Abstract
Solid-phase synthesis underpins many advances in synthetic and combinatorial chemistry, biology, and material science. The immobilization of a reacting species on the solid support makes interfacing of reagents an important challenge in this approach. In traditional synthesis columns, this leads to reaction errors that limit the product yield and necessitates excess consumption of the mobile reagent phase. Although droplet microfluidics can mitigate these problems, its adoption is fundamentally limited by the inability to controllably interface microbeads and reagent droplets. Here, we introduce Dielectrophoretic Bead-Droplet Reactor as a physical method to implement solid-phase synthesis on individual functionalized microbeads by encapsulating and ejecting them from microdroplets by tuning the supply voltage. Proof-of-concept demonstration of the enzymatic coupling of fluorescently labeled nucleotides onto the bead using this reactor yielded a 3.2-fold higher fidelity over columns through precise interfacing of individual microreactors and beads. Our work combines microparticle manipulation and droplet microfluidics to address a long-standing problem in solid-phase synthesis with potentially wide-ranging implications.
Similar content being viewed by others
Introduction
Solid-phase synthesis techniques1,2,3 are indispensable for the de novo synthesis of oligonucleotides (single stranded DNA4,5,6 and RNA7), oligopeptides1,3,8, oligosaccharides9, and combinatorial libraries10. In this approach, long chained polymeric molecules are synthesized by stitching one monomeric unit at a time onto initiator (primer) strands that are bound to insoluble solid supports1,8,11,12. Immobilization of one reacting species on the solid supports brings forth the challenge of adequate reagent interfacing in solid-phase synthesis. The resultant incomplete reactions lead to accumulating deletion errors \((\xi )\) over multiple synthesis cycles that decrease the product purity and yield (\(\eta={\left(1-\xi \right)}^{N}\))4,5,6,13,14 with increasing strand length (larger \(N\)) of the polymeric chain. In general, this is addressed by flushing the solid supports with a large excess of mobile reagents in the fluidic phase in traditional synthesis columns1,8,11,12. However, this entails significant reagent consumption and wastage1,6,8,11,12,15,16. Hence, minimizing reagent consumption and wastage as well as reaction errors (\(\xi\)) is necessary to realize the scale of synthesis effort required to engineer complex molecules through an iterative design-build-test-learn approach5,15,17.
Droplet microfluidics18,19,20,21,22,23,24,25 is a potent platform that can address these issues. The small size of microdroplet reactors (\(1 \, \upmu {{\rm{m}}}-500 \, \upmu {{\rm{m}}}\) in diameter) can minimize reagent wastage19,21,23,25. It can enable superior interfacing of mobile reagents with solid supports due to faster mixing (faster diffusion times in smaller droplets) of reacting species18,19,20,22,25,26,27. Furthermore, due to the advent of on-demand droplet generation, a single droplet can be dispensed into the reaction chamber at rates determined by the synthesis and detection process. Unlike the continuous stream droplet generation, this prevents the fluid flow driving the droplet generation from impacting the chemical reaction and detection processes25,28,29,30,31,32. These advantages have attracted the attention of chemists33,34, biologists35,36,37,38,39, and material scientists40,41,42 alike towards developing droplet reactors in a microfluidic platform which has led to the tremendous growth of the field in the last few decades. However, the reagents in all these instances are entirely in the fluidic phase33,34,35,36,37,38,39,40,41,42. Despite its many decisive advantages, droplet microfluidic analog for solid-phase synthesis, though highly desirable15,43, remains an unmet scientific and engineering challenge due to the inability to controllably interface solid supports with reagent droplets in a droplet microfluidic platform.
Here we combine dielectrophoresis44,45,46,47,48,49,50 with on-demand droplet generation25,28,29,30,31,32 and state-of-the-art device fabrication to introduce Dielectrophoretic Bead-Droplet Reactor (DBDR). This is an approach to implement solid-phase synthesis reactions in droplet microfluidic systems by controllably encapsulating and ejecting (interfacing) individual microbeads (of radius \({R}_{b}\)) from single reagent microdroplets (of radius \({R}_{d}\)). The capillary force of interfacial tension45,51,52 (\({\gamma }_{{ow}}\)), which is significant at this length scale, impedes the bidirectional motion of the bead across the interface. It drives the bead towards the interfacial energy minimum, the position of which is governed by the intrinsic interfacial properties of the bead, droplet and the medium45,51,52. Therefore, an external force is required to manipulate the bead entirely into and out of the droplet across the droplet-medium interface by overcoming the capillary force. Micromanipulation techniques53,54 such as dielectrophoretic, magnetic, optical, and acoustic tweezers, in which forces scale with the particle volume (\(\propto {R}_{b}^{3}\)), have traditionally been employed to maneuver microparticles in a single fluidic suspension medium against the viscous drag force (\(\propto {R}_{b}v\)) at a velocity v relative to the medium45. However, manipulation of microparticles across micron scale immiscible fluidic interfaces remains challenging due to the large capillary force. It scales linearly with the particle size45,51,52 (\(\propto {R}_{b}\)) compared to the volume scaling (\(\propto {R}_{b}^{3}\)) of the micromanipulation body forces53,54. Here we show that by judicious design of the interfacial, and geometrical properties of the bead, droplet, and the suspension medium the balance between the counteracting dielectrophoretic and capillary forces can be modulated by simply tuning the supply voltage (\({V}_{s}\)) to completely encapsulate and eject the bead out of the droplet (Fig. 1a) in a droplet microfluidic device (Fig. 1b, c). Specifically, we show that when sufficiently large voltage (\({V}_{s}\)) is applied across the electrodes, the dielectrophoretic force on the droplet (\(\propto {{R}_{d}^{3}\left|{V}_{s}\right|}^{2}\)) overcomes the capillary forces (\(\propto {R}_{b}{\gamma }_{{ow}}\)) to move the hydrophobic bead into the droplet (across the aqueous reagent-oil medium interface). At low voltages (\({V}_{s}\)), the dominant capillary force ejects the hydrophobic bead out of the droplet. In all prior reports of microparticle encapsulation, beads pre-suspended in the dispersed (droplet generating) phase are either engulfed within the droplet during its formation55,56 or are injected into another droplet57. In these approaches, once encapsulated, the beads remain confined within the droplet either by the capillary force (hydrophilic beads) or by acoustic forces which push the beads to the center of the droplet (away from the droplet-medium interface). In all these processes, the bead does not move across an interface of immiscible fluids. While external fields and microchannel architectures may be used to trigger droplet breakup57, the beads do not move across any fluidic interface. On the contrary, by employing counteracting capillary and dielectrophoretic force, DBDR ensures bidirectional manipulation of the bead across the droplet-medium interface for its encapsulation within the droplet (and thus interfacing with the reagents) as well as its ejection from it (and thus separation from the reagents). Ejection will allow the fluorescent characterization of the products bound to the beads after a reaction step.
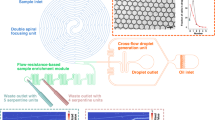
a In DBDR reactions are implemented by encapsulating and ejecting individual beads from reagent droplets. This is achieved by simply tuning the voltage supply on the trap electrode that modulates the balance between counteracting dielectrophoretic and capillary force. Inside the droplet, the fluorescently labeled nucleotides (dCTP-AF647) couple onto the initiator strands on the bead. b The device footprint depicts the alignment of the droplet generator and the electrodes in the reaction chamber and the spatial ___location of the bead and the dispensed droplet. The dashed box shows the section of the device in (a). c Fabricated silicon-on-glass microfluidic device with ITO electrodes. Purple color is overlayed on the transparent ITO electrodes to distinguish them from the device background. The strong electric field gradient of the electrodes enables dielectrophoretic trapping. The arrows indicate the direction of the electric field. d Representation of chemical reactions on the solid support: (i) Binding the initiator strand on the bead through streptavidin-biotin hydrogen bonds, (ii) Enzymatic coupling of fluorescently labeled nucleotides (dCTP-AF647) onto the 3′ end of the initiator strands on the bead inside the reagent droplet.
We use DBDR to make a proof-of-concept demonstration of the enzymatic coupling of fluorescently labeled nucleotides to the 3′ end of the initiator strands bound to the bead surface (Fig. 1d). Furthermore, fluorescence-based comparisons of synthesis reactions implemented using DBDR with synthesis columns indicate improved coupling fidelity due to superior interfacing of beads with reagents.
Results
Device and reaction design
The silicon-on-glass microfluidic device developed for demonstration of DBDR consists of Indium Tin Oxide (ITO) electrodes (\(\approx \,15\,{{{{\upmu }}}} {{\rm{m}}}\) wide, Fig. 1b, c and Supplementary Fig. 1) in the reaction chamber (\(3\,{{\rm{{mm}}}} \times 10\,{{\rm{{mm}}}} \times 0.2\,{{\rm{{mm}}}}\)). These electrodes are suitably aligned with the on-demand droplet generator microchannel34,37,38,39,40,41 (\(\approx \, 25 \, \upmu {{\rm{m}}}\) wide and \(\approx \, 20\, \upmu {{\rm{m}}}\) height, Fig. 1b, c and Supplementary Fig. 1) to ensure that the droplet (\({R}_{d} \, \approx \, 25\,\upmu {{\rm{m}}}\)) dispensed into the reaction chamber lies within their trapping range. A piezo driven pressure controller is used to drive fluid flow through the microchannel. The abrupt release of microchannel confinement at the intersection with the reaction chamber leads to sharp change in capillary pressure which triggers the droplet breakup31. This avoids the impact of flow rate variations (due to the external noise from fluidic controller hardware) on the droplet size (which depends solely on the device geometry) to generate monodisperse droplets. The electrodes are made smaller than the channel (Fig. 1c) to ensure that the trapped droplet encapsulates the trapped bead as it covers the electrode (Fig. 1a). ITO and glass ensure optical transparency for imaging the trapping and manipulation process as well as fluorescence detection of the coupling reaction (Fig. 1c).
Streptavidin-coated fluorescent green polystyrene beads (\({R}_{b}=3\,\upmu {{\rm{m}}}\), Fig. 1d) are used as solid supports. 5′ biotinylated oligos with 25 nucleotides bound to the microbead using streptavidin-biotin linkages (Fig. 1a, d(i)) serve as initiators. The enzyme terminal deoxynucleotidyl transferase (TdT)58,59,60,61,62 couples the nucleotides fluorescently labeled with Alexa Fluor 647 (dCTP-AF647) to the 3′ end of the initiators (Fig. 1d(ii)) when the bead is encapsulated within the reagent droplet (Fig. 1a). A red LED excites AF647 to detect the coupling of dCTP-AF647 to the initiated bead63,64 (Fig. 1a, d(ii), Supplementary Fig. 2).
