Abstract
Multi-resonance thermally activated delayed fluorophores have been actively studied for high-resolution photonic applications due to their exceptional color purity. However, these compounds encounter challenges associated with the inefficient spin-flip process, compromising device performance. Herein, we report two pure-blue emitters based on an organoboron multi-resonance core, incorporating a conformationally flexible donor, 10-phenyl-5H-phenophosphazinine 10-oxide (or sulfide). This design concept selectively modifies the orbital type of high-lying excited states to a charge transfer configuration while simultaneously providing the necessary conformational freedom to enhance the density of excited states without sacrificing color purity. We show that the different embedded phosphorus motifs (phosphine oxide/sulfide) of the donor can finely tune the electronic structure and conformational freedom, resulting in an accelerated spin-flip process through intense spin-vibronic coupling, achieving over a 20-fold increase in the reverse intersystem crossing rate compared to the parent multi-resonance emitter. Utilizing these emitters, we achieve high-performance pure-blue organic light-emitting diodes, showcasing a top-tier external quantum efficiency of 37.6% with reduced efficiency roll-offs. This proposed strategy not only challenges the conventional notion that flexible electron-donors are undesirable for constructing narrowband emitters but also offer a pathway for designing efficient narrow-spectrum blue organic light-emitting diodes.
Similar content being viewed by others
Introduction
The pursuit of organic materials capable of emitting narrow-spectrum luminescence in the three primary colors—red, green, and blue (RGB)—is critical for advancing the next generation of organic light-emitting diode (OLED) displays1,2,3. Although thermally activated delayed fluorescence (TADF) materials have garnered attention for achieving 100% exciton utilization, most exhibit broad-spectrum emission due to multiple vibrational transitions4. The emergence of boron/nitrogen (B/N)-based polycyclic multi-resonance (MR) TADF materials has sparked a paradigm shift in electroluminescence (EL) material design, aiming for narrowband emission and high luminescence efficiency5,6. However, a significant hurdle for the development of MR-TADF materials lies in their slow spin–flip process7,8,9,10,11. Most MR-TADF structures exhibit comparable locally excited (LE) features in their singlet and triplet excited states12. According to El-Sayed’s rule13, the spin–orbit coupling (SOC) matrix element between singlet and triplet states with the same type of orbital is vanishingly tiny, making this spin flipping (1LE → 3LE) in MR-TADF structures forbidden. This results in ultralow reverse intersystem crossing (RISC) rates (kRISC), ranging from 103 to 105 s−114, leading to significant triplet accumulation and detrimental exciton losses at high excitation densities.
Modifying MR cores with electron-deficient15,16 or -rich17,18,19,20 moieties is a proven approach that can markedly boost kRISC by enhancing long-range charge transfer (CT) character in excited states. Nevertheless, because of the unmanageable electron push-pull effect, these methods of CT excited-state regulation often suffer from a series of drawbacks, for instance, decreased photoluminescence quantum yields (PLQYs)21,22, redshifted emission18,23,24, deteriorative color purities induced by broadened emission bandwidth25,26, shoulder peak or tail at long-wavelength regions of emission17,27. The negative influences of CT excited state on the luminescence color purity of MR chromophores, especially the pure-blue light-emitting ones, substantially impede their functionalization and further EL performance improvement.
Recently, accelerating spin-flip via combining spin–orbit and vibronic effects, collectively termed the spin–vibronic coupling (SVC), has received much attention28. This kind of coupling requires a small energy difference between the states involved, as well as the existence of coupling vibrational modes, which would invoke quantum-mechanically forbidden RISC at non-adiabatic crossings and provide a stepwise spin–flip process with reduced activation energy barrier29. Nevertheless, there is only a small fraction of MR-TADF compounds that exhibit close-lying triplet states30. Moreover, due to the inherent rigidity of MR chromophores, the suppression of numerous vibronic modes diminishes the efficacy of the SVC process, especially when compared to twisted donor−acceptor (D−A) materials that offer greater conformational freedom31. As a result of this constraint, conventional SVC-involved spin–flip in the MR-TADF system remains sporadic and inefficient, leading to kRISC values far <105 s−1 32,33,34.
Herein, we introduce a conformationally flexible-donor-incorporation (CFDI) strategy, combining CT excited-state modulation and SVC as illustrated in Fig. 1 The CFDI strategy involves the deliberate design of conformationally flexible donors, 10-phenyl-5H-phenophosphazinine 10-oxide (NPO) and 10-phenyl-5H-phenophosphazinine 10-sulfide (NPS), which skillfully avoids S1 state involvement while utilizing electron pull-push effects and multiconformation to optimize the spin-flip process. We validate the CFDI design with two pure-blue MR-TADF emitters, BNCz-NPO and BNCz-NPS, featuring NPO and NPS as functional donors, respectively. Substituting NPO (NPS) selectively switches the triplet locally excited (3LE) character to a charge transfer one (3CT), allowing the spin–flip process. The flexibility of the donors can appropriately render certain conformational freedom that generates dense excited states to realized intense SVC-mediated RISC channels compared to reported SVC tactics. Our findings indicate that while the embedded phosphorus motifs (phosphine oxide/sulfide) finely tune the electronic structure and conformational freedom, the introduction of NPO/NPS only adds vibrational modes in the low-frequency region, thereby preserving the narrowband emission attribute. As a result, we realized a significantly improved kRISC coupled with highly efficient pure-blue luminescence with a peak at 476 nm, a small FWHM of 20 nm, and a superior PLQY approaching unity. Impressively, our champion OLEDs achieved a top-ranking external quantum efficiency (EQE) of 37.6%, representing one of the highest reported efficiencies to date in the field of blue OLEDs.
