Abstract
Most patients with advanced hepatocellular carcinoma (HCC) ultimately experience tumor progression after first-line systemic therapies. Systemic therapy is generally recommended as second-line treatment for advanced HCC in the major guidelines. Combining apatinib with hepatic arterial infusion chemotherapy (HAIC) likely drives synergistic activity on advanced HCC with extrahepatic metastasis. This phase II trial (ChiCTR2000029082) aimed to assess efficacy and safety of this combination in patients with HCC with extrahepatic metastasis who have progressed after first-line systemic therapies. The primary end point was the objective response rate (ORR). The secondary endpoints were progress-free survival (PFS), disease control rate (DCR), 6- and 12-month survival rates, overall survival (OS), and adverse events (AEs). Thirty-nine patients received oral treatment with apatinib, and hepatic artery infusion oxaliplatinplus raltitrexed. Per RECIST v1.1, the ORR and DCR was 53.8% and 89.7% in the patients population, respectively. The median PFS and OS was 6.2 months and 11.3 months, respectively. The 6- and 12-month survival rates were 81.7% and 44.1%, respectively. All AEs were manageable by medication or dose modifications. Apatinib plus HAIC for second-line therapy in advanced HCC with extrahepatic metastasis shows promising efficacy and manageable toxicities.
Similar content being viewed by others
Introduction
According to Barcelona Clinical Liver Cancer (BCLC) staging system, hepatocellular carcinoma (HCC) with extrahepatic metastasis is classified as advanced stage (BCLC stage C) along with portal vein invasion1. Extrahepatic metastases are depicted by imaging at the time of initial diagnosis in 13–37% of patients with HCC2,3. Although there are different treatment methods for HCC in different stages worldwide, systemic therapies are usually the recommended treatment options in the major HCC guidelines for HCC with extrahepatic metastasis4,5,6,7.
Systemic therapy for advanced HCC has made great progress since sorafenib, and many systemic therapies have been successfully used as second-line treatments for advanced HCC, such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs)8,9,10,11. However, compared to systemic therapy alone, the combination of systemic therapy and loco-regional therapy may have a better efficacy for advanced HCC, especially for advanced HCC who have progression of intrahepatic lesions after systematic therapy12.
Apatinib is a small-molecule TKI, which blocks tumor angiogenesis and inhibits tumor growth by targeting VEGFR-213. In a series of trials14,15,16, apatinib has shown encouraging antitumor efficacy and acceptable toxicities in some malignant tumor. In a randomized, placebo-controlled, phase III trial (AHELP study)17, apatinib monotherapy, as a second-line or later treatment, was administered to patients with advanced HCC. The objective response rate (ORR) was 11% (95% CI, 7–15), and the median progress-free survival (PFS) and overall survival (OS) were 4.5 months (95% CI, 3.9–4.7) and 8.7 months (95% CI, 7.5–9.8), respectively. Moreover, apatinib was recommended as a second-line treatment for patients with advanced HCC by the Chinese HCC guideline18.
Hepatic arterial infusion chemotherapy (HAIC) can directly provide sustained high concentrations of chemotherapy drugs to liver tumors, which is related to local anti-tumor effects. The efficacy of HAIC in the application of advanced HCC with lesions limited to the liver has been clarified, and recently the combination therapy of HAIC and TKI has shown satisfactory antitumor outcomes19,20. Moreover, a retrospective study has shown that HAIC, as a loco-regional treatment method, also showed a certain efficacy in HCC with extrahepatic metastasis21.