Physical working principle of DBDR
The device, filled with \(\,\approx \, 2.5\%\,{{{{\rm{w}}}}}/{{{{\rm{w}}}}}\) solution of Span 80 in silicone oil (kinematic viscosity\(=1 \; {{\rm{cSt}}}\)), is mounted on the sample holder with relevant electrical and fluidic connections (Supplementary Fig. 3). Beads functionalized with appropriate initiator strands (Fig. 1d) are suspended in the oil solution and introduced into the device through the bottom fluidic port (Fig. 1c). When a single bead enters the vicinity of the electrodes it gets dielectrophoretically trapped (Fig. 2b) by applying a voltage (\({{V}_{s}=V}_{{BD}}=40 \, {{\rm{V}}}\) amplitude) across the electrodes (E1 and GND in Fig. 1c and Fig. 2b). The voltage supply is then lowered. Subsequently, a reagent droplet is dispensed into the reaction chamber (Fig. 2c) by applying a pressure pulse using a piezoelectric pressure controller (Elveflow OB1 MK3+). The magnitude (\(\approx \, 10 \, {{\rm{mbar}}}\)) and the duration of the pulse (\(\approx \, 300\, {{\rm{ms}}}\)) are critical to generate a single droplet. Then the voltage supply is switched back on (\({V}_{s}={V}_{{DD}}=20\,{{\rm{V}}}\)) to trap the droplet adjacent to the bead (Fig. 2d).
a Schematic plot of the sequential change in the supply voltage and pressure required to drive the physical process underlying DBDR. The voltage supply is used to manipulate the bead and the droplet in the reaction chamber. The pressure pulse dispenses a droplet on-demand into the reaction chamber. \({T}_{{ENC}}\) represents the time for which the bead remains encapsulated within the droplet. b A single bead in the vicinity of the electrode is dielectrophoretically trapped by raising the voltage to \({V}_{S} \, \approx \, 40\,{{\rm{V}}}\). c The voltage is lowered, and a pressure pulse is exerted on the microchannel to dispense a single droplet into the reaction chamber. d The pressure pulse is stopped and a voltage \({V}_{{DD}} \, \approx \, 20 \, {{\rm{V}}}\) is applied to trap the generated droplet adjacent to the trapped bead. e (i) Experimental implementation and (ii) numerical electrohydrodynamic phase field simulation depicts the encapsulation of the bead by the droplet under high supply voltage (\({V}_{s}\approx \, 120 \, {{\rm{V}}}\)) and its ejection from the droplet under low supply voltage (\({V}_{s} \, \approx \, 0.1\,{{\rm{V}}}\)). The phase field variable (\({{{{\rm{\phi }}}}}\)) has a value of \(-1\) in the silicone oil suspension medium (phase 1) and a value of \(1\) in the reagent droplet (phase 2). \({{{{\rm{\phi }}}}}\) transitions from \(-1\) to \(1\) at the droplet-medium interface. The bead is represented in white. (iii) Electrocapillary potential energy representation of the engulfing and (iv) ejection process. The frames in e(i) are extracted from Supplementary Movie 1 which was recorded at \(30 \, {{\rm{fps}}}\). The scale bars in these frames represent \(50 \, \upmu {{\rm{m}}}\).
When \({V}_{s}\) is increased to \(\approx \, 120 \, {{\rm{V}}}\) at \(200 \, {{\rm{Hz}}}\) supply frequency (\({f}_{s}\)), the bead is fully encapsulated by the reagent droplet (Fig. 2e(i), Supplementary Movie 1). Reducing \({V}_{s}\) to \(\approx \, 0.1 \, {{\rm{V}}}\) ejects the bead from the droplet (Fig. 2e(i), Supplementary Movie 1). Electrohydrodynamic simulations (Fig. 2e(ii), details in Supplementary Notes1–4, Supplementary Figs. 4–6, and Supplementary Table 1) support these observations. It can be understood in terms of the change in the system’s electrocapillary potential energy (\(\triangle U\))51,52 due to a change in the Gibbs free energy (\(\Delta {U}_{{IT}}\approx -{{{{{\rm{\gamma }}}}}}_{{ow}}\cos \theta \triangle {A}_{{ws}}\)) of the interfaces between the bead, droplet and the medium as well as the change in the electrical energy (Δ UE = −Δ(QVs)/2) stored in the system (Fig. 2e(iii), (iv)). Here, \({{{{{\rm{\gamma }}}}}}_{{ow}}=\, 5.5 \, {{\rm{{mN/m}}}}\), \({A}_{{ws}}\) is the area of the bead covered by the reagent droplet, \({{{{\rm{\theta }}}}}\,\approx \,{145}^{\circ }\) is the contact angle that the reagent droplet forms on the surface of the bead and \(Q\) is the charge stored on the electrode E1. The large \({{{{\rm{\theta }}}}}\) means the bead has a propensity towards the oil (hydrophobic). When \({V}_{s}\) is high, \(\left|\Delta {U}_{E}\right| \, \gg \, \left|\Delta {U}_{{IT}}\right|\). To attain the minimum energy configuration, the droplet moves towards the electrode (\(\Delta U \, \approx \,\Delta {U}_{E} \, < \, 0\)) and engulfs the bead in the process (Fig. 2e(iii)). When \({V}_{s}\) is low, \(\left|\Delta {U}_{E}\right| \, \ll \, \left|\Delta {U}_{{IT}}\right|\). To attain the minimum energy configuration, the droplet ejects the hydrophobic bead, which is touching the droplet-medium interface from the inside, as it moves away from the electrode (\(\Delta U \; \approx \; \Delta {U}_{{IT}} \; < \; 0\), Fig. 2e(iv)). Once the bead completely separates from the droplet, \(\Delta {U}_{{IT}}\) is negligible (Fig. 2e(iv)). The process of encapsulation (Supplementary Movie 2) and ejection (Supplementary Movie 3) proceed over \(\approx \, 200\!-\!400 \, {{\rm{{ms}}}}\). This fast transition time can ensure that the synthesis rate is not limited by the physical sample handling process. The pressure pulse for on-demand single droplet generation (Fig. 2a) was exerted when the electrical voltage was low. This ensures bead and droplet trapping as well as encapsulation and ejection proceeds without any undue effect of the fluid flow in the device or additional droplet generation. The time (\({T}_{{ENC}}\)) for which the bead and the droplet remain in contact depends on the time for which the voltage is high (Supplementary Movie 1 and Supplementary Movie 4).
Many design choices are crucial towards the successful working of the above process. While a larger droplet (\({R}_{d}=50 \, \upmu {{\rm{m}}}\)) requires a lesser voltage (\({V}_{s} \, \approx \, 85 \, {{\rm{{V}}}}\)) to exert significant dielectrophoretic force (\(\propto {R}_{d}^{3}{\left|{V}_{s}\right|}^{2}\)) to encapsulate the bead (shown in Fig. 3a) it would also mean more reagent usage per reaction and longer diffusion times. On the other hand, a smaller droplet (\({R}_{d}=20\,\upmu {{\rm{m}}}\)) would require a larger voltage (\({V}_{s}\,\approx \,135\,{{\rm{{V}}}}\)) to exert significant dielectrophoretic force (\(\propto {R}_{d}^{3}{\left|{V}_{s}\right|}^{2}\)) to move the bead across the interface (Fig. 3b). In fact, this emphasizes our choice to drive the encapsulation and ejection process by exerting dielectrophoretic force on the droplet. It will experience a much larger dielectrophoretic force than the bead due to its larger size and larger permittivity contrast with the suspension medium (\(\frac{{F}_{{DEP},\, {droplet}}}{{F}_{{DEP},\, {bead}}}{\propto \left(\frac{{R}_{d}}{{R}_{b}}\right)}^{3}\times \left(\frac{{K}_{1{rd}}}{{K}_{1b}}\right)\)) that can help overcome the large capillary force. Here, \({K}_{1}\) is the well-known Clausius-Mossotti factor (Supplementary Note 2 and Supplementary Fig. 5). This requires the droplet to experience a non-zero dielectrophoretic force when not encapsulating the bead (Fig. 1a, b). The alignment of the droplet generator and electrodes (Fig. 1b, c) ensures that the droplet is dispensed into the vicinity of the electrode where it can be successfully trapped by its electric force field. Once the voltage (\({V}_{s}\)) is lowered, the dominant capillary force ejects the bead touching the interior surface of the droplet.
a Larger droplet \(\left({R}_{d}=50 \, \upmu {{\rm{m}}}\right)\) can encapsulate the bead at a much lesser supply voltage (\({V}_{s}\approx 85V\)). b Smaller droplet \(\left({R}_{d}=20 \, \upmu {{\rm{m}}}\right)\) requires a larger supply voltage (\({V}_{s}\approx 135V\)) to overcome the capillary force and encapsulate the bead. At the lower voltage (\({V}_{s} \, \approx \, 117 \, {{\rm{V}}}\)) the dielectrophoretic force cannot overcome the capillary force to encapsulate the bead into the smaller droplet (It was enough to encapsulate the bead in the \({R}_{d}=25 \, \upmu {{\rm{m}}}\) droplet). c The low viscosity of silicone oil 1 cSt enables the ejection of the bead from the droplet at \({V}_{s} \, \approx \, 0.1\, {{\rm{V}}}\). With increase in oil viscosity the increased dissipation of the kinetic energy of the droplet prevents complete separation from the bead. d Silicone oil 1 cSt (dashed line) renders the hydrophilic (in air, dotted line) streptavidin surface slightly hydrophobic. The surfactant Span80 further reduces the interfacial tension between the silicone oil and the reagent and increases the contact angle (solid line) that the reagent droplet forms on a streptavidin surface making it hydrophobic. It is critical for the ejection of the bead from the droplet. Interfacial tension and contact angle measurements are recorded with time variations to account for the surface adsorption. (Refer to Experimental Procedure for Bead-Droplet Interaction subsection of Methods for details.) The streptavidin and glass thicknesses are not drawn to scale. The scale bars on experimental frames in (a, b) represent \(40 \, \upmu {{\rm{m}}}\). The symbol ϕ in the color plots is the phase variable in the phase field simulations. \(\phi=-1\) in the oil medium (represented in blue) and \(\phi=1\) in the aqueous medium (represented in red). \(-1 < \phi < 1\) represents the boundary region between the droplet and the oil medium.
Silicone oil \(1 \, {{\rm{{cSt}}}}\) acts as a water insoluble, chemically inert, and non-ionic suspension medium with low viscosity, high dielectric breakdown strength and low electrical conductivity that prevents the rapid evaporation of the tiny reagent microdroplets due to their high surface-to-volume ratio29,45 (detailed discussion in Supplementary Note 4). Low viscosity of silicone oil \(1 \, {{\rm{{cSt}}}}\) minimizes viscous dissipation of the kinetic energy45 of the moving droplet as it moves away from the electrode (towards the minimum Gibbs free energy configuration). This ensures complete ejection of the bead (Fig. 3c). Its high dielectric breakdown electric field strength (\(\approx \, 13.8 \, {{\rm{{V}}}}/\upmu {{\rm{m}}}\)) allows to exert a large enough voltage across the electrodes to ensure encapsulation of the bead within the droplet without causing dielectric breakdown of the suspension medium. Its low electrical conductivity (\({\sigma }_{o} \, \approx \, {10}^{-14} \, {{\rm{{S/m}}}}\)) minimizes spatially non-uniform joule heating (\(\propto {\sigma }_{o}{\left|E\right|}^{2}\)) to limit unwanted electrothermal flow of the suspension medium65,66. Its low ionic concentration also limits electro-osmotic fluid flow66. The latter two factors ensure that dielectrophoresis is the dominant electric field effect and prevent unwanted motion of the bead and droplet under electric field driven fluid flow that could impede the encapsulation and ejection process. If oils with identical physical properties except for higher viscosities were to be used, the higher dissipation of kinetic energy would prevent complete ejection of the bead from the droplet.
