Abstract
Soil extractable nitrate, ammonium, and organic nitrogen (N) are essential N sources supporting primary productivity and regulating species composition of terrestrial plants. However, it remains unclear how plants utilize these N sources and how surface-earth environments regulate plant N utilization. Here, we establish a framework to analyze observational data of natural N isotopes in plants and soils globally, we quantify fractional contributions of soil nitrate (fNO3-), ammonium (fNH4+), and organic N (fEON) to plant-used N in soils. We find that mean annual temperature (MAT), not mean annual precipitation or atmospheric N deposition, regulates global variations of fNO3-, fNH4+, and fEON. The fNO3- increases with MAT, reaching 46% at 28.5 °C. The fNH4+ also increases with MAT, achieving a maximum of 46% at 14.4 °C, showing a decline as temperatures further increase. Meanwhile, the fEON gradually decreases with MAT, stabilizing at about 20% when the MAT exceeds 15 °C. These results clarify global plant N-use patterns and reveal temperature rather than human N loading as a key regulator, which should be considered in evaluating influences of global changes on terrestrial ecosystems.
Similar content being viewed by others
Introduction
Nitrogen is a vital nutrient element for life on Earth. Vascular plants dominate biomass and carbon (C) capture on land where N limitation is widespread1. Accordingly, a better understanding of plant N-use mechanisms is critical for assessing and predicting primary productivity of terrestrial ecosystems2,3. Global changes such as climate warming and increasing atmospheric N deposition have significantly impacted the soil N cycle and plant N utilization and consequently terrestrial primary productivity4,5. Nevertheless, the exact contributions of soil N sources to terrestrial plants (i.e., how plants utilize the available soil N sources) remain unquantified and their variations among global terrestrial environments remain unclear6. This knowledge gap is preventing an accurate evaluation of N-cycle effects on biodiversity and the C cycle, as well as their responses to projected environmental changes3,7.
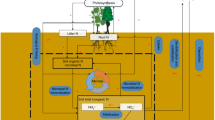
Non-N2-fixing plants are assumed to primarily acquire bioavailable N from soils via roots1. The total extractable N (TEN) pool accessible to microbes and plants includes nitrate (NO3-), ammonium (NH4+), and organic N (EON) (Fig. 1)3,6,8. Plant roots acquire soil extractable N directly or via mycorrhizal associaton9. Acquired N is allocated and assimilated among leaves, stems, and roots, which combined constitutes the whole-plant N pool (Fig. 1)10,11. Before the 1980s, soil inorganic N was recognized as the exclusive plant N source, and thus soil N mineralization has long been used to evaluate the plant-used N in soils (PUN; Fig. 1)12. Nevertheless, subsequent findings of root absorption of EON molecules (such as amino acids, peptides, proteins, and even microbes) in ecosystems under different climate contexts indicate that soil EON is a non-negligible contributor of PUN and soil N mineralization could not fully account for PUN13,14,15. Since then, the concentrations and pool sizes of NO3-, NH4+, and EON have been measured and used in combination with plant C/N ratios to model the plant-soil N and C cycle6,7,16. However, parallel evidence on the complex N competition between plants and microbes and intrinsic N preference of plants among NO3-, NH4+, and EON indicates that TEN cannot be simply taken as PUN8,12. For example, specific chemical fractions of the TEN pool that are immobilized and transformed by soil microbes can differ substantially from those observed in soil TEN17. Also, plant acquisition of soil NO3-, NH4+, and EON do not follow their proportions in soil TEN because of the verified plant N preference18,19,20. These two factors result in different estimates of pool sizes and chemical proportions between TEN and PUN in soils. Among the existing studies, the 15N isotope dilution method is effective at partitioning contributions of NO3-, NH4+, and EON to PUN, respectively8. However, the high cost of 15N tracers and artificial injection makes it most applicable for incubating plants and small-scale experiments. Further, asynchrony in N species, time, and space among short-term 15N additions, microbial turnover, and root absorption caused substantial uncertainties in evaluating the integrative long-term mechanisms of plant N utilization (Table s1)8,12. For natural ecosystems, the 15N-tracer application with water can strongly alter soil chemistry and disturb microbe-soil-plant N relationships13,14. For EON, the 15N tracer is restricted to few molecules (e.g., free amino acids, which only account for <5% of EON in soil21) and thus cannot accurately elucidate plant EON utilization. Therefore, an integrative and non-invasive approach is strongly needed for deciphering ‘in-situ’ N-use mechanisms of terrestrial plants.
