Abstract
The redox state of a planetary mantle affects its thermal evolution. The redox evolution of lunar mantle, however, remains unclear due to limited oxygen fugacity (fO2) constraints from young lunar samples. Here, we report vanadium (V) oxybarometers on olivine and spinel conducted on 27 Chang’e-5 basalt fragments from 2.0 billion years ago. These fragments yield an average fO2 of ΔIW -0.84 ± 0.65 (2σ), which closely aligns with the Apollo samples from 3.6–3.0 billion years ago. This temporal uniformity indicates the lunar mantle remained reduced. This observation reveals that the processes responsible for oxidizing mantles of Earth and Mars either did not occur or had negligible oxidizing effects on the Moon. The long-term reduced mantle may lead to a distinctive volatile degassing pathway for the Moon. It could also make the lunar mantle more difficult to melt, preventing internal heat dissipation and consequently resulting in a slow cooling rate.
Similar content being viewed by others
Introduction
Oxygen fugacity (fO2), a measure of the redox conditions of rocks, is a leading factor controlling several planetary properties and the speciation of volatiles during volcanic outgassing1,2,3,4. The mantles of large differentiated terrestrial bodies, such as Earth and Mars, have undergone oxidation over time from an originally reduced state. Such increases in oxygen fugacity have profoundly impacted both the composition of their mantles as well as the two planets’ surface environments1,2,3,4,5,6. However, it is still unclear if such an oxidation evolution is widespread among differentiated terrestrial bodies. Based on lunar samples from ca. 3.6–3.0 billion years ago (Ga), a fO2 of ~ΔIW -1 was estimated for the lunar mantle6. However, the redox evolution of the lunar mantle remains unknown owing to the absence of younger samples. China’s Chang’e-5 (CE-5) mission, delivering the first samples from the Moon in over four decades after the Apollo and Luna missions, successfully retrieved 1731 g of lunar soil samples from northern Oceanus Procellarum7. Petrographic and geochronological analyses of the basalt fragments in the CE-5 soil reveal that they formed during volcanic eruptions at ca. 2.0 Ga (in refs. 8,9). Based on their homogeneous 87Sr/86Sr ratios, εNd(t) values, and comparable REE patterns, the CE-5 basaltic fragments were likely derived from a series of lava flows10,11. These relatively young basalt samples thus provide a unique opportunity to investigate the redox evolution of the lunar mantle over time.
Various approaches have been applied for estimating the oxygen fugacity of lunar basalts and pyroclastic glasses, such as solid electrolytes12,13, mineral assemblages14, melt and mineral compositions15, and the oxidation state of multiple valence cations16. Although each approach has its limitations, most results suggest that the lunar basalts and pyroclastic glasses crystallized at relatively reduced conditions ranging from the ilmenite breakdown reaction (ilmenite = rutile+iron) to the iron-wüstite buffer (IW)12,13,14,15,16. To determine the fO2 at reduced conditions, vanadium (V) oxybarometers have been developed17,18,19,20. Varying in different valence states (V2+, V3+, V4+, and V5+), the behavior of V differs considerably between the Earth, Moon, and Mars: in terrestrial basalts, V4+ is dominant; in lunar basalts, V3+ is dominant; and in Martian basalts, V3+ and V4+ are both abundant19,20,21. Empirical studies have demonstrated that the partitioning behavior of V (DV) between olivine/spinel and a melt is redox-sensitive22,23. The DVolivine/melt and DVspinel/melt values tend to increase as the oxygen fugacity decreases24,25. Applying both of these oxybarometers requires that the olivine/spinel and melt are in equilibrium. In addition, the partitioning of V between spinel and olivine can also be employed20. The advantage of the DVspinel/olivine method is that it does not require samples representing melts or samples with remnant melt present. It only requires that the spinel-olivine pairs co-crystallized or were in equilibrium20. Based on these V oxybarometers, the fO2 of the Apollo lunar mare basalts and pyroclastic glasses has been determined from ΔIW -1.9 to −0.9 (refs. 18,19).