Addition of the surfactant (Span80) to silicone oil 1 cSt further reduces \({\gamma }_{{ow}}\) to \(5.5 \, {{\rm{mN/m}}}\) and increases the contact angle (\(\theta\)) that the aqueous droplet forms on the streptavidin coated surface of the bead to \(145^{\circ}\) (Fig. 3d and Sample Preparation subsection of Methods). This makes the otherwise hydrophilic streptavidin surface of the bead (water droplet forms a contact angle of \(50^{\circ}\) on a streptavidin surface in air) hydrophobic. Therefore, the choice of silicone oil and surfactant is critical for the spontaneous ejection of the bead from the droplet on touching its interior surface in the absence of an applied voltage. The surfactant also increases the contact angle (\(\beta\)) of the aqueous reagent droplet on the silanized interior surface of the device to \(\approx \, 138^{\circ}\) (Experimental Procedure for Bead-Droplet Interaction subsection in Methods). This ensures that the device walls are predominantly wet by the suspension medium, and the aqueous reagent phase does not stick to the device walls. This is necessary for droplet generation (Fig. 2c) and its subsequent dielectrophoretic manipulation (Fig. 2d, e). The non-wetting of the device walls by the droplet phase also avoids reactor fouling (evident by the absence of any significant remnant fluorescence trail along the path of motion of the droplet phase in Fig. 2c, d, e). This would be critical to detect reactions without signal interference from unwanted sources.
Enzymatic coupling of nucleotide in DBDR
After establishing the physical working principle of DBDR, it was used for a proof-of-concept demonstration of enzymatic coupling of nucleotides onto the initiator strands bound to a microbead. A droplet size of \({R}_{d}=25 \, \upmu {{\rm{m}}}\) was chosen (through the design of microchannel dimensions, Fig. 1b, c) to minimize reagent usage and their diffusion times (associated with larger droplets) while still ensuring dielectrophoretic encapsulation of the bead (increasingly difficult with smaller droplets). The droplet has red fluorescently tagged nucleotides (dCTP-AF647) at a \(5 \, \upmu {{\rm{M}}}\) concentration. Therefore, the bead with \(\approx \, 100 \, {{\rm{attomoles}}}\) of initiator strands on its surface has access to \(\approx \, 325 \, {{\rm{attomoles}}}\) nucleotides within the volume of the droplet (forms a contact angle \(\beta \,\approx \,138^{\circ}\) with the device surface, Experimental Procedure for Bead-Droplet Interaction subsection in Methods) which is51,52 \(\frac{(2-3\cos \beta+{\cos }^{3}\beta )}{3}\pi {R}_{d}^{3}=65 \, {{\rm{pl}}}\) (A slightly excess quantity of reagent was chosen to ensure ample supply of nucleotides to the initiators subject to variations arising due to sample preparations and device fabrication). The encapsulation and ejection process were triplicated along with fluorescent imaging of the bead immediately before encapsulating and after ejecting it from the droplet (Fig. 4a, Experimental Procedure for Chemical Coupling subsection in Methods and Supplementary Fig. 7a–f). While there was no red fluorescence from the bead prior to encapsulation, we observed a clear red fluorescence from it after ejection. This confirmed the binding of the fluorescently tagged nucleotides (dCTP-AF647) to the bead surface57,63,64. This is a further confirmation that the bead was enveloped within the droplet when \({V}_{s}\) was high. The three repeats of this experiment (Supplementary Fig. 7g) were performed with encapsulation time of ≈ 300 s to ensure reproducibility. To eliminate any false positives due to unincorporated nucleotides non-specifically bound to the surface of the bead and confirm chemical binding of nucleotides to the 3′ end of the initiator strands, we reiterated the above process using beads devoid of initiator strands and reagent solution without TdT (control experiment in Fig. 4b). Lack of red fluorescence from the bead after ejection confirmed the absence of non-specific binding of nucleotides to the bead surface (Fig. 4b, Experimental Procedure for Chemical Coupling subsection in Methods, and Supplementary Fig. 7h–m). This shows DBDR can be a robust approach for enzymatic DNA synthesis on individual beads in droplet reactors within droplet microfluidic devices. It brings together the disparate fields of droplet microfluidics and solid-phase synthesis.
a Enzymatic coupling of fluorescently labeled nucleotides (dCTP-AF647) onto the initiator strands tethered to the green fluorescent microbead. The bead fluoresces red only after encapsulation and ejection from the droplet indicating binding of the nucleotides to its surface. b Control experiment was performed without initiator strands on the bead surface and enzymes in the droplet. The absence of red fluorescence from the bead after ejection from the droplet confirms that the red fluorescence from the bead in (a) can be attributed to the chemical coupling of the nucleotide to the initiator strands. c Reactions are implemented in synthesis columns by flushing reagents through the bead stack. d The top pane represents the average fluorescence intensity of 285 beads which reacted in synthesis columns. These fluorescences were collected across multiple frames (A few sample frames are shown in Supplementary Fig. 9a.). A few sample beads collected from all the frames are shown in the inset on the top. The left gray band with brightness gradient in the bottom pane represents the line fluorescence intensity of these beads reacted in the column taken along the yellow lines drawn across the beads. The band edges represent the line fluorescence of beads that demarcate the quartiles of the histogram in the top pane. The dashed curve in the bottom pane represents the fluorescence intensity distribution of the bead with average fluorescence intensity equal to the mean of the histogram. The mean (\({\mu }_{{col}}\)) and the standard deviation (\({\sigma }_{{col}}\)) of the average fluorescence intensities of beads reacted in the synthesis column are 627.6 and 372 respectively. These are evaluated over 285 beads. The three beads which represent the three experimental trials of DBDR are shown as insets in the top pane. Their mean (\({\mu }_{{DBDR}}\)) and standard deviation (\({\sigma }_{{DBDR}}\)) are 2057.5 and 209 respectively. The scatter plots in the bottom pane represent their fluorescence intensities along the yellow lines. The mean and standard deviation of the three distributions is represented by the solid black curve and the right most gray band.
Coupling fidelity enhancement in DBDR
After having demonstrated enzymatic coupling of nucleotides to initiated beads using DBDR, we compared its coupling fidelity with reactions implemented in synthesis columns (Fig. 4c) in which many initiated beads are tightly packed between filters using fluorescence measurements. We observed large variations in fluorescence intensity across beads reacted in the column (Fig. 4d, Supplementary Figs. 8–10). This indicates non-uniform reagent environment in the vicinity of each bead which results in corresponding variations in the reaction fidelities. The small fraction of the beads with the brightest fluorescence closely represent the maximum attainable enzymatic coupling fidelities. The reduced fidelity on an overwhelming majority of the beads leads to an overall decrease in the average coupling efficiency (average of the histogram in Fig. 4) and suggests significant missed couplings. On the other hand, beads reacted using DBDR consistently exhibit fluorescence intensities in the vicinity of the maximum values obtained using synthesis columns. This is quantified using the average fluorescence intensity ratio (\({{FIR}}_{{avg}}\)). \({{FIR}}_{{avg}}=3.2\) in Fig. 4d indicates enhanced access of individual bead surfaces to reagents within the microdroplets which then translates to improved reaction fidelities. While excess concentration of reagents is generally used to compensate for reduced access to reagents and drive-up reaction fidelities in synthesis columns, it is also accompanied by excess reagent wastage as well1,6,8,11,12,13,14. This observed superior performance of DBDR compared to synthesis columns (statistical significance of the observed 3.2-fold fidelity enhancement is indicated by Welch’s t test, Supplementary Table 2, and its rank transformed variant, Supplementary Table 3, described in detail in the Image Processing and Data Analysis subsection of Methods) can be attributed to the superior reagent access to the bead surfaces in DBDR than in synthesis columns where many beads are stacked together.
The stacking of beads in synthesis columns may restrict reagent access to the bead surfaces. Ideally, the beads would be stacked in regularly packed lattice arrangements67 within the column (Supplementary Fig. 11). However, in practice, it is a rarity. Even if the beads are perfectly stacked in the columns at the beginning, on introduction of reagents into the synthesis column (say at \(t=0 \, {{\rm{s}}}\), Fig. 5), the particles are displaced from the perfect stack due to the drag force44 exerted by the turbulent flow68 (\(t=0\).05 s, Fig. 5). The resultant increased interparticle spacing facilitates improved reagent access to the bead surfaces in the columns compared to the perfect stacks. Once the reagent influx stops, the particles settle down under gravity into imperfect stacks (\(t=70 \, {{\rm{s}}}\) and \(t=300 \, {{\rm{s}}}\), Fig. 5). Different particles have different settling times. This explains the varying exposure (spread of the gray band and histogram in Fig. 4d) to reagents during the experimental time (≈ 300 s). Even when the beads are floating up, there are many beads in proximity (\(\approx \, 510\) beads in a region of size equivalent to a droplet in frame \(t=0\).05 s in Fig. 5) competing for the reagent molecules. The \(\approx \, 100 \, {{\rm{attomoles}}}\) of initiators on the surface of the beads which are ideally packed (\(t=70 \, {{\rm{s}}}\) and \(t=300 \, {{\rm{s}}}\) in Fig. 4) in regular lattice arrangements (say in rhombohedral) have access to reagents in an interparticle void volume \(\left({V}_{{void}}\right)\) of \(4\left(\sqrt{2}-\frac{\pi }{3}\right){R}_{b}^{3} \, \approx \, 40 \, {{\rm{fl}}}\)69,70. The \(5 \, \upmu {{\rm{M}}}\) concentration of fluorescently labeled nucleotides implies \(\approx \, 0.2 \, {{\rm{attomoles}}}\) per void in a unit cell in a rhombohedral stacked lattice (Supplementary Fig. 11). On the other hand, in DBDR, the bead has access to the entire reagent volume within the droplet (\(\approx \, 65\, {{\rm{pl}}}\)) and \(\approx \, 325 \, {{\rm{attomoles}}}\) of nucleotides within it. This is confirmed by the much faster diffusion time (\({T}_{{diff},\,{DBDR}} \, \approx \, \frac{2{R}_{d}^{2}}{D} \, \approx \,12.5\,{{\rm{s}}}\)) of single nucleotides (diffusion coefficient \(D \, \approx \, {10}^{-6}\!-\!{10}^{-5}\, {{\rm{cm}}}^{2}/{\rm s}\,\)71,72) across the droplet compared to the reaction time (≈ 300 s). On the other hand, diffusion across the length of the synthesis column (h = 2.28 mm, Supplementary Fig. 13) is expected to occur over much longer time scales (\({T}_{{diff},\, {col}} \, \approx \, \frac{{h}^{2}}{2D} \, \approx \, 25000 \, {{\rm{s}}}\)). So, reagent molecules in the columns that are further away from the stack are less likely to contribute to the reaction by interacting with beads. Therefore, ideally the fluorescence intensity ratio (\({{FIR}}_{{avg}}\)) between beads reacted using DBDR and in a perfectly stacked synthesis column is expected to be 500 (this can be the upper limit of attainable \({{FIR}}_{{avg}}\)). However, due to the imperfect nature of the stacking and the non-zero contribution of diffusion our experiments yielded an \({{FIR}}_{{avg}}=3.2\). Apart from this, the AC electric field driven migration of the charged reacting species in the microdroplet may also contribute to the improved reagent access to the solid supports in DBDR (Supplementary Note 6 and Supplementary Figs. 14–17). While our analysis does not eliminate the possibility of some beads reacted in synthesis columns having the same access to reagents as in DBDR, it emphasizes the inadequate access to reagents over a large number of beads that limits the overall fidelity of these macroscale reactors. Therefore, we see that stacking of beads in synthesis columns which is known to provide the large surface area of solid support for the synthesis of large quantity of oligonucleotides1,2,4,5,8,11,12, also contributes significantly to reaction errors (reduced reaction fidelity). These errors accumulate over multiple synthesis cycles to ultimately negate the advantage of large synthesis quantity achieved in the columns. To compensate for this reduced reagent access and longer diffusion times in synthesis columns, higher reagent concentrations are used1,8,11,12. However, this increases reagent wastage per reaction that can accumulate over multiple synthesis cycles to make the synthesis of longer and more complex oligomer strands inviable. Many approaches have been employed on the macroscale, such as column agitation, to improve interfacing of solid supports with synthesis reagents for higher yields73. Though reliable improvements in reaction fidelity have been obtained in some cases, implementing them at scale would require considerable re-engineering of existing column and titer plate-based synthesizers. Consumption of large amounts of reagents also remains a challenge in these systems, especially for iterative design-build-test-learn approaches. On the other hand, by encapsulating one bead per droplet, DBDR ensures optimal reagent access to the surface of every bead during the experimental duration. Precise dielectrophoretic micromanipulation ensures that individual beads have access to their own packet of reagents in their immediate vicinity thus eliminating the possibility of suboptimal reagent access to the beads (solid supports) as in synthesis columns. Although we demonstrated reaction in a single bead-droplet system, DBDR can open up the possibility of generating large-scale one-bead-one-compound74 libraries while minimizing reagent usage for iterative experimental design cycles by combining parallel droplet generation75,76 with parallel micromanipulation77.