The ratio of natural N isotopes (i.e., 15N/14N, denoted as δ15N values and expressed in per mille units; δ15N = [(15N/14N)sample / (15N/14N)standard) – 1] × 1000, (where the standard is atmospheric N2) has been known as a non-invasive measure to decipher N-cycle processes22,23,24,25,26,27. Hitherto, the combination of leaf δ15N (denoted as δ15Nleaf) with the δ15N of soil NO3-, NH4+, and EON (denoted as δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON, respectively) and Bayesian isotope mixing models has been recognized as a feasible method to quantify respective contributions of soil NO3-, NH4+, and EON to PUN18,19,20. However, two fundamental questions concerning the quantification of soil N sources to PUN remain unresolved at the global scale. First, the intra-plant 15N heterogeneity causes differences between the whole-plant δ15N (denoted as δ15Nplant) and δ15Nleaf28. This difference caused errors in interpreting plant N sources by using δ15Nleaf but has not been constrained to characterize δ15Nplant signatures18. Additionally, the preferential uptake and transformation of 14N from mycorrhiza to plants cause lower δ15Nplant than the corresponding δ15N of PUN (denoted as δ15NPUN)24,26. The discrepancies between δ15Nplant and δ15NPUN (∆m) not only differ among mycorrhiza types, but also vary with environmental conditions influencing the dependence of plant N uptake on mycorrhiza9. However, no study has constrained ∆m values for specific mycorrhizal symbioses across contrasting environments, thus hampering understanding of variation in δ15NPUN of global terrestrial plants.
Second, it is a long-standing question what proportions of soil N sources contribute to PUN under different environmental backgrounds, so that the environmental control mechanisms of the fractional variations remain unclear. Regarding the contributing proportions, some studies showed that plants under lower MAT (<5 °C) or at higher latitudes (> 63 °N) mainly utilized EON (43–66% (c.a. >59%) for tundra plants) (data compiled in Table s1). In contrast, the other studies in these regions estimated much lower contributions of organic N (c.a. <22%), with c.a. 14–61% and 24–63% from NH4+ and NO3-, respectively (Table s1). Similarly, some studies showed that plants under higher MAT (>12 °C) or at lower latitudes (<38 °N) mainly used inorganic N (c.a. 86–95%), but other studies in these regions argued that the contribution of organic N to PUN reached 20-39% (Table s1). These contrasting findings demonstrate that the proportional contributions of soil N sources to PUN remain an open question. Regarding the environmental drivers, global change studies have confirmed that increasing atmospheric N deposition, temperature, and precipitation are three main factors affecting ecosystem N cycling6. However, existing studies have been mostly based on simulations of single or two factors and mainly concerned plant inorganic N utilization29. In the ‘real’ world of global terrestrial ecosystems, it remains uncertain whether and how these factors influence the geographic distribution of the relative contributions of soil NH4+, NO3-, EON to PUN. Based on higher soil inorganic N concentrations, mineralization and nitrification rates under simulated warming and N additions17,30, plant inorganic N and NO3- utilization were assumed to increase with increasing temperature and N deposition31. However, based on a data compilation of sparse observations (data compiled in Table s2), only a temperature effect was observed on plant inorganic N uptake. For precipitation, an observed phenomenon is that the contribution of NH4+ relative to NO3- to PUN increased with mean annual precipitation (MAP) because of inhibition of nitrification and enhanced denitrification18,29. However, due to variation in experimental conditions it remains uncertain which environmental factors are regulating soil N source contributions to PUN and how these contributions change across terrestrial biomes.
Here, this study resolves the long-standing question of natural N abundance isotope methods for constraining δ15NPUN signatures and accomplishes the quantification of soil NO3-, NH4+, and EON contributions to PUN and their global patterns, respectively. First, we update the global δ15Nleaf dataset based on that in Craine et al.26 and the literature published since January 10th, 2018 (Figs. s1a, s2a; Supplementary Text 1). By compiling the global data of δ15Nleaf, stem δ15N (δ15Nstem), and root δ15N (δ15Nroot) measured for the same plant individuals (Figs. s1b, s2; Supplementary Text 2), we establish the relationship between δ15Nleaf and δ15Nplant to constrain the corresponding δ15Nplant values of global δ15Nleaf observations (Fig. s3). Then, we analyze the effects of MAT, MAP, and plant life form on δ15Nplant of different mycorrhizal plants (Table s3, Fig. s4) and establish the relationships of δ15Nplant with MAT for plants with the same mycorrhizal type and life form (Fig. s5). Using these relationships, we constrain ∆m values for global δ15Nplant values and finally obtain the corresponding δ15NPUN signatures (Table s4). Further, we compile a global dataset of δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON (Figs. s1c, s6; Supplementary Text 3), analyze the effects of climate and soil parameters on these source δ15N values of PUN (Table s5), establish their relationships with MAT (Fig. 2) to constrain site-based mean δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON values for the corresponding site-based δ15NPUN values (Fig. 2). Based on site-based δ15NPUN and source δ15N values in each grid cell (0.1° (latitude) × 0.1° (longitude)) and using the Stable Isotope Analysis in R (i.e., the SIAR model) (detailed in Methods), we accomplish the calculations of fractional contributions of soil NO3-, NH4+, and EON to PUN for global 1610 grid cells (Fig. s7). Finally, we examine relationships between the PUN source contributions and major environmental factors to evaluate the environmental controls of plant N utilization across terrestrial ecosystems.
Variations of site-based mean δ15N of leaf N (δ15Nleaf), whole-plant N (δ15Nplant), PUN (δ15NPUN), soil NO3- (δ15Nsoil-NO3-), soil NH4+ (δ15Nsoil-NH4+), and soil EON (δ15Nsoil-EON) with MAT are shown in panels (a)–(f), respectively. Mean and SD values were based on sample replicates at each site (n = 1–2118 for plants, and n = 2–21 for soils). Site distribution is shown in Fig. s1. The regression was analyzed by fitting effects with 95% confidence intervals.