Here, we perform systematic analyses of V oxybarometers for olivine and spinel from 27 CE-5 basalt fragments using their major and trace element compositions to constrain the redox conditions of the lunar mantle source, which can then be compared to other terrestrial bodies in the Solar System.
Results
Petrographic and mineralogical characteristics of CE-5 basalt fragments
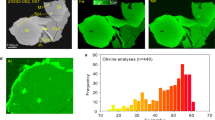
The CE-5 basalt fragments analyzed here show variable textures, including subophitic, poikilitic, porphyritic, and equigranular (Supplementary Table 1), which are consistent with previous observations10. The basalt fragments consist primarily of pyroxene, plagioclase, olivine, and ilmenite, with trace amounts of silica, spinel, apatite, whitlockite, baddeleyite, zirconolite, and tranquillityite (Supplementary Table 1). Olivine and spinel co-crystallized during the cooling of the CE-5 basaltic magma. In the early stage of crystallization, olivine became enriched in magnesium (Mg) with high Mg# [atomic Mg/(Mg + Fe)] and spinel enriched in chromium (Cr) with low Ti# [atomic Ti/(Ti + Cr)]. As the crystallization process continues, Mg# in olivine decreases while Ti# in spinel increases (Fig. 1). The compositions and evolutionary trends of olivine, spinel, pyroxene, and plagioclase are consistent with previous studies8,10,26,27 (Supplementary Figs. 1–4; Supplementary Data 1), supporting the interpretation that these CE-5 basalt fragments probably originated from a similar mantle source10,11,28. The V contents in olivine range from 2.8 ± 0.4 to 47.0 ± 1.7 ppm (Supplementary Data 2) and are positively correlated with Mg#Ol (OlFo) (Supplementary Fig. 1f). The concentration of V in spinel varies from 681.0 ± 4.0 ppm to 4,826.0 ± 30.0 ppm (Supplementary Data 3) and exhibits a negative correlation with Ti# (Supplementary Fig. 2b).
In the early stage of crystallization, olivine has high Mg# and the coexisting spinel has low Ti# (a, b). As the crystallization process continues, Mg# in olivine decreases while Ti# in spinel increases (b, c). In the late stage of crystallization, olivine has low Mg# and the coexisting spinel has high Ti# (d). Mg#= [atomic Mg/ (Mg + Fe)]; Ti#= [atomic Ti/ (Ti + Cr)]. Abbreviations: Cpx, clinopyroxene; Ilm, ilmenite; Pl, plagioclase; Ol, olivine; Spl, Spinel; Tro, troilite.
The fO2 of CE-5 basalts
The V contents in olivine and spinel, combined with the whole-rock V contents previously reported29, allow us to estimate the fO2 of CE-5 basalt. The whole-rock Mg# of CE-5 basalt is estimated to be 28.9 with a V content of 88.9 ppm (refs. 26,29). The OlFo of olivine equilibrated with the CE-5 basalt can be predicted from the forsterite of liquidus phases by the equation (Mg#melt = 1/([1/OlFo-1]/KD + 1)) (ref. 30). The OlFo in equilibrium with the CE-5 basalt ranges roughly from 51.9–58.5 when KD = 0.333 ± 0.044 (ref. 31). According to the olivine V oxybarometer20, the fO2 of CE-5 basalt is calculated to be from ΔIW −1.37 ± 0.10 to ΔIW + 0.16 ± 0.32, with an average of ΔIW -0.95 ± 0.82 (2σ) (Fig. 2a and Supplementary Data 4). Similarly, the spinel V oxybarometer23, based on DV in Cr-spinel and melt, yields fO2 ranging from ΔIW -0.94 ± 0.07 to ΔIW -0.55 ± 0.07 with an average of ΔIW −0.76 ± 0.24 (2σ) (Fig. 2a and Supplementary Data 5). Overall, the fO2 of the CE-5 basalt is estimated to be ΔIW -0.84 ± 0.65 (2σ). This result represents the fO2 of the original melt of the CE-5 basalt because the olivine phenocrysts and Cr-spinel are the earliest crystallizing phases of the CE-5 basalt10,11,29. The fO2 of CE-5 basalt closely resembles the average fO2 (ΔIW -1.0) of the lunar mantle derived from the 3.6–3.0 Ga Apollo basalts and pyroclastic glasses6,12,13,14,15,16,17,18,19, suggesting that the lunar mantle maintained its reduced state from 3.6 Ga to 2.0 Ga.