Stacking of beads prevents reagent access to reaction sites on the bead surface in synthesis columns. Assuming an ideal scenario wherein beads were initially stacked in perfect rhombohedral lattices, particle tracking simulations show that when the reagent is introduced into the column the beads would be displaced from the stack due to turbulent fluid flow and then resettle over the experimental time once the reagent flow stops. This allows enhanced (compared to the perfectly stacked scenario) and varying reagent access to many beads transiently as they settle down, which explains the spread of fluorescence intensities of beads reacted in columns in Fig. 4.
Discussion
In summary, we have demonstrated that microbeads can be controllably encapsulated and then ejected from aqueous microdroplets to carry out high-fidelity solid-phase synthesis reactions. This process has many significances. Firstly, the demonstrated physical process of microbead encapsulation and ejection from microdroplets extends the capability of dielectrophoresis (and more generally any trapping and micromanipulation technique) to drive microparticle transport across immiscible fluidic interfaces, especially with sharp micron-sized radius of curvature. Secondly, this also extends the physical sample handling capability of droplet microfluidic platforms to include solid supports, thus opening a route for the droplet microfluidic implementation of solid-phase synthesis. The counteracting dielectrophoretic and capillary forces enable the voltage-controlled interfacing of individual microbeads (solid supports) with picolitre scale reagent microdroplets to form a bead-droplet reactor. As proof-of-concept, we demonstrated the enzymatic coupling of a fluorescently labeled nucleotide to the 3′ end of the initiator strand bound to the bead surface. Finally, fluorescence measurements suggest that reactions implemented using DBDR exhibit higher fidelities than synthesis columns due to the (i) absence of bead-bead stacking as in synthesis columns, and (ii) faster reagent diffusion across microdroplet reactors. We would also like to emphasize that DBDR is vastly different from previous reports of beads in droplet reactor systems in which the enzyme is immobilized on the bead and the reactants and products are confined to the droplet57.
The proof-of-concept demonstration of enzymatic coupling of nucleotides onto the bead surface using DBDR with a 3.2-fold higher fidelity compared to synthesis column if repeated multiple times (say \(N\)) to generate \(N\) nucleotides long oligomers across many beads. For such \(N\) nucleotide long oligomers, the error free products ratio14 (\({EFPR}\)) synthesized using DBDR and synthesis columns is given by \({EFPR}=\frac{1}{{10}^{6}}\times {3.2}^{N}\)(around a million beads are used in the synthesis column). Therefore, for merely \(N=12 \,\, > \,\, \frac{6}{{\log }_{10}3.2}\), \({EFPR} \, > \,1\) can be achieved. So, higher quantity of intended error-free synthesis product can be achieved on 1 bead using DBDR than on a million beads using synthesis columns for oligomers that are longer than merely 12 nucleotides (\(N=12\)). Furthermore, the reagent usage ratio (\({RUR}\)) between DBDR and synthesis columns would be \({RUR}=\frac{N\,\times 65 \, {{\rm{pl}}}}{N \times 50 \, \upmu {{\rm{l}}}} \,\approx \, {10}^{-6}\). Additionally, the synthesis time ratio (\({STR}\)) between DBDR and synthesis columns would be \({STR}=\frac{N\times 12.5 \, {{\rm{s}}}}{N\,\times 25000 \, {{\rm{s}}}}=5\times {10}^{-4}\). Therefore, DBDR can synthesize significantly higher quantity of error free strands while consuming much less reagents without the need for post synthetic purification or error correction at much faster rates. The synthesis fidelity improvement would be more pronounced with increase in \(N\). This immense potential of DBDR for high fidelity solid-phase synthesis combined with reduced reagent consumption and faster reaction rate can be harnessed by combining parallel droplet generation75,76, particle manipulation77, and automation to scale up our proof-of-concept demonstration for high-throughput generation of solid-phase synthesis products. This can expand the accessible synthetic design space using solid-phase synthesis for a wide range of applications in chemistry, biology, medicine, material science and information technology. The high synthesis fidelity made possible by DBDR is the foremost requirement for error free digital data storage in DNA63,78. Furthermore, instead of extracting the beads from the device, DBDR can be combined with existing approaches for the droplet microfluidic implementations of gene assembly, their transformation into cells, cell growths, and colony screening to develop an entirely droplet microfluidic-based design-build-test-learn cycle for synthetic biology15 that could minimize reagent consumption in the otherwise resource-intensive iterative process.
DBDR can have applications beyond high-fidelity solid-phase synthesis with reduced reagent consumption and wastage. It can be a great way to exploit the basic advances made in microdroplet chemistry79,80 for solid-phase synthesis on an integrated chip-scale droplet microfluidic platform for practical applications. It can also be an excellent platform to study enzymatic reactions in volume (within the microdroplet) and surface (on the bead surface) confined environments81,82. These possibilities make DBDR an exciting platform for on-chip chemistry in droplet microfluidic systems.
Methods
Device fabrication
Fabrication started with \(\sim 500 \, \upmu {{\rm{m}}}\) thick silicon wafers (p-type, \(10\!-\!20 \, \Omega \, {{{{\rm{cm}}}}}\), \(\left\langle 100\right\rangle\)) and borofloat glass wafers \(\sim 550 \, \upmu {{\rm{m}}}\) thick that are \(100 \, {{\rm{mm}}}\) in diameter.
The silicon wafer was etched in 5 layers to define the (1) alignment marks, (2) droplet generation microchannel, (3) reaction chamber (4) and (5) ports for external fluidic and electrical connections (Supplementary Fig. 1a–c). The first four layers were defined by reactive ion etching using SF6 gas. In the 4th layer the ports were etched almost through the wafer from the backside. In the 5th layer, the ports were completed by laser drilling through the ports from the front side. The photoresist for each layer was patterned using a standard photolithographic approach with a maskless exposure system (Heidelberg MLA 150).
Eight hundred nanometer \((800 \, {{{{nm}}}})\) of ITO was sputter deposited on piranha cleaned borofloat glass wafers at LGA Thin Films. This was followed by 2 layers of photolithographic processing: (1) the electrodes and alignment marks were defined by RIE of ITO from the entire wafer (barring the electrodes) using CH4 and H2 gases and (2) the contact pads were defined by evaporative deposition of \(10 \, {{{{\rm{nm}}}}}\) and \(200 \, {{{{\rm{nm}}}}}\) Gold followed by metal liftoff. After each layer, the wafer was wet cleaned using \(5:1:1::{{\rm{{H}}}_{2}{O}:{{H}}_{2}{{O}}_{2}:N{H}_{4}OH}\) at \(70\) °C for 1 h (Supplementary Fig. 1d).
The glass and silicon wafers were then aligned (Supplementary Fig. 1e) and bonded anodically at 350 °C by applying a voltage of 350 V for 6 min. The wafers are then diced into 25 mm × 30 mm chips using a laser cutter (Supplementary Fig. 1f, g, and Fig. 1b). The chips were silanized using vapor phase deposition of Dimethyldichlorosilane (DDMS) at Integrated Surface Technologies to make the device interior surface hydrophobic for the formation of water droplets. The silane on the exterior surface of the chip was stripped using UV ozone treatment to stick fluidic connectors using Loctite 401 adhesives (Supplementary Fig. 1h). Electrical leads are soldered onto the gold contact pads through the electrical ports in the silicon wafer (Supplementary Fig. 1h).
Experimental setup
The experimental setup consists of the device holder interfaced with the fluidic, electrical, and optical subsystems.
Device sample holder
A 3D printed plastic sample holder was used to mount the device on the experimental setup. Copper pins hold the device in place with a \(0.17\,{{\rm{mm}}}\) glass coverslip (\(24 \, {{\rm{mm}}}\times 40 \, {{\rm{mm}}}\)) from SPI below it. The sample holder was screwed onto a X-Y stage from Newport (Model#-406) mounted on a modified Nikon TE2000U inverted microscope (Supplementary Fig. 2a, b).
Fluidic subsystem
A piezo driven pressure controller (OB1 MK3+ from Elveflow) with a \(30 \, {{\rm{{psi}}}}\) input from the house nitrogen supply and a maximum output of \(2000 \, {{\rm{mbar}}}\) was connected to the input of a fluidic tank (\(15 \, {{\rm{ml}}}\) plastic tube) using a \(10 \, {{\rm{mm}}}\) OD tubing (Supplementary Fig. 2c, d). The output of the tank flows into the device via a \(1/16 \, {{\rm{inch}}}\) OD and \(1/32 \, {{\rm{inch}}}\) ID polytetrafluoroethylene (PTFE) tubing from Masterflex (item#-EW06407-41) which was plugged into the fluidic port of the device using the Nanoport Assemby from IDEX Health & Science (part# - N-333).
Electrical subsystem
An A.C. high voltage amplifier (A. A. Lab Systems Ltd. Model#-A-303) amplifies the signal from a function generator (Hewlett Packard Model#-8116A) to generate a maximum output amplitude of \(200 \, {{\rm{V}}}\) (Supplementary Fig. 2e, f). The amplified output was connected to the electrical leads of the device through a single pole double throw (SPDT) switch which connects the supply across either of the electrodes (E1 or E2) and the ground pad.
