Zhang, D. X., Spiering, M. J., Dawe, A. L. & Nuss, D. L. Vegetative incompatibility loci with dedicated roles in allorecognition restrict mycovirus transmission in chestnut blight fungus. Genetics 197, 701–U413 (2014).
Belov, A. A., Witte, T. E., Overy, D. P. & Smith, M. L. Transcriptome analysis implicates secondary metabolite production, redox reactions, and programmed cell death during allorecognition in Cryphonectria parasitica. G3 11, jkaa021 (2021).
Gonçalves, A. P. et al. Allorecognition upon fungal cell-cell contact determines social cooperation and impacts the acquisition of multicellularity. Curr. Biol. 29, 3006–3017.e3 (2019).
Arshed, S. et al. The Bcvic1 and Bcvic2 vegetative incompatibility genes in Botrytis cinerea encode proteins with ___domain architectures involved in allorecognition in other filamentous fungi. Fungal Genet. Biol. 169, 103827 (2023).
Strom, N. B. & Bushley, K. E. Two genomes are better than one: history, genetics, and biotechnological applications of fungal heterokaryons. Fungal Biol. Biotechnol. 3, 4 (2016).
Loubradou, G., Begueret, J. & Turcq, B. MOD-D, a G alpha subunit of the fungus Podospora anserina, is involved in both regulation of development and vegetative incompatibility. Genetics 152, 519–528 (1999).
Paoletti, M., Saupe, S. J. & Clavé, C. Genesis of a fungal non-self recognition repertoire. PLoS ONE 2, e283 (2007).
Liu, S. et al. Fungal DNA virus infects a mycophagous insect and utilizes it as a transmission vector. Proc. Natl Acad. Sci. USA 113, 12803–12808 (2016).
Kondo, H., Botella, L. & Suzuki, N. Mycovirus diversity and evolution revealed/inferred from recent studies. Annu. Rev. Phytopathol. 60, 307–336 (2022).
Marquez, L. M., Redman, R. S., Rodriguez, R. J. & Roossinck, M. J. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315, 513–515 (2007).
Myers, J. M. & James, T. Y. Mycoviruses. Curr. Biol. 32, R150–R155 (2022).
Heiniger, U. & Rigling, D. Biological control of chestnut blight in Europe. Annu. Rev. Phytopathol. 32, 581–599 (1994).
Milgroom, M. G. & Cortesi, P. Biological control of chestnut blight with hypovirulence: a critical analysis. Annu. Rev. Phytopathol. 42, 311–338 (2004).
Choi, G. H. et al. Molecular characterization of vegetative incompatibility genes that restrict hypovirus transmission in the chestnut blight fungus Cryphonectria parasitica. Genetics 190, 113–127 (2012).
Zhang, D. X. & Nuss, D. L. Engineering super mycovirus donor strains of chestnut blight fungus by systematic disruption of multilocus vic genes. Proc. Natl Acad. Sci. USA 113, 2062–2067 (2016).
Mu, F. et al. Virome characterization of a collection of Sclerotinia sclerotiorum from Australia. Front. Microbiol. 8, 2540 (2017).
Jia, J. et al. Interannual dynamics, diversity and evolution of the virome in Sclerotinia sclerotiorum from a single crop field. Virus Evol. 7, veab032 (2021).
Ruiz-Padilla, A., Rodríguez-Romero, J., Gómez-Cid, I., Pacifico, D. & Ayllón, M. A. Novel mycoviruses discovered in the mycovirome of a necrotrophic fungus. mBio 12, e03705–e03720 (2021).
Ghabrial, S. A., Caston, J. R., Jiang, D., Nibert, M. L. & Suzuki, N. 50-plus years of fungal viruses. Virology 479–480, 356–368 (2015).
Brusini, J. & Robin, C. Mycovirus transmission revisited by in situ pairings of vegetatively incompatible isolates of Cryphonectria parasitica. J. Virol. Methods 187, 435–442 (2013).
Yaegashi, H. et al. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol. Ecol. 83, 49–62 (2013).
Deng, Y. et al. Viral cross-class transmission results in disease of a phytopathogenic fungus. ISME J. 16, 2763–2774 (2022).