The anti-tumor mechanism of raltitrexed is to selectively inhibit thymidylate synthase, and synthesize polyglutamic acid compounds through folate based polyglutamic acid synthase in cells. Its inhibitory effect on thymidylate synthase is strong, and it stays in cells for a long time22. Compared to the extremely short plasma concentration half-life of fluorouracil (only 5–20 min)23, the plasma concentration of raltitrexed showed a three-phase decrease after administration, with a final half-life of 8.2 to 105 h24. Raltitrexed can be administered for HCC patients in a shorter period of time (1 h), while fluorouracil requires a longer period of continuous administration (usually ≥46 h)19,25. This is an advantage of raltitrexed, which may improve the patient’s tolerance and adherence. Like HAIC with cisplatin, 5-fluorouracil (5-FU), cisplatin plus 5-FU, or oxaliplatin plus 5-FU, HAIC with oxaliplatin plus raltitrexed was also an efficacious and safe treatment for patients with advanced HCC26,27.
Here we report a phase II, prospective clinical trial of apatinib and HAIC with oxaliplatin plus raltitrexed in patients with HCC with extrahepatic metastasis who had progressed in intrahepatic lesions after first-line systemic therapy. The study showed that combining apatinib with HAIC resulted in encouraging efficacy in patients with HCC with extrahepatic metastasis.
Results
Patient population
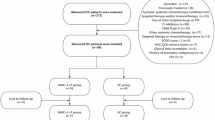
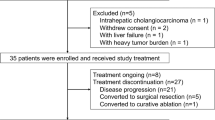
From April 2021 and September 2022, a total 47 patients were screened. Among them, 39 patients (31 males and 8 females; median age, 55 years; range, 28–73 years) were enrolled in the patient population (Fig. 1). The distant metastasis sites were lung, adrenal gland, bone, and abdominal cavity. Among the sites of extrahepatic metastasis, lung and lymph nodes were the most common sites, with 25 patients (64.1%) of lung metastasis and 16 patients (41.0%) of lymph node metastasis. There were 13 patients (33.3%) with multiple systemic metastases and 26 patients (66.7%) with single metastasis (Table 1).
By the data cutoff date of January 31, 2023, the median follow-up period was 20.0 months (95% CI: 16.5–23.5). Out of 39 patients, 32 (82.1%) had discontinued the trial, while 7 (17.9%) patients were still receiving the study treatment, including one patient who was still receiving apatinib plus HAIC and six patients who were still receiving apatinib monotherapy (Fig. 1).
Efficacy
The primary outcome of the study was ORR according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). In the first stage of the studies Simon’s two-stage design, nine of the 19 patients who could be evaluated in the first stage of this study showed an objective response (47.4%), meeting the threshold to continue the study into the second stage.
After the second stage, objective response was achieved in 21 (53.8%) of the 39 patients in the patient population. 21 patients achieved partial response, 14 patients achieved stable disease, and 4 patients achieved progressive disease. Disease control was achieved in 35 (89.7%) of 39 patients in the patient population (Table 2).
Based on modified RECIST (mRECIST), objective response was achieved in 24 (61.5%) of the 39 patients in the patient population. 21 patients achieved partial responses, 11 patients achieved stable disease, and 4 achieved progressive disease. Disease control was achieved in 35 (89.7%) of the 39 patients (Table 2; Figs. 2 and 3).
Target intrahepatic and extrahepatic lesions shrinkage were noted in 34 (87.2%) and 29 (74.4%) of 39 patients after treatment, and the mean best percentage change of the target intrahepatic and extrahepatic lesions size from the baseline were −24.2% (SD 0.28) and −18.8% (SD 0.37) (Figs. 4 and 5).
The median PFS was 6.2 months (95% CI, 4.8–7.6) (Fig. 6). The median intrahepatic control period and extrahepatic control period were 7.0 (95% CI, 4.8-9.2) months and 6.2 (95% CI, 5.0–7.4) months, respectively. There was no significant difference in the median intrahepatic control period and extrahepatic control period (P = 0.383) (Fig. 7).
The 6- and 12-month survival rates were 81.7% (SD, 5.9%) and 44.1% (SD, 8.1%), respectively. The median OS was 11.3 months (95% CI, 8.5–14.1) (Fig. 8).