a The fO2 estimates of the CE-5 basalt fragments derived from V oxybarometers. The grey bar represents the average fO2 of the original melt of the CE-5 basalt. The gray lines indicate the trend of increased fO2 during late-stage crystallization of the CE-5 basalt when Mg#equilibrium melt ≤ 20. b The mantle fO2 of the Moon, Mars, and Earth. The fO2 data for the Apollo samples are from refs. 18,19; the fO2 data for Mars are from refs. 5,65,67,68,69; the fO2 data for Earth are from refs. 3,70,71. The average fO2 (ΔIW-1.0 ± 0.9) of the lunar mantle was calculated based on the CE-5 basalt (ΔIW -0.84 ± 0.65) and the Apollo samples18,19; the average fO2 of the mantles of Earth and Mars are from refs. 5,33, respectively, noting that shergottite is considered the most accurate approximation for bulk silicate Mars, and others are excluded from the average calculation (Methods); The grey bars represent the average fO2 of the mantles of Earth, Mars and Moon. The error bar represents 2 standard deviations for each point. Abbreviations: CE-5, Chang’e-5; Ol, olivine; Spl, spinel; VLT, very low Ti. Source data are provided as a Source Data file.
Furthermore, the variation of fO2 throughout the entire crystallization of the CE-5 basalts was measured using the spinel-olivine V oxybarometer20. The fO2 increases from ΔIW -1.86 ± 0.15 to ΔIW + 2.18 ± 0.54 during late-stage crystallization when the Mg# of the equilibrium melt drops to <20 (Fig. 2a; Supplementary Data 6). This rise in fO2 during the late-stage magma evolution has also been observed in Apollo basalts, which is suggested to result from hydrogen degassing21. This process also occurred during the late-stage crystallization of the CE-5 basalt, as indicated by hydrogen isotopic variations in melt inclusions in ilmenite32. Nevertheless, the average fO2 during the early-stage crystallization (Mg# of the equilibrium melt ≥29) is ΔIW -0.92 ± 0.15, which is consistent with those inferred from olivine and spinel V oxybarometry (Fig. 2a; Supplementary Data 6). Therefore, the rise in fO2 during the late-stage magma evolution does not affect the fO2 estimation of the original melt of the CE-5 basalt.
Discussion
Based on our result and the published data on the Apollo basalts and pyroclastic glasses, the fO2 variation of the lunar mantle can be constrained through time. The fO2 estimates of the lunar mantle vary within a quite restricted range from ΔIW -1.90 ± 0.20 to ΔIW + 0.16 ± 0.32. First, just in terms of variation, the lunar range is smaller than those of the mantles of Earth and Mars (Fig. 2b). Second, in terms of the fO2 values themselves, the lunar mantle exhibits the most reduced state with an average fO2 of ΔIW -1.0. In contrast, Earth’s mantle is the most oxidized (average ΔIW + 3.5), followed by Mars’ mantle at a little less than half that value (average ΔIW + 1.4). It can be inferred that the original fO2 values of Earth, Mars, and the Moon were all initially reduced enough (ΔIW -1.5 to ΔIW -2.0; refs. 33,34) to reach a state of equilibrium with iron metal in order to have segregated their metallic cores. As such, the mantles of Earth and Mars must have undergone considerable oxidation from their early reduced conditions1,2,3,5. In stark contrast, the redox state of the lunar mantle changed little for about 2.0 billion years from ca. 3.6 Ga to 2.0 Ga, from ΔIW -1.90 to ΔIW -0.84, respectively (Fig. 3a). The preservation of this long-term reduced lunar mantle suggests that the processes that oxidized the mantles of Earth and Mars either did not occur or had negligible oxidizing effects on the Moon.