Paleg, L. G. & Aspinall, D. The Physiology and Biochemistry of Drought Resistance in Plants (Academic Press, 1981).
Qamar, A., Mysore, K. S. & Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 6, 503 (2015).
Zhao, P. et al. Targeted and untargeted metabolomics profiling of wheat reveals amino acids increase resistance to fusarium head blight. Front. Plant Sci. 12, 762605 (2021).
Cecchini, N. M., Monteoliva, M. I. & Alvarez, M. E. Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol. 155, 1947–1959 (2011).
Patriarca, E. J. et al. The multifaceted roles of proline in cell behavior. Front. Cell Dev. Biol. 9, 728576 (2021).
Chen, C. & Dickman, M. B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl Acad. Sci. USA 102, 3459–3464 (2005).
Derbyshire, M. C., Newman, T. E., Khentry, Y. & Owolabi Taiwo, A. The evolutionary and molecular features of the broad‐host‐range plant pathogen Sclerotinia sclerotiorum. Mol. Plant Pathol. 23, 1075–1090 (2022).
Xie, J. & Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 52, 45–68 (2014).
Mu, F. et al. Nine viruses from eight lineages exhibiting new evolutionary modes that co-infect a hypovirulent phytopathogenic fungus. PLoS Pathog. 17, e1009823 (2021).
Liu, L. et al. Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc. Natl Acad. Sci. USA 111, 12205–12210 (2014).
Hai, D. et al. Discovery and evolution of six positive-sense RNA viruses co-infecting the hypovirulent strain SCH733 of Sclerotinia sclerotiorum. Phytopathology 112, 2449–2461 (2022).
Xie, J. et al. A novel mycovirus closely related to hypoviruses that infects the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 418, 49–56 (2011).
Xie, J. et al. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J. Gen. Virol. 87, 241–249 (2006).
Xiao, X. et al. A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J. Virol. 88, 10120–10133 (2014).
Yu, X. et al. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl Acad. Sci. USA 110, 1452–1457 (2013).
Attanayake, R. N., Carter, P. A., Jiang, D., Del Río-Mendoza, L. & Chen, W. Sclerotinia sclerotiorum populations infecting canola from China and the United States are genetically and phenotypically distinct. Phytopathology 103, 750–761 (2013).
Liu, H. et al. A novel mycovirus that is related to the human pathogen hepatitis E virus and rubi-like viruses. J. Virol. 83, 1981–1991 (2009).
Miller, G. et al. Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J. Biol. Chem. 284, 26482–26492 (2009).
Hu, C. A., Delauney, A. J. & Verma, D. P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl Acad. Sci. USA 89, 9354–9358 (1992).
Takagi, H. Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol. 81, 211–223 (2008).
Ben Rejeb, K., Abdelly, C. & Savoure, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80, 278–284 (2014).
Szabados, L. & Savoure, A. Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97 (2010).
Wu, S. et al. Virus-mediated suppression of host non-self recognition facilitates horizontal transmission of heterologous viruses. PLoS Pathog. 13, e1006234 (2017).
Alvarez, M. E., Savoure, A. & Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 27, 39–55 (2022).
Dementhon, K., Iyer, G. & Glass, N. L. VIB-1 is required for expression of genes necessary for programmed cell death in Neurospora crassa. Eukaryot. Cell 5, 2161–2173 (2006).
Stauder, C. M. et al. Enhanced hypovirus transmission by engineered super donor strains of the chestnut blight fungus, Cryphonectria parasitica, into a natural population of strains exhibiting diverse vegetative compatibility genotypes. Virology 528, 1–6 (2019).
Thapa, V. & Roossinck, M. J. Determinants of coinfection in the mycoviruses. Front. Cell Infect. Microbiol. 9, 169 (2019).
Arthur, K. & Pearson, M. Geographic distribution and sequence diversity of the mycovirus Botrytis virus F. Mycol. Prog. 13, 1249–1253 (2014).
Vainio, E. J., Piri, T. & Hantula, J. Virus community dynamics in the conifer pathogenic fungus Heterobasidion parviporum following an artificial introduction of a partitivirus. Microb. Ecol. 65, 28–38 (2013).
Khalifa, M. E. & MacDiarmid, R. M. A novel totivirus naturally occurring in two different fungal genera. Front. Microbiol. 10, 2318 (2019).