Results
Molecular design, synthesis, and characterization
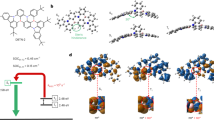
A prototypical MR framework, BNCz, was employed as the light-emitting core due to its excellent optical properties35. Despite several proposed modification strategies to enhance its performance, most BNCz derivatives emit in the blue-green spectrum, presenting a challenge to simultaneously achieving efficient RISC and pure-blue emission. Herein, we introduce an NPO/NPS unit onto the para-position of the BNCz skeleton with respect to the B atom, to yield two MR-TADF emitters, namely BNCz-NPO and BNCz-NPS, are depicted in Fig. 1 Compared to previously reported modifying building blocks for MR cores, NPO/NPS serves as a versatile excited-state modifier with distinct advantages. The NPO/NPS unit exhibits moderate-to-weak electron-donating ability due to the negative inductive effect of the embedded P = O/P = S (refer to Supplementary Fig. 1). It is anticipated that the mild CT formed between NPO/NPS and the MR core will compete with the intrinsic short-range CT of the MR structure, leading to blue-shifted emission36. In addition, The C-N linking style between BNCz and NPO/NPS is designed to minimize undesired π-bonding features, restricting conjugation extension and bond stretching37, thereby facilitating narrow-spectrum blue light emission. Moreover, by leveraging the thermal pyramidal inversion behaviors commonly observed in arylphosphines38, the incorporation of NPO/NPS is expected to enhance the degree of conformational freedom39. The larger atomic radius of phosphorus compared to sulfur hints that the NPO and NPS units exhibit multiple metastable conformers similar to phenothiazine40, further promoting dense excited state alignment and proper vibration modes. Under these circumstances, a significantly improved SVC-mediated RISC process can be realized, given the small energy differences with coupled vibrational modes in such a dense excited-state system with both LE and CT natures.
The synthetic routes to BNCz-NPO and BNCz-NPS are shown in Supplementary Fig. 2. The double Br/Li exchange of Boc-protected bis(2-bromophenyl)amine formed a bis-lithiated species and was then treated with dichloro(phenyl)phosphane to afford dihydrophenophosphanizine. Without further purification, the dihydrophenophosphanizine intermediate was oxidized by H2O2 or S8 before Boc detachment by trifluoroacetic acid to yield NPO or NPS, respectively. Finally, BNCz-NPO and BNCz-NPS were synthesized using Hartwig-Buchwald C–N coupling reactions of a brominated BNCz derivative with NPO and NPS, respectively. The target materials were purified by column chromatography and temperature-gradient vacuum sublimation and were characterized by 1H/13C/31P NMR, high-resolution mass spectroscopies, and X-ray crystallographic analyses. According to the cyclic voltammetry results (Supplementary Fig. 3), the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels were calculated to be –5.6 and –3.1 eV for BNCz-NPO and –5.6 and –3.0 eV for BNCz-NPS, respectively. BNCz-NPO and BNCz-NPS demonstrate high decomposition temperatures (5% weight loss) of 503 and 463 °C (Supplementary Fig. 4), respectively, without glass transition points (scanning room temperature to 350 °C), thermally stable for the fabrication of vacuum-deposited OLEDs.
Crystallographic analysis
The influences of NPO/NPS substituent on molecular conformation and intermolecular interaction are displayed in single-crystal structures. We obtained two distinct types of single crystals for BNCz-NPO and BNCz-NPS, designated as BNCz-NPO-α/BNCz-NPO-β and BNCz-NPS-α/BNCz-NPS-β, by employing an anti-solvent vapor diffusion method with various solvent systems. The selected crystallographic information are summarized in Supplementary Tables 1 and 2. The polymorphism of BNCz-NPO and BNCz-NPS confirms their conformational flexibility under ambient conditions. As shown in Fig. 2, the N–P–C angles are 116.80°, 120.01°, 115.60° and 124.97° for BNCz-NPO-α, BNCz-NPO-β, BNCz-NPS-α, and BNCz-NPS-β, respectively. Notably, substantial differences exist in the dihedral angles between the C1–N–C4 and the C2–P–C3 planes (θ). For instance, BNCz-NPO-β exhibits a larger θ of 11.70° when compared with BNCz-NPO-α (0.92°). As sulfur is more sizable than oxygen, BNCz-NPS is expected to exhibit greater conformational variation than BNCz-NPO. Compared to BNCz-NPO, BNCz-NPS shows larger distortion with θ of 3.58° and 22.37° for its α and β type crystals, respectively.
The existence of diverse conformers could help to promote multiple excited states and enrich vibration modes in accordance with our design principle. Equally important, unlike phenothiazine derivatives showing a distorted boat-chair conformation40, the more crowded configuration of phosphine oxide/sulfide imparts a nearly coplanar geometry to the heterocycle skeleton of NPO (NPS). This characteristic may suppress high-amplitude structural deformation and vibration, mitigating potential spectral broadening. Moreover, the bulky NPO/NPS, with significant steric hindrance, induces a nearly orthogonal conformation with an MR-NPO/NPS dihedral angle exceeding 80°, effectively suppressing strong interchromophore interactions (Supplementary Fig. 5). We employed Hirshfeld surface analysis to scrutinize the packing mode in single crystals of BNCz-NPO and BNCz-NPS (Supplementary Fig. 6a). The analysis revealed sparser contact densities and larger contact distances compared to BNCz, indicating weaker intermolecular interactions and reduced interchromophore contacts.
Theoretical investigation
Theoretical calculations based on density functional theory (DFT) have been conducted to elucidate the electronic structures. Geometry optimizations in the ground states were adapted from crystallographic data. As illustrated in Supplementary Fig. 7, the LUMOs of BNCz-NPO and BNCz-NPS closely resemble that of BNCz, exhibiting a regular distribution in accordance with the MR effect. However, their HOMO distributions exhibit variations between cases. The HOMOs of BNCz-NPO-α, BNCz-NPO-β, and BNCz-NPS-β align with BNCz’s HOMO distribution, while that of BNCz-NPS-α delocalizes to the NPS unit. Despite its minimal impact on HOMOs, the electron-donating effect of NPO/NPS causes the HOMO-1 to shift from the MR core to the NPO/NPS units. This underscores the notable influence of conformational changes on electronic configuration imparted by the CFDI strategy.
Figure 3a depicts the flexible potential energy curve scan of BNCz-NPO and BNCz-NPS at the ground state. It is found that a shallow potential well, with an energy difference lower than thermal energy at room temperature (kBT ≈ 0.026 eV, kB: Boltzmann constant, T: temperature), exists in a wide range of θ from −40° to 47° for BNCz-NPO. This range becomes larger from −45° to 50° for BNCz-NPS with higher conformational flexibility. Additionally, we found that the α conformer of BNCz-NPO (BNCz-NPS) exhibits a minimal potential energy difference compared to the β conformer, resulting in a nearly equal Boltzmann distribution ratio between them at room temperature (Fig. 3b). This helps elucidate the observed polymorphism of BNCz-NPO and BNCz-NPS.
