Abstract
The reinforcing nature of social interactions is necessary for the maintenance of appropriate social behavior. However, the neural substrates underlying social reward processing and how they might differ based on the sex and internal state of the animal remains unknown. It is also unclear whether these neural substrates are shared with those involved in nonsocial rewarding processing. We developed a fully automated, two choice (social-sucrose) operant assay in which mice choose between social and nonsocial rewards to directly compare the reward-related behaviors associated with two competing stimuli. We performed cellular resolution calcium imaging of medial prefrontal cortex (mPFC) neurons in male and female mice across varying states of water restriction and social isolation. We found that mPFC neurons maintain largely non-overlapping, flexible representations of social and nonsocial reward that vary with internal state in a sex-dependent manner. Additionally, optogenetic manipulation of mPFC activity during the reward period of the assay disrupted reward-seeking behavior across male and female mice. Thus, using a two choice operant assay, we have identified sex-dependent, non-overlapping neural representations of social and nonsocial reward in the mPFC that vary with internal state and that are essential for appropriate reward-seeking behavior.
Similar content being viewed by others
Introduction
Animals, including humans, are capable of flexibly choosing between various rewarding stimuli such as food, water, and social interactions. Although considerable progress has been made toward understanding the neural substrates underlying nonsocial reward-related behaviors, less is known about social reward-related behaviors due to the inherent complexity of social interactions1. Across species, affiliative social interactions appear to engage the same neural circuits, including the canonical reward-related mesolimbic system, as nonsocial rewards2,3,4,5. Additionally, social interactions, like nonsocial rewards, can act as positive reinforcers, with rodents returning to the context of a social interaction6,7 and engaging in operant tasks for access to a conspecific8,9,10,11,12. Despite these commonalities, it is unclear if social and nonsocial reward-related information is encoded by the same or distinct populations of neurons in various brain regions. Some lines of evidence support the common currency view13,14, which proposes that the same reward circuitry is used to process both social and nonsocial rewards. For example, neurons in the primate amygdala respond similarly to both juice and pictures of monkeys15. In contrast, other evidence supports social specificity, the idea that social stimuli are processed by distinct populations of neurons in the brain16,17,18. Thus, it remains unresolved whether social and nonsocial reward-related behaviors are mediated by shared or separate neuronal populations, specifically in the medial prefrontal cortex (mPFC).
The mPFC is a central node in an interconnected network of brain regions that has been implicated in both nonsocial reward processing19,20,21,22,23,24 and a wide range of social behaviors25,26,27,28,29,30. While the role of the mPFC has been extensively studied in the context of nonsocial reward-related behaviors19,20,21,22,23,24, less is known regarding its contribution to social reward-seeking. In rodents, recent work has shown that mPFC neurons are active in the proximity of conspecifics and show increased synchrony when mice engage in affiliative social interactions25,29,31. Furthermore, mPFC dysfunction is thought to underlie the social behavioral deficits associated with Autism Spectrum Disorders (ASD)32,33,34. These social behavioral deficits are often accompanied by deficits in the evaluation and processing of nonsocial reward-related stimuli35,36,37. Consequently, determining whether social and nonsocial rewards share neural substrates within the mPFC has important implications for improving therapeutic options for individuals with ASD.
The internal state of an animal also plays a large role in determining its motivation to engage in certain rewarding behaviors and the perceived value of the chosen reward38,39,40. While there is emerging literature to suggest that brainwide changes in neural dynamics result from changes in internal state through different types of deprivation such as thirst41 and social isolation42, how the internal state of an animal modulates reward representations in the mPFC is poorly understood. Despite evidence that sex differences may contribute to how animals engage with and process rewarding stimuli, sex is an often overlooked component when determining how an animal’s internal state modulates reward-related behaviors. In fact, recent studies have shown that male and female mice exhibit divergent learning and decision-making strategies in reward-related behaviors22,43,44. Moreover, sex differences in mPFC reward representations were shown to underlie some of the behavioral differences observed in nonsocial reward-seeking45. However, it is unknown if these sex differences extend to social reward-related behaviors. Additionally, even though studies have shown differential impacts of thirst and social isolation on reward-related behaviors in male versus female mice, it is unclear if there are sex differences in how reward representations are affected by varying internal state.
To address these questions, we developed a two choice (social-sucrose) operant assay in which mice can freely choose between social and sucrose (nonsocial) rewards in order to directly compare these two types of reward-related behavior. We found that, under control conditions (no social, food, or water deprivation), both male and female mice showed a comparable preference for social and sucrose rewards on the two choice assay, with female mice preferring social reward slightly more than sucrose reward. We then sought to determine if distinct ensembles of neurons in the mPFC encode social versus nonsocial rewards. Using cellular resolution calcium imaging, we identified largely non-overlapping ensembles of mPFC neurons that responded to social versus sucrose reward. While the neurons that responded to social reward displayed mostly excitatory responses, the neurons modulated by sucrose reward exhibited both inhibitory and excitatory responses. Interestingly, the neurons that displayed an inhibitory response to sucrose reward were also excited in response to social reward. These social and sucrose reward ensembles were less overlapping in female mice compared to male mice. After characterizing these distinct social and nonsocial reward representations in the mPFC, we optogenetically manipulated mPFC neurons during the reward period of the two choice operant assay and found that it disrupted reward-seeking behavior in both male and female mice. We then examined how varying the internal state of the animal affected reward representations in the mPFC by either water depriving or socially isolating mice prior to imaging them on the two choice operant assay. Water deprived mice of both sexes showed increased sucrose reward-seeking behavior and a greater proportion of mPFC neurons that were modulated by sucrose reward relative to control conditions. By tracking the same neurons across imaging sessions, we found that the newly recruited sucrose reward-responsive neurons were largely derived from previously latent, reward-unresponsive neurons. We also found that acute social isolation differentially affected reward-seeking behavior in male and female mice. However, it did not affect the proportion of mPFC neurons that were modulated by social reward. Instead, social isolation altered the amplitude of the neural responses to social but not sucrose reward in a sex-dependent manner, with increased amplitude in socially-isolated female mice and decreased amplitude in socially-isolated male mice relative to control conditions. Overall, these findings suggest that although male and female mice behave similarly while flexibly choosing between social and nonsocial rewards on a two choice operant assay, there are sex differences in how social and nonsocial rewards are represented and in how changing internal state affects these representations in the mPFC.
Results
Male and female mice demonstrate positive reinforcement for social and sucrose reward stimuli
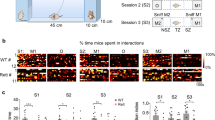
In order to directly compare nonsocial and social reward-related behavior and the neural circuits underlying these behaviors, we developed a self-paced, automated two choice (social-sucrose) operant assay in which mice were trained to associate two separate choice ports with access to either a sucrose reward or a same sex conspecific (social reward) over 1 hour daily sessions (Fig. 1a–c).
a, b Schematic of the automated two choice (social-sucrose) operant assay in which a mouse can freely choose (nose-poke) to obtain either a sucrose (right, top blue panel) or social (right, bottom red panel) reward. c Timeline of an example behavioral session showing social (red squares) and sucrose (blue squares) choices followed by the respective reward consumption (red or blue arrowheads) or failure to enter the reward zone (yellow circles) over an hour. Fully trained male mice (d) complete an equivalent number of successful sucrose and social trials, while female mice (h) complete more successful social than sucrose trials. Paired t test (male: number of trials: p = 0.73; female: p = 4.91*10−4). Male and female mice (e, i) show a similar choice latency for sucrose and social choices. Paired t test (male: p = 0.12; female: p = 0.93). Male mice (f) were slightly faster to consume sucrose reward than social reward, while female mice (j) had equivalent latencies to consume social and sucrose reward. Paired t test (male: p = 2.04*10−8; female: p = 0.093). Male (g) and female (k) mice made fewer social reward fails than sucrose reward fails. Paired t test (male: p = 1.00*10−7; female: p = 4.10*10−6). N = 21 male mice, 12 female mice, 3 behavioral sessions per mouse. l Under full water access conditions, mice run on the two choice operant assay with a social target (SG) completed fewer successful sucrose trials when compared to mice from the object group (OG) and a similar number of successful sucrose trials when compared to mice from the empty cage group (ECG). One-way ANOVA (p = 1.34*10−8) with post-hoc t tests (SG vs OG: p = 8.44*10−9; SG vs ECG: p = 0.052; OG vs ECG: p = 0.10). There was no difference in sucrose choice latency (m), sucrose reward latency (n) or number of sucrose reward fails (o) between groups. One-way ANOVA (m, p = 0.080; n, p = 0.19; o, p = 0.078). p Mice run on the two choice assay with a social target (SG) completed more social trials than mice run with an empty cage (ECG) or an object (OG). One-way ANOVA (p = 3.30*10−10) with post-hoc t tests (SG vs OG: p = 2.63*10−9; SG vs ECG: p = 1.17*10−4; OG vs ECG: p = 0.87). Across all conditions, mice showed similar social choice latency (q) and number of social reward fails (s). One-way ANOVA (q, p = 0.92; s, p = 0.44). r Mice run with a social target (SG) showed similar social reward latency to mice run with an empty cage (ECG) and decreased social reward latency compared to mice run with an object (OG). One-way ANOVA (p = 2.00*10−4) with post-hoc t tests (SG vs OG: p = 1.04*10−4; SG vs ECG: p = 0.46; OG vs ECG: p = 0.19). SG: n = 21 mice, ECG: n = 4 mice, OG: n = 10 mice, 3 sessions per mouse. Box plots: center line denotes median, box edges indicate the 25th and 75th percentiles, and whiskers extend to ±2.7σ. *p < 0.05. Behavioral assay schematic (a) was created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en. Mouse schematic (b) adapted from Open Clipart by lemmling.
Initially, mice on restricted water access are trained to associate nose-poking a choice port with sucrose reward delivery at a reward port located on the adjacent wall of the operant chamber through a series of increasingly difficult assays (Supplementary Fig. 1 and see Methods). Mice showed a consistent decrease in the time from nose-poking the choice port to accessing the reward port (sucrose reward latency) with training (Supplementary Fig. 1h: one-way ANOVA with post-hoc t tests, p = 4.01*10−8, for detailed statistics see Supplementary Data 1). During the final training stage, a social component was added to the assay to allow mice to choose between a social and sucrose reward (Supplementary Fig. 1a, Supplementary Fig. 2). At the start of each trial on the two choice operant assay, two choice ports on one wall of the operant chamber illuminate (Fig. 1b, trial start). Nose-poking one of the illuminated choice ports gives mice access to 10 μl of 10% sucrose at the sucrose reward port for up to 8 s (Fig. 1b, sucrose trial, blue panel), while nose-poking the other illuminated choice port opens an automated gate to give mice access to a novel, same sex conspecific (Fig. 1b, social trial, red panel). Both male and female mice showed a decrease in their social reward latency across five training sessions on the two choice operant assay, as mice learned to associate the social choice port with a social reward (Supplementary Fig. 2g, o: one-way ANOVA with post-hoc t tests, male: p = 8.19*10−6, female: p = 0.016). Male mice also showed a decrease in social reward fails, defined as failure to enter the social zone when the gate is open, with training (Supplementary Fig. 2h: one-way ANOVA with post-hoc t tests, p = 0.0011). Additionally, both sexes showed a stable number of successful social trials across five training sessions (Supplementary Fig. 2e, m: one-way ANOVA with post-hoc t tests, male: p = 0.11, female: p = 0.98).
After five training sessions on the two choice operant assay, mice were removed from restricted water access and run for an additional five sessions (post-training, full water access). Post-training, we found that male mice in control conditions (not water, food, or socially deprived) completed an equivalent number of successful sucrose and social trials (Fig. 1d: paired t test, p = 0.73), while female mice completed slightly more successful social than sucrose trials (Fig. 1h: paired t test, p = 4.91*10−4) at comparable choice latencies (Fig. 1e, i: paired t test, male: p = 0.12, female: p = 0.93). Male mice were slightly slower to engage with social compared to sucrose reward (Fig. 1f, paired t test, p = 2.04*10−8), likely reflecting their longer exposure to sucrose reward across all training assays. In contrast, female mice demonstrated equivalent reward latencies for both trial types (Fig. 1j: paired t test, p = 0.093). Male and female mice made more sucrose reward fails than social reward fails (Fig. 1g, k: paired t test, male: p = 1.0*10−7, female: p = 4.10*10−6).
Although female mice completed slightly more successful social trials and slightly fewer successful sucrose trials than male mice (Supplementary Fig. 3i: two-factor ANOVA with sex (male/female) and trial type (sucrose/social) as factors, interaction: p = 8.00*10−4, sex: p = 0.78, trial type: p = 0.0035, with post-hoc unpaired t tests comparing sex within trial type, sucrose: p = 0.011, social: p = 0.029), both sexes completed significantly more social and significantly fewer sucrose trials when compared to partial water access conditions during training (Supplementary Fig. 3a, e: two-factor ANOVA with water condition (PW/FW) and trial type (sucrose/social) as factors; male — interaction: p = 2.63*10−26, water condition: p = 9.54*10−13, trial type: p = 1.22*10−27, with post-hoc unpaired t tests comparing water condition within trial type, sucrose: p = 1.04*10−20, social: p = 2.32*10−5; female — interaction: p = 4.93*10−20, water condition: p = 5.76*10−6, trial type: p = 3.02*10−9, with post-hoc unpaired t tests comparing water condition within trial type, sucrose: p = 4.49*10−13, social: p = 4.14*10−8). Additionally, male mice displayed shorter reward latencies to consume sucrose reward and made fewer sucrose reward fails compared to female mice (Supplementary Fig. 3k, l: two-factor ANOVA with sex (male/female) and trial type (sucrose/social) as factors, reward latency-interaction: p = 0.032, sex: p = 4.00*10−4, trial type: p = 1.64*10−7, with post-hoc unpaired t tests comparing sex within trial type, sucrose: p = 1.29*10−5, social: p = 0.35; reward fails — interaction: p = 0.010, sex: p = 0.0048, trial type: p = 2.79*10−15, with post-hoc unpaired t tests comparing sex within trial type, sucrose: p = 0.0073, social: p = 0.28), male and female mice otherwise performed similarly when seeking social rewards on the two choice operant assay (Supplementary Fig. 3j). Furthermore, female reward-seeking behavior on the two choice operant assay was not affected by estrous cycle (Supplementary Fig. 6a, b) or mouse strain (Supplementary Fig. 6c–f). These findings suggest that when male and female mice have ad libitum access to water, they are motivated to seek both social and nonsocial rewards (Fig. 1, Supplementary Fig. 3).
Importantly, mice trained on the two choice operant assay without a social target present (empty cage group, ECG) or with a novel object instead of a social target (object group, OG) significantly decreased the number of successful empty cage/object trials, while increasing the number of sucrose trials over five consecutive sessions (Supplementary Fig. 4b, f: one-way ANOVA with post-hoc t tests, number of sucrose trials — ECG: p = 0.01, OG: p = 1.25*10−7; number of social trials — ECG: p = 5.51*10−6, OG: p = 3.0*10−4). When these mice had ad libitum water access, they continued to complete significantly fewer social, but not sucrose, trials when there was no social target (empty cage or object) compared to mice run on the two choice operant assay with a social target (Fig. 1l, p: one-way ANOVA with post-hoc t tests, number of sucrose trials: p = 1.34*10−8; number of social trials: p = 3.30*10−10), which indicates that social reward-seeking behavior is positively reinforced by the presence of a social target and not solely by the gate opening or novelty-seeking behavior (Fig. 1l–s, Supplementary Fig. 4).
To further confirm the goal-directed nature of the social reward-seeking behavior observed on the two choice operant assay, we developed an additional multi-choice assay in which a third choice port was introduced that was not associated with any reward (Supplementary Fig. 5a, b). Mice run on this assay preferentially increased the number of successful social but not null trials completed over time and were significantly faster to enter the corresponding reward zone on sucrose and social trials but not null trials (Supplementary Fig. 5c, d: two-factor ANOVA with training day (day 1/day 9) and trial type (sucrose/social/null) as factors, interaction: p = 0.0059, training day: p = 0.68, trial type: p = 5.02*10−13, with post-hoc unpaired t tests comparing trial type within training day, day 1 — suc v soc: p = 1.02*10−4, suc v null: p = 1.45*10−4, soc v null: p = 0.48, day 9 — suc v soc: p = 0.078, suc v null: p = 5.21*10−4, soc v null: p = 0.013; Supplementary Fig. 5e: two-factor ANOVA with choice type (sucrose/social/null) and reward zone (sucrose/social) as factors, interaction: p = 2.44*10−13, choice type: p = 0.41, reward zone: p = 0.10, with post-hoc unpaired t tests comparing reward zone within choice type, sucrose: p = 2.19*10−8, social: p = 1.82*10−5, null: p = 0.41). Finally, when mice were run on a social extinction assay for six consecutive sessions during which the social target was removed after five consecutive sessions of full water access (FW) on the two choice operant assay (Supplementary Fig. 6g), mice preferentially decreased the number of successful social trials and increased the number of successful sucrose trials (Supplementary Fig. 6h, i: two-factor ANOVA with social condition (FW/extinction) and trial type (sucrose/social) as factors, interaction: p = 0.0047, social condition: p = 0.55, trial type: p = 0.66, with post-hoc paired t tests comparing social condition within trial type social: p = 0.0295, sucrose: p = 0.0063). These findings are among the first to demonstrate that social interaction can promote positive reinforcement of reward-seeking behavior similarly to sucrose consumption in an operant assay.