Results
δ15N values of leaf, stem, root, and the whole plant
The δ15Nleaf, δ15Nstem, and δ15Nroot of terrestrial plants showed substantial differences from each other (p < 0.05, Fig. s2a–c). Based on the same plant individuals, δ15Nleaf was higher by 0.5 ± 2.5‰ than δ15Nroot and by 0.4 ± 1.2‰ than δ15Nstem (Fig. s2d, e), positive correlations between δ15Nleaf and δ15Nroot or δ15Nstem showed slope values of 0.57 and 0.90, respectively (Fig. s3). There was a linear correlation between δ15Nleaf and the corresponding δ15Nplant for herbs, shrubs, and trees (Fig. s3c), which were used to calibrate global δ15Nleaf observations to the corresponding δ15Nplant values. Globally, δ15Nplant values differed distinctly among herb, shrub, and tree (Table s3). Moreover, δ15Nplant values of the same life form differed among mycorrhizal types, showing a general order of nonmycorrhizal (NM) > arbuscular mycorrhizal (AM) > ectomycorrhizal (ECM) > ericoid mycorrhizal (ERM) (Fig. s4). For the same life form and mycorrhizal type, δ15Nplant values varied linearly with the MAT (Fig. s5), which was used to calculate the corresponding δ15NPUN values (Table s4).
δ15N values of PUN and soil N sources
The δ15NPUN values averaged 3.4 ± 3.3‰ (−15.0‰–21.7‰) (Fig. s6a) and increased linearly with MAT (δ15NPUN = 0.09 × MAT + 1.98; Fig. 2). Similarly, soil δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15NEON values were also mainly influenced by MAT (Table s5) and increased linearly with MAT (Fig. 2), showing different slope and intercept values (δ15Nsoil-NO3- = 0.18 × MAT – 4.51, δ15Nsoil-NH4+ = 0.37 × MAT + 0.01, and δ15NEON = 0.28 × MAT + 1.63; Fig. 2) from that of δ15NPUN. For sites with simultaneous N concentration and isotope observations in both plant and soil, the site-based mean δ15N values of TEN (δ15NTEN) (5.4 ± 2.7‰) were higher by 2.3 ± 2.7‰ on average than the corresponding δ15NPUN (3.1 ± 2.6‰) and the differences generally increased with MAT (Fig. s8).
Fractional contributions of soil NO3 -, NH4 +, and EON to PUN
The plant fNO3-, fNH4+, and fEON values averaged 29 ± 19%, 42 ± 18%, and 29 ± 19%, respectively (Fig. s7). Generally, the fNO3- and fNH4+ increased while the fEON decreased with the latitude (Figs. 3, s9). Neither plant fNO3-, fNH4+, nor fEON values showed a clear relationship with MAP and the flux of atmospheric N deposition, instead they varied with the MAT nonlinearly (Figs. 4, s10). Across the observed temperature spectrum, the fNO3- increases with MAT and reaches a peak of 46% at 28.5 °C (fNO3- = 0.01 × MAT2 + 0.65 × MAT + 20.8; Fig. 4a). The fNH4+ also increased with MAT, achieving a maximum of 46% at 14.4 °C, followed by a decline as temperatures further increased (fNH4+ = −0.05 × MAT2 + 1.52 × MAT + 35.6; Fig. 4b). Meanwhile, the fEON gradually decreased with MAT, stabilizing at about 20% when the MAT exceeded 15 °C (fEON = 0.04 × MAT2 – 2.20 × MAT + 48.6; Fig. 4c).
ƒNO3- (a, b), ƒNH4+ (c, d), and ƒEON (e, f) are fractional contributions of soil NO3-, NH4+, and EON to PUN, respectively. In panels (a), (c), and (e), gray areas are deserts, glaciers, perennial snow-covered areas, bare ground, and agricultural land etc. The mapping was conducted by ArcGIS version 10.8 (Esri Inc., USA) using the Kriging spatial interpolations based on the 0.1° (latitude) × 0.1° (longitude) grid-based data. The base map was downloaded from https://hub.arcgis.com/datasets/esri::world-countries-generalized. In panels (b), (d), and (f), red lines and gray areas show mean and SD values.
Discussion
Differing δ15Nleaf, δ15Nstem, and δ15Nroot of terrestrial plants at both site (Fig. s2a–c) and individual levels (Fig. s2d–f) demonstrated substantial N isotope effects of intra-plant N assimilation/allocation28,32,33 and none of them can exactly represent the corresponding δ15Nplant signatures28,34. The generally higher δ15Nleaf than the corresponding δ15Nplant (Figs. 2a, b, s3c) points to a potential risk of overestimating the contribution of 15N-enriched soil N sources to terrestrial plants when neglecting the intra-plant N isotope effects on δ15Nleaf values. Accordingly, the δ15Nleaf vs δ15Nplant correlation based on the same plant individuals (Fig. s3c) provides a transformation of global δ15Nleaf measurements (Fig. 2a) to the corresponding δ15Nplant values (Fig. 2b).