Optical subsystem
The optical subsystem (Supplementary Fig. 2g, h) was built around a modified Nikon inverted TE2000U microscope. A blue LED (SOLIS-445C from Thorlabs, \(445 \, {{\rm{nm}}}\) and \(5.4 \, {{\rm{W}}}\) min) with a band pass excitation filter (D480/30x from Chroma) images the bead (\(6 \, \upmu {{\rm{m}}}\) diameter fluorescent green streptavidin coated polystyrene beads with excitation maxima at \(441 \, {{\rm{nm}}}\) and emission maxima at \(486 \, {{\rm{nm}}}\), Catalog#-24157) and the fluid flow in the device onto a sCMOS camera from Thorlabs (Part#-CS2100M-USB). The experiments were recorded at 33 frames per second. A red LED (M625L4 from Thorlabs, \(625 \, {{\rm{nm}}}\) and \(700 \, {{\rm{mW}}}\)) excites the Alexa-647 to detect nucleotides labeled with the fluorophore (dCTP-AF647) in the reagent droplet. A sCMOS camera from PCO (PCO edge 5.5) was used to capture the low light intensity levels emanating from the nucleotides coupled to the initiator strands on the beads at \(2 \, {{\rm{s}}}\) integration time. Appropriate bandpass excitation (Item#-86-988 from Edmund Optics, \(640 \, {{\rm{nm}}}\) center wavelength, \(14 \, {{\rm{nm}}}\) bandwidth, OD - 6) and emission (Item#-86-987 from Edmund Optics, \(676 \, {{\rm{nm}}}\) center wavelength, \(29 \, {{\rm{nm}}}\) bandwidth, optical density - 6) filters were used to ensure non-overlap of the excitation and emission spectrum. A Nikon objective (ELWD-20, 20x mag, 0.45 NA) with a correction collar for spherical aberration correction (set at \(0.7 \, {{\rm{mm}}}\) which is \(0.17 \, {{\rm{mm}}}\) thick glass coverslip \(+0.53 \, {{\rm{mm}}}\) thick borofloat glass of the device) was used for imaging.
Sample preparation
Preparing the oil solution by adding surfactant
\(4 \, {{\rm{ml}}}\) of Span 80 (S6760 from Sigma Aldrich) was added to \(200 \, {{\rm{ml}}}\) of \(1 \, {{\rm{cSt}}}\) silicone oil (PSF – \(1 \, {{\rm{cSt}}}\) from Clearco Products) to make a \(2.5\%w/w\) solution. It was sonicated for \(30 \, {{\rm{mins}}}\) to ensure complete dissolution of the surfactant.
Attaching initiator strand to beads
The initiator strand, which was a biotinylated oligomer with \(25\) bases (T25mer, 5′ biotin, IDT) was attached to the \(6\upmu {{\rm{m}}}\) diameter (Rb = 3 μm) streptavidin coated green-fluorescent polystyrene beads using the strong biotin-streptavidin hydrogen bond. The reaction was carried out for \(60\min\) at 2 °C, 14 RPM. The approximate starting yield (140 attomoles per bead (T25mer bound)) was determined by measuring the optical density at \(260\) nm (Nanodrop) before and after initial binding, then subtracting the supernatant and wash OD values from the starting yield. Binding/ wash buffer: \(20 \, {{\rm{mM}}}\) Tris pH \(7.5\), \(1 \, {{\rm{M}}}\) NaCl, \(1 \, {{\rm{{mM}}}}\) EDTA, \(0.0005\%\) Triton-X 100 (45 μl (plus 5 μl 100 μM T25) for binding reaction, and 500 μl for wash steps). Based on a particle concentration of \(1.4\%\), the number of beads was ~867,000 per 25 μl reaction (accounting for a \(20\%\) loss due to mixing and washing steps). Beads with initiator strands were then spun down using an Eppendorf Minispin (Catalog#-022620100) to remove the supernatant and were segregated into two parts (i) for Dielectrophoretic Bead-Droplet Reaction in the fabricated chip, and (ii) for benchtop synthesis in columns.
Suspending initiated beads in oil solution
The spun down initiated beads are suspended in the oil solution by sonication. The concentration of the beads in the silicone oil solution were tuned to ensure mostly a single bead floats in the vicinity of the electrodes within the field of view of the objective.
Preparing reagent solution
The reagent solution was prepared by mixing \(25 \, \upmu {{\rm{l}}}\) of reagents consisting of the fluorescently labeled base (dCTP-AF647) in a buffer solution of \(50 \, {{\rm{mM}}}\) Potassium Acetate, \(20 \, {{\rm{mM}}}\) Tris-acetate, \(10\,{{\rm{mM}}}\) Magnesium Acetate, and \(0.25 \, {{\rm{mM}}}\) Cobalt Chloride with the enzyme (TdT) solution consisting of \(3 \, \upmu {{\rm{l}}}\) of \(50 \, {{\rm{mM}}}\) KPO4, \(100 \, {{\rm{mM}}}\) Sodium Chloride, \(1.43 \, {{\rm{mM}}}\) β-ME, \(50\%\) glycerol, and \(0.1\%\) Triton X-100 solution in an Eppendorf tube. This reagent solution was formulated by initial benchtop experiments as described in the subsequent experimental procedure section. A trace amount of sodium salt of fluorescein (F6377 from Sigma Aldrich) was added to the reagent using a toothpick to discriminate it from the continuous phase inside the microfluidic device.
Filling device with oil solution as continuous phase
The device was completely immersed in \(50 \, {{\rm{ml}}}\) of the \(2.5\%\, {{\rm{w/w}}}\) solution of Span80 in \(1 \, {{\rm{cSt}}}\) silicone oil contained in a glass jar inside a vacuum desiccator. As the desiccator was evacuated the air inside the device was drawn out. When the desiccator is refilled with air, the silicone oil solution gushes into the device to fill it completely without any trapped air bubbles.
Mounting device on sample stage
The device was then removed from the glass jar, its outer surface was cleaned by thoroughly wiping with isopropanol, and then mounted on the sample holder (Supplementary Fig. 3a). The objective was focused on the output of the droplet generation channel and the ITO electrodes.
Making electrical and fluidic connections to device ports
Electrical connections are made from the output of the amplifier to the ground pad and to the trap electrodes through the SPDT switch (Supplementary Fig. 3a). The fluidic tank was filled with 15 ml of the above oil solution. Pressure was applied using the pressure controller to fill the output PTFE tubing from the tanker with oil solution which is dipped at the other end inside the 1.5 ml tube containing the reagent. Just before oil starts dripping from the tubing into the reagent tube, the height of the PTFE tubing and reagent tube were raised to suck the reagent into the tubing. Then the tubing was lowered again into another Eppendorf tube containing the oil solution. As the reagent solution started dripping, the height was raised again to fill the PTFE tubing with the oil solution while ensuring there are no trapped air bubbles. The tubing was then connected to the device inlet while pushing out the oil solution at the bottom to ensure no air gaps and fluidic continuity (the oil solution inside the device and at the bottom of the tubing are the same). This approach prevented immediate flow of the reagent solution through the droplet generation channel as soon as the PTFE tubing was connected thus allowing time for experimental setup and control (Supplementary Fig. 3b).
Experimental procedure for bead-droplet interaction
Encapsulation and ejection of bead from droplet
Beads suspended in the oil solution were introduced into the device through the oil inlet (Supplementary Fig. 1a). The voltage supply was switched on and set to around \(40 \, {{\rm{V}}}\) amplitude at \(200 \, {{\rm{Hz}}}\) to dielectrophoretically trap a bead floating near the top electrode. Then the voltage supply was switched off. Following this a pressure of \(60 \, {{\rm{mbar}}}\) was applied on the pressure controller to drive reagent flow in the device. As the reagent approached the entrance of the microfluidic channel, a sudden pressure pulse of approximately \(10 \, {{\rm{mbar}}}\) for approximately \(300 \, {{\rm{ms}}}\) was exerted to dispense a single droplet into the reaction chamber (Supplementary Movie 5). A higher-pressure pulse or a longer pulse duration would lead to the generation of multiple droplets (Supplementary Movie 6). Then the voltage supply was switched on again at \(20 \, {{\rm{V}}}\) amplitude and \(200 \, {{\rm{Hz}}}\) to trap the droplet adjacent to the bead on the top electrode.
At this point the supply voltage was gradually increased to \(\sim \, 120 \, {{\rm{V}}}\) amplitude. The droplet moves toward the electrode to encapsulate the bead. Subsequently the voltage was reduced to \(0.1 \, {{\rm{V}}}\) amplitude and the bead was ejected out of the droplet. Supplementary Movie 1 depicts the encapsulation and ejection process. The bead can undergo multiple encapsulations and ejection (Supplementary Movie 7) as well by switching the voltage between high and low. The images extracted from these videos were suitably processes for enhanced clarity without altering the data and results.
Estimating interfacial tension and contact angle
The interfacial tension values reported in the main text were measured using the standard Wilhelmy plate method. The contact angles were measured by capturing the droplet shape on a streptavidin coated glass slide (GS-SV-5 from Nanocs) and silanized glass surface and then estimating the angle it forms on the surface through shape fitting.
Estimating electrical conductivity of reagent
The conductivity of the reagent droplet was measured using an Orion 3 Star Conductivity Portable.
Modeling of bead-droplet Interaction
Coupled electrohydrodynamic simulations
The electric field driven encapsulation of the microbead into the microdroplet and its ejection out of it was modeled by using coupled electrohydrodynamic simulations in COMSOL Multiphysics (Supplementary Fig. 4). The fluid flow (aqueous droplet motion in silicone oil medium) was modeled as two-phase fluid flow using the Navier-Stokes equation45. The phase variable \(\phi\) tracked the fluidic interface between the droplet and the suspension medium using the phase field method83. \(\phi=-1\) within the silicone oil medium and \(\phi=1\) in the droplet. It transitions from \(-1\) to \(1\) at the interface of the droplet and the suspension medium. To model the driving electric force, the electric charge continuity equation45,51,84 was used to solve for the non-uniform electric field distribution when an AC voltage was supplied across a pair of electrodes of different dimensions. The electric force density on the fluids was evaluated by using \(\vec{\nabla }\cdot {T}^{\leftrightarrow}\). Here, \({T}^{\leftrightarrow}\) is the Maxwell Stress Tensor44,51,84,85,86,87. This force sets the fluid flow in motion. As the droplet moves, the boundary condition of the electric charge continuity equation changes. This changes the electric field distribution and \(\vec{\nabla }\cdot {T}^{\leftrightarrow}\) in turn. This underlines the basic coupling between the Navier Stokes equation and the charge continuity equation. In the simulations, the bead is modeled as a stationary polarizable dielectric particle with a fixed contact angle (\(\theta=145^{\circ}\)) that the droplet forms on its surface in the silicone oil medium. This leads to an additional capillary force acting on the bead-droplet system when the bead and the droplet are in contact. For simplicity, axis symmetric simulations were adopted. This helps focus on the essential physical interaction without getting into the nuances of device design.
The encapsulation and ejection process can be modeled as a balance between the electric and capillary force. To understand the scaling laws underlying these forces, we derived approximate analytical equations for the electric and capillary forces.