Safety
We analyzed the safety data in all 39 patients only presenting treatment-related adverse events (AEs). A treatment-related AE included any adverse event that in the investigator’s opinion may have been caused by the study drugs or HAIC procedure with reasonable possibility. There was no report of death. Grade 4 AEs included thrombocytopenia 1 patient (2.6%) and elevated aspartate aminotransferase 1 patient (2.6%). Grade 3 AEs were leukopenia (2 [5.1%]), thrombocytopenia (3 [7.7%]), alanine aminotransferase increased (2 [5.1%]), aspartate aminotransferase increased (3 [7.7%]), hypertension (10 [25.6%]), hand-foot syndrome (5 [12.8%]), proteinuria (3 [7.7%]), and sepsis (1 [2.6%]) (Table 3). Two cases of infectious diseases, including cholecystitis and sepsis, occurred in the combination stage of apatinib plus HAIC and were resolved within 2 weeks of antibiotic treatment.
Thirty (77%) of 39 patients had apatinib dose modifications, of whom 16 (53%) patients only needed to reduce the dose once, and 14 (47%) patients needed to reduce the dose twice. Seventeen (57%) of 30 patients reduced the dose of apatinib during or at the end of treatment in the first cycle, and the first dose reduction of apatinib was recorded in three (10%) patients in the second cycle, five (17%) patients in the third cycle, two (7%) patient in the fourth cycle, two (7%) patient in the fifth cycle, and one (3%) patient in the sixth cycle. Twelve (31%) of 39 patients had dose modifications of two chemotherapeutic drugs, of whom 5 (42%) patients reduced the dose once and 7 (58%) patients reduced the dose twice. Among the 7 patients with two chemotherapeutic drugs dose reductions, three (43%) patients terminated HAIC prematurely after achieving a partial response and were considered by the researchers to be benefiting from treatment and remained in the trial.
At the follow-up end point, 39 patients received a total of 150 cycles of HAIC (mean, 3.8; SD, 1.7). Nine (23.1%) patients completed six cycles of HAIC. The median exposure times of apatinib were 7.2 months, ranging from 1.0 to 22.0 months.
Discussion
In Asian countries, the staging and treatment of advanced HCC with and without extrahepatic metastasis (portal vein invasion) are usually differentiated, and loco-regional therapies including HAIC were performed as treatment option for advanced HCC with portal invasion following the management guidelines of HCC5,6,28. However, in most of trials, the two types of advanced HCC had not been distinguished, or extrahepatic metastasis was merely mentioned in the stratified analysis of advanced HCC. Both systemic and loco-regional therapies are suitable for advanced HCC with portal vein invasion, but loco-regional therapy for advanced HCC with extrahepatic metastasis is undoubtedly not the best choice. This phase II study is to evaluate the combination of a VEGFR TKI and HAIC for advanced HCC with extrahepatic metastasis.
Nowadays, systemic therapy, which was recommended second-line treatment for advanced HCC in the major guidelines, mainly focuses on TKIs and ICIs, including combination therapy or monotherapy8,9,10,11,29,30,31,32. In the several trials of ICI alone or with TKI as second-line therapies for advanced HCC, the ORR of the combination of nivolumab (PD-1 inhibitor) and ipilimumab (cytotoxic T-lymphocyte associated protein 4) was the highest, reaching 32%32,33. However, the curative benefits for patients with advanced HCC remained unsatisfactory. The ORR in this study was significantly higher, and although this was not a head-to-head comparative study, the outcomes in this study were still worth looking forward to.