Cellular resolution imaging of mPFC neurons in male and female mice during the two choice operant assay
To determine what information mPFC neurons encode during social and nonsocial reward-related behavior, we performed cellular resolution calcium imaging of mPFC neurons during the two choice operant assay (Supplementary Movie 1). We injected an AAV expressing the calcium indicator GCaMP6f and implanted a gradient-index (GRIN) lens into the mPFC of 9 male and 6 female mice (Fig. 2a). All lens-implanted mice were then successfully trained on the two choice operant assay. After 3 weeks of training, the lens was coupled to a head-mounted miniscope to record activity of GCaMP6f-expressing mPFC neurons while mice completed the two choice operant assay. CNMFe was used to detect the activity of individual neurons46. Post-training, in full water access conditions, lens-implanted imaging mice were run on a minimum of 3 daily sessions prior to imaging mPFC neural activity in the two choice operant assay. We imaged a total of 459 neurons from 9 male mice and 570 neurons from 6 female mice in control conditions (Fig. 2b, d). GRIN lens placement and viral expression were confirmed on post-hoc histology (Fig. 2c, e).
a Top row: Schematic of the viral strategy used to label mPFC neurons with GCaMP6f. Bottom row: Imaging setup of GRIN lens placement in the mPFC, including representative images showing GRIN lens placement (left), GCaMP6f expression (middle, GCaMP6f in green; DAPI in blue) in the mPFC at (from left to right) 4x, 20x, and 60x magnification. Right panel shows nuclear exclusion of GCaMP6f. Scale bars: 500 µm, 250 µm and 25 µm. A pie chart showing the number of neurons recorded from each male (b, n = 459 neurons, 9 mice) and female mouse (d, n = 570 neurons, 6 mice). Reconstruction of GRIN lens placement in the nine male (c) and six female (e) mice using WholeBrain software84. Each colored line corresponds to the same colored slice in the pie chart and shows the position of the lens in the Allen Mouse Brain Common Coordinate Framework. Coordinates are relative to bregma. f A schematic of the two choice operant assay events to which mPFC neuronal activity was aligned, from left to right, trial start, social choice, sucrose choice, social reward, and sucrose reward. Top row: Heatmaps of average normalized fluorescence of all mPFC neurons that are significantly modulated (excited or inhibited) by each task event in male (g) and female (h) mice. Neurons are sorted by the time of maximum fluorescence across each task event. Bottom row: Average normalized fluorescence traces of the neurons from the corresponding heatmap that are significantly modulated by each task event. Shaded error regions indicate ± SEM. Proportions of total recorded neurons that are modulated by the various task events in male (i) and female (j) mice (male: n = 459 neurons, 9 mice; female: n = 570 neurons, 6 mice). Mouse schematic (f) adapted from Open Clipart by lemmling.
Neurons were classified as task-modulated if the maximum or minimum of their average activity differed from a null distribution generated by randomly shuffling neural activity (see Methods, example task-modulated neurons Supplementary Fig. 7a) within a three second window around the occurrence of a task event (trial start, social or sucrose choice, social or sucrose reward, Fig. 2f)20. We found that many mPFC neurons showed significantly modulated time-locked responses to specific behavioral events in the two choice operant assay (Fig. 2g, h, Supplementary Fig. 7b–e). In fact, across all mPFC neurons recorded in male mice during the two choice operant assay, 3.27% (n = 15/459) were significantly modulated by trial start, 8.71% (n = 40/459) were significantly modulated by social choice, 9.37% (n = 43/459) were significantly modulated by sucrose choice, 29.19% (n = 134/459) were significantly modulated by social reward and 19.17% (n = 88/459) were significantly modulated by sucrose reward (Fig. 2i). Across all mPFC neurons recorded from female mice during the two choice operant assay, 1.05% (n = 6/570) were significantly modulated by trial start, 16.67% (n = 95/570) were significantly modulated by social choice, 9.30% (n = 53/570) were significantly modulated by sucrose choice, 35.44% (n = 202/570) were significantly modulated by social reward and 17.54% (n = 100/570) were significantly modulated by sucrose reward (Fig. 2j). We found similar fractions of task-modulated neurons when using an encoding model to temporally separate calcium activity kernels associated with various behavioral events (Supplementary Fig. 8; proportion z test, male — choice: p = 0.86, reward: p = 0.39; female — choice: p = 0.12, reward: p = 0.15). Previous literature has shown that mPFC neurons respond more to action (i.e., nose-poke, reward consumption) than stimulus (i.e., trial start) events21. Consistent with these findings, we found that a greater proportion of mPFC neurons responded to choice and reward consumption than to trial start (Supplementary Fig. 9a: male — stimulus events: 3.27%, 15/459, action events: 66.45%, 305/459; female — stimulus events: 1.05%, 6/570, action events: 78.95%, 450/570; proportion z test, male and female: p < 0.00001). Additionally, we found that more mPFC neurons were modulated by reward than choice (Supplementary Fig. 9b, c: male — choice: 18.08%, 83/459, reward: 48.37%, 222/459; female — choice: 25.96%, 148/570, reward: 52.98%, 302/570; proportion z test, male and female: p < 0.00001).
Sex differences in selectivity of social and nonsocial reward-related neural representations
Given the well-defined role of the mPFC in decision-making47,48, we next examined the responses of choice-modulated neurons during the two choice assay. We found that choice-modulated neurons reliably showed peak fluorescence around the time of choice port entry and that the timing of peak fluorescence did not vary with reward latency (Fig. 3a, c). In contrast, the timing of the peak fluorescence of reward-modulated neurons varied with reward latency when aligned to choice port entry (Fig. 3b, d). Furthermore, we found that a similar proportion of neurons responded to social (n = 8.71%, 40/459) and sucrose (n = 9.37%, 43/459) choice in male mice (Fig. 2i, proportion z test, p = 0.73), while a significantly greater proportion of neurons responded to social choice (n = 16.67%, 95/570) than sucrose choice (n = 9.30%, 53/570) in female mice (Fig. 2j, proportion z test, p = 2.15*10−4). The neurons that were excited by sucrose choice were more selective for sucrose choice in female mice (Supplementary Fig. 9h: paired t test, p = 3.61*10−6) relative to male mice (Supplementary Fig. 9e: paired t test, p = 0.45), while neurons that were excited by social choice (Supplementary Fig. 9d, g) as well as those that were inhibited by sucrose choice (Supplementary Fig. 9f, i) showed similar responses across both sexes. These findings suggest that mPFC neurons differentially represent social and sucrose choice in male versus female mice.
Normalized fluorescence of an example neuron significantly modulated by social choice aligned to social choice (a) compared to that of an example neuron significantly modulated by social reward aligned to social choice (b). Normalized fluorescence of an example neuron significantly modulated by sucrose choice aligned to sucrose choice (c) compared to that of an example neuron significantly modulated by sucrose reward aligned to sucrose choice (d). Top row shows average fluorescence ± SEM. Bottom row shows a heatmap of normalized fluorescence on each trial aligned to choice (dashed line at zero indicates choice onset) and sorted by reward latency (dark dotted line indicates reward onset). mPFC choice neurons are largely non-overlapping in their responsiveness to social and sucrose choice in both male (e) and female (f) mice (% non-overlapping, male: 83.82%, n = 57/68; female: 93.02%, n = 80/86). g mPFC population activity accurately decoded the subsequent choice earlier in female (green) mice relative to male (purple) mice indicated by shaded gray region. Unpaired t test (−3.0 to −2.5 s: p = 0.0027, −2.5 to −2.0 s: p = 0.0032, −2.0 to −1.5 s: p = 0.019). Choice decoding accuracy in female and male mice becomes equivalent at 1.5 s before choice port entry and both are greater than chance decoding accuracy (colored asterisks and bolded lines indicate where choice decoding is significantly greater than chance). Shaded error regions indicate ± SEM. Dashed line at zero indicates choice port entry. h mPFC population activity in female mice more accurately decoded the choice made on a particular trial compared to male mice. Unpaired t test (p = 0.0090). i mPFC population activity in a 3 s window around social and sucrose choice was equivalently sufficient to decode the sex of the animal and decoded with significantly higher accuracy than shuffled data (dashed line) on both trial types. Unpaired t test (social versus sucrose: p = 0.19; social versus shuffled: p = 2.08*10−19; sucrose versus shuffled: p = 6.84*10−12). j Across all mice, mPFC neural representations resulted in greater decoding accuracy for reward compared to choice. Paired t test (p = 7.90*10−8). N = 9 male mice, 9 female mice. Trial-averaged population neural activity traces of sucrose (blue) and social (red) trials in male (k, n = 459 neurons, 9 mice) and female (m, n = 423 neurons, 5 mice) mice plotted on the first 3 PCs in state space. Arrowhead indicates direction of time. Filled green circle indicates choice onset. Euclidean distance separating PC-projected population vectors in a 3 s window around choice is significantly greater between social and sucrose trials than within each trial type in both male (l, n = 9 mice) and female (n, n = 6 mice) mice. Paired t test (male: p = 5.76*10−4; female: p = 0.0048). All decoding was significantly greater than shuffled data, indicated by a dashed line at 50%. Box plot: center line denotes median, box edges indicate the 25th and 75th percentiles and whiskers extend to ±2.7σ. *p < 0.05.
In fact, we observed that neural representations of social and sucrose choice in the mPFC are largely non-overlapping in both male and female mice and significantly more non-overlapping than expected by chance when compared to a shuffled distribution (Fig. 3e, f: % non-overlapping, male: 83.82%, n = 57/68, shuffled: 66.37 ± 3.29; female: 93.02%, n = 80/86, shuffled: 72.37 ± 2.81, ± standard deviation, unpaired t test, male v shuffled: p = 1.56*10−74; female v shuffled: p = 3.51*10−88; see “Task modulation analysis” in Methods). Although neural representations are non-overlapping in both male and female mice (proportion z test, p = 0.07), we were able to decode subsequent choice at an earlier time point in female mice compared to male mice (Fig. 3g: female: 3.5 s, male: 1.5 s prior to choice). We were also able to decode the choice made by the mouse from mPFC population activity with higher accuracy in female mice compared to male mice (Fig. 3h: average decoding accuracy, male: 56.09 ± 2.23, female: 67.03 ± 2.43; unpaired t test, p = 0.0090). Additionally, we were able to decode the sex of the animal from mPFC population activity associated with choice on both trial types. (Fig. 3i: average decoding accuracy, social choice: 63.70 ± 1.03, sucrose choice: 61.68 ± 1.13; unpaired t test, p = 0.19; Supplementary Fig. 9j: average F1 scores, social choice: 0.61 ± 0.014, sucrose choice: 0.61 ± 0.017; unpaired t test, p = 0.55). The largely non-overlapping nature of the choice responses was corroborated by plotting the neural trajectories of mPFC population activity on the first 3 principal components (PCs) in a 3 s window around choice port entry in both male and female mice (Fig. 3k, m: total variance explained, male: 59.2%, female: 57.6%) and calculating pairwise Euclidean distances of population vectors within trial type versus between trial type (Fig. 3l, n). In both sexes, we found that the Euclidean distance within trial type was significantly less than the distance between trial type across social and sucrose trials (Fig. 3l, n: male — average distance within: 54.71 ± 4.76, between: 60.87 ± 5.13; paired t test, p = 5.76*10−4; female — average distance within: 49.89 ± 2.55, between: 71.93 ± 2.99; paired t test, p = 0.0048). Additionally, we used Mahalanobis distance to determine if social and nonsocial choice responses were different at a population level (Supplementary Fig. 9k, l). We found that there was a significantly larger population response to social choice compared to sucrose choice in male and female mice (Supplementary Fig. 9k, l: unpaired t test, male: p = 1.09*10−4; female: p = 0.0042). Thus, population activity even at a single-trial level showed separable subspace representations of social and nonsocial trials. These findings suggest that mPFC neural activity associated with choice port entry is distinct for social and nonsocial trials despite the mice engaging in similar behaviors (nose-poking a choice port). Consequently, neural representations of social and nonsocial reward-related behaviors are highly selective and sex-dependent even prior to reward consumption.
Since we found that the largest proportion of mPFC neurons was modulated by reward (Supplementary Fig. 9b, c) and that trial type decoding was greater for reward than choice in male and female mice (Fig. 3j: average decoding accuracy, choice: 60.0 ± 2.18, reward: 86.38 ± 2.78; paired t test, p = 7.90*10−8), we further characterized the mPFC reward responses. In both sexes, we found that the majority of neurons that were responsive to social reward were positively modulated or excited by social reward (Fig. 4a, male: 96.27%, n = 129/134; female: 87.62%, n = 177/202), compared to those that were negatively modulated or inhibited by social reward (Fig. 4a, male: 3.73%, n = 5/134; female: 12.38%, n = 25/202; proportion z test, male and female: p < 0.00001). In contrast, in all mice, more neurons were inhibited by sucrose reward (Fig. 4b, male: 64.77%, n = 57/88; female: 60.0%, n = 60/100) than excited by sucrose reward (Fig. 4b, male: 35.23%, n = 31/88; female: 40.0%, n = 40/100; proportion z test, male: p = 8.87*10−5; female: p = 0.0047). Thus, it appears that mPFC neurons differ in their responses to social and nonsocial rewards, with a largely excitatory response to social reward and a mixed inhibitory and excitatory response to sucrose reward.
a In both male (left) and female (right) mice, a larger proportion of mPFC neurons are positively modulated (excited) in response to social reward (male: 28.11%, n = 129/459; female: 31.10%, n = 177/570) compared to those that are negatively modulated (inhibited) by social reward (male: 1.09%, n = 5/459; female: 4.39%, n = 25/570). Proportion z test (male: p < 0.00001; female: p < 0.00001). b In contrast to social reward responses (a), mPFC neurons in male (left) and female (right) mice are more likely to be inhibited (male: 12.42%, n = 57/459; female: 10.53%, n = 60/570) rather than excited (male: 6.75%, n = 31/459; female: 7.02%, n = 40/570) in response to sucrose reward. Proportion z test (male: p = 0.0036; female: p = 0.036). c mPFC reward neurons differ in the selectivity of their reward responsiveness in male mice. Social excite neurons are largely exclusive in their response to social reward (64.34%, n = 83/129), with a significantly smaller subset of social excite neurons also responding to sucrose reward (35.66%, 46/129). Proportion z test (p = 4.08*10−6). In contrast, most sucrose excite neurons also responded to social reward (70.97%, n = 22/31). Around half of sucrose inhibit neurons also responded to social reward (42.11%, n = 24/57). Proportion z test (sucrose excite: p = 9.60*10−4, sucrose inhibit: p = 0.092). d mPFC reward neurons are largely non-overlapping in their reward responsiveness in female mice. Social excite neurons are largely exclusive in their response to social reward (80.62%, n = 104/129), with a significantly smaller subset of social excite neurons also responding to sucrose reward (19.38%, n = 25/129). Proportion z test (p < 0.00001). The majority of sucrose excite neurons (70.0%, n = 28/40) and sucrose inhibit neurons did not respond to social reward (78.3%, n = 47/60). Proportion z test (sucrose excite: p = 3.45*10−4, sucrose inhibit: p = 5.38*10−10). e Largely non-overlapping populations of mPFC neurons respond to social and sucrose reward in both male (n = 73.10%, 125/171) and female (n = 87.75%, 179/204) mice. The populations are more distinct in female mice relative to male mice. Proportion z test (p = 3.11*10−4). f Average responses of social reward excite neurons to social (red) and sucrose (blue) reward in male (left) and female (right) mice. g Average responses of sucrose reward excite neurons to social (red) and sucrose (blue) reward in male (left) and female (right) mice. h Average responses of sucrose reward inhibit neurons to social (red) and sucrose (blue) reward in male (left) and female (right) mice. i Heatmaps of the average fluorescence of mPFC neurons in male (left, n = 129 neurons) and female (right, n = 129 neurons) mice that are significantly excited by social reward aligned to social reward (top row) and sucrose reward (bottom row). Neurons are sorted by the time of peak response to social reward. j Heatmaps of the average fluorescence of mPFC neurons in male (left, n = 31 neurons) and female (right, n = 40 neurons) mice that are significantly excited by sucrose reward aligned to social reward (top row) and sucrose reward (bottom row). Neurons are sorted by the time of peak response to sucrose reward. k Heatmaps of the average fluorescence of mPFC neurons in male (left, n = 57 neurons) and female (right, n = 60 neurons) mice that are significantly inhibited by sucrose reward aligned to social reward (top row) and sucrose reward (bottom row). Neurons are sorted by the time of minimum response to sucrose reward. A row on the top and bottom panels of each heatmap (i, j, k) corresponds to the same neuron. l Comparison of the peak amplitude of responses of social reward excite neurons to social and sucrose reward in male (purple, n = 129 neurons, 9 mice) and female (green, n = 129 neurons, 5 mice) mice shows that these neurons on average have a robust excitatory response to social but not sucrose reward in both sexes. Two-factor ANOVA with sex and trial type as factors (interaction: p = 0.067, sex: p = 0.17, trial type: p = 4.11*10-58) with post-hoc t tests comparing trial type within sex (male: p = 1.20*10−33, female: p = 4.15*10−26). m Comparison of the peak amplitude of responses of sucrose reward excite neurons to social and sucrose reward in male (purple, n = 31 neurons, 9 mice) and female (green, n = 40 neurons, 5 mice) mice shows that sucrose reward excite neurons are selective for sucrose reward in female but not male mice. Two-factor ANOVA with sex and trial type as factors (interaction: p = 0.0042, sex: p = 0.014, trial type: p = 1.87*10−4) with post-hoc t tests comparing trial type within sex (male: p = 0.58, female: p = 4.79*10−9). n Comparison of the peak amplitude of responses of sucrose reward inhibit neurons to social and sucrose reward in male (purple, n = 57 neurons, 9 mice) and female (green, n = 60 neurons, 5 mice) mice shows that these neurons on average have a higher amplitude response to social reward compared to sucrose reward across both sexes. Two-factor ANOVA with sex and trial type as factors (interaction: p = 0.011, sex: p < 0.00001, trial type: p = 0.057) with post-hoc t tests comparing trial type within sex (male: p = 5.48*10−18, female: p = 7.60*10−12).
