Abstract
Sustainable photoactivated room temperature phosphorescent materials exhibit great potential but are difficult to obtain. Here, we develop photoactivated room temperature phosphorescent materials by covalently attaching lignin to polylactic acid, where lignin and polylactic acid are the chromophore and matrix, respectively. Initially the phosphorescence of the lignin is quenched by residual O2. However, the phosphorescence is switched on when the residual oxygen is consumed by the triplet excitons of lignin under continuous UV light irradiation. As such, the lifetime increases from 3.0 ms to 221.1 ms after 20 s of UV activation. Interestingly, the phosphorescence is quenched again after being kept under an atmosphere of air for 2 h in the absence of UV irradiation due to the diffusion of oxygen into the materials. Using these properties, as-developed material is successfully used as a smart anti-counterfeiting logo for a medicine bottle and for information recording.
Similar content being viewed by others
Introduction
Organic RTP materials are particularly useful for a wide range of applications, such as, bioimaging, anti-counterfeiting applications, X-ray scintillators and information encryption, due to the intrinsic flexibility, easy chemical modification and tunable optical properties1,2,3,4,5,6,7,8. To design effective organic RTP materials, two rules should be followed. First, the triplet excitons of the chromophores must be effectively populated by facilitating intersystem crossing (ISC) from singlet excitons to triplet excitons, which typically requires efficient spin-orbit coupling. Second, nonradiative deactivation of the resulting triplet excitons needs to be suppressed, which is normally achieved by rigidifying the chromophores. Following these principles, effective RTP materials have been developed, including molecular crystals, supramolecules, polymer composites, MOF, COF, and carbon dots9,10,11,12,13,14,15.
To further expand the application of these RTP materials, recent effort has been directed toward the introduction of extra functionality to the RTP materials. As a result, a series of multifunctional RTP materials including chiral RTP materials, stimuli-responsive RTP materials and mechanical robust RTP materials have been developed16,17,18,19,20,21. Amongst these materials, photo-activated RTP materials have received particular attention due to their crucial role in optical printing, 3D displays and information storage22. Generally, the materials exhibit negligible or no RTP before light irradiation. While, a long-lived RTP emission is observed after exposure of the materials to a light source for a certain period of time (seconds to minutes). These photoactivated RTP materials include small molecules and polymer composites23,24,25,26,27,28,29,30. Due to the advantages of easy processability and broad applicability, polymer composite-based photoactivated RTP materials are particularly important31,32,33,34,35. Most of these polymer composites are binary systems and prepared via physically mixing chromophores with the polymer matrix. The whole preparation process requires multiple steps and is complicated. Additionally, the materials used are typically generated using non-renewable petrol-derived resources. Moreover, only weak non-covalent interactions are formed in these binary systems, which can easily deteriorate and result in a compromised RTP performance36. For example, high humidity or water can quench the RTP when polyvinyl alcohol or similar hydrophilic polymers are integrated with these systems37.
To address the weak non-covalent interactions between the chromophore and matrix, Li et al. have co-polymerized 4-vinylpyridine (4VP) and (9H-carbazol-9-yl)(4-vinylphenyl) methanone to produce a single-component polymer with photoactivated afterglow emission38. However, all the components used for this system are derived from petrol resources which raises sustainability concerns.
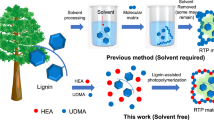
Utilizing natural biomass as a replacement to petrol resources for producing functional RTP materials is a promising method to overcome challenges in this area, since biomass resources are abundant, cheap, and sustainable3,39,40,41,42,43. Lignin, as the main byproduct in the pulping and paper industry, is produced in about 60-70 Mt per year. Approximately 95% of the lignin is then directly burned. Thus, it is particularly important to seek a value-added approach to use lignin resources. As a result of the aromatic chromophores contained in lignin, the main byproduct in the pulping and paper industry, is regarded as a very promising resource for developing RTP materials44,45,46,47,48,49,50. Nevertheless, photoactivated RTP materials from lignin or biomass resources have rarely been reported. Additionally, PLA is a biodegradable thermoplastic polyester produced by the condensation polymerization of lactic acid, which is derived from the fermentation of sugars from carbohydrate sources such as corn, sugarcane or tapioca. From an energy consumption, CO2 emissions and end of life options, PLA is superior to many petroleum-based polymers51. Motivated by this, we prepared photo-activated RTP materials (PLA-Lig) by grafting lignin with polylactic acid via in situ polymerization (Fig. 1). Lignin served as the RTP chromophore and the grafted polylactic acid was the matrix for PLA-Lig.