Dielectrophoretic force
The dielectrophoretic force44,51,84,85,86,87 on a generic charge neutral polarizable particle (of radius \({R}_{p}\)) can be evaluated by expressing its polarizability and the non-uniform electric field as an infinite series of multipolar expansions87. We base our approximate analysis on the dipolar/first (\(n=1\)) term of this series. The approximate dielectrophoretic force (\({\vec{F}}_{{DEP}}\)) then scales as \({\vec{F}}_{{DEP}}\propto {K}_{1}{R}_{p}^{3}\). Here \({K}_{1}\) is the familiar Classius-Mossotti factor and \({R}_{p}\) is the radius of the polarizable particle which can be the droplet or the bead. \({K}_{1}\) is given by \(\frac{{\tilde{\varepsilon }}_{p}-{\tilde{\varepsilon }}_{o}}{{\tilde{\varepsilon }}_{p}+2{\tilde{\varepsilon }}_{o}}\). Here \({\tilde{\varepsilon }}_{p/o}\) is the complex permittivity of the particle or the suspension oil medium which is a function of their respective relative permittivities (\({\varepsilon }_{p/o}\)) and conductivities (\({\sigma }_{p/o}\)). Therefore, the water droplet experiences a much larger force than the bead due to its larger permittivity contrast with the oil medium as well as much larger size (Supplementary Fig. 5). So, if multiple droplets are suspended in the reaction chamber, the primary electric field driven effect is the merger of the droplets.
Capillary force
The capillary45,51,52 force on the bead-droplet system arises due to the change in the total interfacial energy of the system as the bead is encapsulated/ejected within the droplet. For our case in which \({R}_{b} \; \ll \; {R}_{d}\), the change in total interfacial energy is given as \(\triangle {U}_{{IT}}\approx -{\gamma }_{{ow}}\cos \theta \triangle {A}_{{ws}}\). Here, \({\gamma }_{{ow}}\) is the oil-water interfacial tension and \({A}_{{ws}}\) is the interfacial area of the water/aqueous droplet and the solid bead surface (Supplementary Fig. 6). From this the scaling of the interfacial capillary force can be approximated as \({\vec{F}}_{{IT}}\propto {R}_{b}{\gamma }_{{ow}}\cos \theta\).
Experimental procedure for chemical coupling
Chemical coupling of base and control on the device
The above physical process was used (with a 300 s encapsulation time) for the enzymatic coupling of fluorescently tagged nucleotides onto the initiator strand on the bead with a few additional intermediate images of the area around the reaction zone captured as enlisted below.
-
Before loading the beads into the device an image to estimate the background noise under red illumination (Supplementary Fig. 7a, b).
-
After the bead was dielectrophoretically trapped on the top electrode an image each with the blue and red excitation are captured to measure the level of the red fluorescence signal from the site of the bead just prior to the reaction (Supplementary Fig. 7c, d).
-
Finally, after the encapsulation and ejection process another set of images under blue and red excitation were captured (Supplementary Fig. 7e, f).
These images were captured as 16-bit Tiff files. Supplementary Fig. 7c, f are used in Fig. 4a of the main text. These steps were repeated to see the repeatability of the chemical coupling reaction on our platform. The three different reacted beads under red excitation are depicted separately in Supplementary Fig. 7g and are also used in Fig. 4 of the main text.
The control experiment was repeated using the above process but with beads without initiator strands and reagent droplets without the enzymes (Supplementary Fig. 7h–m). Supplementary Fig. 7h, m are used in Fig. 4b of the main text.
Column synthesis
Firstly, free solution reactions are implemented to develop optimal room temperature protocol (Supplementary Fig. 8a, b) for translation into DBDR. Results were analyzed using reverse-phase high performance liquid chromatography (HPLC) (Supplementary Fig. 8b).
To simulate an enzymatic synthesis reaction using a column, an open-top nylon syringe filter (Omicron SFNY04XB, \(4 \, {{\rm{mm}}}\), \(0.45 \, \upmu {{\rm{m}}}\)) was used. To the bottom filter (\(0.45 \, \upmu {{\rm{m}}}\) pore size) which was held in place by a plastic ring, \(15 \, \upmu {{\rm{l}}}\) of beads (\(1.2 \, {{\rm{M}}}\)) were added. A top filter was then positioned above the reagent bed. Between the filters the reaction volume was about \(15 \, \upmu {{\rm{l}}}\). A \(1 \, {{\rm{ml}}}\) syringe was used to push the bead medium (\(10 \, {{\rm{mM}}}\) HCL, \(2 \, {{\rm{M}}}\) NaCl, \(1 \, {{\rm{mM}}}\) EDTA, \(0.0005\%\) Triton-X 100, pH \(7.3\)) passed the bottom filter until it completely exited the drip director. \(50\,\upmu {{\rm{l}}}\) (\(6 \, \upmu {{\rm{l}}}\) TdT (\(20\,{{\rm{U}}}/\upmu {{\rm{l}}}\)), \(5 \, \upmu {{\rm{l}}}\) \(10x\) TdT buffer (\(50 \, {{\rm{mM}}}\) potassium acetate, \(50 \, {{\rm{mM}}}\) Tris-acetate, \(10\,{{\rm{mM}}}\) magnesium acetate, pH 7.9 @ 25 °C), 5 μl 10× (2.5 mM) solution of \({{\rm{Co}}{{Cl}}_{2}}\), 0.25 μl 1 mM Alexa Fluor™ 647-aha-dCTP, 33.75 μl water) were added to the top filter, and an empty 1 ml syringe was used to push the reagent passed the top filter (flow rate at \(50 \, \upmu {{\rm{l}}}\) per second), into the reaction area until the reagent could be seen inside the drip director. The column was kept upright for \(5 \, {{\rm{mins}}}\). Once the reaction was completed, the empty 1 ml syringe was used to push the spent reagents through the column until the drip director was clear. Afterwards, \(1 \, {{\rm{ml}}}\) of bead suspension medium (described above) was used to wash the beads. These beads were then taken in a \(1.5 \, {{\rm{ml}}}\) Eppendorf tube for analysis using fluoroscopy.
As a control, synthesis was performed on beads without initiators and reagents without TdT and the reaction was analyzed through fluorescence measurements.
Measuring fluorescence from beads reacted in columns
About 3 µl of the reacted bead suspension in the buffer was taken in an Eppendorf tube and was diluted to ensure the bead concentration was small enough to prevent signal interference from beads in different planes while being large enough to have ample beads within the field of view to get a statistically significant inference about fluorescence intensity distribution. The beads were introduced into the device filled with MilliQ water. The same chips were used for fluorescence measurements to ensure identical optical environment for comparison between on-chip experiments with their column counterparts. Once the beads settled down (imaged using blue excitation) the excitation was switched to red to image the fluorescence intensity of the beads. Many such frames of red fluorescent beads were collected with a large number of beads (\(285\)). Some are shown in Supplementary Fig. 9a. A few representative beads spanning the entire range of fluorescence intensities are used in Fig. 4d of the main text.
The control experiments implemented using DBDR (Fig. 4b of main text) were reiterated on the columns. The fluorescence of these beads was measured following the same procedure as discussed in the previous paragraph (Supplementary Fig. 9b).
Image processing and data analysis
Encapsulation and ejection of bead from droplet
The recorded video (Supplementary Movie 1) of the encapsulation and ejection process was analyzed frame by frame using ImageJ and snapshots that best represent the processes were selected and labeled for Fig. 2 of the main text.
Establishing enzymatic coupling of base to the initiator strands on the bead in DBDR
Maintaining the same scale of \(250-3300\) across the red fluorescence images, the difference in brightness of the bead with enzymatic coupling (Fig. 4a main text and Supplementary Fig. 7f) and the control bead (Fig. 4b main text and Supplementary Fig. 7m) was obvious.
Analyzing fluorescence intensity distribution
Each frame in Supplementary Fig. 9a was analyzed using predefined image processing functions in Matlab to detect the beads (Supplementary Fig. 10), binarize them, evaluate their mean fluorescence intensity, and evaluate fluorescence intensity distribution across a horizontal line passing through the bead center. Average fluorescence values across frames were collected to plot a histogram of the fluorescence intensity distribution of all beads reacted on the benchtop using synthesis columns.
The average fluorescence intensity of the three beads reacted using DBDR (Supplementary Fig. 7g) were found to be \(2184.5\), \(2171.7\), \(1816.2\) (same unit as was used for column fluorescence data). These values are higher than the fluorescence intensity (represented in Supplementary Fig. 10d) of all the \(285\) beads reacted in columns whose fluorescence data was collected. This indicates that the reaction fidelity using DBDR is higher than synthesis columns.
Analyzing statistical distribution of data
To establish the statistical significance of our fluorescence comparison-based claim that the solid-phase synthesis reaction fidelity achieved using DBDR is higher than synthesis columns we resort to statistical hypothesis testing. We seek to establish that the mean fluorescence intensity of beads reacted using DBDR is higher than the mean fluorescence intensity of beads reacted using synthesis columns at significance level of \(0.05\) or a confidence level of \(95\%\). The t-test which tests for the null hypothesis of equivalence of sample means for both the synthesis methods using the following test statistic88,89,90,91,92 (\(t\) value) is appropriate for our purpose.
Here, \({\mu }_{{DBDR}/{Column}}\) is the sample mean of the respective synthesis methods, \({\sigma }_{{DBDR}/{Column}}\) is the sample standard deviation of the respective synthesis method, and \({N}_{{DBDR}/{Column}}\) is the number of bead samples over which the mean and the standard deviation were evaluated in the respective synthesis methods. For columns, \({N}_{{column}}=285\). This is the total number of beads that were accounted for in the histogram in Supplementary Fig. 10d. For DBDR, \({N}_{{DBDR}}=3\). These are the 3 beads represented in Supplementary Fig. 7g. The relevant values are summarized in Supplementary Table 2a. As the standard deviations and the number of bead samples are unequal in the two synthesis methods, we use the Welch’s t-test for the statistical significance analysis89,90,93. The degree of freedom for the Welch t-test which is given by the Welch-Satterthwaite equation is90,94,95:
The values of \(t\) and \({df}\) evaluated using Eq. 1 and eq. 2 are \(11.65\) and \(2.14\) (summarized in Supplementary Table 2b). Therefore, \(2 \, < \,{df}=2.14 \, < \, 3\). Using a standard t-test table for two-tailed testing we see that if \({df}=2\) for a two-tailed significance level (\(\alpha\)) of \(0.01\) the critical t-value (\({t}^{*}\)) is \(9.925 \, < \, t\) and for a two-tailed significance level (\(\alpha\)) of \(0.002\) the critical t-value (\({t}^{*}\)) is \(22.327 \, > \,t\). On the other hand, if \({df}=3\) for a two-tailed significance level (\(\alpha\)) of \(0.002\) the critical t-value (\({t}^{*}\)) is \(10.215 \, < \, t\) and for a two-tailed significance level (\(\alpha\)) of \(0.001\) the critical t-value (\({t}^{*}\)) is \(12.924\, > \,t\). Thus, we can safely say that our null hypothesis can be rejected at significant level of \(\alpha=0.01\) or at a confidence level of \(99\%\). Hence, our result is definitely significant at \(\alpha=0.05\) or a confidence level of \(95\%\). This was confirmed using the inbuilt ttest2 function in Matlab for Welch’s t-test which rejected the null hypothesis. To evaluate if the sample sizes (\({N}_{{DBDR}}\) and \({N}_{{column}}\)) were sufficient for statistical testing, we calculate the power of the statistical test96 using the inbuilt sampsizepwr function in Matlab for \(\alpha=0.05\). We obtain a statistical power of almost \(1\) (a power of \(0.8\) at \(\alpha=0.05\) is generally considered adequate96). Therefore, our sample size suffices for statistical testing.