Excited state calculations were performed using the spin-component scaling second-order approximate coupled-cluster (SCS-CC2) method with the cc-pVDZ basis set30. Supplementary Fig. 8 illustrates a comparatively low ΔEST value of ~0.12 eV estimated in vacuum for the compounds, similar to that of BNCz. Natural transition orbital (NTO) analyses were conducted to gain insights into the excited-state nature. Consistent with BNCz (Supplementary Fig. 9), the S1 and T1 states of BNCz-NPO and BNCz-NPS predominantly localize on their MR core (Fig. 3c, d), indicating LE characteristics (1LE and 3LE). In contrast, some higher-lying excited states, such as S2, T2, and T4, of BNCz-NPO and BNCz-NPS exhibit long-range CT or hybridized local and charge-transfer (HLCT) features, while those of BNCz remain dominated by LE. These CT characteristics are quantified by larger values of the distance of charge transfer (DCT) and the amount of charge transferred (qCT)41, as shown in Supplementary Table 3. Due to the involvement of CT excited states distinct from the original LE-typed orbital feature of the MR core, both BNCz-NPO and BNCz-NPS display significantly larger SOC matrix elements than BNCz (Supplementary Fig. 10). Notably, BNCz-NPS exhibits larger DCT and qCT values than BNCz-NPO, resulting in larger SOC matrix elements than BNCz-NPO. These SOC values notably surpass those of most MR-type TADF emitters42.
Excited states with a CT nature are typically more sensitive to the polarity of the environment compared to those with an LE nature. Thus, while BNCz-NPO and BNCz-NPS exhibit comparable excited state energy levels to BNCz due to the weak electron-donating nature of NPO/NPS, their S2, T2, and T4 excited states become more stabilized than those of BNCz in polar CH2Cl2 (Supplementary Fig. 8). Additionally, due to subtle differences in excited-state energies among different conformers, BNCz-NPO and BNCz-NPS actually have a much richer landscape of excited states. Furthermore, the conformationally flexible NPO/NPS facilitates appropriate vibrational overlap between the nearly degenerate excited states, promoting reverse internal conversion. In this scenario, although the spatial orbital occupation (LE) of the S1 and T1 excitations results in vanishing direct SOC, the close-lying upper CT (or HLCT) excited states of BNCz-NPO and BNCz-NPS provide a feasible channel for efficient RISC, wherein higher-order SOC involving 3LE (or 3CT) and 1CT (or 1LE) in thermal equilibrium plays a crucial role43.
Photophysical properties
The absorption and photoluminescence (PL) spectra of BNCz-NPO and BNCz-NPS in 10−5 M toluene are shown in Fig. 4a. BNCz-NPO and BNCz-NPS exhibit intense short-range CT absorption bands peaking at ca. 462 nm. Compared to the case of BNCz (λabs = 468 nm), hypochromatic shifts in absorption were observed. Following this trend, BNCz-NPO and BNCz-NPS display shorter-wavelength blue emissions with a peak at 476 and 475 nm, respectively (BNCz: λem = 483 nm), resulting from the reduced short-range CT characteristic. The PLQYs of BNCz-NPO and BNCz-NPS in dilute toluene solution are as high as 98.3% and 91.8%, respectively, which are comparable to that of BNCz (93.2%). It is worth noting that the PL spectra of the MR-TADF emitters are unstructured, with a very narrow FWHM of 20 and 21 nm for BNCz-NPO and BNCz-NPS, respectively. Such small FWHM values were roughly the same as the BNCz MR-core (23 nm) without spectral shoulders, reflecting that the NPO/NPS subunit does not add detrimental vibrations. Polarity-sensitive long-range CT character involvement in the S1 state often impairs emission color purity18. Negligible solvatochromism was observed in absorption (Supplementary Fig. 11) and PL spectra (Supplementary Fig. 12), implying weak long-range CT characters in the ground and S1 states. The emission peak redshifts from nonpolar n-hexane to polar acetonitrile are 17, 13, and 13 nm for BNCz, BNCz-NPO, and BNCz-NPS, respectively (Supplementary Table 4), indicating reduced short-range CT character upon NPO/NPS modification, which is in good accordance with the theoretical results.
a Absorption and PL spectra of BNCz, BNCz-NPO, and BNCz-NPS in toluene (1 × 10–5 M) at room temperature. b transient PL decay curves of BNCz, BNCz-NPO, and BNCz-NPS in oxygen-free toluene solution using a variable pulsed laser (λex = 375 nm). c Comparison between the calculated rate constants of BNCz, BNCz-NPO, and BNCz-NPS. d Reorganization energy versus the normal mode wavenumbers of BNCz, BNCz-NPO, and BNCz-NPS (insert: pie charts illustrating the contribution to the total reorganization energy from the bond length, bond angle, and dihedral angle). e Calculated Huang–Rhys factors with values > 0.02 from S1 to S0 transition for BNCz, BNCz-NPO, and BNCz-NPS (inset: selected vibration modes with the most significant contribution to the Huang–Rhys factor).
From the fluorescence and phosphorescence spectra in toluene measured at 77 K (Supplementary Fig. 13), the S1 and T1 energy levels are estimated to be 2.67/2.52 and 2.64/2.50 eV for BNCz-NPO and BNCz-NPS, respectively. Subsequently, the ΔESTs of BNCz-NPO and BNCz-NPS are determined to be 0.15 and 0.14 eV, respectively, which align well with the calculation results. These values are sufficiently small to support the exciton upconversion from the T1 to the S1 state, indicative of TADF. PL decays of BNCz, BNCz-NPO and BNCz-NPS consist of ns-scale prompt fluorescence (Supplementary Fig. 14) and μs-scale delayed fluorescence components (Fig. 4b). The corresponding prompt (τPF) and delayed (τDF) lifetimes were fitted to be 4.8 ns/13.9 μs for BNCz-NPO and 4.7 ns/9.7 μs for BNCz-NPS, respectively. In contrast, BNCz demonstrated a significantly longer τDF of 66.8 μs, indicating a much more efficient RISC process in the presence of a CFDI strategy. Temperature-dependent transient PL measurements (Supplementary Fig. 15) unambiguously confirm the involvement of triplet excitons in light emission through an endothermic RISC process. It is noteworthy that BNCz-NPO and BNCz-NPS exhibit efficient TADF in solution states without the aid of host materials, distinguishing them from most reported MR chromophores with LE-featured singlet and triplet excited states5,12,44. The photophysical results demonstrate that the CFDI strategy not only prevents the involvement of NPO/NPS in the S1 states to retain the excellent photophysical properties of the MR core but also simultaneously induces a mild electron push-pull effect to accurately modulate long-range CT features of the high-lying singlet and triplet excited states for an allowed RISC process. The fluorescence radiative decay rate constants (kF) of BNCz-NPO and BNCz-NPS are notably high at 6.5 × 107 and 5.3 × 107 s–1, respectively (Fig. 4c, Table 1). These values exceed the corresponding knr values (1.1 × 106 and 4.8 × 106 s–1), indicating negligible energy loss during the S1 → S0 transition controlled by the rigid MR core. Importantly, compared to the small kRISC of the prototypical BNCz (1.8 × 104 s–1), those of BNCz-NPO and BNCz-NPS are increased by 12.2 and 20.5 folds, reaching 2.2 × 105 and 3.7 × 105 s–1, respectively.