After exposure of PLA-Lig to a UV light source for 20 s, the lifetime of the RTP emission increased from 3.0 ms to 221.1 ms. However, the RTP of PLA-Lig was quenched when the film was kept under an atmosphere of air for 2 h. As such the activation and deactivation of the RTP of PLA-Lig was switchable.
Results
Preparation and RTP emission of PLA-Lig
Precursor molecules of PLA-Lig were synthesized by grafting lactide to lignin via ring-opening polymerization catalyzed by 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD)52,53. Specifically, the lignin served as alcohol macroinitiator and lactide was used as monomers (Supplementary Fig. 1). Signals assigned to lignin and PLA were observed in 1H NMR spectra of PLA-Lig, confirming a successful reaction (Supplementary Fig. 2). Furthermore, GPC analysis was used to determine the molecular weight of the as-obtained precursor molecules. The precursor molecules exhibited an increased molecular weight when compared to raw lignin as determined by GPC analysis, confirming that polymerization had occurred (Supplementary Fig. 3). The precursor molecules were then processed into a film (PLA- Lig) using a solvent processing method. As-obtained Lig-PLA exhibited good thermal stability and it did not degrade significantly as the temperature was increased to 283.8 °C (Supplementary Fig. 4). Subsequently, the optical properties of the PLA-Lig film were evaluated. Significantly the aromatic units of lignin, PLA-Lig enabled an enhanced absorbance over the 300–400 nm range in CH2Cl2. Also, PLA-Lig in a film state exhibited an absorbance over the 200–380 nm range (Supplementary Fig. 5)54.
In addition PLA-Lig exhibited fluorescence at 435 nm and almost no RTP emission was observed from untreated PLA-Lig (Fig. 2a and Supplementary Fig. 6). Surprisingly, an obviously increased RTP emission was observed on exposure of PLA-Lig to UV (365 nm, 0.02 mW cm−2) for 5 s (Fig. 2b). Further increasing the exposure time led to a continuous enhancement of RTP emission. Meanwhile, the RTP lifetime of PLA-Lig also increased from 3.0 ms to 221.1 ms upon exposure of PLA-Lig to a UV light source for 20 s (Supplementary Fig. 7 and Supplementary Movie 1). The quantum yield of the RTP for activated PLA-Lig was 2.95%. Notably, the quantum yield of PLA-Lig did not obviously change during the process (5.10% before irradiation and 5.07% after irradiation). Interestingly, both the activated RTP emission and lifetime of PLA-Lig decreased in an ambient environment after removing the UV light source. Almost no RTP emission from PLA-Lig was observed after 2 h (Fig. 2c). Significantly, the “RTP increase-decrease” of PLA-Lig can be cycled several times upon switching on and off the UV excitation sources (Fig. 2d and Supplementary Fig. 8), indicating the optical robustness of the materials. Notably, photo-activated PLA-Lig maintains an almost unchanged lifetime after 1-month storage under ambient conditions (Supplementary Fig. 9). Moreover, the RTP emission of PLA-Lig was not obviously quenched by humidity and the lifetime only decreased from 223.3 ms to 222.4 ms when the sample was kept in a 90% humidity environment (Supplementary Fig. 10). The PLA-Lig was stable because water cannot easily enter the PLA-Lig, which was verified by contact angle measurements (Supplementary Fig. 11). Additionally, attributed to the thermosetting properties, PLA-Lig was easily converted into different 3D shapes via thermal processing. Notably, the PLA-Lig maintained the UV activated RTP properties and the RTP lifetime was not compromised before and after thermal processing (Supplementary Fig. 12). As a control experiment the PLA in the absence of lignin only exhibited very weak RTP emission either before or after UV activation, attributed to the clustering-induced emission of carbonyl and oxygen atoms in PLA (Supplementary Fig. 13)55. This result confirms that lignin was the RTP chromophore in PLA-Lig. Subsequently, the RTP performance of PLA-Lig was investigated upon varying the amount of lignin incorporated. All the samples with varying amounts of lignin exhibited photoactivated RTP (Supplementary Fig. 14).