Compared with the AHELP study of apatinib monotherapy for advanced HCC as a second-line treatment17, apatinib plus HAIC had a better ORR, a longer PFS, and a higher 6-month survival rate in this study. In this study, the proportion of patients with intrahepatic lesions shrinkage was higher than that with extrahepatic lesions (87.2% vs. 74.4%), moreover, the median intrahepatic control period was longer than the median extrahepatic control period (7.0 months vs. 6.2 months). Therefore, there was potential synergistic effects between HAIC and apatinib for advanced HCC. Although these were not head-to-head comparative studies, the results still indicated that HAIC treatment for intrahepatic lesions potentially accelerated the reduction of the burden of intrahepatic lesions, which might lead to better tolerance of patients to apatinib, prolonging the exposure time of apatinib (7.2 months vs. 3.6 months), and might help improve the antitumor effect of apatinib. Furthermore, more patients had the opportunity to receive subsequent antitumor treatments, due to the better tumor control by apatinib, thus obtaining better survival benefits. The addition of HAIC treatment to anti-angiogenic treatment has been shown to improve the outcomes of advanced HCC. In previous studies19,20, the combination therapy of HAIC and sorafenib showed better antitumor outcomes than sorafenib alone. Additionally, a previous retrospective study34 showed that for patients with advanced HCC, the efficacy of apatinib combined with transcatheter arterial chemoembolization (TACE) was superior to that of TACE alone, indicating that the combination of apatinib with loco-regional therapy of liver could effectively improve the clinical efficacy for advanced HCC.
In this study, lung and lymph nodes were the most common sites of extrahepatic metastasis of advanced HCC. This finding was consistent with that of Sanjeev K et al. who analyzed the ___location of extrahepatic metastasis in 403 patients with advanced HCC2.
In the TRIPLET phase II trial35, HAIC combined with apatinib and PD-1 inhibitors were used to treat advanced HCC patients as a first-line treatment. The triple therapy had better ORR (77.1%) and disease control rate (97.1%), as well as longer PFS (10.38 months), and 17.1% of patients achieved tumor downstaging and received curative therapy. There was no patient who underwent curative therapy due to tumor downstaging after treatment in this study. Combined immunotherapy might further enhance the efficacy of HAIC plus apatinib in the treatment of advanced HCC patients, and further comparative studies were needed to demonstrate its effectiveness.
The safety profile of our trial was composed of AEs related to apatinib and HAIC. Hypertension, hand-foot syndrome, oral ulcer and proteinuria were considered to be related to apatinib as an anti-angiogenic TKI agent, and most cases of AEs were grade 1 to 3 in this trial, which were generally consistent with previous studies in HCC with orally administered apatinib17,36. AEs attributable to HAIC with oxaliplatin plus raltitrexed have been reported in a previous study26. In this trial, the incidences of 29.5% for abdominal pain were observed, and two cases (4.5%) of infectious diseases occurred after treatment, one case of sepsis and one case of cholecystitis, which were considered to be related to HAIC. Occurrence of other AEs, including gastrointestinal toxicity, hematological toxicity and liver dysfunction, might be associated with the combination treatment. Compared with the AHELP study of apatinib monotherapy, the incidence of liver injury was low, with hyperbilirubinemia and elevated levels of ALT and AST in this study. In the SILIUS study37, HAIC combined with sorafenib also showed slightly lower liver injury compared to sorafenib alone (including elevated ALT, AST, and total bilirubin). Furthermore, HAIC plus sorafenib significantly reduced the incidence of hyperbilirubinemia compared to sorafenib alone in the FOHAIC-1 study (any grade: 17.2% vs 35.7%)38. This might be related to the rapid reduction of intrahepatic lesion burden by HAIC, which enables patients to have better tolerance to apatinib. All AEs were manageable by medication or dose reduction.
This study has several limitations. Firstly, due to the lack of a control arm in our single-arm design, it is difficult to attribute the observed benefits solely to the addition of HAIC to apatinib. Secondly, the proportion of participants with dose modifications of the study agents, especially apatinib, was higher than expected. However, almost all participants continued to receive study treatment after medication or dose modifications.
In conclusion, apatinib plus HAIC has promising efficacy and manageable toxicities in this trial, making it a promising option for second-line treatment in patients with advanced HCC with extrahepatic metastases. Multicenter phase III randomized controlled trials are warranted to further demonstrate these findings.