a The temporal evolution of mantle fO2 for the Moon, Mars, and Earth. The fO2 data for the Earth’s mantle are from mid-ocean ridge basalts and ultramafic lavas1,2. The fO2 data for the Martian mantle are from shergottites5. The fO2 data for the lunar mantle are from the Apollo basalts and pyroclastic glasses18,19. The age data are from refs. 1,2,5,72,73. The red star represents the original average fO2 values of Earth, Mars, and the Moon derived from refs. 33,34. The error bar represents 2 standard deviations for each point. b Mechanism diagrams by terrestrial body. The late accretion mass of Earth, Mars, and Moon are from ref. 38. The late accretion mass ratio represents the ratio of late accretion mass to the body mass. Abbreviations: CE-5, Chang’e-5; Ga, Giga annum; GPa, Giga Pascal. Source data are provided as a Source Data file.
[7] Three potential processes could oxidize planetary mantles. One is Fe-disproportionation caused by the crystallization of bridgmanite [(Mg,Fe)(Al,Si)O3)], the predominant mineral of the lower mantle. Under high pressures (>10 GPa), Fe2+ in bridgmanite undergoes disproportionate conversion to Fe3+ and Fe0, following the equation 3Fe2+ = 2Fe3+ + Fe0 (refs. 33,35). The separation of precipitated iron metal from the crystallizing lower mantle and its migration into the core may have increased the overall oxygen concentration of the mantle. The elevated fO2 of Earth’s mantle has been proposed to thus result from the loss of disproportionated Fe triggered at high pressures33,35 (Fig. 3b). Mars is smaller than Earth, and thus has a relatively limited area at depth where its lower mantle can reach pressures high enough to cause Fe-disproportionation5 (Fig. 3b). For the even smaller Moon, Fe-disproportionation could be absent entirely because the mantle pressure (<4 GPa) is insufficient to trigger the conversion33,35 (Fig. 3b), facilitating the maintenance of the reduced state.
The second candidate mechanism is late accretion. If late-accreted materials with high fO2 were implanted in the mantle during impacting, the oxidation state of the mantle would increase36,37. Although the Earth and Mars have large late accretion mass ratios (0.33% and 0.31%, respectively)5,38, it is unclear whether the late accreted materials were oxidizing36,37 or not39,40. Nevertheless, the Moon has accreted a significantly smaller volume of materials over its evolutionary history with a late accretion mass ratio as low as 0.02% (ref. 38; Fig. 3b). Even if we assume that all the late-accreted materials were oxidized materials (e.g., pure water ice) and can only exhaust ~0.06% iron metal, which may still be insufficient to oxidize the lunar mantle.
Hydrogen loss presents a third possibility. A substantial loss of a strong reducing agent, i.e., hydrogen, would have elevated the mantle fO2. The loss of hydrogen can occur by hydrodynamic escape under an H2-dominated (compared to other H-species) atmosphere3 or by faster escape of H than O from a water vapor-dominated (compared to other H-species) atmosphere41,42. In the case of the Moon, if an early atmosphere was present, the total amount of hydrogen should mainly occur as H243. The degassing of H2 from the mantle can result in an oxidizing effect through the reaction H2O + Fe0 = H2 + FeO. Once the Fe0 was exhausted, continued H2 loss would have oxidized the mantle. However, there is no significant change in the fO2 of the lunar mantle from the earliest constraints at 3.6 Ga to the eruption of the CE-5 basalt at 2.0 Ga (Fig. 3a), indicating that hydrogen degassing of the lunar mantle during its evolution (e.g., magma ocean and mare basalt volcanism) was not sufficient to exhaust Fe0 and increase oxygen fugacity. Because the relatively small size of the Moon theoretically permits a greater degree of degassing than larger planetary bodies44, it can be posited that the long-term reduced lunar mantle possessed a relatively low initial water content.