The generally increasing δ15Nplant of the same life form and mycorrhizal type with MAT (Fig. s5) revealed temperature as an effective predictor of mycorrhizal N isotope effects on δ15Nplant on the global scale (Table s4). The decreasing mycorrhizal N isotope effects with increasing MAT for most plants (Table s4) confirmed a weaker mycorrhizal mediation of the whole-plant N acquisition under warmer climate conditions9,24. This tendency might be related to the generally increasing soil bioavailable N (particularly inorganic N sources) with MAT for the direct N absorption via plant roots9,24. The correlation between mycorrhizal N isotope effects and MAT for the same life form and mycorrhizal type identified in our study (Table s4) allowed a transformation of global δ15Nplant measurements (Fig. 2b) to the corresponding δ15NPUN values in soils (Fig. 2c).
Globally, δ15Nsoil-TEN (Fig. s8b), δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON all increased linearly with MAT (Fig. 2d–f). Increasing temperature generally enhances rates of microbial N mineralization, nitrification, and denitrification in soils30. The openness of the soil N cycle would further increase the production and losses of 15N-depleted N species, such as the N-containing gases (e.g., NO, N2O, N2, NH3)22,25,35. This mechanism was well supported by increasing 15N abundances of soil bulk N pools with MAT25,36. Because the N nutrition of terrestrial plants depends on acquiring NO3-, NH4+, and EON from soils, the δ15NPUN naturally followed soil N sources to increase with MAT (Fig. 2c). These results demonstrate a temperature-induced openness of the soil N cycle25 and offer δ15NPUN as an indicator of the plant-soil N cycle.
Based on simultaneous N concentration and isotope observations in both plants and soils (Figs. s8, s11–12), we confirmed distinctly lower δ15N values in PUN than in TEN (Fig. s8). Clearly, neither δ15Nplant nor δ15NPUN can exactly represent soil δ15NTEN to elucidate the soil or ecosystem bioavailable N, also soil δ15NTEN cannot be simply taken as δ15NPUN. These results reflected differing sizes and compositions between the total bioavailable N and PUN pools in soils (Fig. 1). The generally lower δ15NPUN than δ15NTEN in soils found in our study (Fig. s8c) demonstrated that plants utilized relatively more 15N-depleted NO3- than 15N-enriched NH4+ and EON in soils (Fig. s12). Interestingly, we found that δ15NPUN increased by a lower magnitude than the δ15Nsoil-TEN with increase MAT (Fig. s8a, b), which caused an increasing δ15NTEN-PUN value with MAT (Fig. s8c). In principle, δ15NPUN values were controlled by both δ15N values and fractional contributions of the corresponding soil NO3-, NH4+, and EON18,20,23. Because δ15N values of soil N sources are mainly regulated by microbial N cycles17,37 and they increase with MAT (Fig. 2d–f), the lower sensitivity of δ15NPUN than δ15NTEN to MAT (Fig. s8) indicates changing fractional contributions of soil NO3-, NH4+, and EON to PUN, i.e., changing plant N-use strategies with MAT.
Globally, plant fNO3-, fNH4+, and fEON values averaged 29%, 42%, and 29%, respectively (Fig. s7). Clearly, plants mainly utilized inorganic N, though organic N is an important N source for plants6,13,14. Moreover, significant changes in fNO3-, fNH4+, and fEON with MAT, rather than with MAP or atmospheric N deposition flux (Figs. 4, s10), indicate a global temperature-dependent plant N-use pattern. This suggests that N deposition and MAP do not significantly affect plant N utilization, whereas MAT plays a critical role in driving the geographical distribution of plant N-use patterns at the global scale. From polar to tropical regions, both fNO3- and fNH4+ increased, and fEON decreased, with decreasing latitude (Figs. 3, s9) and the increase in MAT (Fig. 4). These results provide quantitative evidence confirming that plants in relatively colder climate conditions (<0 °C) utilized soil EON in higher proportions (>50%) than plants in relatively warmer climate (Fig. 4c). Fractional contributions of three N forms to PUN changed with MAT nonlinearly (Fig. 4), which revealed that plant utilization of soil NO3-, NH4+, and EON differed in responsiveness and sensitivity between colder and warmer climates. In colder climates (MAT of −18.7 to −2.0 °C), plant utilization of soil NO3- was proportionally low (below 20%), attributed to significantly reduced nitrification rates6,19. In contrast, in warmer climates at sites with MAT ranging from 11.4 to 28.5 °C, soil NO3- contribution to PUN exceeded the global average (29%), reaching up to 46% (Fig. 4a). Additionally, the contribution of soil NH4+ to PUN increased with rising MAT, peaking at 46% at 14.4 °C (Fig. 4b) due to increased N mineralization rates12,30. However, fNH4+ declined with MAT when exceeding 14.4 °C (Fig. 4b), likely due to enhanced nitrification rates38 and the toxic effects of excessive NH4+, causing lower intracellular pH and ionic imbalance in plants3.