Next, we examined the selectivity of mPFC reward responses by determining the overlap between these populations. In both male (Fig. 4c) and female (Fig. 4d) mice, largely non-overlapping populations of mPFC neurons responded to social and sucrose reward, significantly more non-overlapping than expected by chance when compared to a shuffled distribution (% non-overlapping: male: 73.10%, n = 125/171, shuffled: 61.15 ± 1.45; female: 87.75%, n = 179/204, shuffled: 72.20 ± 1.47, ± standard deviation, unpaired t test, male v shuffled: p = 4.82*10−93; female v shuffled: p = 9.50*10−110, see “Task modulation analysis” in Methods). The social and nonsocial reward ensembles in the mPFC were even more distinct in female mice relative to male mice (Fig. 4e: proportion z test, p = 3.11*10−4). Thus, both male and female mice showed largely non-overlapping social and nonsocial reward responses in the mPFC.
We then evaluated how social and sucrose reward-responsive neurons were modulated by the alternative reward stimulus. We compared the social and sucrose reward responses of neurons that were excited by social reward (Fig. 4f, i, l), excited by sucrose reward (Fig. 4g, j, m) and inhibited by sucrose reward (Fig. 4h, k, n). Both social reward excite and sucrose reward inhibit neurons showed significantly higher responses to social reward compared to sucrose reward in male and female mice (Fig. 4l, n: two-factor ANOVA with sex (male/female) and trial type (sucrose/social) as factors, social excite — interaction: p = 0.067, sex: p = 0.17, trial type: p = 4.11*10−58, with post-hoc unpaired t tests comparing trial type within sex, male: p = 1.20*10−33, female: p = 4.15*10−26; sucrose inhibit — interaction: p = 0.011, sex: p < 0.00001, trial type: p = 0.057, with post-hoc unpaired t tests comparing trial type within sex, male: p = 5.48*10−18, female: p = 7.60*10−12). In contrast, sucrose reward excite neurons showed significantly higher responses to sucrose reward relative to social reward in female mice but not male mice (Fig. 4m: two-factor ANOVA with sex (male/female) and trial type (sucrose/social) as factors, interaction: p = 0.0042, sex: p = 0.014, trial type: p = 1.87*10−4 with post-hoc unpaired t tests comparing trial type within sex, male: p = 0.58, female: p = 4.76*10−9). These sex differences in reward selectivity were further supported by evidence that reward decoding accuracy is greater in female mice than in male mice (Fig. 5b: average decoding accuracy, male: 82.54 ± 3.71, female: 93.29 ± 1.47; unpaired t test, p = 0.022). Additionally, mPFC activity during the reward period could decode animal sex with greater accuracy on social trials compared to sucrose trials (Fig. 5a: average decoding accuracy, social: 79.45 ± 1.34, sucrose: 68.10 ± 1.74; unpaired t test, p = 1.70*10−6; Supplementary Fig. 7f: average F1 scores, social: 0.77 ± 0.020, sucrose: 0.63 ± 0.026; unpaired t test, p = 4.88*10−5). We used Mahalanobis distance to determine if social and nonsocial reward responses were different at a population level (Fig. 5c, d). We found that there was a significantly larger population response to social rewards compared to sucrose rewards in male and female mice (Fig. 5c, d: unpaired t test, male: p = 1.71*10−8; female: p = 2.07*10−5). Sucrose and social reward trials also occupy distinct neural subspaces (Fig. 5e, f, left panel: total variance explained, male: 63.8%; female: 62.8%; Supplementary Fig. 7g, h; Supplementary Movie 2) in both sexes as measured by Euclidean distance between and within trial type population vectors (Fig. 5e, f, right panel: male — average distance within: 45.63 ± 4.21, between: 55.14 ± 5.19; paired t test, p = 5.81*10−4; female — average distance within: 47.16 ± 3.22, between: 67.17 ± 5.80; paired t test, p = 0.0020). Across sexes, PC1 weights are higher for neurons classified as either social or sucrose reward-responsive compared to those that are reward unresponsive, further supporting the encoding of distinct social and nonsocial reward information at the population level in the mPFC (Fig. 5e, f, middle panel: one-way ANOVA with post hoc t tests, male: p = 9.22*10−17, female: p = 2.93*10−8). Non-overlapping social and nonsocial reward representations in the mPFC were also seen in mice that were passively exposed to either a social target or sucrose solution (Supplementary Fig. 10m-o) and when the nonsocial reward stimulus was water instead of sucrose (Supplementary Fig. 10a-l).
a mPFC population activity in a 3 s window around reward was able to decode the sex of the animal with higher accuracy on social than on sucrose trials. Unpaired t test (p = 1.70*10−6). N = 9 male mice, 5 female mice. b Decoders trained on female (n = 5 mice) mPFC neural reward responses decoded the reward type with higher accuracy than decoders trained on male (n = 9 mice) reward responses. Unpaired t test (p = 0.022). Decoding accuracy was calculated for each animal from all recorded neurons, with a trial-matched number of sucrose and social trials. All decoding was significantly greater than shuffled data, indicated by a dashed line at 50%. Mahalanobis distance was greater for social than sucrose reward in male (c, n = 101 social trials, 153 sucrose trials, 9 mice) and female (d, 104 social trials, 83 sucrose trials, 5 mice) mice. Unpaired t test (male: p = 1.71*10−8; female: p = 2.07*10−5). Trial-averaged population neural activity traces of sucrose (blue) and social (red) reward trials in male (e, left panel, n = 459 neurons, 9 mice) and female (f, left panel, n = 423 neurons, 5 mice) mice plotted on the first 3 PCs in state space. Arrowhead indicates direction of time. Filled green circle indicates reward onset. PC1 weights are higher for both social and sucrose reward neurons compared to other neurons in both male (e, middle panel) and female (f, middle panel) mice. One-way ANOVA (male: p = 9.22*10−17; female: p = 2.93*10−8) with post-hoc t tests (male — soc v other: p = 2.46*10−17, suc v other: p = 3.14*10−6, soc v suc: p = 0.076; female — soc v other: p = 6.21*10−7, suc v other: p = 2.11*10−5, soc v suc: p = 0.96). Euclidean distance separating PC-projected population vectors in a 3 s window around reward is significantly greater between social and sucrose trials than within each trial type in both male (e, right panel) and female (f, right panel) mice. Paired t test (male: p = 5.81*10−4; female: p = 0.0020). *p < 0.05. Shaded error regions indicate ± SEM. Dashed line at zero indicates reward onset. Box plots: center line denotes median, box edges indicate the 25th and 75th percentiles and whiskers extend to ±2.7σ.
These data suggest that in male mice, reward-responsive neurons fall into three broad categories: one that is exclusively excited by social reward (Fig. 4f, i, l: left column), a second that is excited by both social and sucrose reward (Fig. 4g, j, m: left column) and a third that is excited by social reward and inhibited by sucrose reward (Fig. 4h, k, n: left column). However, in female mice, there is increased selectivity amongst the categories of responses such that the non-specific reward excite category seen in male mice is exclusively excited by sucrose reward in female mice (Fig. 4g, j, m: right column). The sex-dependent differences in social and nonsocial reward representations in the mPFC are also observed at the population level.
Optogenetic manipulation of mPFC neurons disrupts reward-seeking behavior
Since we found that the largest proportion of mPFC neurons were reward-responsive during the two choice operant assay, we then wanted to determine if optogenetic activation of mPFC activity during the reward period could alter reward-seeking behavior. We injected male and female mice with channelrhodopsin (ChR2) or GFP (control), implanted ferrules bilaterally in the mPFC (Fig. 6b, c) and then trained these mice on the two choice assay (Fig. 6a). Once mice were trained, we used blue light to activate mPFC neurons during the reward period on a random 50% of trials over the course of 7 sessions (Supplementary Fig. 11a). We found that activation of mPFC neurons during the reward period increased both reward latency and number of reward fails on both trial types when compared to controls across sexes (Fig. 6d, e, h, i: two-factor ANOVA with virus (ChR2/GFP) and trial type (sucrose/social) as factors, male: reward latency — interaction: p = 0.21, virus: p = 7.52*10−9, trial type: p = 5.62*10−13 with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 1.95*10−6, social: p = 5.51*10−5; reward fails — interaction: p = 6.57*10−6, virus: p = 2.38*10−10, trial type: p = 3.21*10−8 with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 2.83*10−8, social: p = 0.0042; female: reward latency — interaction: p = 0.064, virus: p = 4.09*10−13, trial type: p = 2.05*10−19, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 7.92*10−10, social: p = 3.81*10−7; reward fails — interaction: p = 0.0026, virus: p = 1.14*10−9, trial type: p = 0.0002, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 9.98*10−7, social: p = 1.63*10−4). We also found that optogenetic activation of mPFC neurons increased the average distance traveled by male and female mice in the operant arena during the reward period (Fig. 6f, j: two-factor ANOVA with virus (ChR2/GFP) and trial type (sucrose/social) as factors, male — interaction: p = 0.19, virus: p = 1.41*10−18, trial type: p = 5.50*10−98, with post-hoc unpaired t tests comparing virus within trial types, sucrose: p = 3.67*10−15, social: p = 3.64*10−8; female — interaction: p = 0.97, virus: p = 2.00*10−11, trial type: p = 9.23*10−114, with post-hoc unpaired t tests comparing virus within trial types, sucrose: p = 2.71*10−6, social: p = 2.75*10−6). This effect on distance traveled was not seen on trials without laser stimulation in male mice or on sucrose trials without laser stimulation in female mice (Supplementary Fig. 11d, i: two-factor ANOVA with virus (ChR2/GFP) and trial type (sucrose/social) as factors, male — interaction: p = 0.15, virus: p = 0.037, trial type: p = 4.71*10−89; female — interaction: p = 0.084, virus: p = 0.0052, trial type: p = 5.49*10−120, with post-hoc unpaired t tests comparing virus within trial types, sucrose: p = 0.86, social: p = 0.0076). Activating mPFC neurons outside of the reward period had no effect on the average distance traveled by mice in the operant arena (Supplementary Fig. 11f, k: two-factor ANOVA with virus (ChR2/GFP) and stimulation (on/off) as factors, male — interaction: p = 0.55, virus: p = 0.28, stimulation: p = 0.74; female — interaction: p = 0.36, virus: p = 7.46*10−4, stimulation: p = 0.10), which suggests that the effects on distance traveled observed during the reward period are not solely the result of increased motor behavior due to mPFC activation. Additionally, we found that mPFC activation caused ChR2-expressing mice to spend less time in the social zone during stimulated social trials compared to controls (Fig. 6g, k: unpaired t test, male: p = 0.015; female: p = 2.69*10−6). This effect was also seen in female, but not male mice, in trials without laser stimulation (Supplementary Fig. 11e, j: unpaired t test, male: p = 0.29; female: p = 8.02*10−4). These findings are consistent with decreased social investigation observed with optogenetic activation of mPFC neurons in previous studies25,26.
a Experimental timeline showing training and optogenetic stimulation schedule. b Schematic of the viral strategy (left panel) used to label mPFC neurons with either channelrhodopsin (ChR2) or GFP (control). Example histology showing ChR2 expression (ChR2 in green, DAPI labeling of cell nuclei in blue) and ferrule placement in the mPFC at 4x magnification (right panel). Scale bar: 500 µm. c Reconstruction of optic ferrule placement in male (left, n = 14 mice) and female (right, n = 14 mice) mice using WholeBrain software84. Each colored dot shows the position of the optic ferrule in the Allen Mouse Brain Common Coordinate Framework. Blue dots indicate ChR2 mice (male: n = 8 mice; female: n = 8 mice), black dots indicate GFP mice (male: n = 6 mice; female: n = 6 mice). Coronal slice is 1.945 mm anterior to bregma. Optogenetic activation of mPFC neurons during the reward period increases reward latency (d, h), reward fails (e, i) and average distance traveled (f, j) on sucrose and social trials compared to GFP controls in both male (d, e, f) and female (h, i, j) mice. Two-factor ANOVA with virus (ChR2/GFP) and trial type (sucrose/social) as factors (d, interaction: p = 0.21, virus: p = 7.52*10−9, trial type: p = 5.62*10−13; e, interaction: p = 6.57*10−6, virus: p = 2.38*10−10, trial type: p = 3.21*10−8; f, interaction: p = 0.12, virus: p = 1.41*10−18, trial type: p = 5.50*10−98; h, interaction: p = 0.064, virus: p = 4.09*10−13, trial type: p = 2.05*10−19; i, interaction: p = 0.0026, virus: p = 1.14*10−9, trial type: p = 0.0002; j, interaction: p = 0.97, virus: p = 2.00*10−11, trial type: p = 9.23*10−114) with post-hoc unpaired t tests comparing virus within trial type (d, sucrose: p = 1.95*10−6, social: p = 5.51*10−5; e, sucrose: p = 2.83*10−8, social: p = 0.0042; f, sucrose: p = 3.67*10−15, social: p = 3.64*10−8; h, sucrose: p = 7.92*10−10, social: p = 3.81*10−7; i, sucrose: p = 9.98*10−7, social: p = 1.63*10−4; j, sucrose: p = 2.71*10−6, social: p = 2.75*10−6). Optogenetic activation of mPFC neurons also caused a decrease in the time spent in the social zone during the reward period when compared to GFP controls in male (g) and female (k) mice. Unpaired t test (male: p = 0.015; female: p = 2.69*10−6). l Schematic of the viral strategy (left panel) used to label mPFC neurons with either GtACR inhibitory opsin (GtACR) or mCherry (control). Example histology showing GtACR expression (GtACR in red, DAPI labeling of cell nuclei in blue) and ferrule placement in the mPFC at 4x magnification (right panel). Scale bar: 500 µm. m Reconstruction of optic ferrule placement in male (left, n = 13 mice) and female (right, n = 13 mice) mice using WholeBrain software84. Each colored dot shows the position of the optic ferrule in the Allen Mouse Brain Common Coordinate Framework. Red dots indicate GtACR mice (male: n = 7 mice; female: n = 7 mice), black dots indicate mCherry mice (male: n = 6 mice; female: n = 6 mice). Coronal slice is 1.945 mm anterior to bregma. Optogenetic inhibition of mPFC neurons during the reward period increases reward latency on both social and sucrose trials compared to mCherry controls in male (n) and female (r) mice. Two-factor ANOVA with virus (GtACR/mCherry) and trial type (sucrose/social) as factors (n, interaction: p = 0.67, virus: p = 0.0004, trial type: p < 0.00001; r, interaction: p = 0.83, virus: p = 2.46*10−8, trial type: p = 2.29*10−12) with post-hoc unpaired t tests comparing virus within trial type (n, sucrose: p = 0.0014, social: p = 0.021; r, sucrose: p = 5.46*10−10, social: p = 0.0014). Optogenetic inhibition also increases the number of social reward fails compared to mCherry controls in male mice (o) and both sucrose and social reward fails in female mice (s). Two-factor ANOVA with virus (GtACR/mCherry) and trial type (sucrose/social) as factors (o, interaction: p = 0.50, virus: p = 0.0039, trial type: p = 0.37; s, interaction: p = 0.055, virus: p = 0.0002, trial type: p = 0.0001) with post-hoc unpaired t tests comparing virus within trial type (o, sucrose: p = 0.13, social: p = 0.0084; s, sucrose: p = 0.002, social: p = 0.026). Optogenetic inhibition of mPFC neurons had no effect on average distance traveled during social or sucrose trials in both sexes. Two-factor ANOVA (p, interaction: p = 0.18, virus: p = 0.16, trial type: p = 3.01*10−106; t, interaction: p = 0.76, virus: p = 0.42, trial type: p = 1.97*10−89). Optogenetic inhibition of mPFC neurons caused a decrease in the time spent in the social zone during the reward period when compared to mCherry controls in male (q) but not female (u) mice. Unpaired t test (male: p = 0.013; female: p = 0.31). *p < 0.05. Box plots: center line denotes median, box edges indicate the 25th and 75th percentiles and whiskers extend to ± 2.7σ.