a Images of PLA-Lig upon UV irradiation and after removing the UV excitation (upper), Images of UV-activated PLA-Lig upon UV irradiation and after removing the UV excitation (lower), scale bar = 1 cm. b Phosphorescence spectra of PLA-Lig upon UV irradiation (0.02 mW cm−2, 365 nm) for 0 s (black line), 5 s (red line), 10 s (blue line), 15 s (green line), 20 s (purple line), 25 s (yellow line) and 30 s (cyan line). c Phosphorescence spectra of PLA-Lig upon UV irradiation (black line) and phosphorescence spectra of PLA-Lig activated by UV irradiation in an ambient environment for 10 min (red line), 30 min (blue line), 60 min (green line) and 120 min (purple line). d RTP lifetime of PLA-Lig upon UV irradiation and after switching off the UV light source. (Conditions of all phosphorescence measurements in the Fig. 2: Excitation wavelength = 310 nm, delay time = 10 ms, temperature = 20 °C). All the lignin concentrations in the PLA-Lig were 0.1%.
Mechanism
In order to explore the mechanism of the RTP of PLA-Lig the performance was compared in an air and argon (Ar) environment. PLA-Lig exhibited enhanced initial RTP performance in Ar and the lifetime was 258.9 ms, ~86 times longer than the lifetime in air (3.0 ms) (Fig. 3a, b). Generally, O2 and humidity in the air are two main reasons for RTP quenching. Previous results indicated that the RTP of PLA-Lig was not sensitive to humidity. Thus, the initial lack of phosphorescence for PLA-Lig was attributed to quenching caused by O2 in the air. The quenching of PLA-Lig was enabled since the chains of PLA-Lig lack hydrogen bonding groups such as -OH, -COOH, etc. and as such could not form a tight hydrogen bonding networks. The oxygen permeability value of PLA-Lig was 41918.58 cm3 mm−2 Pa−1 day−1. As a result, it was hard for these molecular chains to form an oxygen barrier and the diffusion of oxygen into the PLA-Lig occurs readily. Subsequently, the diffused oxygen quenched the RTP emission of the lignin in the PLA-Lig. To confirm this a control sample (PVA-Lig) was prepared by embedding lignin into PVA which contains abundant hydroxyl moieties. The oxygen permeability value of PVA-Lig was 1.52 cm3 mm−2 Pa−1 day−1. As expected, benefiting from the barrier formed by the hydroxyl groups of PVA, PVA-Lig immediately exhibited RTP emission without UV activation (Supplementary Fig. 15). Further experimental measurements also verifies this conclusion.
a Phosphorescence spectra of PLA-Lig in air (red line) and Ar (black line) without UV activation. b Phosphorescence lifetime of PLA-Lig in air and Ar without UV activation. c EPR spectra of PLA-Lig in the presence of TEMP in the dark (black line) and upon UV irradiation (red line). d Simulated results of conformation in PLA/Lig. e Simulated results of conformation in PLA-Lig. f The distance between adjacent aromatic units in PLA/Lig (red line) and PLA-Lig (black line). (Conditions of all phosphorescence measurements in Fig. 3: Excitation wavelength = 310 nm, delay time = 10 ms, temperature = 20 °C). All the lignin concentrations in the PLA-Lig were 0.1%.