Further investigation of the photophysical properties in doped films (3 wt% in 9-(2-(9-phenyl-9H-carbazol-3-yl) phenyl)9H−3,9′-bicarbazole, PhCzBCz) is detailed in Supplementary Fig. 16 and Table 1. The BNCz-NPO and BNCz-NPS films exhibit pure-blue emission, peaking at 478 and 481 nm, respectively. The FWHMs are slightly broadened to 26 nm. The doped films do not show broadband emission from excimer or exciplex. The PLQYs of the films are as high as 95.5% and 92.0% for BNCz-NPO and BNCz-NPS, respectively. The τPFs of BNCz-NPO and BNCz-NPS are 2.8 and 3.0 ns, while their τDFs are 28.5 and 21.9 μs, respectively. The kRISCs of BNCz-NPO and BNCz-NPS in the doped film state were estimated to be 1.6 × 105 and 2.7 × 105 s−1, respectively. Although these values are slightly lower than those measured in solution due to the reduced effectiveness of the SVC mechanism in the rigid solid state, they still surpass the kRISC values of conventional MR-TADF emitters, which typically fall below 105 s−1.
The investigation of photophysical properties for our emitters extended to various doping levels (Supplementary Fig. 17). The findings revealed that BNCz-NPO and BNCz-NPS displayed reduced excimer emission and were less susceptible to concentration-induced emission quenching compared to BNCz. Additionally, the lifetimes of BNCz-NPO and BNCz-NPS exhibited slower decreasing trends with increasing doping concentrations, indicative of their superior solid-state luminescence properties. Furthermore, we investigated the PL behaviors in THF/water mixtures with varying water fractions to highlight the capability to mitigate aggregation-caused quenching (ACQ), as shown in Supplementary Fig. 18. The results showed that while BNCz exhibited typical ACQ behavior with increasing water fractions, BNCz-NPO and BNCz-NPS maintained stable emission intensities and FWHM values, indicating noteworthy anti-quenching characteristics imparted by the NPO/NPS functionalization. These observations are consistent with the insights gleaned from crystallographic and computational analyses.
It is widely recognized that introducing flexible units in a conjugated emitter typically results in pronounced structural deformation during electronic transitions, leading to spectral broadening and reduced luminescence efficiency45. Therefore, it is intriguing to observe that incorporating the flexible NPS/NPS moiety into the MR chromophore manages to preserve the merits of narrowband emission and a high PLQY. Structural changes during the S1 → S0 transitions were evaluated using root-mean-square deviations (RMSDs). The RMSDs for BNCz-NPO and BNCz-NPS were calculated to be 0.093 and 0.139 Å, respectively, compared to 0.084 Å for BNCz (Supplementary Fig. 19). As anticipated, the larger RMSD values originate from the fluctuation of the flexible NPO and NPS groups at the peripheral terminal. Quantitative analysis of the intramolecular motions of BNCz, BNCz-NPO, and BNCz-NPS has been conducted through reorganization energy calculation (Fig. 4d). Surprisingly, BNCz-NPO and BNCz-NPS exhibit remarkably small total reorganization energies of 425.5 and 441.7 cm–1, respectively, even surpassing the value for BNCz without NPO/NPS (559.5 cm–1). This indicates that the CFDI strategy may increase the number of vibration modes, but the effect on the total reorganization energies is limited since the triphenylphosphine backbone of NPO/NPS is immobilized by a C–N–C locking and strong intramolecular motions of Ph-P = O(S) units (refer also to Supplementary Fig. 19). The flexibility introduced by the NPO/NPS modification increases the share of low-frequency modes related to bond angle changes from 37.8% (BNCz) to 44.8%/43.7%. In contrast, high-frequency modes associated with changes in bond length, which are closely linked to spectral broadening and the appearance of shoulder peaks, are effectively suppressed.
The emission spectra of BNCz-NPO and BNCz-NPS were simulated by the Frank-Condon analysis for the S1 → S0 transition, and Huang–Rhys factors (S) of the vibrational modes were calculated to elucidate the spectral progression. The simulated emission wavelengths and profiles are in accordance with the experimental results (Supplementary Fig. 20). The principal vibrational modes of BNCz-NPO and BNCz-NPS are identified at frequencies of 11.49 and 3.38 cm−1, respectively, primarily arising from the twisting and rotation vibrations of the NPO and NPS peripheral units. A more detailed inspection might even suggest that the additional vibrations introduced by NPO/NPS are predominantly located in the low-frequency region with wavenumbers less than 250 cm–1, while the high-frequency vibrational modes are limited (refer to Supplementary Fig. 21 and Supplementary Table 5). The restrained high-frequency stretching vibrations, coupled with the structural reorganization between S0 and S1, contribute to the preservation of small overall reorganization energies responsible for the ultrasmall FWHM values. It has been emphasized that concurrently enhancing low-frequency vibrations and reducing high-frequency vibrations is pivotal for achieving narrow-spectrum emission in organic emitters45. The experimental and calculation results collectively illustrate that the CFDI strategy can not only enrich the excited states and vibration modes for realizing efficient SVC-mediated RISC but also retain highly efficient narrow-spectrum emissions.