We next sought to determine if optogenetic inhibition of mPFC activity during the reward period of the two choice operant assay could alter reward-seeking behavior. We injected male and female mice with an inhibitory opsin (GtACR) or mCherry (control), implanted ferrules bilaterally in the mPFC (Fig. 6l, m) and then trained these mice on the two choice assay (Fig. 6a). Once mice were trained, we used blue light to inhibit mPFC neurons during the reward period on a random 50% of trials over the course of 7 sessions (Supplementary Fig. 11a). We found that inhibition of mPFC neurons during the reward period increased reward latency on both trial types when compared to controls across sexes (Fig. 6n, r: two-factor ANOVA with virus (GtACR/mCherry) and trial type (sucrose/social) as factors, male — interaction: p = 0.67, virus: p = 0.0004, trial type: p < 0.00001, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.0014, social: p = 0.021; female — interaction: p = 0.83, virus: p = 2.46*10−8, trial type: p = 2.29*10−12, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 5.46*10−10, social: p = 0.0014). Additionally, optogenetic inhibition caused an increase in the number of sucrose reward fails in male mice and in both sucrose and social reward fails in female mice compared to controls (Fig. 6o, s: two-factor ANOVA with virus (GtACR/mCherry) and trial type (sucrose/social) as factors, male — interaction: p = 0.50, virus: p = 0.0039, trial type: p = 0.37, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.13, social: p = 0.0084; female — interaction: p = 0.055, virus: p = 0.0002, trial type: p = 0.0001, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.002, social: p = 0.026). Unlike optogenetic activation, we found that despite disrupting reward-seeking behavior, optogenetic inhibition of mPFC neurons did not affect the average distance traveled by male or female mice in the operant arena during or outside of the reward period across all trials with and without laser stimulation (Fig. 6p, t: two-factor ANOVA with virus (GtACR/mCherry) and trial type (sucrose/social) as factors, male — interaction: p = 0.18, virus: p = 0.16, trial type: p = 3.01*10−106, female — interaction: p = 0.75, virus: p = 0.42, trial type: p = 1.97*10−89; Supplementary Fig. 11n, s: two-factor ANOVA with virus (GtACR/mCherry) and trial type (sucrose/social) as factors, male — interaction: p = 0.69, virus: p = 0.57, trial type: p = 2.00*10−67, female — interaction: p = 0.67, virus: p = 0.20, trial type: p = 2.94*10−84; Supplementary Fig. 11p, u: two-factor ANOVA with virus (GtACR/mCherry) and stimulation (on/off) as factors, male — interaction: p = 0.38, virus: p = 0.85, stimulation: p = 0.25; female — interaction: p = 0.22, virus: p = 0.45, stimulation: p = 0.22). In male mice, optogenetic inhibition of mPFC neurons during the reward period resulted in decreased time spent in the social reward zone (Fig. 6q: unpaired t test, p = 0.013), an effect that was also seen in trials without laser stimulation (Supplementary Fig. 11o: unpaired t test, p = 0.020). This effect was not observed in female mice on trials with or without laser stimulation (Fig. 6u: unpaired t test, p = 0.31; Supplementary Fig. 11t: unpaired t test, p = 0.12). Overall, these data support a causal role for the mPFC in modulating social and nonsocial reward-seeking behavior and demonstrate that intact mPFC activity during the reward period is crucial for animals to associate choice with reward during the two choice operant assay.
Optogenetic manipulation of mPFC neurons seemed to have an effect on reward-seeking behavior outside of trials in which there was laser stimulation, which resulted in increased reward latency and number of reward fails compared to controls on non-stimulated social trials in all male mice (Supplementary Fig. 11b, c, l, m: two-factor ANOVA with virus (ChR2 or GtACR/GFP or mCherry) and trial type (sucrose/social) as factors, ChR2: reward latency — interaction: p = 0.016, virus: p = 8.00*10−5, trial type: p = 4.62*10−16 with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.074, social: p = 5.31*10−4; reward fails — interaction: p = 0.96, virus: p = 6.82*10−5, trial type: p = 0.0015 with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.025, social: p = 2.50*10−5; GtACR: reward latency — interaction: p = 0.0004, virus: p = 0.0001, trial type: p < 0.00001, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.60, social: p = 2.14*10−5; reward fails — interaction: p = 0.22, virus: p < 0.00001, trial type: p = 0.38, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.027, social: p = 3.04*10−5). Female mice across optogenetic manipulations showed increased reward latency and reward fails on both sucrose and social trials without laser stimulation compared to controls. (Supplementary Fig. 11g, h, q, r: two-factor ANOVA with virus (ChR2 or GtACR/GFP or mCherry) and trial type (sucrose/social) as factors, ChR2: reward latency — interaction: p = 0.11, virus: p = 2.61*10−9, trial type: p = 5.12*10−23, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 1.61*10-8, social: p = 6.04*10−5; reward fails — interaction: p = 0.024, virus: p = 2.23*10−6, trial type: p = 0.83, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.044, social: p = 1.07*10−5; GtACR: reward latency — interaction: p = 1.78*10−12, virus: p = 2.51*10−4, trial type: p = 2.24*10−14, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 1.94*10−4, social: p = 4.25*10−9; reward fails — interaction: p = 0.74, virus: p < 0.00001, trial type: p = 0.31, with post-hoc unpaired t tests comparing virus within trial type, sucrose: p = 0.0076, social: p = 3.12*10−5). These data suggest that transiently altering mPFC activity has persistent effects on social and nonsocial reward-seeking behavior during the two choice operant assay.
Reward-seeking behavior varies with the internal state of mice
We next asked if motivation to seek social versus nonsocial reward could be modulated by changing the internal state of the animal. We changed the level of thirst that animals experienced by restricting their access to water while also monitoring the activity of mPFC neurons. We first determined if thirsty mice (on restricted water access, RW) would change their behavior on the two choice operant assay (Fig. 7a). We found that all mice completed significantly more sucrose trials than social trials when on restricted water access, compared to full water access (Fig. 7b, f: two-factor ANOVA with water condition (FW/RW) and trial type (sucrose/social) as factors, male — interaction: p = 1.99*10−55, water condition: p = 5.14*10−32, trial type: p = 1.22*10−56 with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 1.16*10−38, social: p = 8.97*10−13; female — interaction: p = 1.81*10−23, water condition: p = 2.16*10−11, trial type: p = 8.73*10−15, with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 6.51*10−6, social: p = 6.76*10−17). They also performed the task more quickly with significantly decreased choice latency across all trials (Fig. 7c, g: two-factor ANOVA with water condition and trial type as factors, male — interaction: p = 0.88, water condition: p = 7.37*10−18, trial type: p = 0.071 with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 1.11*10−15, social: p = 6.99*10−7; female — interaction: p = 0.29, water condition: p = 3.97*10−7, trial type: p = 0.18, with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 1.00*10−5, social: p = 0.0054) and decreased sucrose reward latency (Fig. 7d, h: two-factor ANOVA with water condition and trial type as factors, male — interaction: p = 0.49, water condition: p = 3.48*10−9, trial type: p = 1.48*10−19 with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 7.61*10−13, social: p = 0.004; female — interaction: p = 0.46, water condition: p = 3.10*10−10, trial type: p = 2.89*10−6, with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 5.77*10−6, social: p = 0.014) when compared to full water access. Male mice also showed decreased social reward latency on restricted water access compared to full water access (Fig. 7d), while female mice showed increased social reward latency with water restriction (Fig. 7h). All mice on restricted water access made significantly fewer sucrose reward fails compared to mice on full water access and made very few social reward fails overall (Fig. 7e, i: two-factor ANOVA with water condition and trial type as factors, male — interaction: p = 1.24*10−6, water condition: p = 2.47*10−7, trial type: p = 5.31*10−9 with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 7.34*10−7, social: p = 0.36; female — interaction: p = 3.88*10−5, water condition: p = 0.0016, trial type: p = 0.0014 with post-hoc unpaired t tests comparing water conditions within trial types, sucrose: p = 3.01*10−4, social: p = 0.025). This may be the result of increased arousal with thirst41,49. Taken together, these findings demonstrate that thirst significantly alters the reward-seeking behavior of mice on the two choice operant assay with mice preferentially seeking sucrose over social reward.
a Experimental timeline showing water restriction and imaging schedule. During restricted water access conditions (RW), both male (b) and female (f) mice completed significantly more successful sucrose trials and significantly fewer successful social trials than during full water access conditions (FW). Two-factor ANOVA with water condition (FW/RW) and trial type (sucrose/social) as factors (b, interaction: p = 1.99*10−55, water condition: p = 5.14*10−32, trial type: p = 1.22*10−56; f, interaction: p = 1.81*10−23, water condition: p = 2.16*10−11, trial type: p = 8.73*10−15) with post-hoc unpaired t tests comparing water conditions within trial types (b, sucrose: p = 1.16*10−38, social: p = 8.97*10−13; f, sucrose: p = 6.76*10−17, social: p = 6.51*10−6). Male (c) and female (g) mice on RW demonstrated significantly decreased choice latency compared to FW. Two-factor ANOVA with water condition and trial type as factors (c, interaction: p = 0.88, water condition: p = 7.37*10−18, trial type: p = 0.071; g, interaction: p = 0.29, water condition: p = 3.97*10−7, trial type: p = 0.18) with post-hoc unpaired t tests comparing water conditions within trial types (c, sucrose: p = 1.11*10−15, social: p = 6.99*10−7; g, sucrose: p = 1.00*10−5, social: p = 0.0054). Male (d) and female (h) mice on RW significantly decreased their sucrose reward latency compared to FW. Male mice also decreased their social reward latency on RW, while it increased in female mice. Two-factor ANOVA with water condition and trial type as factors (d, interaction: p = 0.49, water condition: p = 3.48*10−9, trial type: p = 1.48*10−19; h, interaction: p = 0.46, water condition: p = 3.10*10−10, trial type: p = 2.89*10−6) with post-hoc unpaired t tests comparing water conditions within trial types (d, sucrose: p = 7.61*10−13, social: p = 0.004; h, sucrose: p = 5.77*10−6, social: p = 0.014). Male (e) and female (i) mice on RW made significantly fewer sucrose reward fails compared to FW, while female mice on RW also made significantly more social reward fails compared to FW. Two-factor ANOVA with water condition and trial type as factors (e, interaction: p = 1.24*10−6, water condition: p = 2.47*10−7, trial type: p = 5.31*10−9; i, interaction: p = 3.88*10−5, water condition: p = 0.0016, trial type: p = 0.0014) with post-hoc unpaired t tests comparing water conditions within trial types (e, sucrose: p = 7.34*10−7, social: p = 0.36; i, sucrose: p = 3.01*10−4, social: p = 0.025). FW: n = 21 male mice, 12 female mice; RW: n = 19 male mice, 6 female mice, 3 sessions per mouse. Top row: Heatmaps of average normalized fluorescence of mPFC neurons in male (j) and female (k) mice that are significantly modulated by sucrose choice (left, male: n = 99 neurons; female: n = 159 neurons) and sucrose reward (right, male: n = 225 neurons; female: n = 250 neurons) during the two choice operant assay under RW conditions. Rows are sorted by maximum fluorescence in each heatmap. Bottom row: Average normalized fluorescence traces of the neurons from the corresponding heatmaps in male (j) and female (k) mice that are significantly modulated by sucrose choice (left) and sucrose reward (right). During RW conditions, a greater proportion of mPFC neurons in both male (l) and female (m) mice are significantly modulated by sucrose choice (male: 22.30%, n = 99/444; female: 27.18%, n = 159/585) and sucrose reward (male: 50.68%, n = 225/444; female: 42.74%, n = 250/585) when compared to full water access conditions. Proportion z test with correction for multiple comparisons (male — sucrose choice: p = 9.53*10−8, sucrose reward: p < 0.00001; female — sucrose choice: p = 4.22*10-15, sucrose reward: p < 0.00001). Trial-averaged population neural activity traces of sucrose and social reward trials in male (n, left panel) and female (o, left panel) mice during FW (sucrose trials: dark blue, social trials: red) and RW (sucrose trials: light blue, social trials: pink) plotted on the first 3 PCs in state space. Arrowhead indicates direction of time. Filled green circle indicates reward onset. Euclidean distance separating PC-projected population vectors in a 3 s window around reward is significantly greater between water restriction conditions for social than for sucrose trials in both male (n, right panel) and female (o, right panel) mice. Paired t test (male: p = 7.93*10−5; female: p = 0.0030). N = 8 male mice, 5 female mice. *p < 0.05 Shaded error regions indicate ± SEM. Dashed line at zero indicates reward onset. Box plots: center line denotes median, box edges indicate the 25th and 75th percentiles and whiskers extend to ±2.7σ.
mPFC sucrose reward responses change with thirst through the recruitment of previously latent neurons
To determine if changes in behavior corresponded with changes in mPFC neural representations on the two choice operant assay, we performed cellular resolution calcium imaging of mPFC neurons when mice were on restricted water access (RW, male: n = 444 neurons, 8 mice; female: n = 585 neurons, 6 mice) and compared that to full water access (FW) conditions.
We compared the neural representations of operant assay events (Fig. 7j–m; Supplementary Fig. 12j–l,n–p), specifically those related to sucrose reward-seeking behavior, across water access conditions. We found that across both sexes, a greater proportion of mPFC neurons were modulated by sucrose choice (Fig. 7l, m: male — RW: 22.30%, 99/444, FW: 9.37%, 43/459; female — RW: 27.18%, 159/585, FW: 9.30%, 53/570; proportion z test, male: p = 9.53*10−8, female: p = 4.22*10−15) and sucrose reward (Fig. 7l, m: male — RW: 50.68%, 225/444, FW: 19.17%, 88/459; female — RW: 42.74%, 250/585, FW: 17.54%, 100/570; proportion z test, male and female: p < 0.00001) in restricted water access conditions compared to full water access conditions. The patterns of selectivity for sucrose and social reward seen in male and female mice during full water access were maintained during restricted water access (Supplementary Fig. 12a–i). We also observed a decrease in the number of social reward-responsive neurons in male and female mice following water deprivation (Supplementary Fig. 12l, p: male — RW: 4.28%, 19/444, FW: 29.19%, 134/459; female — RW: 25.60%, 150/585, FW: 35.44%, 202/570; proportion z test, male: p < 0.00001, female: p = 2.99*10−4). At a population level, sucrose and social reward trials in both FW and RW conditions continued to occupy distinct trajectories in the first 3 PCs of state space (Fig. 7n, o, left panel: total variance explained, male: 55.9%; female: 52.7%) in both sexes. However, the Euclidean distance separating population vectors between water restriction conditions was significantly greater for social trials than for sucrose trials (Fig. 7n, o, right panel: male — average distance between FW and RW for sucrose trials: 21.03 ± 2.85, social trials: 40.26 ± 2.96, paired t test, p = 7.93*10−5; female — average distance between FW and RW for sucrose trials: 26.33 ± 2.97, social trials: 44.06 ± 2.85, paired t test, p = 0.0030). Despite the changes seen in neural responsiveness, mPFC activity during the reward period decoded trial type with comparably high accuracy across water access conditions (Supplementary Fig. 12m, q: average decoding accuracy, male — FW: 77.67 ± 4.55, RW: 69.84 ± 4.06; female — FW: 90.58 ± 1.26, RW: 90.11 ± 3.10; unpaired t test, male: p = 0.21; female: p = 0.91).
These findings raise the question of how the mPFC is able to maintain flexible neural representations of social and nonsocial rewards that are dependent on the internal state of the animal. One possibility is that individual mPFC neurons may change their identity to encode social or nonsocial reward information depending on the internal state of the animal. Alternatively, previously latent populations of mPFC neurons may be activated depending on the internal state of the animal. To distinguish between these possibilities, we utilized the high spatial resolution of calcium imaging to track individual mPFC neurons across water restriction conditions (Fig. 8b)50. We compared the activity of neurons across three conditions, two conditions in which mice had full water access and one condition of restricted water access (Fig. 8a). Across male mice, we tracked 178 mPFC neurons between the first full water access imaging session and the restricted water access imaging session (Fig. 8c, n = 8 mice), 158 mPFC neurons between the first full water access imaging session and the second full water access imaging session (Fig. 8d, n = 7 mice) and 164 neurons between the restricted water access imaging session and the second full water access imaging session (Fig. 8e, n = 7 mice). In female mice, we tracked 226 mPFC neurons between the first full water access imaging session and the restricted water access imaging session (Fig. 8f, n = 5 mice), 256 mPFC neurons between the first full water access imaging session and the second full water access imaging session (Fig. 8g, n = 5 mice) and 253 neurons between the restricted water access imaging session and the second full water access imaging session (Fig. 8h, n = 5 mice).
a Experimental timeline showing water restriction and imaging schedule. b mPFC neurons were tracked across three different imaging sessions using CellReg, a cell registration software50. The top row depicts the CNMFe-generated field of view (FOV) of all identified neurons from each imaging session of a representative animal across various water access conditions. The bottom row depicts individual neurons that were successfully tracked across all three imaging sessions from the same animal. Individual neurons are represented by different colors. Venn diagrams showing the number of tracked neurons across various water restriction conditions in male (c–e) and female (f–h) mice. Time locked responses (top row: average fluorescence trace, bottom row: heatmap showing individual trials) of example neurons that showed stable sucrose reward excite (i) and sucrose reward inhibit (j) responses across both full water access (left panel) and restricted water access imaging sessions (right panel). Pi charts depicting the previous identity of sucrose reward excite (k) and sucrose reward inhibit neurons (l) in the restricted water access condition in male (left panel) and female (right panel) mice. Proportion of tracked reward-unresponsive neurons that convert to sucrose reward excite (m) or sucrose reward inhibit (n) neurons in the subsequent imaging session in male (left panel) and female (right panel) mice. Proportion z test with correction for multiple comparisons (m, male — FWvFW2 (1) compared to FWvRW (2): p = 0.011, FWvFW2 (1) compared to FW2vRW (3): p = 0.003, FWvRW (2) compared to FW2vRW (3): p = 0.64; female — 1 to 2: p = 0.036, 1 to 3: p = 0.27, 2 to 3: p = 0.26; n, male — 1 to 2: p = 2.25*10−6, 1 to 3: p = 1.65*10−6, 2 to 3: p = 0.98; female — 1 to 2: p = 0.0054, 1 to 3: p = 6.37*10−8, 2 to 3: p = 0.009). *p < 0.05. Shaded error regions indicate ± SEM.