Having determined the process for the initial quenching of the RTP emission of PLA-Lig, the UV activation process was further investigated. As determined above, oxygen was the reason for the RTP quenching of PLA-Lig. Thus, a possible mechanism for UV-activation of RTP was proposed to be the consumption of oxygen in PLA-Lig caused by UV irradiation. Generally, oxygen can be consumed and sensitized to excited singlet state O2 assisted by triplet excitons. As such, it is essential to capture the signals of excited singlet state O2 to verify this hypothesis. To this end, 2,2,6,6-tetramethylpiperi-dine (TEMP) was added into PLA-Lig. If excited singlet O2 was present in the system, TEMP would be oxidized to 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), which is an electron paramagnetic resonance (EPR) positive species (Supplementary Fig. 16). As expected, the sample exhibited almost no signals in the dark (Fig. 3c). While a strong EPR signal was observed after irradiating in an air atmosphere. As a control experiment, no obvious change was detected when irradiating in Ar, further confirming the generation of excited singlet state O2 during irradiation in an air atmosphere (Supplementary Fig. 17).
We then evaluated the correlation between the structure and RTP performance of PLA-Lig. To this end, PLA/Lig was prepared via physically mixing PLA with lignin. The PLA/Lig exhibited no RTP emission before or after UV irradiation (Supplementary Fig. 18). These results suggested that the covalent linkage between lignin and PLA was crucial for the RTP emission. Molecular dynamic simulations indicated that the average distance between the aromatic units of PLA-Lig were 4.2 Å. While, the average distance between aromatic units for PLA/Lig was 7.5 Å. Thus, the distance in PLA-Lig was much closer than the distances between aromatic units for PLA/Lig (Fig. 3d–f). The shorter distance between PLA-Lig is beneficial for π–π intermolecular interactions. Such interactions reduce the triplet energy level/T1 and stabilize triplet excitons56. As such, PLA-Lig exhibited RTP emission after UV activation while PLA/Lig does not.
To further verify this, PLA-Lig with different concentrations of lignin was prepared. As expected for PLA-Lig with an increased concentration of lignin (from 0.05% to 0.1%) an enhanced RTP performance was observed, since the distance between the aromatic units of lignin is reduced and the π–π interactions are promoted (Supplementary Fig. 19). Notably, both RTP intensity and lifetime decreased when the lignin concentration in PLA-Lig was further increased from 0.1% to 1%. This result was attributed to aggregation-induced quenching of aromatic units in lignin caused by the high concentrations57,58. Additionally, the red-shifted wavelength of PLA-Lig when compared with PLA/Lig illustrates that RTP in PLA-Lig was caused by the inhibition of molecular motion due to the covalent bonds between PLA and lignin (Supplementary Fig. 20).
Taking all these results together, the RTP mechanism of PLA-Lig can be explained as follows: PLA restricts the motion of lignin via the covalent linkage in PLA-Lig, enabling the potential use as an RTP material. However, the initial RTP of PLA-Lig was weak due to quenching by residual O2 in the PLA-Lig, due to the poor oxygen rejection barrier provided by PLA-Lig. After the residual O2 was converted to excited singlet state O2 assisted by the excited triplet state of lignin, the phosphorescence of lignin in PLA-Lig was activated. The as-obtained singlet state O2 reacts with the β-O-4 bonds or is scavenged by the phenolic moieties present in lignin59,60. Consequently, the phosphorescence was quenched again after UV light irradiation was ceased due to the diffusion of oxygen back into the PLA-Lig polymer.