The Welch’s t-test, which is a parametric test is generally robust for normal distributions with unequal sample sizes and standard deviations89,90,93. For deviations from normal distributions (Supplementary Fig. 10d), nonparametric tests (which do not assume any specific distribution profile) operating on the ranks of the experimentally observed values rather than the actual values themselves are more robust93,97. It is established in statistical literature that a rank transformation on the conventional Welch’s t-test would counter the combined effects of unequal standard deviations as well as non-normal distributions93. Therefore, we apply the above statistical testing procedure to the combined ranks of the average fluorescence intensities of beads reacted using the synthesis column and DBDR. The \(3\) beads reacted using DBDR have higher fluorescence intensities than the \(285\) beads reacted in synthesis columns. So, the beads reacted in columns have ranks from \(1\) to \(285\). While the beads reacted using DBDR have ranks from \(286\) to \(288\). The respective means (\({\mu }_{{DBDR}/{column}}\)) and standard deviations (\({\sigma }_{{DBDR}/{column}}\)) are summarized in Supplementary Table 3a. Using Eqs. 1 and 2, we evaluate \(t=29.41\) and \({df}=285.79\). We see that at \(\alpha=0.05\), \({t}^{*} \,=\, 1.9683 \; < \;t=29.41\) for \({df}=285\) or \(286\). Therefore, the null hypothesis of equivalence of means of DBDR and columns can be safely rejected at the confidence level of \(95\%\). The inbuilt ttest2 function in matlab confirms this. Furthermore, a statistical power of 0.9729 is obtained which confirms that the sample size suffices for the statistical inference. The results are summarized in Supplementary Table 3b.
Modeling of bead stacking in column and comparison of reagent access with bead-droplet reactor
Analytical modeling of ideal case of beads stacked in perfect lattices
To understand how stacking of beads in synthesis columns limits reagent access to the bead surfaces, we begin by considering the ideal case scenario of stacking of beads into perfect lattices. In such a case, effectively one bead is enclosed in a unit cell and access the reagents within the void of the unit cell. The simple cubic is one of the most loosely packed lattices while the rhombohedral is one of the most tightly packed67. The volume of a bead of radius \({R}_{b}\) is \(\frac{4}{3}\pi {R}_{b}^{3}\) (Supplementary Fig. 11). The volume of a simple cubic unit cell is \(8{R}_{b}^{3}\) while that of a rhombohedral unit cell is \(4\sqrt{2}{R}_{b}^{3}\). Therefore. the void volume in these unit cells is \(4\left(2-\frac{\pi }{3}\right){R}_{b}^{3} \, \approx \, 103\,{{\rm{{fl}}}}\) and \(4\left(\sqrt{2}-\frac{\pi }{3}\right){R}_{b}^{3} \, \approx \,40\,{{\rm{fl}}}\) respectively69,70. The reagent solution has 5 μM concnetration of nucleotides (2.5 × 10−7 mmoles of nucleotides were added to 50 μl of reagent solution). Therefore, within the void volume of the simple cubic and rhombohedral unit cells there would be around \(103 \, {{\rm{{fl}}}}\times 5\,\upmu {{\rm{M}}}=0.52 \, {{\rm{attomoles}}}\), and \(40\,{{\rm{fl}}}\times 5\,\upmu {{\rm{M}}}=0.2\,{{\rm{attomoles}}}\) of fluorescently labeled nucleotides respectively which is \(\approx \, 280\) and \(\approx \, 700\) times more than the number of nucleotides in the voids of the simple cubic and rhombohedral lattice respectively. On the other hand, the volume of the droplet (\({R}_{d}=25\mu m\)) which forms a contact angle (\(\beta\)) of \(138^{\circ}\) on the device surface is \(\frac{(2-3\cos \beta+{\cos }^{3}\beta )}{3}\pi {R}_{d}^{3}=65 \, {{\rm{pl}}}\)51,52. At the same \(5 \, \upmu {{\rm{M}}}\) concentration of fluorescently labeled nucleotides, the nearly \(100 \, {{\rm{attomoles}}}\) of initiator strands will have access to \(65 \, {{\rm{pl}}}\times 5 \, \upmu {{\rm{M}}}=325 \, {{\rm{attomoles}}}\) of fluorescently labeled nucleotides within the droplet.
Particle tracking modeling for imperfect tracks
For simplicity of modeling as well as to reduce memory and time requirements, a 2D mirror symmetric simulation was set up combining turbulent fluid flow and particle tracking simulations. The simulation space mimicked the dimensions of the reaction column. A rhombohedral stack of beads was defined at the bottom of the simulation space. Fluid flow equations for turbulent flow68 were simulated to mimic fluid flow in the column when reagents are introduced into it. This fluid flow exerted a drag force on the particles which set them in motion within the column dispersing the perfect stack in the process. The beads have increased and varying access to reagents. Once the reagent inflow subsides, the beads eventually settle down under the influence of gravity into imperfect stacks. Further modeling details can be found in Supplementary Note 5 and Supplementary Fig. 12.
Reagent diffusion
The synthesis columns used in the reaction are 2.28 mm long whereas the microdroplets used in DBDR are \(50 \, \upmu m\) (\(5\times {10}^{-5}{{\rm{m}}}\)) in diameter (Supplementary Fig. 13). The diffusion time is approximated as \({t}_{D}\approx \frac{{l}^{2}}{2D}\)45. Here, \(D\) is the well-known diffusion coefficient which is \(\approx 1.3\times {10}^{-5}{{\rm{cm}}}^{2}/{{\rm{s}}}\)71,72 for single nucleotides. Using this we obtain \({t}_{D,\,{col}} \, \approx \, 2000\, {{\rm{s}}}\) and \({t}_{D,\,{DBDR}}\,\approx \,1\,{{\rm{s}}}\).
Electric field driven enhancement of reagent concentration in DBDR
The reagent droplet in DBDR consists of many positive and negatively charged species which are necessary for the enzymatic coupling of nucleotides to the initiator strands. The applied AC electric field for dielectrophoretic trapping of the droplet and bead also drives ion migration closer to and further away from the encapsulated bead during alternate phases of the AC cycle. This changes the time averaged concentration of ions that the initiators on the bead are exposed to over an entire AC cycle. This migration of ions is modeled using the Nernst-Planck equation45,98 in COMSOL Multiphysics. We model the effect of the AC supply voltage amplitude and frequency. Details can be found in Supplementary Note 6, Supplementary Table 4, and Supplementary Figs. 14–17.
Data availability
All data generated and/or analyzed in this study are included in the manuscript. Source Data is available as a Source Data file. Source data are provided with this paper.
Materials availability
Material requests should be addressed to P.P. or M.A.J.
Code availability
MATLAB codes used to analyze data have been provided as .m files with supporting Readme files together with the Source Data file.
References
Merrifield, R. B. Solid phase synthesis. Science 232, 341–357 (1986).
Seneci, P. Solid-Phase Synthesis and Combinatorial Technologies (Wiley Interscience, New York, U.S.A., 2000).
Merrifield, R. B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85, 2149–2154 (1963).
Kosuri, S. & Church, G. M. Large-scale de novo DNA synthesis: technologies and applications. Nat. Methods 11, 499–507 (2014).
Hughes, R. A. & Ellington, A. D. Synthetic DNA synthesis and assembly: putting the synthetic in synthetic biology. Cold Spring Harb. Perspect. Biol. 9, a023812 (2017).
Hoose, A., Vellacott, R., Storch, M., Freemont, P. S. & Ryadnov, M. G. DNA synthesis technologies to close the gene writing gap. Nat. Rev. Chem. 7, 144–161 (2023).
Marshall, W. S. & Kaiser, R. J. Recent advances in the high-speed solid phase synthesis of RNA. Curr. Opin. Chem. Biol. 8, 222–229 (2004).
Coin, I., Beyermann, M. & Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2, 3247 (2007).
Plante, O. J., Palmacci, M. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Lam, K. S., Lebl, M. & Krchňák, V. The “one-bead-one-compound” combinatorial library method. Chem. Rev. 97, 411–447 (1997).
Krchňák, V. & Holladay, M. W. Solid phase heterocyclic chemistry. Chem. Rev. 102, 61–92 (2002).
Guillier, F., Orain, D. & Bradley, M. Linkers and cleavage strategies in solid-phase organic synthesis and combinatorial chemistry. Chem. Rev. 100, 2091–2157 (2000).
Ma, S., Tang, N. & Tian, J. DNA synthesis, assembly and applications in synthetic biology. Curr. Opin. Chem. Biol. 16, 260–267 (2012).
Ma, S., Saaem, I. & Tian, J. Error correction in gene synthesis technology. Trends Biotechnol. 30, 147–154 (2012).
Gach, P. C., Iwai, K., Kim, P. W., Hillson, N. J. & Singh, A. K. Droplet microfluidics for synthetic biology. Lab Chip 17, 3388–3400 (2017).
Baker, M. Microarrays megasynthesis. Nat. Methods 8, 457–460 (2011).
Ren, C., & Lee, A. Droplet Microfluidics (The Royal Society of Chemistry, Croydon, 2021).
Teh, S., Lin, R., Hung, L. & Lee, A. P. Droplet microfluidics. Lab Chip 8, 198–220 (2008).
Shang, L., Cheng, Y. & Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 12, 7964–8040 (2017).
Solvas, X. C. & deMello, A. Droplet microfluidics: recent developments and future applications. Chem. Commun. 47, 1936–1942 (2011).
Guo, M. T., Rotem, A., Heyman, J. A. & Weitz, D. A. Droplet microfluidics for high-throughput biological assays. Lab Chip 12, 2146–2155 (2012).
Ding, Y., Howes, P. D. & DeMello, A. J. Recent advances in droplet microfluidics. Anal. Chem. 92, 132–149 (2020).
Joensson, H. N. & Svahn, H. A. Droplet microfluidics—a tool for single cell analysis. Angew. Chem. Int. Ed. 51, 12176–12192 (2012).
Kaminski, T. S., Scheler, O. & Garstecki, P. Droplet microfluidics for microbiology: techniques, applications and challenges. Lab Chip 16, 2168–2187 (2016).
Schneider, T., Kreutz, J. & Chiu, D. T. The potential impact of droplet microfluidics in biology. Anal. Chem. 85, 3476–3482 (2013).
Zhong, X., Chen, H. & Zare, R. N. Ultrafast enzymatic digestion of proteins by microdroplet mass spectrometry. Nat. Commun. 11, 1049 (2020).
Wei, Z., Li, Y., Cooks, R. G. & Yan, X. Accelerated reaction kinetics in microdroplets: overview and recent developments. Annu. Rev. Phys. Chem. 71, 31–51 (2020).
Lorenz, R. M., Edgar, J. S., Jeffries, G. D. & Chiu, D. T. Microfluidic and optical systems for the on-demand generation and manipulation of single femtoliter-volume aqueous droplets. Anal. Chem. 78, 6433–6439 (2006).
Chiu, D. T., Lorenz, R. M. & Jeffries, G. D. M. Droplet for ultrasmall-volume analysis. Anal. Chem. 81, 5111–5118 (2009).
Chiu, D. T. & Lorenz, R. M. Chemistry and biology in femtoliter and picoliter volume droplets. Acc. Chem. Res. 42, 649–658 (2009).
Zhu, P. & Wang, L. Passive and active droplet generation with microfluidics: a review. Lab Chip 17, 34–75 (2017).