Electroluminescence properties
To evaluate EL properties of the proposed emitters, a set of OLEDs were first prepared with a structure of indium tin oxide (ITO)/dipyrazino[2,3-f:2′,3′-h] quinoxaline-2,3,6,7,10,11-hexacarbonitrile (HATCN, 5 nm)/4,4’-cyclohexylidenebis[N,N-bis(p-tolyl)aniline] (TAPC, 50 nm)/ 4,4’,4”-tris(carbazol-9-yi)triphenylamine (TCTA, 5 nm)/1,3-bis(carbazol-9-yl)benzene (mCP, 5 nm)/ 1-5 wt% BNCz-NPO or BNCz-NPS: PhCzBCz (EML, 20 nm)/2,8-bis(diphenyl-phosphoryl)-dibenzo[b,d]furan (PPF, 5 nm)/1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene (TmPyPB, 30 nm)/LiF (1 nm)/Al (120 nm), as displayed in Fig. 5a. The EL performances are depicted in Fig. 5, and relevant key parameters are summarized in Table 2. The 1 wt% doped OLEDs based on BNCz-NPO and BNCz-NPS, respectively, exhibit pure-blue emission at 478 and 474 nm with an FWHM of ~26 nm (Fig. 5b), and the corresponding Commission Internationale de I’Éclairage (CIE) coordinates are (0.11, 0.17) and (0.12, 0.14). This human-friendly blue light, with minimal photon energy in wavelengths <455 nm, aligns with the Bio-Blue display concept proposed by Samsung46, reducing the risk of retinal damage (blue light hazard)47.
All devices exhibited an onset voltage of ~3.2 V (Von at 1 cd m–2), indicative of efficient carrier injection and transport (Fig. 5c). A marginal redshift in the emission maxima was observed with an increase in doping concentration, while the FWHM remained nearly unchanged, owing to the bulky orthogonal molecular geometries of BNCz-NPO and BNCz-NPS. Both emitters achieved their optimal EL performance at a 3 wt% doping concentration. The EQEmax of the BNCz-NPO-based device is as high as 32.1%, while that of the BNCz-NPS-based device is 29.6% (Fig. 5d). The EQEmax of 32.1% is among the highest values for all non-sensitized blue MR-TADF OLEDs48,49,50. More importantly, these OLEDs exhibit much-reduced efficiency roll-offs under high exciton density. The EQEs of BNCz-NPO and BNCz-NPS maintain a high level of 21.7% and 23.5% (EQE100) at a display relevant luminance of 100 cd m–2, over 12.9% of BNCz without functionalization51. The alleviated efficiency roll-offs in our devices can be attributed to the enhanced RISC of BNCz-NPO and BNCz-NPS, effectively mitigating triplet-involved exciton quenching. Supplementary Fig. 22 illustrates the EL stabilities of BNCz-NPO and BNCz-NPS. We observed that the BNCz-NPO-based OLED exhibits a significantly prolonged T50 (defined as the time when the brightness diminishes to half of its initial value), defined as the time when the luminance diminishes to half of its initial value, surpassing three-fold that of the BNCz-NPS-based device (15.2 h compared to 3.0 h). We speculate that the lower EL stability of BNCz-NPS could be attributed to the weaker bonding of the P = S group in its molecular structure.
Hyperfluorescence (HF) OLEDs using 2,3,4,5,6-pentakis-(3,6-di-tert-butyl-9H-carbazol-9-yl) benzonitrile (5TCzBN) as a TADF sensitizer in EML were fabricated to further optimize the EL performances52. Great overlap between the PL of 5TCzBN and absorption of BNCz-NPO/BNCz-NPS guarantees efficient Förster energy transfer (Supplementary Fig. 23). The OLEDs were constructed with the configuration of ITO/HATCN (5 nm)/TAPC (50 nm)/TCTA (5 nm)/mCP (5 nm)/3 wt% BNCz-NPO or BNCz-NPS: 10–20 wt% 5TCzBN: PhCzBCz (20 nm)/PPF (5 nm)/TmPyPB (30 nm)/LiF (1 nm)/Al (120 nm) (HF-I type). The EL characteristics are depicted in Fig. 6a and Supplementary Fig. 24, with key parameters summarized in Table 2 and Supplementary Table 6. The optimal concentration of 5TCzBN is 10 wt%. Compared with the non-sensitized devices, the efficiency roll-offs of the devices are significantly suppressed. Impressively, the EQE retained as high as 31.6% and 26.4% at high luminance of 100 and 1000 cd cm–2, respectively, for BNCz-NPS (Fig. 6a). Though the efficiency roll-offs are significantly suppressed, the improvement of maximum EQE in HF-I OLEDs are not obvious. Thus, further optimization was performed using an interlayer sensitization structure53 with a more efficient TADF sensitizer, 9-(5′-(4,6-diphenyl-1,3,5-triazin-2-yl) [1,1′:3′,1′′-terphenyl]−2′-yl)−3,6-diphenyl-9H-carbazole (PPCz-Trz)54. Another set of HF OLEDs was fabricated with a device configuration of ITO/HATCN (5 nm)/TAPC (50 nm)/TCTA (5 nm)/3 wt% BNCz-NPO (BNCz-NPS): PhCzBCz (10 nm)/10 wt% PPCz-TRZ: PPF (2 nm)/PPF (5 nm)/TmPyPB (30 nm)/LiF (1 nm)/Al (120 nm) (HF-II). The device structure and characteristics are shown in Supplementary Fig. 25. In HF-II OLEDs, the PPCz-Trz sensitizer was doped into PPF with high polarity to harvest exciton energy more efficiently, thereby enhancing sensitizing efficiency through a long-range Förster energy transfer process55. As shown in Fig. 6b and Table 2, an ultrahigh EQEmax (EQE100) was achieved with a value of 37.6% (30.2%) for the BNCz-NPO-based HF-II device with the corresponding CIE coordinates of (0.11, 0.18) and with a narrow FWHM of 26 nm.
EQE-luminance characteristics of a HF-I and b HF-II OLEDs. c EQEmax comparison in terms of CIEy value to reported blue MR-TADF OLEDs with FWHM < 40 nm; circle and triangle represent the EL data for mono- and multiple-boron emitters, respectively (references for the plotted data are given in Supplementary Table 7).
The EL performances of representative narrow-spectrum blue MR-TADF emitters are summarized in Fig. 6c and Supplementary Table 7. Notably, the highest efficiencies of narrow-spectrum blue OLEDs are based on multiple-boron MR-TADF motifs that often need intricate synthetic procedures, and only a small fraction of MR-TADF emitters meet the requirements of pure-blue narrow-spectrum emission (CIEy < 0.25, FWHM < 40 nm) and high efficiency (EQE > 30%) at the same time. The molecular design presented here, involving a simple conformationally flexible donor modulation on a mono-boron MR core, allows our MR-TADF emitters to fulfill these demands, enabling the development of robust pure-blue OLEDs. Moreover, BNCz-NPO OLEDs achieve record-setting efficiencies among mono-boron MR-TADF OLEDs and prove competitive with the most efficient multiple-boron counterparts56,57.