We then characterized the identity of neurons that we tracked based on their reward responses in both imaging sessions. In particular, we identified neurons as either social excite, sucrose excite, sucrose inhibit or reward-unresponsive. Neurons were considered stable if they maintained their response (excite or inhibit) to sucrose reward across imaging sessions (see example neurons Fig. 8i, j). Excluding stable neurons, we found that a significantly greater proportion of mPFC neurons that were sucrose reward-responsive during the restricted water access imaging session were previously unresponsive as opposed to converting from social excite neurons during the first full water access imaging session in both male (n = 71.21%, 47/66 neurons, 8 mice) and female (n = 70.45%, 31/44 neurons, 5 mice) mice (Fig. 8k, l: proportion z test, male: p = 1.09*10−6; female: p = 1.20*10−4). As a result, we next quantified the proportion of unresponsive neurons that converted to either sucrose reward excite (Fig. 8m) or sucrose reward inhibit (Fig. 8n) neurons across imaging sessions. We found that a significantly larger proportion of unresponsive neurons converted to either sucrose reward excite (Fig. 8m: proportion z test with correction for multiple comparisons, male — FWvFW2 (1) compared to FWvRW (2): p = 0.011, FWvFW2 (1) compared to FW2vRW (3): p = 0.003, FWvRW (2) compared to FW2vRW (3): p = 0.64; female — 1 to 2: p = 0.036, 1 to 3: p = 0.27, 2 to 3: p = 0.26) or sucrose reward inhibit (Fig. 8n: proportion z test with correction for multiple comparisons, male — 1 to 2: p = 2.25*10−6, 1 to 3: p = 1.65*10−6, 2 to 3: p = 0.98; female — 1 to 2: p = 0.0054, 1 to 3: p = 6.37*10−8, 2 to 3: p = 0.009) neurons across dissimilar water access conditions (full water access/full water access 2 and restricted water access) compared to similar water access conditions (full water access and full water access 2). These findings indicate that the increased proportion of sucrose reward-responsive neurons in the mPFC seen with water restriction were driven largely by the recruitment of previously reward-unresponsive neurons and less by social reward neurons changing identity or by turnover due to the passage of time.
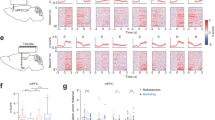
Neural representations of social reward change with social isolation in a sex-dependent manner
The two choice operant assay allows for the direct comparison of social and nonsocial reward-seeking behavior, which enables us to systematically change access to either type of reward outside of the assay and characterize how this affects motivation to seek either reward. Since water restriction preferentially drove reward-seeking behavior and mPFC neural representations toward sucrose reward, we then wanted to determine if social isolation would similarly drive preference for social reward. Consequently, we socially isolated mice for 7 days after they had been trained on the two choice operant assay and imaged mPFC neurons before and after social isolation in male and female mice (Fig. 9a). We found that social isolation had opposing effects on male and female reward-seeking behavior. Specifically, we found that social isolation caused an increase in the proportion of social trials completed by male mice relative to sucrose trials prior to social isolation (Fig. 9b, two-factor ANOVA with isolation (pre/post) and trial type (sucrose/social) as factors; interaction: p = 0.020). In contrast, social isolation caused a relative decrease in the number of social trials completed by female mice compared to sucrose trials prior to social isolation (Fig. 9i, two-factor ANOVA with isolation (pre/post) and trial type (sucrose/social) as factors; interaction: p = 0.0035). We did not observe effects of isolation on the other behavioral parameters in both sexes (Fig. 9c–e,j–l).
a Experimental timeline showing social isolation and imaging schedule. b Male mice showed a relative increase in the number of successful social trials compared to sucrose trials completed after social isolation. Two-factor ANOVA with isolation (pre/post) and trial type (sucrose/social) as factors (interaction: p = 0.020, isolation: p = 0.97, trial type: p = 0.52) with post-hoc unpaired t tests comparing isolation within trial types (sucrose: p = 0.21, social: p = 0.0079). Male mice did not show any significant difference in choice latency (c), reward latency (d) and reward fails (e) of either trial type before and after social isolation. Two-factor ANOVA with isolation (pre/post) and trial type (sucrose/social) as factors (c, interaction: p = 0.69, isolation: p = 0.56, trial type: p = 0.40; d, interaction: p = 0.81, isolation: p = 0.21, trial type: p = 1.08*10−6; e, interaction: p = 0.91, isolation: p = 0.81, trial type: p = 0.0075) with post-hoc unpaired t tests comparing isolation within trial types (d, sucrose: p = 0.36, social: p = 0.34; e, sucrose: p = 0.95, social: p = 0.39). N = 4 male mice. f Male mice did not show a difference in the proportion of mPFC neurons significantly modulated by social reward before social isolation. Proportion z test (p = 0.91). g Average normalized fluorescence traces of mPFC neurons significantly excited by social reward before (red) and after (gray) social isolation in male mice time-locked to social reward shown (left panel). h Comparison of peak fluorescence before (n = 76 neurons) and after (n = 89 neurons) social isolation shows that neural responses to social reward decreased with social isolation in male mice (right panel). Unpaired t test (p = 4.24*10−4). i Female mice showed a relative decrease in the number of successful social trials compared to sucrose trials completed after social isolation. Two-factor ANOVA with isolation and trial type as factors (interaction: p = 0.0035, isolation: p = 0.29, trial type: p = 0.30) with post-hoc unpaired t tests comparing isolation within trial types (sucrose: p = 0.017, social: p = 0.10). Female mice did not show a difference in choice latency (j), reward latency (k) and reward fails (l) of either trial type before and after social isolation. Two-factor ANOVA with isolation and trial type as factors (j, interaction: p = 0.61, isolation: p = 0.29, trial type: p = 0.41; k, interaction: p = 0.24, isolation: p = 0.13, trial type: p = 1.00*10−4; l, interaction: p = 0.36, isolation: p = 0.29, trial type: p = 0.0005) with post-hoc unpaired t tests comparing isolation within trial types (k, sucrose: p = 0.069, social: p = 0.81; l, sucrose: p = 0.42, social: p = 0.33). N = 6 female mice. m Female mice did not show a difference in proportion of mPFC neurons significantly modulated by social reward before and after social isolation. Proportion z test (p = 0.86). n Average normalized fluorescence traces of mPFC neurons significantly excited by social reward before (red) and after (gray) social isolation in female mice time-locked to social reward (left panel). o Comparison of peak fluorescence before (n = 177 neurons) and after (n = 193 neurons) social isolation shows that neural responses to social reward increased with social isolation in female mice (right panel). Unpaired t test (p = 7.58*10−11). *p < 0.05. Shaded error regions indicate ± SEM. Dashed line at zero indicates reward onset. Error bars indicate ± SEM. Box plots: center line denotes median, box edges indicate the 25th and 75th percentiles and whiskers extend to ±2.7σ.
We next determined the effects of social isolation on mPFC neuronal activity. Across all mice, we found that the proportion of social reward neurons in the mPFC did not change with social isolation in male (pre: n = 20.59%, 84/408, post: n = 20.90%, 98/469, 4 mice) or female (pre: n = 35.44%, 202/570, post: n = 35.95%, 206/573, 6 mice) mice (Fig. 9f, m: proportion z test, male: p = 0.91; female p = 0.86). However, when we compared the peak amplitude of social reward excite neuron responses, we found sex-dependent changes in peak amplitude following social isolation. Specifically, in male mice, the peak amplitude of social reward excite neurons significantly decreased after social isolation (Fig. 9g, h: average peak amplitude, pre: 0.27 ± 0.0094, post: 0.22 ± 0.0083; unpaired t test, p = 4.24*10−4), while in female mice the peak amplitude of social reward excite neurons significantly increased after social isolation (Fig. 9n, o: average peak amplitude, pre: 0.23 ± 0.0069, post: 0.30 ± 0.0080; unpaired t test, p = 7.58*10−11). This sex-dependent change in the peak amplitude of the response of social reward excite neurons following social isolation was specific to social reward neurons. We compared the peak amplitude of sucrose reward excite (Supplementary Fig. 14a, c: unpaired t test, male: p = 0.17; female: p = 0.66), and sucrose reward inhibit neurons (Supplementary Fig. 14b, d: unpaired t test, male: p = 0.077; female: p = 0.30) before and after social isolation and found that there was no change in peak amplitude. To rule out the effects of change with time on neural responses, we compared the proportion of social reward neurons and the peak amplitude of their social reward responses across the partial water access (Supplementary Fig. 13) and the full water access imaging sessions before social isolation. We also observed no difference in the proportion of social reward excite neurons between partial water access and full water access pre-isolation sessions (Supplementary Fig. 14e, g: male — pre: 20.59%, n = 84/408, PW: 21.28%, n = 90/423; female — pre: 35.44%, n = 202/570, PW: 32.37%, n = 180/556, proportion z test, male: p = 0.81, female: p = 0.28). However, unlike with social isolation, there was no change in the amplitude of the responses of social reward excite neurons (Supplementary Fig. 14f, h: unpaired t test, male: p = 0.74, female: p = 0.21). These data demonstrate that the sex-dependent changes in peak amplitude seen with social isolation are specific to social reward excite neurons as a result of social isolation.
Discussion
Using an automated two choice operant assay, we found that largely non-overlapping populations of neurons in the mPFC respond to social and sucrose reward and that these representations are more distinct in female mice compared to male mice. We additionally showed that manipulating mPFC activity through both optogenetic activation and inhibition during the reward period disrupts the ability of male and female mice to successfully engage with both types of reward, demonstrating that the mPFC plays a causal role in modulating social and nonsocial reward-seeking behavior. Finally, we found that changing internal state through either water deprivation or social isolation differentially modulates sucrose and social reward representations in a sexually dimorphic manner. Together, these findings provide new insights into how social and nonsocial reward information is organized in the mPFC.
Advantages offered by the two choice (social-sucrose) operant assay
The use of operant assays in rodent models has significantly contributed to our understanding of the neural circuitry underlying the seeking and processing of nonsocial reward-related behaviors, such as food rewards51,52,53. While we know that mice find social interactions rewarding, the complexity of social interactions has made understanding how the brain represents social interactions challenging1. Most studies determine whether a conspecific is perceived as rewarding by comparing the time mice spend in the proximity of a novel mouse relative to an object or an empty cage3,4. Although these assays are straightforward, ascertaining social reward-seeking behavior from free ranging interactions is challenging. These assays also rarely compare social reward to other strongly appetitive stimuli, such as sucrose. As a result, similarities and differences in how social and nonsocial reward-seeking behaviors are encoded in the brain remain poorly understood. To overcome these challenges, we developed a low-cost, fully automated two choice operant assay in which mice can freely choose between social and sucrose rewards. This assay allows for unsupervised high throughput data collection, permitting us to collect hundreds of social and sucrose trials per day across several animals.
Additionally, the operant nature of the assay allows us to constrain social behavior and monitor social reward-seeking with high temporal precision. Using this assay, we found that in control conditions (not social isolation or water deprivation), male mice showed an equal preference for social and nonsocial rewards and female mice slightly preferred social rewards over sucrose rewards (Fig. 1d, h; Supplementary Fig. 3i). Our results confirmed recent findings that adult mice find social stimuli positively reinforcing and will work to gain access to a social stimulus in operant assays8,9,10,12. Building off this work, we were able to directly compare the responses of mPFC neurons to social and nonsocial rewards while mice demonstrated similar motivation to seek both rewarding stimuli. This approach can be used to systematically map how social and nonsocial reward representations are transformed across the reward circuitry, by imaging or recording in regions such as the basolateral amygdala, ventral tegmental area, and the nucleus accumbens during the two choice operant assay. In the future, this assay can also be used to vary dimensions of social reward, such as proximity of the social reward, valence, and reward duration independently and parametrically in freely behaving mice. Finally, although we did not observe strain differences in social and nonsocial reward-seeking behavior in female mice on our assay (Supplementary Fig. 6), others have recently found strain differences in social reward-seeking behavior, with female CD1 mice showing a stronger preference for the social reward compared to C57BL/6J (used in this study)9. This raises interesting questions about how neural circuit differences across strains and species might give rise to varied behavioral outcomes.
Social and nonsocial reward representations in the medial prefrontal cortex
There is little consensus regarding how social and nonsocial reward-related behaviors are mediated within the same brain region. One theory proposes that social and nonsocial behaviors are represented by discrete and mutually inhibitory neural populations54. Support for this theory comes from a recent study that found that neurons in the orbitofrontal cortex that are responsive to social interactions suppress feeding behavior when activated16. Another study also found that different populations within the medial amygdala represented distinct and antagonistic social and asocial behaviors55. Consistent with the idea that distinct and antagonistic populations of neurons mediate social and nonsocial reward, we observed largely non-overlapping populations of neurons within the mPFC that encoded social and nonsocial reward-related information (Figs. 3, 4 and 5). We also observed non-overlapping social and nonsocial neural representations on a passive exposure paradigm (Supplementary Fig. 10m–o). This mPFC reward-related activity was sufficient to decode the trial type during both the choice and reward periods (Fig. 3g, h and Fig. 5b), as well as, the sex of the animal (Fig. 3i and Fig. 5a). Additionally, we found evidence that these populations may regulate social and nonsocial reward information in an antagonistic manner. In particular, using peak amplitude analysis, we identified a subset of mPFC neurons that were inhibited in response to sucrose reward and excited in response to social reward (Fig. 4h, k, n). In contrast, there were very few mPFC neurons that were inhibited by social reward (Fig. 4a). These differences could arise as a result of non-mutual inhibition between social and sucrose ensembles driven by local inhibitory interneurons in the mPFC. Alternatively, they could result from differences in external inputs to the social and nonsocial reward-responsive neurons. Future cell-type specific imaging and optogenetic experiments are essential to begin to distinguish between these possibilities.
Furthermore, we showed that mPFC activity during the reward period plays a causal role in mediating reward-seeking behavior on the two choice operant assay. We found that altering mPFC activity through either optogenetic excitation or inhibition during the reward period increased reward latency and the number of reward fails across trial type in male and female mice (activation: Fig. 6d, e, h, i and inhibition: Fig. 6n, o, r, s). Although the role of the mPFC in nonsocial reward-seeking has been well established, these experiments are the first to suggest a causal role for the mPFC in shaping social reward-seeking behavior and not solely disrupting ongoing social interactions25,26. In the future, combining activity-dependent neuron tagging methods (e.g., TRAP-Cre56) with optogenetic tools could allow for the selective activation and silencing of social or nonsocial reward neurons. These experiments could help provide insight into how selective activity of social and nonsocial reward neurons shape reward-seeking and decision-making behavior.
Effects of internal state and sex on neural representations of reward
There has been increasing evidence that male and female animals differ in their reward-seeking behavior, including differences in reward-associated learning strategies43,57 and sensitivity to reward outcomes22,58. Studies in rodents have also shown that there are sex differences in motivation to seek social reward, with female rodents demonstrating more robust social reward-seeking behavior than male rodents59,60. These findings raise the possibility that there might be differences in how social and nonsocial rewards are represented in male and female mice. In this study, we combined a two choice operant assay with cellular resolution calcium imaging to identify the neural substrates that might underlie some of these sex-dependent behavioral differences.
Although male and female mice demonstrated similar levels of motivation to seek social and sucrose rewards under control conditions during the two choice operant assay, we observed sex differences in how social and nonsocial rewards were represented in the mPFC. Specifically, female mice had more distinct representations of social and nonsocial reward in the mPFC (Fig. 4d, e). As a result, we were able to decode reward type with higher accuracy from the mPFC neural activity of female mice relative to that of male mice (Fig. 5b). We were also able to decode the sex of the animal from the mPFC neural activity around both choice and reward (Fig. 3i and Fig. 5a). Interestingly, we were able to decode subsequent choice at earlier time points in female mice than in male mice (Fig. 3g). Additionally, sucrose reward-responsive neurons in female mice were significantly less responsive to social reward, whereas sucrose reward-responsive neurons in male mice were equally responsive to social reward (Fig. 4m). These findings raise the intriguing possibility that sex differences in mPFC activity could drive divergent reward-seeking behaviors when animals choose between competing social and nonsocial rewards.