Application
PLA-Lig can be used as a smart anti-counterfeiting logo for medicine bottles (Fig. 4a). We prepared logos for a medicine bottle from PVA-Lig, PLA and PLA-Lig which exhibited blue, purple and blue emission upon UV excitation, respectively. Afterglow emission from PVA-Lig was observed and no afterglow emission was observed from PLA and PLA-Lig upon switching off the UV irradiation immediately. However, long-lived afterglow emission was observed from PLA-Lig upon irradiation using UV for 20 s. This indicates that PLA-Lig has great potential as a smart anti-counterfeiting logo for medicine bottles. Using the responsive nature of PLA-Lig, the material could be used for information recording and encryption. Specifically, a “NEFU” photomask was placed between PLA-Lig and a UV light source. After irradiating for 20 s, no variation was detected in both the bright and UV field. However, a clear “NEFU” pattern was observed after removing the UV light due to the activated phosphorescence in the area activated by light irradiation (Fig. 4b). Additionally, a “lantern-shape” photomask was placed between PLA-Lig and a UV light source. After irradiation for 20 s, a clear “lantern” RTP pattern was observed on the PLA-Lig (Fig. 4c). Interestingly, the information was erased gradually in an ambient environment and disappeared completely after 5 h. After the information was erased completely, new information can be written again by irradiating the film using a new photomask. Due to the excellent fatigue resistance, this writing-erasing-rewriting cycle could be repeated many times with no obvious residual information remaining in the film.
a Smart anti-counterfeiting logo for medicine bottle made from PVA-Lig, PLA and PLA-Lig (activated by UV light at 0.02 mW cm-2, 20 s), scale bar = 1 cm; b Fabricating RTP images with PLA-Lig using UV activation (0.02 mW cm-2, 20 s), scale bar = 1 cm; (c) Recycling (lantern to deer) RTP images in PLA-Lig using UV activation (0.02 mW cm−2, 20 s). All the lignin concentrations in the PLA-Lig were 0.1%.
Discussion
In summary, photoactivated RTP materials were developed by modifying lignin with PLA. Initially the as-prepared PLA-Lig exhibited weak RTP with a lifetime of 3.0 ms. Then following photoactivation for 20 s, the RTP lifetime increased to 221.1 ms. Subsequently, the RTP was quenched again after UV light irradiation was ceased due to the diffusion of oxygen into the PLA-Lig. The reversible nature of PLA-Lig could be recycled 7 times without optical fatigue. The optical performance was attributed to the structure of PLA-Lig. Specifically, PLA restricts the motion of lignin via covalent interactions in PLA-Lig, enabling its potential as an RTP material. However, the initial RTP of PLA-Lig was weak due to the quenching effect of residual O2 in the PLA-Lig. As such after the residual O2 was converted to excited singlet state O2 assisted by the excited triplet state of lignin, the phosphorescence of lignin in PLA-Lig was activated. Moreover, the PLA-Lig exhibits great potential as a smart anti-counterfeiting logo for medicine bottles. Using these properties, PLA-Lig was successfully used to produce luminescent images from photomasks for information recording and encryption. We anticipate that the ease of preparation, low cost, convenient processing and sustainability of PLA-Lig, will ensure the practical utility of this material on an industrial scale.
Methods
Preparation of PLA-Lig
Alkali lignin (5 mg), L-(-)-Lactide (4.995 g) and TBD (50 mg) were added to a 25 ml flask with an N2 balloon. The reaction was kept at 130 °C for 3.5 h. After cooling to room temperature, a mixture of acetic acid (50 mg) and dichloromethane (5 mL) was added to the reaction. The reaction mixture was then added to methanol (500 mL). The as-obtained precipitate was PLA-Lig. To further process the PLA-Lig into a film, the precipitate was dissolved in methylene chloride. The solution was then evenly spread in a PTFE container. The PLA-Lig film was obtained via natural evaporation of the solvent over 3–5 days. Prior to measuring the RTP, the PLA-Lig film was dried in an oven at 60 °C for 24 h.
Preparation of PVA-Lig
Polyvinyl alcohol (4.995 g) and alkaline lignin (5 mg) were added to water (20 mL). The mixture was then heated to 90 °C for 2 h until the polyvinyl alcohol was completely dissolved. The mixture was then placed in a glass container. PVA-Lig film was obtained by the natural evaporation of water. Prior to measuring the RTP, the PVA-Lig film was dried in an oven at 60 °C for 24 h.