Discussion
In summary, this study introduces two blue MR-TADF materials, BNCz-NPO and BNCz-NPS, meticulously designed through the CFDI strategy. Through extensive photophysical and theoretical analyses, we have revealed that the incorporation of the medium-to-weak electron-donating NPO (NPS) unit into the MR core selectively tunes the high-lying 3LE states to 3CT ones, enabling allowed RISC, while the narrow-spectrum emissive S1 → S0 transition can be perfectly retained. Crucially, this strategy imparts appropriate conformational freedom to the MR-TADF molecules, enriching dense excited states and their vibrational coupling, thereby facilitating RISC via the SVC mechanism. As a result, BNCz-NPO and BNCz-NPS exhibit significantly improved RISC, with kRISC values of 2.2 × 105 and 3.7 × 105 s–1, respectively, compared to the NPO/NPS-free parent molecule. A device based on BNCz-NPO demonstrates highly efficient pure-blue emission, peaking at 476 nm with a narrow FWHM of 20 nm and a PLQY approaching unity. The pure-blue OLED based on BNCz-NPO achieves a top-ranking EQE of 37.6%, coupled with a reduced efficiency roll-off.
This work underscores the efficacy of the CFDI strategy in simultaneously preserving narrow-spectrum characteristics and improving RISC rates for MR-TADF emitters. Typically, flexible building blocks are avoided in constructing narrowband emitters due to the potential for inducing violent structural deformation and spectral broadening. The proposed CFDI strategy challenges this conventional notion, opening an avenue toward the realization of high-performance, narrow-spectrum blue OLEDs. Additionally, the CFDI strategy may offer a viable approach to enhancing the film-forming properties of rigid, planar MR-TADF emitters, extending their application in large-area solution-processed manufacturing.
Methods
Characterization of the MR-TADF compounds
1H, 13C, 31P NMR measurements were recorded with a Bruker AVANCE III HD-400 NMR spectrometer with chemical shifts reported relative to tetramethylsilane (δ = 0 ppm). High-resolution mass spectra (HRMS) were recorded by the Electrospray Ionization (ESI) method with a Thermo Scientific Q Exactive instrument.
X-ray crystallography
Single crystals were grown in CH2Cl2/n-hexane or CHCl3/MeOH mixtures, and the crystallographic data were collected at 100, 150, or 170 K on a Rigaku Oxford Diffraction Supernova Dual Source diffractometer equipped with an AtlasS2 CCD using Cu Kα radiation. Data reduction was carried out with the diffractometer’s software. The structures were solved by direct methods using Olex2 software, and the non-hydrogen atoms were located from the trial structure and then refined anisotropically with SHELXL-2014 using a full-matrix least-squares procedure based on F2. The weighted R factor, wR and goodness-of-fit S values were obtained based on F2. The hydrogen atom positions were fixed geometrically at the calculated distances and allowed to ride on their parent atoms.
Thermal properties
A STA409PC Thermogravimetric Analyzer and a NETZSCH thermal analyzer (DSC 204 F1) were used to measure the decomposition temperature (5% weight loss, Td) and glass transition temperature (Tg), respectively. The tests were performed at a heating rate of 10 °C min−1 under an N2 atmosphere.
Photophysical measurements
The UV-vis absorption spectra were recorded by Shimadzu UV-2700 UV–vis spectrophotometer equipped with a xenon flash lamp. Steady-state and time-resolved PL spectra were recorded with an Edinburgh FLS980 spectrophotometer. Temperature-dependent measurements were conducted within an Optistat DN optical cryostate (Oxford Instruments). The prompt and delayed transient PL decay profiles were performed using a picosecond pulsed diode laser (EPL-375) and a variable pulse length diode laser (VPL-375) as excitation sources, respectively. Absolute PLQYs were measured with an integrating sphere incorporated into the FLS980 spectrofluorometer.
Cyclic voltammetry measurements and HOMO/LUMO determination
Cyclic voltammetry (CV) was recorded in acetonitrile of tetra-n-butylammoniumhexafluorophosphate (Bu4NPF6) (0.1 M) with a scan rate of 100 mV s−1 in an Autolab PGSTAT302N electrochemical workstation using a three-electrode cell (Ag/AgNO3 reference electrode, Pt wire counter electrode, and glassy carbon working electrode). Fc/Fc+ (0.18 eV against Ag/AgNO3) was used as an external standard for calibrating the reference electrode. The energy level of HOMO and LUMO were calculated according to the equation of [HOMO = −(Eonset + 4.8) eV] and [LUMO = (HOMO + Eg) eV], where Eonset is the onset oxidation potentials, and Eg is the optical bandgap obtained from the absorption onset.
OLED fabrication and measurements
The glass substrates precoated with a 90-nm layer of ITO with a sheet resistance of 15–20 Ω per square was successively cleaned in an ultrasonic bath of acetone, isopropanol, detergent, and deionized water, respectively, taking 10 min for each step. Then, the substrates were dried in a 70 °C oven. Before the fabrication processes, to improve the hole injection ability of ITO, the substrates were treated with O2 plasma for 10 min. The vacuum-deposited OLEDs were fabricated under a pressure of <5 × 10‒4 Pa in the Suzhou Fangsheng OMV-FS380 vacuum deposition system. Organic materials, LiF and Al were deposited at rates of 1–2, 0.1, and 5 A s‒1, respectively. The effective emitting area of the devices was 9 mm2, determined by the overlap between the anode and cathode. The luminance–voltage–current density and external quantum efficiency were characterized with a dual-channel Keithley 2614B source meter and a PIN-25D silicon photodiode. The EL spectra were obtained via an Ocean Optics USB 2000+ spectrometer with a Keithley 2614B source meter. All the characterizations were conducted at room temperature in ambient conditions without any encapsulation, as soon as the devices were fabricated.
Data availability
All the data and methods are present in the main text and the supplementary materials. The X-ray crystallographic data for structures reported in this study have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 2253324 (BNCz-NPO-α), 2282192 (BNCz-NPO-β), 2285218 (BNCz-NPS-α) and 2247937 (BNCz-NPS-β). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Mischok, A., Hillebrandt, S., Kwon, S. & Gather, M. C. Highly efficient polaritonic light-emitting diodes with angle-independent narrowband emission. Nat. Photon. 17, 393–400 (2023).
Joo, W.-J. et al. Metasurface-driven OLED displays beyond 10,000 pixels per inch. Science 370, 459–463 (2020).