We also found that social isolation differentially affected reward-seeking behavior in male and female mice. We observed a relative increase in the number of social trials completed by male mice and a relative decrease in the number of social trials completed by female mice compared to sucrose trials following acute social isolation (Fig. 9b, i). These differences are consistent with previous findings that social isolation increases social approach behaviors in male mice while causing social withdrawal in female mice61. In addition, we observed that there were sex-dependent changes in how mPFC neurons responded to social reward but not sucrose reward following social isolation. In male mice, we found that social reward-responsive mPFC neurons showed a decrease in peak amplitude in response to social reward following social isolation (Fig. 9g, h). In contrast, in female mice, we observed an increase in the peak amplitude of social reward-responsive neurons in response to social reward following social isolation (Fig. 9n, o). These changes in amplitude were socially specific, suggesting that the neural changes resulting from acute social isolation (~1 week) act specifically on mPFC ensembles that are sensitive to social reward. These findings are consistent with a recent neuroimaging study in humans which found that acute social and food deprivation had distinct effects on social versus nonsocial craving responses in cortical regions62. Thus, acute social isolation may not affect nonsocial reward representations in the mPFC. However, it remains unclear if the changes observed in the neural representations of social reward in the mPFC causally contribute to the behavioral differences observed in male and female mice following social isolation. Furthermore, the cause of these sex-dependent changes in mPFC activity following social isolation remain unknown. Hormones63, neuromodulators64 and neuropeptides65, all of which have been shown to change in a sex-dependent manner following social isolation, may contribute to the observed changes in neural representations of social reward.
Although we found that acute social isolation did not alter mPFC responses to sucrose reward, there is substantial evidence that chronic social isolation affects nonsocial reward-seeking behaviors in rodents66,67,68 and humans69. Additionally, there is evidence that chronic social isolation affects mPFC activity61,70,71. Consequently, 7 days of social isolation may not be sufficient to produce the neural and behavioral changes seen with more chronic forms of social isolation. In fact, the social homeostasis theory of social isolation postulates that social isolation activates homeostatic mechanisms that drive prosocial or antisocial behavior depending on the duration of isolation72. Thus, further work is needed to systematically vary the duration of social isolation to determine the precise timing of prosocial versus antisocial behaviors and the neural mechanisms underlying these changes. The two choice operant assay, as presented in this study, is uniquely suited to determine the impacts of social isolation on different reward-related behaviors, since it allows for the quantification and direct comparison of social and nonsocial reward-seeking behaviors following social isolation.
Unlike with social isolation, we did not find sex differences in changes of reward-related behavior or mPFC reward representations with water deprivation. Instead, all mice robustly changed their behavior in favor of sucrose reward-seeking. The neural representations of reward reflected these changes in behavior, with a larger proportion of mPFC neurons responding to sucrose choice and sucrose reward following water restriction (Fig. 7j–m). By longitudinally tracking neurons across imaging sessions, we found that this increase in the number of sucrose reward-responsive neurons was driven by the recruitment of previously reward-unresponsive neurons (Fig. 8k–n). These findings show that the reward selectivity of individual mPFC neurons rarely changes with internal state (i.e., a “social neuron” rarely converts to a “sucrose neuron” in a thirsty animal), demonstrating how thirst shapes reward-related neural activity in the mPFC. Similarly, thirst has been shown to cause widespread changes in brain activity41,73 and level of thirst is known to affect task performance on goal-directed behaviors49. A recent study also found that ventral tegmental area dopamine neurons track satiety and cause changes in dopamine dynamics in distributed brain networks74. In contrast, a separate dopaminergic system in the dorsal raphe nucleus has been shown to mediate behavioral changes following social isolation75. These findings suggest that distinct neuromodulatory and neuropeptide systems may influence the changes seen in social and nonsocial reward representations following water deprivation and social isolation. Future studies are needed to determine how different neuromodulators and hormones act on neurons in the mPFC to modulate social and nonsocial reward representations with varying internal state in a sex-dependent manner.
Broader implications
The ability to perceive social interactions as rewarding is necessary for appropriate social behavior. As such, a prevailing theory for the social dysfunction seen in ASD, known as the social motivation theory, suggests that people with ASD find social interactions less rewarding76. However, it remains unresolved how social reward processing is disrupted in ASD. Human studies have shown that people with ASD have impairments in processing both social and nonsocial rewards77,78, a finding that has also been corroborated in rodent models of ASD79,80,81. In particular, one study found decreased differentiation between social and nonsocial odor cue encoding in the mPFC of mice with a genetic model of ASD28. Another study showed that social deficits in the same mouse model of ASD could be rescued by either increasing excitability of parvalbumin (PV) interneurons or decreasing excitability of pyramidal neurons in the mPFC82. We also found that optogenetically manipulating mPFC activity during the reward period disrupted reward-seeking behavior on the two choice operant assay (Fig. 6). Thus, further interrogation of the neural mechanisms underlying social and nonsocial reward-related behavior, specifically within the mPFC, is imperative to further our understanding of diseases such as ASD. The two choice operant assay described in this study offers a means by which to study two competing reward-related behaviors and to provide further insight into how the brain processes social and nonsocial reward.
In summary, we developed a two choice (social-sucrose) operant assay that allowed us to characterize how social and nonsocial rewards are represented in the mPFC. Using this assay, we demonstrated that social and nonsocial reward representations are non-overlapping and differentially modulated by social isolation and thirst in a sex-dependent manner. Finally, we showed that these representations are essential for appropriate reward-seeking behavior.
Methods
Experimental subjects
For all experiments, C57BL/6J male and female mice aged 6–10 weeks from Jackson laboratories were used. Mice were maintained on a reverse light dark cycle (12-h light off-12-h light on schedule). All experimental procedures were approved by the Emory Institutional Animal Care and Use Committee.
Viruses
The viruses used in this study were purchased from Addgene: AAV5-Syn-GCaMP6f-WPRE-SV40 (100835-AAV5), AAV5-hSyn-hChR2(H134R)-EYFP (26973-AAV5), AAV5-CAG-GFP (37825-AAV5), AAV1-CKIIa-stGtACR2-FusionRed (105669-AAV1), and AAV5-CKIIa-mCherry (114469-AAV5).
Stereotaxic surgeries
For imaging surgeries, mice aged ~6–8 weeks (n = 19 male mice, 6 female mice) were anesthetized with 1–2% isoflurane and placed in stereotactic setup (Kopf). A microsyringe (Nanoject) was used to inject 0.75 µL of AAV5-Syn-GCaMP6f-WPRE-SV40 (Addgene, injected titer of 1.3 × 1013 parts/ml) unilaterally into the mPFC (1.6 mm anterior, 0.5 mm lateral and 2.4 mm in depth). Mice were then implanted with a 0.6 mm diameter, 7.3 mm length GRIN lens (Product ID:1050-004597, Inscopix) over the mPFC (1.8 mm anterior, 0.5 mm lateral and 2.1 mm in depth). A metal cap was cemented over the lens to protect it. Mice were group housed following lens implant. 3–4 weeks following viral injection and lens implant, a baseplate (Product ID: 1050-004638, Inscopix) attached to the miniature microscope (nVista, Inscopix) was positioned ~0.45 mm above the lens such that blood vessels and neurons were in focus. The baseplate was then cemented in place using dental cement (Metabond) and a baseplate cover (Product ID: 1050-004639, Inscopix) was secured over the baseplate to protect the lens.
For optogenetic activation surgeries, mice aged ~6–8 weeks (n = 14 male mice, 14 female mice) were anesthetized with 1–2% isoflurane and placed in stereotactic setup (Kopf). A microsyringe (Nanoject) was used to inject 0.35 µL of AAV5-hSyn-hChR2(H134R)-EYFP (experimental, Addgene, injected titer of 2.4 × 1013 parts/ml, n = 8 male mice, 8 female mice) or AAV5-CAG-GFP (control, Addgene, injected titer of 1.3×10^13 parts/ml, n = 6 male mice, 6 female mice) bilaterally into the mPFC (1.6 mm anterior, 0.5 mm lateral and 2.4 mm in depth). Mice were then implanted with custom-made optic ferrules consisting of a 2.5 mm diameter metal ferrule sleeve (Product ID: MM-FER2003SS-3300, Precision Fiber Products) and a 0.3 mm diameter optic core (Product ID: FT300UMT, ThorLabs) bilaterally at a 10 degree angle into the mPFC above the viral injection (1.8 mm anterior, 0.7 mm lateral and 2.15 mm in depth).
For optogenetic inhibition surgeries, mice aged ~6–8 weeks (n = 13 male mice, 13 female mice) were anesthetized with 1–2% isoflurane and placed in stereotactic setup (Kopf). A microsyringe (Nanoject) was used to inject 0.35 µL of AAV1-CKIIa-stGtACR2-FusionRed (experimental, Addgene, injected titer of 1.3 × 1013 parts/ml, n = 7 male mice, 7 female mice) or AAV5-CKIIa-mCherry (control, Addgene, injected titer of 2.3 × 1013 parts/ml, n = 6 male mice, 6 female mice) bilaterally into the mPFC (1.6 mm anterior, 0.5 mm lateral and 2.4 mm in depth). Mice were then implanted with custom-made optic ferrules consisting of a 2.5 mm diameter metal ferrule sleeve (Product ID: MM-FER2003SS-3300, Precision Fiber Products) and a 0.3 mm diameter optic core (Product ID: FT300UMT, ThorLabs) bilaterally at a 10 degree angle into the mPFC above the viral injection (1.8 mm anterior, 0.7 mm lateral and 2.15 mm in depth).
Behavioral assays
Automated operant assay
We developed an automated, closed-loop operant assay to quantitatively assess social and nonsocial reward behaviors. The assay consists of an acrylic chamber (12 × 12 × 12 inches) with social and nonsocial (sucrose) reward access on opposing sides and two nose-poke ports (choice ports) on the wall adjacent to both reward access sites (Fig. 1a). The two choice ports (Product ID: 1009, Sanworks Bpod) are located 3 inches from the corners and 1 inch from the base of the chamber. The sucrose reward port (Product ID: 1009, Sanworks Bpod) is centered on one adjacent wall and contains a solenoid valve connected to a liquid reservoir containing 10% sucrose in water. On the wall opposite from the sucrose reward port, a 2 × 2 inch square cutout provides access to the social target chamber. The cutout is covered on the exterior side of the chamber by a flat sheet of aluminum fixed to a conveyor belt with a stepper motor which acts as a movable gate, controlled by an Arduino Uno. Behind this cutout, is a smaller acrylic chamber (3 × 3 × 3 inch) with an exposed side blocked by 6 aluminum rods (0.2 cm in diameter and 0.5 cm apart) that holds the target animal. Social target animals were non-cagemate, age- and sex-matched conspecifics. Nose pokes are detected by infrared beam break sensors. A trial begins when both choice ports illuminate and is followed by nose-poking in either choice port to initiate social or nonsocial reward delivery. Nose-poking of the choice port furthest from the sucrose reward port illuminates the reward port and makes 10 µL of sucrose available upon entry into the reward port for up to 8 s. Alternatively, nose-poking of the choice port furthest from the social target chamber, opens the gate for 20 s, which allows the experimental animal to interact with the social target through the aluminum bars without permitting entry into each other’s chamber. Following sucrose or social reward consumption, the trial ends and after a 30 s intertrial interval (ITI), a new trial begins with the illumination of both choice ports. Each behavioral session is 1 h in duration. Our social operant assay consists of several phases: training (4 stages, ~2 weeks) and post-training (up to 3 weeks).
Training paradigms
Mice were co-housed and placed on a restricted water access schedule prior to the start of training. At the end of each day mice were provided 1.5 mL of water in addition to their consumption during training.
Training stage 1: Operant conditioning
In the first stage (operant conditioning), mice were trained to associate poking the reward port with a sucrose reward. Nose port entry triggered the port LED light to turn on and resulted in the delivery of a sucrose reward of 10 µL of 10% sucrose in water. Each session lasted an hour and animals were run through the assay once a day. Mice had to reach a minimum of 50 pokes in a single session to progress to the next training stage. If the animal did not engage with the reward port for more than 2 min after the start of the trial, then the reward port delivered 30 µL liquid without engaging the LED. This automatic reward delivery also occurred at the beginning of the first trial in each session to prime the animal to engage with the port.
Training stage 2: Single port one choice
In the second stage (single port one choice), mice were conditionally rewarded with 10 µL of sucrose for poking the reward port within 8 s of the reward port LED turning on. Trials were separated by a 30 s ITI, and the animal had to refrain from entering the port for 3 s for the next trial to start. A trial was considered a sucrose reward fail if the mouse failed to enter the sucrose reward port after it had been illuminated for 8 s. Mice had to reach a minimum of 40 successful trials during one session to proceed to the next training stage.
Training stage 3: Opposing port one choice
In the third stage (opposing port one choice), the choice port LED furthest from the sucrose reward port (sucrose choice port) turned on to indicate trial start. This assay is self-paced, so mice had an indefinite amount of time to nose-poke the illuminated sucrose choice port. A successful choice port poke resulted in the LED flashing on and off successively 3 times in 0.1 s intervals, after which the reward port LED turned on for up to 8 s. Mice had to nose-poke the reward port within this window to receive a 10 µL sucrose reward. Failure to do so resulted in a reward fail and initiation of a new trial. Trials were separated by a 15 s ITI. In addition, the current trial ended if the animal engaged with the other inactive port instead of the sucrose choice port during the choice period. Mice had to refrain from entering the sucrose choice port for 3 s for the next trial to start. Mice had to perform 40 successful sucrose reward trials to proceed to the final stage of training.
Training stage 4: Two choice (social-sucrose) operant assay
In the final stage (two choice operant), mice on the restricted water access schedule were given 500 µl of water prior to the start of each session (partial water access condition). As with the previous training stage, trial start was indicated by the choice port LED turning on. However, unlike the previous training stage, a second choice port was activated so that the animal could choose between social and nonsocial rewards. Nose-poking the illuminated choice port furthest from the sucrose reward port (sucrose choice port) resulted in the LED flashing on and off successively 3 times in 0.1 s intervals, after which the sucrose reward port LED turned on for 8 s. Mice had to enter the sucrose reward port within this window to receive a 10 µL sucrose reward. Failure to do so resulted in a sucrose reward fail. Nose-poking the illuminated choice port furthest from the social target chamber (social choice port) resulted in the LED flashing on and off successively 3 times in 0.1 s intervals, after which an automated gate opened for 20 s to provide the mouse access to a social target (non-cagemate, sex- and age-matched conspecific) in a smaller chamber. The social target chamber contains an open side covered with aluminum bars that are 0.5 cm apart. This allows mice to freely interact, smell and investigate each other without permitting either animal to enter the other chamber. The gate remained open for 20 s before closing. Failure to investigate the social target in this time resulted in a social reward fail. Trials were separated by a 30 s ITI. Mice were randomly assigned to one of two configurations of the operant chamber in which the social and sucrose rewards were on different sides in order to account for baseline side preferences that may arise.
Post-training: Two choice (social-sucrose) operant assay
Once fully trained, mice were removed from the partial water access schedule and had ad libitum access to water outside of behavioral sessions. They were maintained on this full water access (FW) schedule and were run daily on the two choice operant assay for a week. For restricted water access (RW) experiments, animals were water restricted (1.5 mL of water/day) without access to 500 µL of water before sessions and run daily on the two choice operant assay for a week. For social isolation experiments, mice were fully trained and run daily for 1 week on the full water access schedule. Mice were then single housed for 1 week and not run on the assay. After 1 week, mice were run again for one session on the two choice operant assay.
Empty cage/object control
After mice had been run through the first three training stages, a subset of the mice were run on the final stage of training with either an empty social target cage (empty cage group) or a novel object (object group) (Supplementary Fig. 4). Mice were then removed from the limited water schedule, given ad libitum water access, and run daily for several days on the full water access schedule without a social target and with either an empty cage or a novel object. One mouse from the empty cage group was excluded from full water access analysis due to failure to engage with the task.
Multi-choice operant assay
A third choice port (null port) was added to the two choice operant assay (Supplementary Fig. 5a). Nose-poking the null port resulted in neither a social nor a sucrose reward (null trial). A subset of mice were run on this assay through similar training stages as the two choice operant assay. Mice were then removed from the limited water schedule, given ad libitum water access, and run daily for 9 days on the full water access schedule.
Estrous cycle monitoring
A subset of the female mice underwent vaginal lavage after each behavioral session. The cytological evidence from each lavage was used to determine the daily estrous phase of these female mice (Supplementary Fig. 6a, b).
Water reward control
After mice had been run through the first three training stages, a subset of the mice were run on the final stage of training in which the sucrose reward had been replaced with water (Supplementary Fig. 10a–l). When the mouse nose-poked the nonsocial reward port, 10 µL of water were dispensed instead of 10 µL of sucrose solution. Mice were then removed from the limited water schedule, given ad libitum water access, and run daily for several days on the full water access schedule while receiving a water reward when performing a nonsocial trial.
Passive exposure
Mice were placed in a behavioral arena and passively exposed to either a non-cagemate, sex- and age-matched social target through the opening of an automated gate or a 10 µL droplet of a sucrose solution from a sucrose port (Supplementary Fig. 10m–o). Passive exposure type was randomized and there were at least 30 s between exposures.