Data availability
All relevant data are included in this article and its Supplementary Information files. Source data are provided with this paper. All data underlying this study are available from the corresponding author Zhijun Chen upon request. Source data are provided with this paper.
References
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Dai, X., Huo, M. & Liu, Y. Phosphorescence resonance energy transfer from purely organic supramolecular assembly. Nat. Rev. Chem. 7, 854–874 (2023).
Luo, X. et al. Room-temperature phosphorescent materials derived from natural resources. Nat. Rev. Chem. 7, 800–812 (2023).
Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photonics 15, 187–192 (2021).
Wu, T., Huang, J. & Yan, Y. From aggregation-induced emission to organic room temperature phosphorescence through suppression of molecular vibration. Cell Rep. Phys. Sci. 3, 100771 (2022).
Baryshnikov, G., Minaev, B. & Agren, H. Theory and calculation of the phosphorescence phenomenon. Chem. Rev. 117, 6500–6537 (2017).
Sun, S., Ma, L., Wang, J., Ma, X. & Tian, H. Red-light excited efficient metal-free near-infrared room-temperature phosphorescent films. Natl Sci. Rev. 9, nwab085 (2022).
Ma, X. & Tian, H. Stimuli-responsive supramolecular polymers in aqueous solution. Acc. Chem. Res. 47, 1971–1981 (2014).
Hirata, S. et al. Efficient persistent room temperature phosphorescence in organic amorphous materials under ambient conditions. Adv. Funct. Mater. 23, 3386–3397 (2013).
Yang, X. & Yan, D. Long-afterglow metal-organic frameworks: reversible guest-induced phosphorescence tunability. Chem. Sci. 7, 4519–4526 (2016).
Guo, J., Yang, C. & Zhao, Y. Long-lived organic room-temperature phosphorescence from amorphous polymer systems. Acc. Chem. Res. 55, 1160–1170 (2022).
Tao, S. et al. Design of metal-free polymer carbon dots: a new class of room-temperature phosphorescent materials. Angew. Chem. Int. Ed. 57, 2393–2398 (2018).
Ye, W. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539–1544 (2021).
Zhang, Z.-Y. & Liu, Y. Ultralong room-temperature phosphorescence of a solid-state supramolecule between phenylmethylpyridinium and cucurbit 6 uril. Chem. Sci. 10, 7773–7778 (2019).
Kuila, S. & George, S. J. Phosphorescence energy transfer: ambient afterglow fluorescence from water-processable and purely organic dyes via delayed sensitization. Angew. Chem. Int. Ed. 59, 9393–9397 (2020).
Zhou, Q., Yang, C. & Zhao, Y. Dynamic organic room-temperature phosphorescent systems. Chem 9, 2446–2480 (2023).
Xie, Y. & Li, Z. The development of mechanoluminescence from organic compounds: breakthrough and deep insight. Mater. Chem. Front. 4, 317–331 (2020).
Chen, B., Huang, W. & Zhang, G. Observation of chiral-selective room-temperature phosphorescence enhancement via chirality-dependent energy transfer. Nat. Commun. 14, 1514 (2023).
Hirata, S. & Vacha, M. Circularly polarized persistent room-temperature phosphorescence from metal-free chiral aromatics in air. J. Phys. Chem. Lett. 7, 1539–1545 (2016).
Han, J., You, J., Li, X., Duan, P. & Liu, M. Full-color tunable circularly polarized luminescent nanoassemblies of achiral AIEgens in confined chiral nanotubes. Adv. Mater. 29, 1606503 (2017).
Zhan, L. et al. A simple organic molecule realizing simultaneous TADF, RTP, AIE, and mechanoluminescence: understanding the mechanism behind the multifunctional emitter. Angew. Chem. Int. Ed. 58, 17651–17655 (2019).
Sun, H., Shen, S. & Zhu, L. Photo-stimuli-responsive organic room-temperature phosphorescent materials. ACS Mater. Lett. 4, 1599–1615 (2022).