Liu, J. et al. Toward a BT.2020 green emitter through a combined multiple resonance effect and multi-lock strategy. Nat. Commun. 13, 4876 (2022).
Valeur, B. & Berberan-Santos, M. N. Molecular Fluorescence: Principles and Applications (John Wiley & Sons, 2012).
Hatakeyama, T. et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 28, 2777–2781 (2016).
Kondo, Y. et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photon. 13, 678–682 (2019).
Hu, Y. X. et al. Efficient selenium-integrated TADF OLEDs with reduced roll-off. Nat. Photon. 16, 803–810 (2022).
Wang, Q. et al. Precise regulation of emission maxima and construction of highly efficient electroluminescent materials with high color purity. Angew. Chem. Int. Ed. 62, e202301930 (2023).
Wang, X. et al. Mesityl-functionalized multi-resonance organoboron delayed fluorescent frameworks with wide-range color tunability for narrowband OLEDs. Angew. Chem. Int. Ed. 61, e202206916 (2022).
Madayanad Suresh, S. et al. A deep-blue-emitting heteroatom-doped MR-TADF nonacene for high-performance organic light-emitting diodes. Angew. Chem. Int. Ed. 62, e202215522 (2023).
Chen, G. et al. Triphenylamine-functionalized multiple-resonance TADF emitters with accelerated reverse intersystem crossing and aggregation-induced emission enhancement for narrowband OLEDs. Adv. Funct. Mater. 33, 2211893 (2023).
Wu, X. et al. The role of host–guest interactions in organic emitters employing MR-TADF. Nat. Photon. 15, 780–786 (2021).
El-Sayed, M. A. Spin–orbit coupling and the radiationless processes in nitrogen heterocyclics. J. Chem. Phys. 38, 2834–2838 (1963).
Luo, X.-F., Xiao, X. & Zheng, Y.-X. Recent progress in multi-resonance thermally activated delayed fluorescence emitters with an efficient reverse intersystem crossing process. Chem. Commun. 60, 1089–1099 (2024).
Wang, H. et al. A multiple resonance emitter integrating Para-B-π-B′/Meta-N-π-N pattern via an unembedded organoboron decoration for both high-efficiency solution- and vacuum-processed OLEDs. Adv. Funct. Mater. 33, 2306394 (2023).
Xu, Y. et al. Highly efficient electroluminescent materials with high color purity based on strong acceptor attachment onto B–N-containing multiple resonance frameworks. CCS Chem. 4, 2065–2079 (2021).
Huang, Z. et al. Charge transfer excited state promoted multiple resonance delayed fluorescence emitter for high-performance narrowband electroluminescence. J. Am. Chem. Soc. 145, 12550–12560 (2023).
He, Y.-H. et al. Red-shift emission and rapid up-conversion of B,N-containing electroluminescent materials via tuning intramolecular charge transfer. Mater. Chem. Front. 7, 2454–2463 (2023).
Wang, Q., Xu, Y., Yang, T., Xue, J. & Wang, Y. Precise functionalization of a multiple‐resonance framework: constructing narrowband organic electroluminescent materials with external quantum efficiency over 40%. Adv. Mater. 35, 2205166 (2023).
Jin, J.-M. et al. Synergetic modulation of steric hindrance and excited state for anti-quenching and fast spin-flip multi-resonance thermally activated delayed fluorophore. Angew. Chem. Int. Ed. 63, e202401120 (2024).
Song, X. et al. Efficient narrowband organic light-emitting devices based on multi-resonance TADF emitters with secondary donor. Chem. Eng. J. 467, 143557 (2023).
Fan, X. et al. Managing intersegmental charge‐transfer and multiple resonance alignments of D3‐A typed TADF emitters for red OLEDs with improved efficiency and color purity. Adv. Opt. Mater. 10, 2101789 (2021).
He, Y.-H. et al. Acceptor–donor–acceptor-cond delayed fluorescence emitters for efficient orange-red and white devices with low roll-off. Adv. Funct. Mater. 33, 2304006 (2023).
Liu, Y. et al. Space-confined donor–acceptor strategy enables fast spin–flip of multiple resonance emitters for suppressing efficiency roll-off. Angew. Chem. Int. Ed. 61, e202210210 (2022).
Luo, S. et al. Regulation of multiple resonance delayed fluorescence via through-space charge transfer excited state towards high-efficiency and stable narrowband electroluminescence. Angew. Chem. Int. Ed. 62, e202310943 (2023).
Wu, Q. et al. Dual emission from donor-modified MR-TADF emitter: evidence for coexistence of TICT and MR excited states. Dyes Pigm. 217, 111421 (2023).
Huang, X. et al. Donor-modified multiple resonance emitters with accelerated reverse intersystem crossing towards high-efficiency and narrowband deep-blue OLEDs. J. Mater. Chem. C. 11, 11885–11894 (2023).
Etherington, M. K., Gibson, J., Higginbotham, H. F., Penfold, T. J. & Monkman, A. P. Revealing the spin–vibronic coupling mechanism of thermally activated delayed fluorescence. Nat. Commun. 7, 13680 (2016).
Gibson, J., Monkman, A. P. & Penfold, T. J. The importance of vibronic coupling for efficient reverse intersystem crossing in thermally activated delayed fluorescence molecules. ChemPhysChem 17, 2956–2961 (2016).
Hall, D. et al. Modeling of multiresonant thermally activated delayed fluorescence emitters─properly accounting for electron correlation is key! J. Chem. Theory Comput. 18, 4903–4918 (2022).
Northey, T. & Penfold, T. J. The intersystem crossing mechanism of an ultrapure blue organoboron emitter. Org. Electron. 59, 45–48 (2018).
Patil, V. V. et al. Purely spin‐vibronic coupling assisted triplet to singlet up‐conversion for real deep blue organic light‐emitting diodes with over 20% efficiency and y color coordinate of 0.05. Adv. Sci. 8, 2101137 (2021).
Lee, H. L. et al. Multiple-resonance extension and spin-vibronic-coupling-based narrowband blue organic fluorescence emitters with over 30% quantum efficiency. Adv. Mater. 34, 2202464 (2022).
Kang, J. et al. Expanded multiple-resonance structure for highly efficient narrowband deep-blue organic light-emitting diodes. Mater. Today 69, 88–96 (2023).
Xu, Y. et al. Molecular-structure and device-configuration optimizations toward highly efficient green electroluminescence with narrowband emission and high color purity. Adv. Opt. Mater. 8, 1902142 (2020).