Pfeiffer, I., Kucsera, J., Varga, J., Párducz, A. & Ferenczy, L. Variability and inheritance of double-stranded RNA viruses in Phaffia rhodozyma. Curr. Genet. 30, 294–297 (1996).
Romaine, C., Ulhrich, P. & Schlagnhaufer, B. Transmission of La France isometric virus during basidiosporogenesis in Agaricus bisporus. Mycologia 85, 175–179 (1993).
Zhang, H. et al. A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for brassica protection and yield enhancement. Mol. Plant 13, 1420–1433 (2020).
Zhou, L. et al. A mycovirus modulates the endophytic and pathogenic traits of a plant associated fungus. ISME J. 15, 1893–1906 (2021).
Kovács, G. E. et al. The physiological and biochemical responses of European chestnut (Castanea sativa L.) to blight fungus (Cryphonectria parasitica (Murill) Barr). Plants 10, 2136 (2021).
Suzuki, N. et al. In-tree behavior of diverse viruses harbored in the chestnut blight fungus, Cryphonectria parasitica. J. Virol. 95, e01962–01920 (2021).
Hogan, E. & Griffin, G. Spread of Cryphonectria hypovirus1 into 45 vegetative compatibility types of Cryphonectria parasitica on grafted American chestnut trees. For. Pathol. 32, 73–85 (2002).
Robin, C., Lanz, S., Soutrenon, A. & Rigling, D. Dominance of natural over released biological control agents of the chestnut blight fungus Cryphonectria parasitica in south-eastern France is associated with fitness-related traits. Biol. Control 53, 55–61 (2010).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Kolde, R. & Kolde, M. R. Package ‘pheatmap’. R Package 1, (2018).
Xie, C. et al. Early secretory pathway-associated proteins SsEmp24 and SsErv25 are involved in morphogenesis and pathogenicity in a filamentous phytopathogenic fungus. mBio 12, e03173–03121 (2021).
Rollins, J. A. The Sclerotinia sclerotiorum pac1 gene is required for sclerotial development and virulence. Mol. Plant Microbe Interact. 16, 785–795 (2003).
Li, G. et al. Biological control of sclerotinia diseases of rapeseed by aerial applications of the mycoparasite Coniothyrium minitans. Eur. J. Plant Pathol. 114, 345–355 (2006).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (32072475 and 32130087) (J.X.), the National Key Research and Development Program of China (2022YFA1304400) (D.J.), the Fundamental Research Funds for the Central Universities (2021ZKPY005 and 2662023PY006) (J.X.), and the Earmarked Fund for CARS-12 (D.J.). We would like to thank Professor Kenichi Tsuda and Professor Xiaowei Han at Huazhong Agricultural University, and Professor Nemat O Keyhani at the University of Florida for their constructive suggestions and proofreading of the manuscript. We thank the National Key Laboratory of Agricultural Microbiology Core Facility for assistance in microscopy observation.
Author information
Authors and Affiliations
Contributions
D.H., J.X., and D.J. conceived the project. D.H. executed experiments, analyzed data, and prepared the manuscript. J.L. designed and performed the gene knockout experiment. H.Y. and W.C. conceived the LC-MS experiment. J.C., Y.F., X.X., Y.L., T.C., B.L., X.Y., and Q.C. carried out data collection, analysis, and interpretation. I.K. participated in the guidance of manuscript writing and provided comments for the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks María García-Pedraja, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hai, D., Li, J., Jiang, D. et al. Plants interfere with non-self recognition of a phytopathogenic fungus via proline accumulation to facilitate mycovirus transmission. Nat Commun 15, 4748 (2024). https://doi.org/10.1038/s41467-024-49110-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49110-6
This article is cited by
-
Microbiome-mediated plant disease resistance: recent advances and future directions
Journal of General Plant Pathology (2025)
-
Molecular characterization of a novel non-segmented double-stranded RNA mycovirus isolated from the phytopathogenic fungus Lasiodiplodia pseudotheobromae
Archives of Virology (2025)
-
A biocontrol perspective on mycoviruses in fungal pathogen management
Journal of Plant Diseases and Protection (2025)