Yang, Y. et al. Tunable photoresponsive behaviors based on triphenylamine derivatives: the pivotal role of π-conjugated structure and corresponding application. Adv. Mater. 33, 2104002 (2021).
Huang, Z., He, Z., Ding, B., Tian, H. & Ma, X. Photoprogrammable circularly polarized phosphorescence switching of chiral helical polyacetylene thin films. Nat. Commun. 13, 7841 (2022).
Gu, L. et al. Dynamic ultralong organic phosphorescence by photoactivation. Angew. Chem. Int. Ed. 57, 8425–8431 (2018).
Xiong, S. et al. Achieving tunable organic afterglow and UV-irradiation-responsive ultralong room-temperature phosphorescence from pyridine-substituted triphenylamine derivatives. Adv. Mater. 35, 2301874 (2023).
Qian, C. et al. More than carbazole derivatives activate room temperature ultralong organic phosphorescence of benzoindole derivatives. Adv. Mater. 34, 2200544 (2022).
Jia, X. et al. Photoexcitation-controlled self-recoverable molecular aggregation for flicker phosphorescence. Proc. Natl Acad. Sci. 116, 4816–4821 (2019).
Tao, Y. et al. Resonance-induced stimuli-responsive capacity modulation of organic ultralong room temperature phosphorescence. J. Am. Chem. Soc. 144, 6946–6953 (2022).
Su, Y. et al. Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption. Sci. Adv. 4, eaas9732 (2018).
Dou, X. et al. Advances in polymer-based organic room-temperature phosphorescence materials. Adv. Funct. Mater. 34, 2314069 (2024).
Wang, J., Lou, X.-Y., Wang, Y., Tang, J. & Yang, Y.-W. Recent advances of polymer-based pure organic room temperature phosphorescent materials. Macromol. Rapid Commun. 42, 2100021 (2021).
Doi, M., Ishige, R. & Ando, S. Long-lived luminescence emitted from imide compounds dispersed in polymer matrices after continuous ultraviolet irradiation and its relation to oxygen quenching. Chemphotochem 7, e202200310 (2023).
Louis, M. et al. Blue-light-absorbing thin films showing ultralong room-temperature phosphorescence. Adv. Mater. 31, 1807887 (2019).
Gmelch, M., Thomas, H., Fries, F. & Reineke, S. Programmable transparent organic luminescent tags. Sci. Adv. 5, eaau7310 (2019).
Chen, Q. et al. Long lifetimes white afterglow in slightly crosslinked polymer systems. Nat. Commun. 15, 2947 (2024).
Li, T. et al. Long-lived dynamic room temperature phosphorescent carbon dots for advanced sensing and bioimaging applications. Coord. Chem. Rev. 516, 215987 (2024).
Li, H. et al. Achieving stimuli-responsive amorphous organic afterglow in single-component copolymer through self-doping. J. Am. Chem. Soc. 13, 7343–7351 (2023).
Wan, K. et al. Sustainable afterglow room-temperature phosphorescence emission materials generated using natural phenolics. Angew. Chem. Int. Ed. 61, e202202760 (2022).
Zhang, X. et al. Ultralong phosphorescence cellulose with excellent anti-bacterial, water-resistant and ease-to-process performance. Nat. Commun. 13, 1117 (2022).
Shi, M. et al. Confinement-modulated clusterization-triggered time-dependent phosphorescence color from xylan-carbonized polymer dots. J. Am. Chem. Soc. 146, 1294–1304 (2023).
Cai, S. et al. Ultralong organic phosphorescent foams with high mechanical strength. J. Am. Chem. Soc. 143, 16256–16263 (2021).
Sun, Y. et al. Ultralong lifetime and efficient room temperature phosphorescent carbon dots through multi-confinement structure design. Nat. Commun. 11, 5591 (2020).
Zhai, Y. et al. Room temperature phosphorescence from natural wood activated by external chloride anion treatment. Nat. Commun. 14, 2614 (2023).