Fan, T., Zhang, Y., Zhang, D. & Duan, L. Decoration strategy in para boron position: an effective way to achieve ideal multi‐resonance emitters. Chem. Eur. J. 28, e202104624 (2022).
Pei, Z. et al. Elucidating the electronic structure of a delayed fluorescence emitter via orbital interactions, excitation energy components, charge-transfer numbers, and vibrational reorganization energies. J. Phys. Chem. Lett. 12, 2712–2720 (2021).
Machida, T., Iwasa, T., Taketsugu, T., Sada, K. & Kokado, K. Photoinduced pyramidal inversion behavior of phosphanes involved with aggregation-induced emission behavior. Chem. Eur. J. 26, 8028–8034 (2020).
Takeda, Y. et al. Conformationally-flexible and moderately electron-donating units-installed D–A–D triad enabling multicolor-changing mechanochromic luminescence, TADF and room-temperature phosphorescence. Chem. Commun. 54, 6847–6850 (2018).
Okazaki, M. et al. Thermally activated delayed fluorescent phenothiazine–dibenzo[a,j]phenazine–phenothiazine triads exhibiting tricolor-changing mechanochromic luminescence. Chem. Sci. 8, 2677–2686 (2017).
Le Bahers, T., Adamo, C. & Ciofini, I. A qualitative index of spatial extent in charge-transfer excitations. J. Chem. Theory Comput. 7, 2498–2506 (2011).
Naveen, K. R., Palanisamy, P., Chae, M. Y. & Kwon, J. H. Multiresonant TADF materials: triggering the reverse intersystem crossing to alleviate the efficiency roll-off in OLEDs. Chem. Commun. 59, 3685–3702 (2023).
Lee, H. L. et al. Hybridization of short-range and long-range charge transfer excited states in multiple resonance emitter. Nat. Commun. 14, 4818 (2023).
Yuan, Y. et al. The design of fused amine/carbonyl system for efficient thermally activated delayed fluorescence: novel multiple resonance core and electron acceptor. Adv. Opt. Mater. 7, 1801536 (2019).
Ha, J. M., Hur, S. H., Pathak, A., Jeong, J.-E. & Woo, H. Y. Recent advances in organic luminescent materials with narrowband emission. NPG Asia Mater. 13, 53 (2021).
Samsung Demonstrate a Healthier Bio-Blue OLED Display at SID 2016 | OLED Info https://www.oled-info.com/samsung-demonstrate-healthier-bio-blue-oled-display-sid-2016 (2016).
Zhao, Z.-C., Zhou, Y., Tan, G. & Li, J. Research progress about the effect and prevention of blue light on eyes. Int. J. Ophthalmol. 11, 1999–2003 (2018).
Bian, J. et al. Ambipolar self-host functionalization accelerates blue multi-resonance thermally activated delayed fluorescence with internal quantum efficiency of 100. Adv. Mater. 34, 2110547 (2022).
Park, I. S., Min, H. & Yasuda, T. Ultrafast triplet–singlet exciton interconversion in narrowband blue organoboron emitters doped with heavy chalcogens. Angew. Chem. Int. Ed. 61, e202205684 (2022).
Liang, X. et al. Peripheral amplification of multi-resonance induced thermally activated delayed fluorescence for highly efficient OLEDs. Angew. Chem. Int. Ed. 57, 11316–11320 (2018).
Qu, Y.-K. et al. Steric modulation of spiro structure for highly efficient multiple resonance emitters. Angew. Chem. Int. Ed. 61, e202201886 (2022).
Zhang, K. et al. Carbazole-decorated organoboron emitters with low-lying HOMO levels for solution-processed narrowband blue hyperfluorescence OLED devices. Angew. Chem. Int. Ed. 62, e202313084 (2023).
Liu, H., Chen, J., Fu, Y., Zhao, Z. & Tang, B. Z. Achieving high electroluminescence efficiency and high color rendering index for all-fluorescent white OLEDs based on an out-of-phase sensitizing system. Adv. Funct. Mater. 31, 2103273 (2021).
Jeon, S. O. et al. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photonics 15, 208–215 (2021).
Liu, H., Fu, Y., Chen, J., Tang, B. Z. & Zhao, Z. Energy-efficient stable hyperfluorescence organic light-emitting diodes with improved color purities and ultrahigh power efficiencies based on low-polar sensitizing systems. Adv. Mater. 35, 2212237 (2023).
Naveen, K. R., Yang, H. I. & Kwon, J. H. Double boron-embedded multiresonant thermally activated delayed fluorescent materials for organic light-emitting diodes. Commun. Chem. 5, 149 (2022).
Lv, X. et al. Extending the π-skeleton of multi-resonance TADF materials towards high-efficiency narrowband deep-blue emission. Angew. Chem. Int. Ed. 61, e202201588 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Project Nos. U2001222 for Y.H., U23A20594 for Y.H. and Z.Z., U22A20399 for W.-C.C., 22375066 for Z.Z., 22275114 for M.-C.T.), Guangdong Basic and Applied Basic Research Foundation (Project Nos. 2023B1515040003 for Z.Z. and 2022B1515020041 for M.-C.T.), Science and Technology Planning Project of Shenzhen Municipality (Project No: WDZC20220817160017003 for M.-C.T.).
Author information
Authors and Affiliations
Contributions
W.-C.C. and Y.H. initiated and designed the research. W.-C.C. and L.X. designed the MR-TADF compounds. L.X. synthesized the compounds. L.X., B.L., G.C., X.W. J.-X.C., S.J. conducted the characterization, and photophysical measurements of the compounds. J.W. and Z.Z. carried out the OLED fabrication and characterizations. L.X., B.L. J.-H.T., and S.S.C. performed and analyzed the computational calculations. W.-C.C., Z.Z., M.-C.T., and Y.H. supervised the work. All authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xing, L., Wang, J., Chen, WC. et al. Highly efficient pure-blue organic light-emitting diodes based on rationally designed heterocyclic phenophosphazinine-containing emitters. Nat Commun 15, 6175 (2024). https://doi.org/10.1038/s41467-024-50370-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50370-5
This article is cited by
-
Hyperfluorescence circularly polarized OLEDs consisting of chiral TADF sensitizers and achiral multi-resonance emitters
Nature Communications (2025)
-
Design high-performance narrowband emitters for blue light-emitting diodes through manipulating resonance structure of aromatic heterocycles
Science China Chemistry (2025)