Social extinction
Once fully trained, mice were removed from the limited water schedule and had ad libitum access to water outside of behavioral sessions. They were maintained on this full water access (FW) schedule and were run daily on the two choice operant assay for 5 days. After 5 days of full water access, the social target was removed, and mice were run daily for 6 days without a social target (Supplementary Fig. 6g).
Definition of task events and parameters
Trial start—the time point when both choice ports illuminate.
Social choice—the time point when the experimental mouse enters (nose-pokes) the social choice port after a trial starts.
Sucrose choice—the time point when the experimental mouse enters (nose-pokes) the sucrose choice port after a trial starts.
Social choice latency—the time between the trial start and social choice port entry. Entry into port (nose-poke) is detected by an infrared beam break.
Sucrose choice latency—the time between the trial start and sucrose choice port entry. Entry into port (nose-poke) is detected by an infrared beam break.
Social reward zone—a 1 × 3 inch rectangular region within the operant arena adjacent to and centered around the social target access point where the gate opens.
Social reward consumption—the time point when the experimental mouse first enters the social reward zone following a social choice. Entry into the social reward zone is detected from the behavioral video using Bonsai software83. A mouse is considered to have “consumed” a social reward if it enters the social reward zone within 20 s after social choice port entry.
Sucrose reward consumption—the time point when the experimental mouse first enters the sucrose reward port following a sucrose choice. Entry into the sucrose reward port is detected by an infrared beam break. A mouse is considered to have “consumed” a sucrose reward if it enters the sucrose reward port within 8 s after sucrose choice port entry.
Social reward latency—the time between social choice port entry and the first entry into the social reward zone.
Sucrose reward latency—the time between sucrose choice port entry and the first entry into the sucrose reward port.
Social reward fail—a trial in which the experimental mouse fails to enter the social reward zone within 20 s (duration for which the social gate is open) following social choice.
Sucrose reward fail—a trial in which the experimental mouse fails to enter the sucrose reward port within 8 s (duration of sucrose reward port activation) following sucrose choice.
Successful trial—a trial in which the experimental mouse entered the respective reward zone following choice port entry while the reward was available (20 s for social trials, 8 s for sucrose trials). On the multi-choice assay (Supplementary Fig. 5a), all null trials were considered successful.
Behavioral analysis
All behavioral sessions were recorded at 40 Hz using Pylon software. Following behavioral sessions, videos were analyzed and behavior was quantified. Several metrics were used to quantify mouse behavior for a given behavioral session, including number of pokes per reward type, choice latency and reward latency. Poke number was defined as the total number of times that an animal nose-poked a choice port, independent of reward consumption. Number of successful trials was determined as the total number of times that an animal nose-poked a choice port and entered the appropriate reward zone when the reward was available for consumption. Choice latency was defined as the time between when the choice port LEDs turned on (trial start) and the animal nose-poked either choice port. Following each choice port nose-poke, the chosen port would flash and the reward would be made available for consumption. Reward latency was defined as the time from the start of the presentation of the reward to the consumption of the reward. Sucrose reward consumption start was measured as the first entry of the mouse into the reward port after it was activated by a sucrose choice port nose-poke. Social reward consumption start was measured as the first entry of the mouse into a 1 × 3 inch area (social zone) in front of the opened social gate following a social choice port nose-poke. Social zone entries were tracked through behavioral video analysis using Bonsai software83. Failure to consume a reward within the allotted time following reward presentation was considered a reward fail.
To quantify the time to investigate a social target after the reward was presented (social latency), behavioral video data from each session were analyzed using Bonsai software83. We used the software to track start and end of trials by detecting changes in light levels of the choice port LEDs. Additionally, we tracked the body position (centroid) of the mouse in the chamber and the mouse’s entry into either reward area (1 × 3 inches) around the social target and sucrose reward port. Reward latency was quantified as the time elapsed between turning off of the choice port LED and the animal’s first entrance into the corresponding reward zone. This analysis was combined with existing session data from Bpod to quantify the social and nonsocial reward behavior of each mouse on all behavioral (training and post-training) sessions.
Endoscopic calcium imaging experiments and analysis
Approximately 1 week post injection, GRIN lens (0.6 mm diameter, 7.3 mm length) implanted mice were trained sequentially on each behavioral assay. Once the mice had been trained for ~2 weeks, they were implanted with the baseplate and habituated with a tethered dummy microscope (Product ID: 1050-003762, Inscopix) during behavioral sessions for at least 3 days prior to the actual imaging session. On the day of imaging, mice were allowed to habituate to the scope for 10 min prior to the start of the session. During the behavioral imaging session, the LED power was set to 0.7 and the analog gain on the image sensor was set to 1.6–1.8. Images were acquired at 20 Hz using Inscopix nVista software. Following imaging acquisition, data were spatially downsampled by a factor of 4 and motion corrected using Inscopix software. A CNMFe algorithm46 was then used to identify individual neurons and their fluorescence traces. The fluorescence traces were subsequently aligned to the behavioral data and used for task modulation analysis. One animal was excluded from calcium imaging analysis due to failure to express GCaMP6f. Custom MATLAB software was used for all imaging data analysis.
Optogenetic activation experiments
Approximately 1 week post injection and ferrule implants, ChR2- and GFP-expressing mice were trained sequentially on each behavioral assay. Before each behavioral session, optic ferrules on the animal’s head were coupled to patch cords (Product ID: SBP, Doric) attached to a commutator (Product ID: FRJ_1×1_FC, Doric) using ceramic split mating sleeves (Product ID: ADAF, ThorLabs). The commutator was connected to the 473 nm laser (Product ID: BL473T8-200FC, SLOC Lasers) via a patch cable (Product ID: M72L05, ThorLabs). Mice were tethered during behavioral training to habituate them to the patch cables. Mice were run on the two choice operant assay for 5 days on the partial water access schedule followed by 5 days on the full water access schedule. Optogenetic activation experiments were then performed over 7 consecutive behavioral sessions. During each 1 hour behavioral session, mice were stimulated for 8 s during the reward period on a random 50% of trials (473 nm, 20 Hz, 5 ms pulse duration, 1–3 mW at fiber tip). Bpod Sanworks and a custom MATLAB program were used to control laser pulses. Behavioral analysis was performed using Bonsai and custom MATLAB software to compare behavioral task parameters, such as reward latency, between (non-stim versus stim trials) and across (GFP control versus ChR2 experimental) mice.
Optogenetic inhibition experiments
Approximately 1 week post injection and ferrule implants, GtACR- and mCherry-expressing mice were trained sequentially on each behavioral assay. Before each behavioral session, optic ferrules on the animal’s head were coupled to patch cords (Product ID: SBP, Doric) attached to a commutator (Product ID: FRJ_1 × 1_FC, Doric) using ceramic split mating sleeves (Product ID: ADAF, ThorLabs). The commutator was connected to the 473 nm laser (Product ID: BL473T8-200FC, SLOC Lasers) via a patch cable (Product ID: M72L05, ThorLabs). Mice were tethered during behavioral training to habituate them to the patch cables. Mice were run on the two choice operant assay for 5 days on the partial water access schedule followed by 5 days on the full water access schedule. Optogenetic activation experiments were then performed over 7 consecutive behavioral sessions. During each 1 hour behavioral session, mice were stimulated for 8 s during the reward period on a random 50% of trials (473 nm, 8 s pulse duration, 10 mW at fiber tip). Bpod Sanworks and a custom MATLAB program were used to control laser pulses. Behavioral analysis was performed using Bonsai and custom MATLAB software to compare behavioral task parameters, such as reward latency, between (non-stim versus stim trials) and across (mCherry control versus GtACR experimental) mice.
Analysis of optogenetic experiments
We tracked each mouse’s position from the time of choice port entry to the end of the trial (social trials: from nose-poke to 20 s after; sucrose trials: from nose-poke to 8 s after) using its centroid coordinates obtained through Bonsai analysis. With these coordinates, we created a 2-D distribution (256×256 pixels) of each mouse’s exploration of the chamber. To quantify the distance traveled in centimeters during the reward period per trial (Fig. 6f, j, p, t and Supplementary Fig. 11d, i, n, s), we identified the number of non-zero pixels from the time of choice port entry to the end of the trial and scaled the size of each pixel to its size in centimeters. To calculate the time spent in the social zone per social trial (Fig. 6g, k, q, u and Supplementary Fig. 11e, j, o, t), we used Bonsai tracking analysis to identify the mouse’s entry into the social reward zone from the time of choice port entry to the end of the trial (20 s after social choice port entry). To control for locomotor changes with mPFC activation, mice were stimulated for up to 60 s in the operant arena outside of the reward period. The distance traveled (cm) outside of the reward period was quantified in 5 s bins for the duration of the stimulation as described above (Supplementary Fig. 11f, k, p, u).
Histology and microscopy
After all imaging and optogenetic experiments were completed, mice were sacrificed and perfused with 0.5% PBS followed by 4% paraformaldehyde (PFA). The brains were dissected out and fixed in 4% PFA overnight at 4 °F. They were then transferred to 30% sucrose solution and allowed to fix for a minimum of 24 h. To visualize viral expression and GRIN lens/ferrule placement, brains were sliced at 50 µm slices using an Epredia HM430 microtome and the slices containing the target brain regions were mounted in DAPI solution (Product ID: 0100-20, Southern Biotech). Slices were imaged using fluorescence microscopy (Keyence BZ-X800). Images were registered using WholeBrain software84 to determine GRIN lens and ferrule placement (RRID: SCR_015245).
Data analysis and statistics
Task modulation analysis
We used a method described in ref. 20 to classify neurons as significantly modulated by various task events (trial start, social/sucrose choice, social/sucrose reward)20. To do this, we examined the normalized change in fluorescence in each peri-behavioral task window. Given the temporal proximity of task events (especially choice and reward delivery), we designated a 3 s window around each task event and calculated the mean fluorescence within that window. Peri-behavioral task windows were assigned as follows to eliminate overlap between task event responses: trial start: 1.5 s before and after; choice: 2.5 s before 0.5 s after; reward: 0.5 s before and 2.5 s after. For excitatory responses, we compared the maximum value of the mean fluorescence to the maximum value expected by a shuffled distribution (>99.5 percentile). For inhibitory responses, which require a sustained suppression of fluorescence, we compared the mean value of fluorescence across the time window to that expected by a shuffled distribution (<0.5 percentile). The shuffled distribution was generated by circularly shifting (coded in MATLAB using circshift) individual fluorescence traces by random values over 1000 iterations and then averaging shuffled activity within each peri-behavioral time window.
To determine if the percent of non-overlapping reward-responsive neurons observed in male and female mice (Fig. 4e) was greater than chance, we compared the proportion of non-overlapping reward responses found in male and female mice to a shuffled distribution. The shuffled distribution was generated using a conservative shuffling method in which we randomly shuffled labels of social or sucrose reward-responsive neurons while maintaining a constant number of neurons (repeated over 100 iterations). The real data were then compared to the shuffled data using an unpaired t test.
Tracking neurons across imaging sessions
We imaged mPFC neurons on the two choice operant assay during three water access conditions (two full water access and one restricted water access conditions) and tracked these neurons across imaging sessions as described in ref. 50. The average time between imaging sessions in male mice was as follows: FWvRW: 8.13 ± 0.72 days, FWvFW2: 21.29 ± 1.13 days and FW2vRW: 12.14 ± 0.86 days. The average time between imaging sessions in female mice was as follows: FWvRW: 10.80 ± 0.92 days, FWvFW2: 4.0 ± 0.84 days and FW2vRW: 7.0 ± 0.32 days. We then classified neurons based on their reward responses (social reward excite, sucrose reward excite, sucrose reward inhibit or reward unresponsive) and compared their reward response profiles across similar (full water access and full water access 2) and dissimilar (full water access/full water access 2 and restricted water access) water access conditions.
Encoding model and task modulation analysis
To isolate the contribution of each behavioral event (trial start, social choice, sucrose choice, social reward, sucrose reward) to the variation in the fluorescence activity, we used a linear encoding model as previously described to estimate neural response kernels for every neuron21,22 (Supplementary Fig. 8). We modeled the z-scored fluorescence activity of each neuron (chosen as the dependent variable) as a consequence of a behavioral event (chosen as the independent variable), which was designed to be a set of binary arrays of the behavioral event time (1 when the event occurs, 0 otherwise), convolved with an spline basis set (with 10 splines) spanning 3 s. Thus, the independent variables of the multiple-linear regression model capture time-delayed relationships between the behavioral event and the corresponding fluorescence. Mathematically, the regression model is set up as below -
where, T is the total duration of the recording, \(F\left(t\right)\) is the z-scored fluorescence of the neuron at time t, \({N}_{{{\rm{events}}}}\) is the number of behavioral events being modeled, and \({N}_{{{\rm{splines}}}}\) is the number of degrees of freedom used for the spline basis set (\({N}_{{{\rm{splines}}}}\,\)= 10). The regression parameters \({{{{\rm{\beta }}}}}_{{se}}\) and \({{{{\rm{\beta }}}}}_{0}\) are the coefficients for the sth spline for event e and intercepts respectively. Lasso regularization parameter \({{{\rm{\lambda }}}}\) is identified through minimizing the squared distance between the fluorescence and the modeled data, using five fold cross-validation. \(S{p}_{{se}}\left(t\right)\) is the independent variable of the regression, and is calculated as follows -
where, \({ts}\) samples from 1 to Ts = 3 s, \({b}_{e}\left({ts}\right)\) is the binary array for the event e, and \({spline}{s}_{s}\) is the sth bspline basis set, created in MATLAB using the create_bspline_basis function of the MATLAB package fdasrvf (https://github.com/jdtuck/fdasrvf_MATLAB).
Considering the temporal proximity of the events, we chose 3 s windows for the choice events (“Sucrose choice”/“Social choice”) as 2.5 s before, and 0.5 s after the event; reward events (“Sucrose reward”/“Social reward”) as 0.5 s before, and 2.5 s after; and “Trial start” to be 1.5 s before and after the event.
Finally, using the regression parameters \({{{{\rm{\beta }}}}}_{{se}}\) and \({{{{\rm{\beta }}}}}_{0}\), we calculated the kernel for each behavioral event and neuron as
We used this encoding model to identify neurons that significantly encode a behavioral event. To identify significant neurons, we compared the change in the goodness of fit of our encoding model (Eq. 1) to the fluorescence data calculated with all events (full model, with \({N}_{{{\rm{events}}}}\,\)= N) to a reduced model where one of the events is removed (\({N}_{{{\rm{events}}}}\) = N-1). We used F-statistic as a measure of goodness of fit for the models and iteratively calculated this statistic by removing predictors of each event from the model. We then compared this value with 500 instances of shuffled data, obtained by shuffling the fluorescence data in 1-s bins. We obtained a p-value by comparing the F-statistic from the real data to the shuffled data. If the calculated p-value for the response of a neuron to the event is less than 0.01 (account for multiple comparisons), the neuron is considered to encode the event.
Neural trajectories for choice and reward
To determine the population activity trajectories for sucrose and social trials, we considered all recorded neurons across trials and reduced them to a three-dimensional neural subspace using Principal Component Analysis (PCA)85. For visualizing the male and female choice (Fig. 3k, m) and reward (Fig. 5e, f) trajectories, we calculated trial-averaged z-scored fluorescence chosen around choice or reward time windows respectively and concatenated across animals to form a three-dimensional array as [number of neurons x trial type x time window]. We fit a PCA (coded in MATLAB using pca) to the array after collapsing across the last two dimensions and projected the resulting dimensionality-reduced array independently onto social and sucrose trials (score output from pca). To quantify the difference between the neural subspace occupied by these trials (Fig. 3l, n; 5e, f) we repeated the procedure as above for each animal and calculated pairwise Euclidean distance both within and between the first three PC projected vectors of social and sucrose trials31. Average Euclidean distances (Fig. 3l, n; 5e, f) are reported across 9 male mice and 6 female mice. Additionally, in Fig. 5e, f, we identified the contribution of social and sucrose reward neurons to the variability explained by PC1 as compared to the rest of the mPFC population. Average PC1 coefficients (absolute values for the loading of PC1, coeff output from pca) are reported across all neurons identified as significantly encoding social (134 neurons in male, 202 neurons in female), sucrose reward (88 neurons in male, 107 neurons in female) or neither (283 neurons in male, 298 neurons in female). In Fig. 7n, o, we determined if neural subspace occupied by social and sucrose trials varied with changes in the internal state of the animal. We calculated trial-averaged z-scored fluorescence chosen around reward from neurons tracked across both the FW and RW access conditions (see section Tracking neurons across imaging sessions) that were concatenated, dimensionality-reduced, and projected independently onto social and sucrose, FW and RW trials as described previously. We also quantified the Euclidean distances both between and within (Fig. 7n, o) the PC projected vectors of the above four trial types and reported the average across 8 male mice and 5 female mice.