Based on simultaneous N concentration and isotope observations in both plant and soil (Figs. s8, s11-12), we found that fNO3-, fNH4+, and fEON did not differ substantially from those based on non-synchronous observations, showing consistently low ΔfNO3-, ΔfNH4+, and ΔfEON values of −1.1 ± 11.1%, 2.0 ± 9.3%, and −0.9 ± 9.1%, respectively (Fig. s11). Also, calculating results based on simultaneous observations confirmed a globally temperature-dependent plant N-use pattern, i.e., both fNO3- and fNH4+ increased and fEON decreased with MAT (although not significantly, Fig. s11). Moreover, we found that both βNO3- and βNH4+ increased while βEON decreased with the increasing MAT (β represents the preference degree of plant NO3-, NH4+, or EON utilization, detailed in Methods, Fig. s12). This pointed to an important plant N-use strategy that plants in relatively colder climate conditions preferred soil organic N sources over inorganic N sources13,15. Conversely, plants in relatively warmer climates preferred soil inorganic N sources over organic N sources18,20. In general, plants display a clear plasticity of relative N preference in response to increased MAT.
In summary, this study presents a quantitative analysis on levels and spatial variation of soil N source contributions to global terrestrial plants, providing methods and evidence for evaluating plant N-use mechanisms and N cycling of terrestrial ecosystems. By explicitly constraining isotope effects of intra-plant N assimilation/allocation and mycorrhizal N acquisition of different plant life forms under different climates, we transferred the δ15Nleaf to a parameter of δ15NPUN for elucidating plant N utilization. Substantially differing signatures between δ15NPUN and δ15NTEN informed that neither δ15Nleaf nor δ15NTEN can be directly taken as an indicator of soil N availability or the relative availability of PUN to soil N supply. Globally, we found that variations of δ15NPUN and therefore soil N source contributions to terrestrial plants were temperature-dependent and nonlinear, showing increasing plant inorganic N utilization and relative preference while decreasing organic N utilization and preference with increasing MAT. Our finding revealed the important role of plant N-use strategies in regulating plant δ15N records and their responses to warming climate. Besides, due to differing C costs among plant NH4+, NO3-, and EON assimilation, our finding aids further evaluation of the effects of plant N utilization on C cycles.
However, extant δ15N observations on roots and stems were still sparse among terrestrial plants, and simultaneous observations on δ15N, N concentrations, and biomass for leaves, stems, and roots were even less. Particularly, δ15N values of soil extractable N sources have seldom been observed together with those of plants, particularly for sites and ecosystems with relatively lower MAT. Further, δ15N values of soil EON sources that were actually used by plants remain difficult to determine. These are substantial uncertainties in the established relationships and calculated results of this study. Although it might not influence the general patterns of soil N source contributions to global plants found in the present study, to widely conduct simultaneous and point-to-point observations on plant-soil δ15N parameters would provide more precise estimates on plant N sources and availability for biogeochemical and earth system modeling efforts.
Methods
Data compilation of δ15Nleaf, Nleaf, and N deposition
We collected the δ15Nleaf data published after January 10th, 2018, and combined them with the existing δ15Nleaf dataset collected by Craine et al.26. Briefly, we searched literature published since 2018 on the Web of Science and Google Scholar with the terms “nitrogen isotope or 15N” and “leaf or foliar”. The δ15Nleaf data of (1) urban areas, (2) agricultural ecosystems, (3) non-control samples of manipulative experiments, (4) non-vascular plants, (5) fertilized plants, (6) semi-aquatic or aquatic plants, and (7) N2-fixing plants were excluded. Data in figures of publications were extracted using the software of Web Plot Digitizer (Version 4.2, San Francisco, California, USA).
By August 1st of 2022, 37 publications (listed in Supplementary Text 1) with the required δ15Nleaf data were available, and a total of 16494 observations at 1218 sites were newly added to the dataset collected by Craine et al.26 (41669 observations at 5296 sites before January 10th of 2018). Sites of all δ15Nleaf observations (58163 observations at 6514 sites) distribute between 54.5°S and 71.1°N (Figs. s1a, s2a), with the MAT spanning from −18.7 °C to 28.6 °C and MAP ranging from 50.1 mm to 6576.0 mm. The data of MAT and MAP were collected either from the original literature or cited from the climatic database at http://www.worldclim.org using the coordinate information. All δ15Nleaf observations were conducted for 5632 plant species of 2172 herbs, 1156 shrubs, and 2304 trees. The sampling years of δ15Nleaf observations range from 1876 to 2022, with 90% sampled after 1995.
The Nleaf data were also collected from the corresponding literature if available. Besides, to examine atmospheric N deposition effects on plant and soil N variables, we collected the data of deposition fluxes of inorganic and organic N in wet and dry deposition based on the observations in 12 individual years during 1984 − 201639. We used the coordinate information of plant or soil δ15N observation sites in our study to match and extract the corresponding data and then calculated mean annual fluxes of total N deposition.
Constraining terrestrial δ15Nplant signatures
The whole-plant N level (Nplant) is mainly determined by leaf, stem, and root N levels, so we have the following mass-balance Eq. (1).
where Nleaf, Nstem, and Nroot are the N concentrations in the leaf, stem, and root, respectively; Fleaf, Fstem, and Froot are their respective biomass percentages in the whole plant. Then, the δ15Nplant can be expressed by the following δ15N mass-balance Eq. (2).
where we assume that Fleaf + Fstem + Froot = 1.