Methods
Study design and participants
This was a multicenter, open-label, single-arm, phase II trial done at 5 hospitals in China, with Simon’s two-stage optimal design. The trial was approved by the Ethics Committee of Fujian Cancer Hospital (Fuzhou, China) (K2020-016-02). The study design and conduct complied with all relevant regulations regarding the use of human study participants and was conducted in accordance with the criteria set by the Declaration of Helsinki. All patients involved in the trial provided written informed consent. This study was registered with www.chictr.org.cn, number ChiCTR2000029082.
The first patient was enrolled on 1 April 2019 and the last patient on 26 September 2022. Eligible HCC patients were diagnosed based on histological examination, contrast-enhanced CT, or MRI findings7. Patients aged 18–75 years who had progressed to a first-line systemic therapy (defined as the radiological progression of intrahepatic lesions during the initial first-line systemic therapy or within 6 months after a first-line systemic therapy) with advanced HCC with extrahepatic metastasis were eligible to participate the study. Extrahepatic metastasis included distant metastasis or lymph node metastasis. Other inclusion criteria were: (1) Eastern Cooperative Oncology Group performance score of 0–2, (2) Child-Pugh class of A or B (7 points), (3) adequate hematologic values (leukocyte count >3000/mm3, absolute neutrophil count more than 1500/mm3, platelet count more than 75,000/mm3), (4) adequate renal function (serum creatinine up to 1.5 times the upper normal limit, (5) at least one measurable intrahepatic lesion as defined by RECIST 1.1. The main exclusion criteria included any loco-regional or systemic therapy within the last 4 weeks, previous exposure to apatinib, oxaliplatin or raltitrexed, and uncontrolled hypertension. More detailed inclusion and exclusion criteria are listed in the Study Protocol (in Supplementary Note 2 in the Supplementary Information).
Procedures
For the HAIC procedure, the Seldinger technique was used to puncture the femoral artery. Under the guidance of digital subtraction angiography, the catheter and coaxial microcatheter were inserted into the feeding hepatic artery. Specific details were shown in HAIC protocol (in the Supplementary Note 1 in the Supplementary Information). The treatment regimen was administered as follows: oxaliplatin, 85 mg/m2 (continuous infusion for 4 h); raltitrexed, 3 mg/m2 (continuous infusion for 1 h). After HAIC, the catheter and sheath were removed. The treatment was divided into 3-week cycles, with a maximum of 6 cycles, and continued until disease progression, intolerant toxicities, or patient withdrawal.
All patients were orally administered apatinib at an initial dose of 500 mg once daily for the first time 2 days after initial HAIC, and continued until disease progression, intolerant toxicities, or patient withdrawal.
Treatment modifications were allowed for the AE of apatinib and HAIC, including treatment interruption and dose reduction of apatinib and chemotherapeutic drugs. Repeated interruptions of treatment were permitted as required for no more than 14 days each time.
When grade 3 or 4 AE occurred during HAIC treatment, the next HAIC procedure was suspended until the AE decreased to grade 1 or below; the HAIC procedure was resumed, and both chemotherapeutic drugs were reduced by 25%.
For grade 4 non-hematological AE, apatinib was delayed recovery to grade 1 or below, and then the dose was reduced once. When the first non-hematological AE of grade 3 and hematological AE of grade 3 or 4 occurred, apatinib was delayed until recovery to non-hematological AE of grade 1 or below and hematological AE of grade 2 or below, and then the treatment was resumed at the same dose. At the second occurrence of non-hematological AE grade 3 and hematological AE grade 3 or 4, the patient received a dose reduction (500 mg and 250 mg alternately or 250 mg once a day, depending on the dose level at the time of AE). If researchers considered to reduce the dose necessary, then reduce the dose for intolerable grade 2 AE.