Decoding choice and reward using neural data
To decode the identity of choice (Fig. 3h) or reward (Fig. 5b), we used logistic regression (coded in MATLAB using fitclinear) for the neural data from the corresponding choice or reward time window. For each mouse, we used a trial-matched number of sucrose and social trials to construct a three-dimensional array of [number of neurons × number of trials × time window of z-scored fluorescence data]. This ensured that each binary decoder was trained with balanced labels accounting for the variability in the number of sucrose and social trials performed by each mouse. The three-dimensional array was then collapsed across the last two dimensions to use as an input to the regression. This was done to maximize the number of samples that could be used to train our decoder, as described in ref. 86. The input to the decoder was then divided into train and test sets with a 70–30 train-test split and the train set was used to optimize the regression model hyperparameters using Lasso regularization. We evaluated the performance of the decoder, (reported as decoding accuracy) by applying the trained model to predict labels for the test set. We repeated the above steps to calculate decoding accuracy for 500 iterations in which we randomly sampled trials into the train and test set. The average decoding accuracy for each animal was reported as an average of 500 iterations. As a comparison, we also calculated the decoding accuracy for a shuffled dataset that was created by shuffling the z-scored fluorescence data in non-overlapping 1-s bins to maintain the autocorrelation property of the signal but disrupt the activity-aligned neural responses, as described in ref. 87. Average decoding accuracy is reported across 9 male mice and 5 female mice (1 female mouse was excluded due to insufficient number of sucrose trials).
Time-course of choice decoding
To determine the earliest time point at which trial type (sucrose or social) can be successfully decoded from neural activity prior to choice port entry, we modified the aforementioned logistic regression model as follows. In particular, the input to the decoder was the z-scored fluorescence with a time-window of 6 s prior to and 2 s after choice port entry. For trials in which choice latency was <6 s, we time matched by linearly interpolating the neural data from trial start to choice port entry. These data were then binned into non-overlapping 500 ms bins, and decoding accuracy was independently calculated for each time bin. This process was repeated for shuffled data, and significant choice decoding was identified using a two-tailed t test to determine when the decoding accuracy in the time bin of the original data was significantly different from the shuffled data. To compare the time-course of choice decoding between male and female mice (Fig. 3g), we accounted for the differences in the trial numbers and the number of animals in each group. We chose five mice of each sex and matched the number of sucrose and social trials both between and within the groups. Shuffling was performed as described in the “Decoding choice and reward using neural data section” above.
Sex decoding
To determine if sex identity could be decoded from neural activity during the choice and reward periods, we adjusted the aforementioned logistic regression model as follows. For each trial type (social or sucrose trial), we trained binary decoders to predict if the neural responses corresponded to either a male or female mouse. Across the 8 male and 5 female mice (1 male and 1 female mouse were excluded due to insufficient number of trials), we performed 40 pairwise comparisons and calculated decoding accuracies across both unshuffled and shuffled data. We then reported the average accuracy across the 40 comparisons made during social and sucrose choice (Fig. 3i) or reward (Fig. 5a). As an additional measure of decoder performance, we also reported the F1 scores (calculated as the ratio of precision and recall for each decoder) for decoding sex from social and sucrose choice (Supplementary Fig. 9j) and reward (Supplementary Fig. 7f). Similar to our previous decoder models, we matched the number of trials and neurons in each comparison. Shuffling was performed as described in the “Decoding choice and reward using neural data” section above.
Mahalanobis distance
To identify if neural responses to social and sucrose rewards are uniquely represented at a population level, we calculated the Mahalanobis distance between the reward responses of all neurons to their baseline. In line with previous studies27,31,88, we chose the Mahalanobis distance to quantify the difference introduced in the population response due to our two rewarding conditions, while accounting for the underlying baseline variability in the neurons89. For each trial type of social or sucrose, we calculated the Mahalanobis distance as:
where at time t, \(F{\left(t\right)}_{{{\rm{trial type}}}}^{{{\rm{population}}}}\) is the population response vector (number of neurons × 1), \(F{\left(t\right)}_{{{\rm{baseline}}}}^{{{\rm{population}}}}\) is the average population response vector at baseline, \({\Sigma }_{{{\rm{baseline}}}}^{-1}\) and is the covariance matrix calculated over all baseline timepoints. We calculated baseline responses for each mouse as the fluorescence average over the inter-trial-interval period of 30 s and time-matched to the duration of the reward. Mahalanobis distances are calculated as averages over all social and sucrose trials across male (Fig. 5c, Supplementary Fig. 9k: n = 101 social and 153 sucrose trials, 9 mice) and female mice (Fig. 5d, Supplementary Fig. 9l: n = 104 social and 83 sucrose, 5 female mice) from 3 s before to 5 s after social/sucrose reward and baseline. Boxplots on the right panel are average distances for a 3 s window around the reward.
Statistical analyses
All statistical tests were performed using custom MATLAB scripts. Unless otherwise specified, all statistical tests were two-sided and corrected for multiple comparisons. All the statistical tests that were performed and their corresponding values can be found in Supplementary Data 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data shown in the figures are available in the Source Data file. The raw and analyzed calcium imaging datasets collected in this study are too large to be made available online and are available upon request to the corresponding author. The full water access imaging dataset is available here: https://figshare.com/s/794dd9190c2439310955. Behavioral data is available in the Source Data file. Source data are provided with this paper.
Code availability
Custom MATLAB code is available online here: https://github.com/muruganlab/Isaac_et_al_2024_mPFC_sex_diff.
References
Chen, P. & Hong, W. Neural circuit mechanisms of social behavior. Neuron 98, 16–30 (2018).
Dölen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Hung, L. W. et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017).
Rilling, J. et al. A neural basis for social cooperation. Neuron 35, 395–405 (2002).
Panksepp, J. B. & Lahvis, G. P. Social reward among juvenile mice. Genes Brain Behav 6, 661–671 (2007).
Pearson, B. L. et al. Absence of social conditioned place preference in BTBR T+tf/J mice: relevance for social motivation testing in rodent models of autism. Behav. Brain Res. 233, 99–104 (2012).
Hu, R. K. et al. An amygdala-to-hypothalamus circuit for social reward. Nat. Neurosci. 24, 831–842 (2021).
Ramsey, L. A., Holloman, F. M., Hope, B. T., Shaham, Y. & Venniro, M. Waving through the window: a model of volitional social interaction in female mice. Biol. Psychiatry 91, 988–997 (2022).
Solié, C., Girard, B., Righetti, B., Tapparel, M. & Bellone, C. VTA dopamine neuron activity encodes social interaction and promotes reinforcement learning through social prediction error. Nat. Neurosci. 25, 86–97 (2022).
Pierce, A. F., Protter, D. S. W., Chapel, G. D., Cameron, R. T. & Donaldson, Z. R. Nucleus accumbens dopamine release reflects the selective nature of pair bonds. Curr Biol. 34, 519–530.e5 (2024).
Ramsey, L. A., Holloman, F. M., Lee, S. S. & Venniro, M. An operant social self-administration and choice model in mice. Nat. Protoc. 18, 1669–1686 (2023).
Sugrue, L. P., Corrado, G. S. & Newsome, W. T. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat. Rev. Neurosci. 6, 363–375 (2005).
Willmore, L. et al. Overlapping representations of food and social stimuli in mouse VTA dopamine neurons. Neuron 111, 3541–3553.e8 (2023).
Munuera, J., Rigotti, M. & Salzman, C. D. Shared neural coding for social hierarchy and reward value in primate amygdala. Nat. Neurosci. 21, 415–423 (2018).
Jennings, J. H. et al. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565, 645–649 (2019).
Ruff, C. C. & Fehr, E. The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci. 15, 549–562 (2014).
Lockwood, P. L., Apps, M. A. J. & Chang, S. W. C. Is There a ‘Social’ Brain? Implementations and Algorithms. Trends Cogn. Sci. 24, 802–813 (2020).
Starkweather, C. K., Babayan, B. M., Uchida, N. & Gershman, S. J. Dopamine reward prediction errors reflect hidden-state inference across time. Nat. Neurosci. 20, 581–589 (2017).
Cameron, C. M., Murugan, M., Choi, J. Y., Engel, E. A. & Witten, I. B. Increased cocaine motivation is associated with degraded spatial and temporal representations in IL-NAc neurons. Neuron 103, 80–91.e7 (2019).
Parker, N. F. et al. Choice-selective sequences dominate in cortical relative to thalamic inputs to NAc to support reinforcement learning. Cell Rep. 39, 110756 (2022).
Cox, J. et al. A neural substrate of sex-dependent modulation of motivation. Nat. Neurosci. 26, 274–284 (2023).
Otis, J. M. et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 543, 103–107 (2017).
Ferenczi, E. A. et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698 (2016).
Murugan, M. et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677.e16 (2017).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Kingsbury, L. et al. Cortical representations of conspecific sex shape social behavior. Neuron 107, 941–953.e7 (2020).
Levy, D. R. et al. Dynamics of social representation in the mouse prefrontal cortex. Nat. Neurosci. 22, 2013–2022 (2019).
Lee, E. et al. Enhanced neuronal activity in the medial prefrontal cortex during social approach behavior. J. Neurosci. 36, 6926–6936 (2016).
Amadei, E. A. et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301 (2017).
Kingsbury, L. et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell 178, 429–446.e16 (2019).
Castelli, F., Frith, C., Happé, F. & Frith, U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125, 1839–1849 (2002).
Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155, 997–1007 (2013).
Brumback, A. C. et al. Identifying specific prefrontal neurons that contribute to autism-associated abnormalities in physiology and social behavior. Mol. Psychiatry 23, 2078–2089 (2018).
Greene, R. K., Walsh, E., Mosner, M. G. & Dichter, G. S. A potential mechanistic role for neuroinflammation in reward processing impairments in autism spectrum disorder. Biol. Psychol. 142, 1–12 (2019).
Kohls, G. et al. Reward system dysfunction in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 8, 565–572 (2013).
Kohls, G., Antezana, L., Mosner, M. G., Schultz, R. T. & Yerys, B. E. Altered reward system reactivity for personalized circumscribed interests in autism. Mol. Autism 9, 9 (2018).
Anderson, D. J. Circuit modules linking internal states and social behaviour in flies and mice. Nat. Rev. Neurosci. 17, 692–704 (2016).
Tye, K. M. Neural circuit motifs in valence processing. Neuron 100, 436–452 (2018).
Mosberger, A. C., de Clauser, L., Kasper, H. & Schwab, M. E. Motivational state, reward value, and Pavlovian cues differentially affect skilled forelimb grasping in rats. Learn. Mem. 23, 289–302 (2016).
Allen, W. E. et al. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 253 (2019).
Zelikowsky, M. et al. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173, 1265–1279.e19 (2018).
Chen, C. S. et al. Divergent strategies for learning in males and females. Curr. Biol. 31, 39–50.e4 (2021).
Greiner, E. M., Müller, I., Norris, M. R., Ng, K. H. & Sangha, S. Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behav. Brain Res. 368, 111903 (2019).
Becker, J. B. & Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183 (2019).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Elife 7, e28728 (2018).
Rigotti, M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013).
Euston, D. R., Gruber, A. J. & McNaughton, B. L. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012).
Matteucci, G. et al. Cortical sensory processing across motivational states during goal-directed behavior. Neuron 110, 4176–4193.e10 (2022).
Sheintuch, L. et al. Tracking the same neurons across multiple days in Ca2+ imaging data. Cell Rep. 21, 1102–1115 (2017).
van Zessen, R., Phillips, J. L., Budygin, E. A. & Stuber, G. D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012).
Adamantidis, A. R. et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 31, 10829–10835 (2011).
Siemian, J. N., Arenivar, M. A., Sarsfield, S. & Aponte, Y. Hypothalamic control of interoceptive hunger. Curr. Biol. 31, 3797–3809.e5 (2021).
Gangopadhyay, P., Chawla, M., Dal Monte, O. & Chang, S. W. C. Prefrontal-amygdala circuits in social decision-making. Nat. Neurosci. 24, 5–18 (2021).
Hong, W., Kim, D.-W. & Anderson, D. J. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361 (2014).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Chen, C. S., Knep, E., Han, A., Ebitz, R. B. & Grissom, N. M. Sex differences in learning from exploration. Elife 10, e69748 (2021).
Dhingra, I. et al. Sex differences in neural responses to reward and the influences of individual reward and punishment sensitivity. BMC Neurosci 22, 12 (2021).
Borland, J. M., Rilling, J. K., Frantz, K. J. & Albers, H. E. Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology 44, 97–110 (2019).
Vahaba, D. M., Halstead, E. R., Donaldson, Z. R., Ahern, T. H. & Beery, A. K. Sex differences in the reward value of familiar mates in prairie voles. Genes Brain Behav 21, e12790 (2022).
Tan, T. et al. Neural circuits and activity dynamics underlying sex-specific effects of chronic social isolation stress. Cell Rep. 34, 108874 (2021).
Tomova, L. et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat. Neurosci. 23, 1597–1605 (2020).
Senst, L., Baimoukhametova, D., Sterley, T.-L. & Bains, J. S. Sexually dimorphic neuronal responses to social isolation. Elife 5, e18726 (2016).
Campi, K. L., Greenberg, G. D., Kapoor, A., Ziegler, T. E. & Trainor, B. C. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology 77, 208–216 (2014).
Ross, A. P. et al. Sex-dependent effects of social isolation on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5HT) 1a receptor binding and aggression. Horm. Behav. 116, 104578 (2019).
Wallace, D. L. et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 12, 200–209 (2009).
Mastrogiovanni, N. A., Wheeler, A. K. & Clemens, K. J. Social isolation enhances cued-reinstatement of sucrose and nicotine seeking, but this is reversed by a return to social housing. Sci. Rep. 11, 2422 (2021).
Walker, D. M. et al. Crystallin mu in medial amygdala mediates the effect of social experience on cocaine seeking in males but not in females. Biol. Psychiatry.11, 895–906 (2022).
Heilig, M., Epstein, D. H., Nader, M. A. & Shaham, Y. Time to connect: bringing social context into addiction neuroscience. Nat. Rev. Neurosci. 17, 592–599 (2016).
Yamamuro, K. et al. Juvenile social isolation enhances the activity of inhibitory neuronal circuits in the medial prefrontal cortex. Front. Cell. Neurosci. 14, 105 (2020).
Makinodan, M. et al. Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci. Rep. 7, 5481 (2017).
Lee, C. R., Chen, A. & Tye, K. M. The neural circuitry of social homeostasis: consequences of acute versus chronic social isolation. Cell 184, 2794–2795 (2021).
Eiselt, A.-K. et al. Hunger or thirst state uncertainty is resolved by outcome evaluation in medial prefrontal cortex to guide decision-making. Nat. Neurosci. 24, 907–912 (2021).
Grove, J. C. R. et al. Dopamine subsystems that track internal states. Nature 608, 374–380 (2022).
Matthews, G. A. et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016).
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S. & Schultz, R. T. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239 (2012).
Kinard, J. L. et al. Neural mechanisms of social and nonsocial reward prediction errors in adolescents with autism spectrum disorder. Autism Res 13, 715–728 (2020).
Clements, C. C. et al. Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry 75, 797–808 (2018).
Lewis, E. M. et al. Parallel social information processing circuits are differentially impacted in autism. Neuron 108, 659–675.e6 (2020).
Bariselli, S. et al. SHANK3 controls maturation of social reward circuits in the VTA. Nat. Neurosci. 19, 926–934 (2016).
Kim, J., Jung, M. W. & Lee, D. Reward learning improves social signal processing in autism model mice. Cell Rep. 42, 113228 (2023).
Selimbeyoglu, A. et al. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci. Transl. Med. 9, 7 (2017).
Lopes, G. et al. Bonsai: an event-based framework for processing and controlling data streams. Front. Neuroinform. 9, 7 (2015).
Fürth, D. et al. An interactive framework for whole-brain maps at cellular resolution. Nat. Neurosci. 21, 139–149 (2018).
Cunningham, J. P. & Yu, B. M. Dimensionality reduction for large-scale neural recordings. Nat. Neurosci. 17, 1500–1509 (2014).
Lui, J. H. et al. Differential encoding in prefrontal cortex projection neuron classes across cognitive tasks. Cell 184, 489–506.e26 (2021).
Engelhard, B. et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570, 509–513 (2019).
Remedios, R. et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017).
Li, Y. et al. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171, 1176–1190.e17 (2017).
Acknowledgements
We would like to thank Dr. Larry Young, Dr. Robert Liu, and Dr. Nancy Padilla-Coreano for their feedback on the manuscript. We would like to thank Jan Hawes, Dr. Julia Cox, and Dr. Yunmiao Wang for their technical assistance with this project. This work was supported by National Institutes of Health grants R01MH130755 (M.M.) and F31MH133373 (J.I.); the Emory Conte Center Pilot Project Grant (M.M.) and the Emory University Research Committee Grant (M.M.). J.L.J. and M.M. were supported by HHMI Gilliam Fellowship GT15888.
The mouse schematic used in the depiction of the behavioral assay (Figs. 1b and 2f; Supplementary Figs. 1a, 4a, 5a, 5b, 8b, 10m and 11a) was adapted from https://openclipart.org/detail/17558/simple-cartoon-mouse.
Author information
Authors and Affiliations
Contributions
J.I. and M.M. conceived the project and designed the experiments. J.I., S.C.K. and N.S. optimized the behavioral assay. J.I. and S.C.K., with support from J.L.J. and M.R., collected the data. J.I. and H.B. analyzed the data. J.I., S.C.K., H.B. and M.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Isaac, J., Karkare, S.C., Balasubramanian, H. et al. Sex differences in neural representations of social and nonsocial reward in the medial prefrontal cortex. Nat Commun 15, 8018 (2024). https://doi.org/10.1038/s41467-024-52294-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52294-6