The N concentration and δ15N data of stems and roots were collected from the publications of δ15Nleaf observations. In our calculations (Eqs. (1) and (2)), we only used the N concentration and δ15N data simultaneously measured for the same plant individuals (Fig. s3). The biomass data were collected either from the original literature or cited from the global database of Reich et al.10. In total, 103 publications (listed in Supplementary Text 2) with 382 and 1752 observations of δ15Nstem and δ15Nrootwere available, respectively (Fig. s2b, c). Sites of δ15Nstem and δ15Nroot observations distribute between 45.8°S and 74.5°N (Fig. s1b), with the MAT spanning from −16.3 °C to 28.0 °C. The available δ15Nstem and δ15Nroot observations were conducted for a total of 246 plant species, including 120 herbs, 57 shrubs, and 69 trees.
The δ15N values showed difference between leaf and stem or root of the same individuals among the observed plants (Fig. s2d-f). The δ15Nleaf and δ15Nplant were positively correlated for herbs δ15Nplant = 0.77( ± 0.08) × δ15Nleaf – 0.13( ± 0.19) (R = 0.93, p < 0.01), shrubs δ15Nplant = 0.80( ± 0.06) × δ15Nleaf – 0.98( ± 0.10) (R = 0.94, p < 0.01), and trees δ15Nplant = 0.93( ± 0.00) × δ15Nleaf – 0.13( ± 0.00) (R = 0.98, p < 0.01) (Fig. s3c). These relationships were used to calculate δ15Nplant values for each δ15Nleaf observation of each life form in the global dataset (Fig. 2b).
Constraining terrestrial δ15NPUN signatures
The differences between δ15Nplant and δ15NPUN exist (Δm) for plants associated with mycorrhiza due to substantial N isotope effects caused by plant N acquisition via mycorrhizal associations22,24. Thus, we have Eq. (3) to calculate the δ15NPUN.
To constrain the Δm values, we collected the records of the mycorrhizal types of plants either from the original literature or referring to records in other publications40. In this study, a total of 240, 4849, 391, and 106 among 5586 plant species are associated with NM, AM, ECM, and ERM, respectively. The Δm value for NM plant species was assumed as 0‰ because the direct entering processes of soil N sources into plant roots have no substantial 15N discrimination18,23,24. The Δm values for plant species associated with mycorrhiza can be estimated as the δ15Nplant differences between mycorrhizal plants and NM plants, respectively, which have been revealed varying in a general order of AM < ECM < ERM24.
In this study, we newly found that the δ15Nplant variations were influenced by life forms and MAT (Table s3, Fig. s4–5), thus we constructed the δ15Nplant variations of each mycorrhizal type of each life form with MAT (Fig. s5). Then, we calculated the MAT-specific Δm values by using the fitting formula of NM minus the fitting formula of the corresponding each mycorrhizal type plants of the same life form (Table s4, Fig. s5). The negative correlation between MAT and Δm (Table s4) indicates that as MAT increases, soil N availability also increases, reducing plant reliance on mycorrhizae and thereby diminishing their N isotopic effects9,24. Therefore, we calibrated δ15Nplant to δ15NPUN using this relationship to minimize the impact of mycorrhizae. Finally, the MAT-specific δ15NPUN values were calculated (Figs. s6a, 2c).
Calculating contributions of soil NO3 -, NH4 +, and EON to PUN
The δ15NPUN value is determined by δ15N values of soil NO3-, NH4+, and EON (δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON, respectively) and fractional contributions of soil NO3-, NH4+, and EON to PUN (ƒNO3-, ƒNH4+, and ƒEON, respectively)18,19,23, which can be expressed by Eq. (4).
where we assume that ƒEON + ƒNH4+ + ƒNO3- = 1.
To constrain the δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON in terrestrial ecosystems, we searched literature published on the Web of Science and Google Scholar with the terms “nitrogen isotope”, “15N”, “soil ammonium”, “soil NH4+”, “soil nitrate”, “soil NO3-”, “soil DON”, “soil dissolved organic nitrogen”, “soil EON”, “soil extractable organic nitrogen”. Observations for fertilized soils (including 15N-labeling experiments) and soils at depths over 30 cm were excluded from the searched literature. By August 1st, 2022, we obtained 46 publications (listed in Supplementary Text 3) with a total of 321, 356, and 262 observations of δ15Nsoil-NO3- at 56 sites, δ15Nsoil-NH4+ at 62 sites, and δ15Nsoil-EON at 28 sites, respectively (Figs. s1c, s6b-d). Sites of soil δ15N observations distribute between 30.3°S and 68.4°N, with the MAT spanning from −8.4 °C to 28.0 °C (Figs. s1c, 2d–f). To determine the regulating factor of soil δ15N variations, we collected the data on soil density, clay, pH, and organic C from the Harmonized World Soil Database v1.2 (https://www.fao.org/home/en/) using the coordinate information. Besides, soil NO3-, NH4+, EON, and TEN concentrations were also collected for sites with simultaneous observations on δ15Nleaf, δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON (Figs. s8, s11–12).