Assessments
All patients were evaluated and documented within a week before the study. Abdominal contrast-enhanced CT or MRI and Chest CT were performed after two, four, and six cycles of HAIC during the combination stage of apatinib plus HAIC, and subsequently every 2 months during apatinib monotherapy until confirmed disease progression. During the combination stage, patients had to come to the inpatient every 3 weeks. During the aptinib monotherapy, patients had to come to the outpatient clinic every month. Two radiologists with more than 10 years of experience in liver cancer diagnosis independently compared the follow-up images with baseline images without survival data to evaluate best tumor response to treatment. Physical examination, tumor markers, blood tests, including hematologic, liver function and renal function tests, were performed when patients came to the inpatient or outpatient clinic as required, and any dysfunction was evaluated. For patients with suspected of new extrahepatic spread, further examination was performed. The AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Outcomes and endpoints
The primary endpoint was the ORR, which was calculated by dividing the number of patients with a best response of complete or partial response by the total number of patients. Tumor response was assessed according to the RECIST v1.1 and mRECIST. The secondary endpoints were PFS, disease control rate, 6- and 12-month survival rates, OS, and AEs. The PFS was calculated from the initiation of the first treatment to the date of disease progression or death by any cause, or it was censored on the last follow-up day if the patient was still alive. The disease control rate was calculated by dividing the number of patients with a best response of complete response, partial response, or stable disease for ≥6 weeks by the total number of patients. The 6- and 12-month survival rates were calculated as the proportion of surviving patients after 6 and 12 months, respectively. The OS was defined as the time from the initial treatment until death from any cause, or it was censored on the last follow-up day if the patient was still alive. The intrahepatic control period referred to the time from initial treatment to the progression of intrahepatic lesions. The extrahepatic control period referred to the time from initial treatment to the progression of extrahepatic lesions.
Statistics and reproducibility
We used Simon’s two-stage design with a one-sided α error rate of 5% and a power of 80%39. The null hypothesis that the true objective response rate per RECIST v1.1 was ≤30% was tested against a one-sided alternative of >30%. A true objective response rate of 50% was assumed. Under these assumptions, the study should be continued after the first stage if six or more enrolled patients responded out of the first 19. This study would be regarded as a success if 16 or more patients out of the total of 39 showed signs of response.
Categorical data were assessed using the chi-square test. The median PFS, OS and associated 95% CIs, and 6-month and 12-month survival rates were estimated using Kaplan–Meier survival analysis, and the median PFS of intrahepatic and extrahepatic lesions were compared using the log-rank test. All statistical analyses were performed using the SPSS software (version 23.0, IBM Corp., Armonk, NY).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Article and Supplementary Information and Source Data file. The de-identified participant data and statistical analysis plan may also be accessed from the first author Shiguang Chen (Email: [email protected]) within 3 months to 3 years after this article publication. And the data is only used for the research purpose. The HAIC protocol and study protocol are available in the Supplementary Information file under Supplementary Note 1 and 2, respectively. The remaining data are available in the manuscript, Supplementary Information, or Source Data file. Source data are provided with this paper.
References
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 6 (2021).
Katyal, S. et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology 216, 698–703 (2000).
Natsuizaka, M. et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J. Gastroenterol. Hepatol. 20, 1781–1787 (2005).
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Zhou, J. et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 9, 682–720 (2020).
Kudo, M. et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer 10, 181–223 (2021).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296 (2019).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Qin, S. K. et al. Pembrolizumab versus placebo as second-line therapy in patients from asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J. Clin. Oncol. 41, 1434–1443 (2023).
Ding, Z. N. et al. Systemic therapy with or without locoregional therapy for advanced hepatocellular carcinoma: a systematic review and network meta-analysis. Crit. Rev. Oncol. Hematol. 184, 103940 (2023).
Peng, S. et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by apatinib. Cancer Lett. 373, 193–202 (2016).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454 (2016).
Lin, Y. et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: the REALITY randomized clinical trial. JAMA Oncol. 8, 242–250 (2022).
Liu, C. et al. Apatinib in patients with advanced chordoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 21, 1244–1252 (2020).
Qin, S. K. et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 6, 559–568 (2021).
Zhou, J. et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition). Liver Cancer 12, 405–444 (2022).
He, M. K. et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 5, 953–960 (2019).