Wan, K. et al. Structural materials with afterglow room temperature phosphorescence activated by lignin oxidation. Nat. Commun. 13, 5508 (2022).
Yuan, J. et al. Sustainable afterglow materials from lignin inspired by wood phosphorescence. Cell Rep. Phys. Sci. 2, 100542 (2021).
Cao, M. et al. Producing naturally degradable room-temperature phosphorescent materials by covalently attaching lignin to natural polymers. Cell Rep. Phys. Sci. 5, 101811 (2024).
Yin, W. et al. Producing sustainable room temperature phosphorescent materials using natural wood and sucrose. Cell Rep. Phys. Sci. 5, 101792 (2024).
Cao, M. et al. Biobased and biodegradable films exhibiting circularly polarized room temperature phosphorescence. Nat. Commun. 15, 2375–2375 (2024).
Guo, H. et al. Photocured room temperature phosphorescent materials from lignosulfonate. Nat. Commun. 15, 1590–1590 (2024).
Nagarajan, V., Mohanty, A. K. & Misratt, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 4, 2899–2916 (2016).
Chung, Y. et al. A renewable lignin-lactide copolymer and application in biobased composites. ACS Sustain. Chem. Eng. 1, 1231–1238 (2013).
Chile, L., Kaser, S. J., Hatzikiriakos, S. G. & Mehrkhodavandi, P. Synthesis and thermorheological analysis of biobased lignin-graft-poly(lactide) copolymers and their blends. ACS Sustain. Chem. Eng. 6, 1650–1661 (2018).
Boarino, A., Schreier, A., Leterrier, Y. & Klok, H.-A. Uniformly dispersed poly(lactic acid)-grafted lignin nanoparticles enhance antioxidant activity and UV-barrier properties of poly(lactic acid) packaging films. Acs. Appl. Polym. Mater. 4, 4808–4817 (2022).
Zhang, H. et al. Clusterization-triggered emission: uncommon luminescence from common materials. Mater. Today 32, 275–292 (2020).
Zhang, Y. et al. π-π interaction-induced organic long-wavelength room-temperature phosphorescence for in vivo atherosclerotic plaque imaging. Angew. Chem. Int. Ed. 63, e202313890 (2024).
Liu, R., Jiang, T., Liu, D. & Ma, X. A facile and green strategy to obtain organic room-temperature phosphorescence from natural lignin. Sci. China Chem. 65, 1100–1104 (2022).
Nie, X. et al. Broad-band visible-light excitable room-temperature phosphorescence via polymer site-isolated dye aggregates. Adv. Opt. Mater. 10, 2200099 (2022).
Liu, X. et al. Selective removal of phenolic compounds by peroxydisulfate activation: inherent role of hydrophobicity and interface ROS. Environ. Sci. Technol. 56, 2665–2676 (2022).
Liu, X. et al. A photosensitive sustainable lignin nanoplatform for multimodal image-guided mitochondria-targeted photodynamic and photothermal therapy. Mater. Today Chem. 26, 101000 (2022).
Acknowledgements
Z.C. wishes to thank the National Natural Science Foundation of China (31890774) and Fundamental Research Funds for the Central Universities (2572022CG02). T.D.J. wishes to thank the University of Bath and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD01) for support.
Author information
Authors and Affiliations
Contributions
Conceptualization: Z.C. and T.D.J.; Methodology: J.Z., B.T., Y.Z., M.W., and S.Li.; Investigation: J.Z., Y.Z., M.W., and S.Li.; Visualization: J.Z., Y.Z., M.W., S.Li., J.L., and S.Liu.; Supervision: Z.C.; Writing-original draft: All authors; Writing-review and editing: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, J., Tian, B., Zhai, Y. et al. Photoactivated room temperature phosphorescence from lignin. Nat Commun 15, 7198 (2024). https://doi.org/10.1038/s41467-024-51545-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51545-w
This article is cited by
-
Solvent-free processing of lignin into robust room temperature phosphorescent materials
Nature Communications (2025)