Park, S., Wu, T., Chen, Y., Teitell, M. A. & Chiou, P. High-speed droplet generation on demand driven by pulse laser-induced cavitation. Lab Chip 11, 1010–1012 (2011).
Niu, X., Gelen, F., Edel, J. B. & DeMello, A. J. A microfluidic dilutor for high-throughput screening. Nat. Chem. 3, 437–442 (2011).
Sun, A. C. et al. A droplet microfluidic platform for high-throughput photochemical reaction discovery. Nat. Comm. 11, 6202 (2020).
Eastburn, D. J. et al. Microfluidic droplet enrichment for targeted sequencing. Nucleic Acid Res. 43, e86 (2015).
Agresti, J. J. et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Nat. Acad. Sci. 107, 4004–4009 (2010).
Tewhey, R. et al. enrichment for large-scale targeted sequencing. Nat. Biotechnol. 27, 1025–1031 (2009).
Duncombe, T. A., Tentori, A. M. & Herr, A. E. Microfluidics: reframing biological enquiry. Nat. Rev. Mol. Cell Biol. 16, 554–567 (2015).
Larsen, A. C. et al. A general strategy for expanding polymerase function by droplet microfluidics. Nat. Commun. 7, 11235 (2016).
Zhang, J. et al. One-step fabrication of supramolecular microcapsules from microfluidic droplets. Science 335, 690–694 (2012).
Nightingale, A. M., Phillips, T. W., Banock, J. H. & DeMello, J. C. Controlled multistep synthesis in a three-phase droplet reactor. Nat. Commun. 5, 3777 (2014).
Banock, J. H. et al. Continuous synthesis of device-grade semiconducting polymers in droplet based microreactors. Adv. Func. Mater. 23, 2123–2129 (2013).
Ostrov, N. et al. Technological challenges and milestones for writing genomes. Science 366, 310–312 (2019).
Pethig, R. Dielectrophoresis: status of the theory, technology and applications. Biomicrofluidics 4, 022811 (2010).
Bruus, H. Theoretical Microfluidics (Oxford University Press, Oxford, U.K., 2008).
Sher, L. D. & Schwann, H. P. Microelectrophoresis with alternating electric fields. Science 148, 229–231 (1965).
Becker, F. F. et al. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc. Natl Acad. Sci. 92, 860–864 (1995).
Millman, J. R., Bhatt, K. H., Prevo, B. G. & Velev, O. D. Anisotropic particle synthesis in dielectrophoretically controlled microdroplet reactors. Nat. Mater. 4, 98–102 (2005).
Pohl, H. A. & Hawk, I. Separation of living and dead cells by dielectrophoresis. Science 152, 647–649 (1966).
Velev, O. D., Prevo, B. G. & Bhatt, K. H. On-chip manipulation of free droplets. Nat 426, 515–516 (2003).
Mugele, F. & Baret, J. C. Electrowetting: from basics to applications.J. Phys. Condens. Matter 17, R705–R774 (2005).
Berthier, J. Micro-Drops and Digital Microfluidics (Elsevier, Oxford, U.K., 2013).
Ozcelik, A. et al. Accoustic tweezers for the life sciences. Nat. Methods 15, 1021–1028 (2018).
Tenje, M., Fornell, A., Ohlin, M. & Nilsson, J. Particle manipulation methods in droplet microfluidics. Anal. Chem. 90, 1434–1443 (2018).
Clark, I. C. & Abate, A. R. Microfluidic bead encapsulation above 20 kHz with triggered drop formation. Lab Chip 18, 3598–3605 (2018).
Abate, A. R., Chen, C., Agresti, J. J. & Weitz, D. A. Beating Poisson encapsulation statistics using close-packed ordering. Lab Chip 9, 2628–2631 (2009).
Liu, Z., Fornell, A. & Tenje, M. A droplet acoustofluidic platform for time-controlled microbead based reactions. Biomicrofluidics 15, 034103 (2021).
Eisenstein, M. Enzymatic DNA synthesis enters new phase. Nat. Biotechnol. 38, 1113–1115 (2020).
Palluk, S. et al. De novo DNA synthesis using polymerase-nucleotide complex. Nat. Biotechnol. 36, 645–650 (2018).
Lee, H. H., Kalhor, R., Goela, N., Bolot, J. & Church, G. M. Terminator-free template-independent enzymatic DNA synthesis for digital information storage. Nat. Commun. 10, 2383 (2019).
Jensen, M. A. & Davis, R. W. Template independent enzymatic oligonucleotide synthesis (TiEOS): its history, prospects and challenges. Biochemistry 57, 1821–1832 (2018).
Clore, A. A new route to synthetic DNA. Nat. Biotechnol. 36, 593–595 (2018).
Nguyen, B. H. et al. Scaling DNA data storage with nanoscale electrode wells. Sci. Adv. 7, eabi6714 (2021).
Chow, B. Y., Emig, C. J. & Jacobson, J. M. Photoelectrochemical synthesis of DNA microarrays. Proc. Nat. Acad. Sci. 106, 15219–15224 (2009).
Ramos, A., Morgan, H., Green, N. G., Castellanos, A. & Electrokinetics, A. C. A review of forces in microelectrode structures. J. Phys. D Appl. Phys. 31, 2338–2353 (1998).
Green, N. G., Ramos, A. & Morgan, H. A.C. Electrokinetics: A survey of sub-micrometre particle dynamics. J. Phys. D Appl. Phys. 33, 632–641 (2000).
Conway, J. H & Sloane, N. J. H. Sphere Packings, Lattices and Groups (Springer: New York, U.S.A., 1999). .
Garde, R. J. Turbulent Flow (New Age Science Limited, Kent, U.K., 2010).
Gvirtzman, H. & Roberts, P. V. Pore scale spatial analysis of two immiscible fluids in porous media. Water Resour. Res. 27, 1165–1176 (1991).
Graton, L. C. & Fraser, H. J. Systematic packing of spheres-with particular relation to porosity and permeability. J. Geol. 43, 785–909 (1935).
Nkodo, A. E. et al. Diffusion coefficient of DNA molecules during free solution electrophoresis. Electrophoresis 22, 2424–2432 (2001).
Lukacs, G. L. et al. Size dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 275, 1625–1629 (2000).
Alshanski, I. et al. Enhancing the efficiency of the solid-phase peptide synthesis (SPPS) process by high shear mixing. Org. Process. Res. Dev. 22, 1318–1322 (2018).
Lam, K. S., Lebl, M. & Krchňák, V. The “one-bead-one-compound” combinatorial library method. Chem. Rev. 97, 411–448 (1997).
Eggersdorfer, M. L., Seybold, H., Ofner, A., Weitz, D. A. & Studart, A. R. Wetting controls of droplet formation in step emulsification. Proc. Nat. Acad. Sci. 115, 9479–9484 (2018).
Ofner, A. et al. High-throughput step emulsification for the production of functional materials using a glass microfluidic device. Macromol. Chem. Phys. 218, 16000472 (2017).
Chiou, P. Y., Ohta, A. T. & Wu, M. C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 436, 370 (2005).
Goldman, N. et al. Towards practical, high capacity, low-maintenance information storage in synthesized DNA. Nature 494, 77–80 (2013).
Lee, J. K., Samanta, D., Nam, H. G. & Zare, R. N. Spontaneous formation of gold nanostructures in aqueous microdroplets. Nat. Commun. 9, 1562 (2018).
Nam, I., Lee, J. K., Nam, H. G. & Zare, R. N. Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets. Proc. Nat. Acad. Sci. 114, 12396–12400 (2017).
Kuchler, A., Yoshimoto, M., Luginbuhl, S., Mavelli, F. & Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 11, 409–420 (2016).
Grommet, A. B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotech. 15, 256–271 (2020).
Jacqmin, D. Calculation of two-phase Navier-Stokes flows using phase-field modeling.J. Comput. Phys. 155, 96–127 (1999).
Jackson, J. D. Classical Electrodynamics. 3rd edn (John Wiley & Sons, New York, 1999).
Griffiths, D. J. Introduction to Electrodynamics. 4th edn (Cambridge University Press, Cambridge, 2017).
Padhy, P., Zaman, M. A., Jensen, M. A. & Hesselink, L. Dynamically controlled dielectrophoresis using resonant tuning. Electrophoresis 42, 1079–1092 (2021).
Jones, T. B. Electromechanics of Particles. (Cambridge University Press, Cambridge, 2010).
Krzywinski, M. & Altman, N. Significance, p values and t-tests. Nat. Methods 10, 1041–1042 (2013).
Krzywinski, M. & Altman, N. Comparing samples - part 1. Nat. Methods 11, 215–216 (2014).
Derrick, B., Toher, D. & White, P. Why Welch’s t-test is type I error robust. Quant. Meth. Psych. 12, 30–38 (2016).
Ross, A., & Willson, V. L., Basic and Advanced Statistical Tests: Writing results sections and creating tables and figures (Sense Publishers, Rotterdam, 2017).
Schumacker, R., & Tomek, S. Understanding Statistics Using R. (Springer, New York, 2013).
Zimmerman, D. W. & Zumbo, B. D. Rank transformations and the power of the student t test and welch t’ test for non-normal populations with unequal variances. Can. J. Exp. Psychol. 47, 523–539 (1993).
Miles, J., & Banyard, P. Understanding and Using Statistics in Psychology: a Practical Introduction (Sage Publications, London, 2007).
Alfassi, Z. B., Boger, Z., & Ronen, Y. Statistical Treatment of Analytical Data (CRC Press, Boca Raton, 2005).
Krzywinski, M. & Altman, N. Power and sample size. Nat. Methods 10, 1139–1140 (2013).
Krzywinski, M. & Altman, N. Nonparametric tests. Nat. Methods 11, 467–468 (2013).
Kirby, B. J. Micro and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices (Cambridge University Press, Cambridge, 2010).
Acknowledgements
We thank Dr. Paul Christopher Hansen for helpful discussions on numerical simulations. This project was funded by National Human Genome Research Institute (NHGRI) and National Institute of General Medical Sciences (NIGMS) of the National Institute of Health (NIH) under grant numbers 5R21HG009758 and R01GM138716 respectively and by the Stanford SystemX Alliance Seed Funding. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF)/Stanford Nanofabrication Facility (SNF), supported by the National Science Foundation under award ECCS-2026822.
Author information
Authors and Affiliations
Contributions
L.H., R.W.D., P.P., M.A.Z., and M.A.J. conceived the project. L.H. supervised the project. P.P. designed and fabricated the device with process inputs from M.A.Z., Y.C., and L.G. P.P. and M.A.Z designed and built the fluidic, electrical, and optical setups. P.P. designed and conducted the on-chip experiments and data analysis. M.A.J. performed chemical sample preparation and column synthesis experiments. P.P. performed the numerical simulations. M.A.Z. wrote the image processing codes to analyze bead fluorescence. M.W. helped with experimental data analysis. Y.H. performed the contact angle and interfacial tension measurements. P.P. wrote the manuscript with significant inputs from M.A.Z., M.A.J., and L.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Padhy, P., Zaman, M.A., Jensen, M.A. et al. Dielectrophoretic bead-droplet reactor for solid-phase synthesis. Nat Commun 15, 6159 (2024). https://doi.org/10.1038/s41467-024-49284-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49284-z
This article is cited by
-
Self-powered droplet manipulation for full human-droplet interaction in multiple mediums
Nature Communications (2025)