Zheng, K. L. et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology 303, 455–464 (2022).
Lyu, N. et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin in hepatocellular cancer with extrahepatic spread. J. Vasc. Interv. Radiol. 30, 349–357. e2 (2019).
Jarmula, A. Antifolate inhibitors of thymidylate synthase as anticancer drugs. Mini. Rev. Med. Chem. 10, 1211–1222 (2010).
Heggie, G. D., Sommadossi, J. P., Cross, D. S., Huster, W. J. & Diasio, R. B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 47, 2203–2206 (1987).
Clarke, S. J., Hanwell, J., de Boer, M., Planting, A. & Judson, I. R. Phase I trial of ZD1694, a new folate-based thymidylate synthase inhibitor, in patients with solid tumors. J. Clin. Oncol. 14, 1495–1503 (1996).
Lyu, N. et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil / leucovorin versus sorafenib for advanced hepatocellular carcinoma. J. Hepatol. 69, 60–69 (2018).
Chen, S. G., Zhang, K. Z., Liu, W. F. & Yu, W. C. Hepatic arterial infusion of oxaliplatin plus raltitrexed in patients with intermediate and advanced stage hepatocellular carcinoma: a phase II, single-arm, prospective study. Eur. J. Cancer 134, 90–98 (2020).
Zang, M. Y. et al. Hepatic arterial infusion chemotherapy with oxaliplatin plus raltitrexed versus oxaliplatin plus fluorouracil in intermediate and advanced hepatocellular carcinoma: a retrospective study. J. Clin. Oncol. 40, e16166 (2022).
Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC korea practice guidelines for the management of hepatocellular carcinoma. Korean J. Radiol. 23, 1126–1240 (2022).
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952 (2018).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Xu, J. M. et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase ii trial. Clin. Cancer Res. 27, 1003–1011 (2021).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Li, L. et al. Second-line treatment options for hepatocellular carcinoma: current state and challenges for the future. Expert. Opin. Investig. Drugs 31, 1151–1167 (2022).
Chen, S. G., Yu, W. C., Zhang, K. Z. & Liu, W. F. Comparison of the efficacy and safety of transarterial chemoembolization with and without apatinib for the treatment of BCLC stage C hepatocellular carcinoma. BMC Cancer 18, 1131 (2018).
Zhang, T. Q. et al. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): a phase II study. Signal Transduct. Target Ther. 8, 413 (2023).
Yu, W. C., Zhang, K. Z., Chen, S. G. & Liu, W. F. Efficacy and safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: a prospective observation study. Medicine 97, e9704 (2018).
Kudo, M. et al. SILIUS study group. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet. Gastroenterol. Hepatol. 3, 424–432 (2018).
Lyu, N. et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J. Clin. Oncol. 40, 468–480 (2022).
Simon, R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials 10, 1–10 (1989).
Acknowledgements
The authors thank the participating patients and their families for their trust and contributions to the study. This work was supported by funding from the Guiding Project of Science and Technology Planning Project in Fujian Province, China (2019Y0060 to S.C.), the Natural Science Foundation of Fujian Province, China (2023J011248 to S.C.).
Author information
Authors and Affiliations
Contributions
Conceptualization: S.C. and C.C.; data curation: S.C., X.W., B.Y., J.P., Q.X., W.Y., N.G., Z.W., J.H., W.L., and X.W.; funding acquisition, S.C.; investigation, W.Y.: methodology, S.C., and C.C.: project administration, C.C.: software, S.C., X.W.: supervision, C.C.: writing—original draft, all authors; writing—review & editing, all authors. All authors were involved in the approval of the report and the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yanfang Liu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Wang, X., Yuan, B. et al. Apatinib plus hepatic arterial infusion of oxaliplatin and raltitrexed for hepatocellular carcinoma with extrahepatic metastasis: phase II trial. Nat Commun 15, 8857 (2024). https://doi.org/10.1038/s41467-024-52700-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52700-z