The δ15Nsoil-NO3-, δ15Nsoil-NH4+ and δ15Nsoil-EON values are influenced by various production and consumption processes of the corresponding N forms17. These processes, and thus the above soil δ15N parameters, are influenced by climatic and environmental factors37. This study found that variations of δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON were all influenced significantly by MAT (Table s5), increasing linearly with MAT (Fig. 2d–f). This finding is supported by evidence that increasing temperature enhances microbial N processes and the ‘openness’ of soil N cycles, leading to more 15N enrichment in soil N sources22,25,35. Accordingly, we calculated the mean δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON values for each δ15NPUN observation site by using the corresponding MAT and the fitting formula in Fig. 2d–f.
Then, we calculated ƒNO3-, ƒNH4+, and ƒEON values using an isotope-mixing model (named Stable Isotope Analysis in R, SIAR). The SIAR model, which is designed around a Bayesian framework, effectively utilizes a Dirichlet distribution to establish a logical prior for estimating source contributions41,42. This framework not only focuses on managing sample size variability but also prioritizes the data distribution within each sample over the mere number of observations, enhancing the model’s accuracy and robustness in source estimation41,42. In each run of the SIAR model, the δ15NPUN data in a grid cell of 0.1° (latitude) × 0.1° (longitude) in the same sampling year (with 3–1028 observations for each grid cell in each year, referring to Craine et al.26), the mean ± SD of site-based δ15Nsoil-NO3-, δ15Nsoil-NH4+, and δ15Nsoil-EON values in the corresponding grid cell were input into the model. Then, the percentage data of each source (n = 10,000) output from each run of the SIAR model was used to calculate the mean ± SD of grid-based ƒNO3-, ƒNH4+, and ƒEON values (Fig. s7) and to map their spatial distribution (Fig. 3).
For study sites with simultaneous observations on δ15Nleaf, δ15Nsoil-NO3-, δ15Nsoil-NH4+, δ15Nsoil-EON, and δ15Nsoil-TEN, we separately calculated the site-based δ15Nplant, δ15NPUN, fNO3-, fNH4+, and fEON values based on the above methods and examined their variations with MAT (Figs. s8, s11–12). In combination with simultaneous observations on soil NO3-, NH4+, EON, and TEN concentrations at each of the above study sites, we further calculated the relative preference degree (β) of plant N utilization among NO3-, NH4+, and EON by using Eq. (5).
where i represents NO3-, NH4+, or EON, [i] and [TEN] are N concentrations of i and TEN, respectively. Positive, zero, and negative values of β parameters indicated the preference of a given N form over other N forms, no preference, and the preference of the other N forms over a given N form, respectively20,43.
Statistical analyses
General Linear Models were used to examine the effects of major environmental and plant variables on δ15Nplant variations of NM, AM, ECM and ERM plants. Multiple linear regression analyses were used to examine the effects of the major soil and environmental variables on soil δ15N variations and plant N sources contribution. Regression analyses were used to examine variations of plant and soil δ15N parameters and fractional contributions of soil N sources to PUN and TEN with MAT and latitude, respectively. The ArcGIS 10.8 software (Esri Inc., USA) was used to perform spatial interpolations, layers overlay and property sheet processing of data points. Regression analyses were conducted using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
Data availability
The data underlying the findings of this study are available in this article. Source data are provided with this paper.
Code availability
The SPSS package can be downloaded from https://www.ibm.com/products/spss-statistics. The source code for SIAR used in this paper is openly available from https://rdrr.io/cran/siar/.
References
Vitousek, P. M. & Howarth, R. W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry. 13, 87–115 (1991).
McKane, R. B. et al. Resource based niches provide a basis for plant species diversity and dominance in Arctic tundra. Nature. 415, 68–71 (2002).
Britto, D. T. & Kronzucker, H. J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 112, 957–963 (2013).
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature. 451, 293–296 (2008).
Bloom, A. J., Burger, M., Asensio, J. S. R. & Cousins, A. B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Nature. 328, 899–903 (2010).
Chapin, III. F. S., Matson, P. A. & Vitousek, P. M. Principles of Terrestrial Ecosystem Ecology (Springer, 2011).
Melillo, J. M. et al. Global climate change and terrestrial net primary production. Nature. 363, 234–240 (1993).
Kuzyakov, Y. & Xu, X. L. Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol. 198, 656–669 (2013).
Hobbie, E. A. & Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 196, 367–382 (2012).
Reich, P. B. et al. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 111, 13721–13726 (2014).
Sardans, J. et al. Changes in nutrient concentrations of leaves and roots in response to global change factors. Global Change Biol. 23, 3849–3856 (2017).
Schimel, J. P. & Bennett, J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 85, 591–602 (2004).
Chapin, F. S. III., Moilanen, L. & Kielland, K. Preferential use of organic nitrogen for growth by a nonmycorrhizal arctic sedge. Nature. 361, 150–153 (1993).
Näsholm, T. et al. Boreal forest plants take up organic nitrogen. Nature. 392, 914–916 (1998).
Näsholm, T., Kielland, K. & Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 182, 31–48 (2009).
Fisher, J. B. et al. Carbon cost of plant nitrogen acquisition: a mechanistic, globally applicable model of plant nitrogen uptake, retranslocation, and fixation. Global Biogeochem. Cy. 24, GB1014 (2010).
Xu, S. Q. et al. Isotopic elucidation of microbial nitrogen transformations in forest soils. Global Biogeochem. Cy. 35, e2021GB007070 (2022).
Houlton, B. Z., Sigman, D. M., Schuur, E. A. G. & Hedin, L. O. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc. Natl. Acad. Sci. USA 104, 8902–8906 (2007).
Liu, X. Y. et al. Nitrate is an important nitrogen source for Arctic tundra plants. Proc. Natl Acad. Sci. USA 115, 3398–3403 (2018).
Hu, C. C. et al. Plant nitrogen and phosphorus utilization under invasive pressure in a montane ecosystem of tropical China. J. Ecol. 107, 372–386 (2019).
Lipson, D. & Näsholm, T. The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia. 128, 305–316 (2001).
Högberg, P. Tansley Review No. 95 15N natural abundance in soil–plant systems. New Phytol. 137, 179–203 (1997).
Evans, R. D. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 6, 121–126 (2001).
Craine, J. M. et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 183, 980–992 (2009).
Craine, J. M. et al. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil. 396, 1–26 (2015).
Craine, J. M. et al. Isotopic evidence for oligotrophication of terrestrial ecosystems. Nat. Ecol. Evol. 2, 1735–1744 (2018).
Mason, R. E. et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science. 376, eahh3767 (2022).
Kolb, K. J. & Evans, R. D. Implications of leaf nitrogen recycling on the nitrogen isotope composition of deciduous plant tissues. New Phytol. 156, 57–64 (2002).
Wang, L. X. & Macko, S. A. Constrained preferences in nitrogen uptake across plant species and environments. Plant Cell Environ. 34, 525–534 (2011).
Bai, E. et al. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 199, 441–451 (2013).
Lie, Z. Y. et al. Warming leads to more closed nitrogen cycling in nitrogen-rich tropical forests. Global Change Biol. 27, 664–674 (2021).
Yoneyama, T. & Kaneko, A. Variations in the natural abundance of 15N in nitrogenous fractions of komatsuna plants supplied with nitrate. Plant Cell Physiol. 30, 957–962 (1989).
Evans, R. D., Bloom, A. J., Sukrapanna, S. S. & Ehleringer, J. R. Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ. 19, 1317–1323 (1996).
Hu, C. C. et al. A new isotope framework to decipher leaf-root nitrogen allocation and assimilation among plants in a tropical invaded ecosystem. Sci. Total Environ. 806, 151203 (2022).
Fang, Y. T. et al. Microbial denitrification dominates nitrate losses from forest ecosystems. Proc. Natl. Acad. Sci. USA 112, 1470–1474 (2015).
Amundson, R. et al. Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem. Cy. 17, 1031–1041 (2003).
Denk, T. R. A. et al. The nitrogen cycle: a review of isotope effects and isotope modeling approaches. Soil Biol. Biochem. 105, 121–137 (2017).
Dawes, M. A., Schleppi, P., Hättenschwiler, S., Rixen, C. & Hagedorn, F. Soil warming opens the nitrogen cycle at the alpine treeline. Global Change Biol. 23, 421–434 (2017).
Ackerman, D., Millet, D. B. & Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Global Biogeochem. Cy. 33, 100–107 (2019).
Wang, B. & Qiu, Y. L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza. 16, 299–363 (2006).
Evans, J. S., Handley, S. J., Perham, N., Over, D. E. & Thompson, V. A. Frequency versus probability formats in statistical word problems. Cognition. 77, 197–213 (2000).
Parnell, A. C., Inger, R., Bearhop, S. & Jackson, A. L. Source partitioning using stable isotopes: Coping with too much variation. PLoS One. 5, e9672 (2010).
Liu, X. Y. et al. Ammonium first: natural mosses prefer atmospheric ammonium but vary utilization of dissolved organic nitrogen depending on habitat and nitrogen deposition. New Phytol. 199, 407–419 (2013).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (42125301 (X.Y.L.), 42330505 (X.Y.L.), and 42103075 (C.C.H.)) and the Double Thousand Plan of Jiangxi Province (jxsq2023102213) (X.Y.L.). We want to thank all researchers who reported and kindly provided us with observation data on concentrations and isotopes of N in plants and soils.
Author information
Authors and Affiliations
Contributions
X.Y.L. designed the research. C.C.H. and X.Y.L. conducted the research (data collections and analyses) and co-wrote the manuscript. W.S., X.T.L., Y.W.K., C.J.C., R.M., E.N.J.B., A.W.D., B.Z.H., and C.Q.L. commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shuwei Liu and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, CC., Liu, XY., Driscoll, A.W. et al. Global distribution and drivers of relative contributions among soil nitrogen sources to terrestrial plants. Nat Commun 15, 6407 (2024). https://doi.org/10.1038/s41467-024-50674-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50674-6
This article is cited by
-
Effects of Exogenous Melatonin on Alfalfa (Medicago sativa L.) Under High-nitrate Stress
Journal of Soil Science and Plant Nutrition (2025)
-
miRNA-seq analysis revealed a potential strategy underlying poplar root responses to low nitrogen stress
Planta (2025)
-
Global plant nitrogen use is controlled by temperature
Nature Communications (2024)