In summary, the insufficient pressure for Fe-disproportionation, the low mass ratio of late-accreted materials, and the low initial water content of the Moon may collectively contribute to the maintenance of its long-term reduced state, which in turn affects the thermochemical evolution of the Moon, including the degassing pathway and cooling history4,45. The species and behavior of gases in the C-H-O-S system are predominantly controlled by oxygen fugacity. Under high fO2 conditions, volatile species such as CO2, H2O and SO2 dominate the degassing gas. At low fO2, the degassing is dominated by CO, H2 and H2S4,45. Since the diffusion rate of H2 is approximately three orders of magnitude faster than that of H2O46, hydrogen degassing should be more extensive at low fO2. In contrast, sulfur degassing should be suppressed due to the high solubility of sulfur in silicate melt under reduced conditions45,47. This degassing pathway under reduced conditions can well explain the observed low degrees of S degassing (19 ~ 40%) and high degrees of H degassing (98-99%) in the Apollo and CE-5 basalt samples32,48,49.
The redox evolution of the lunar mantle also serves as a probe for understanding the secular cooling of its interior. Based on the bulk composition and fO2 of lunar basalts, the evolution of the mantle potential temperature (Tp) of the Moon with time can be estimated per billion years (Gyr). The Tp derived from the CE-5 basalt at 2.0 Ga is estimated to be 1186 ± 8 °C under the actual reduced condition (average ΔIW -1.0). This result is ~179 °C lower than the ca. 4.4–3.0 Ga lunar samples with an average Tp of 1,365 ± 66 °C (Fig. 4; Supplementary Data 7), indicating a relatively slow cooling rate (75 ± 24 °C Gyr−1) of the lunar mantle. If we assume that the CE-5 basalt derived from a lunar mantle with the same oxidation state as the Earth’s mantle (average ΔIW + 3.5), the estimated Tp should be as low as 1119 ± 9 °C (Fig. 4; Supplementary Data 7), requiring a faster cooling rate of ~103 ± 24 °C Gyr−1 over the same time interval. This prediction is supported by an experimental study showing that rocks with lower fO2 are more resistant to melting4. It can thus be postulated that the elevated melting temperature in the reduced lunar mantle may lead to the observed slow cooling rate due to the suppression of volcanic activity and the subsequent retardation of the dissipation of the Moon’s internal heat.
The mantle potential temperature (Tp) of the lunar mantle over time according to different fO2. The average bulk compositions of low-Ti lunar basalts, meteorites, and CE-5 basalts are from refs. 26,62,63, and their ages are from refs. 1,2,5,72,73. Tp values are calculated based on ref. 31. The error bars are calculated from the uncertainties in the lunar mantle potential temperature (Tp). CE-5, Chang’e-5; Ga, Giga annum. Source data are provided as a Source Data file.
Methods
Sample preparation and characteristics
Twenty-seven basalt fragments were obtained from a series of Chang’e-5 (CE-5) lunar soil samples (CE5CO100YJFMO0103, CE5CO400YJFMO0406, CE5C0600YJFM00402, and CE5Z0303YJFM002) allocated by China National Space Administration. These basalt fragments, ranging in size from ~0.25–1.28 mm, exhibit diverse textures such as subophitic, poikilitic, porphyritic, and equigranular. These textures are comparable to those observed for other basalt fragments in CE-5 soil samples10. They were embedded in one-inch epoxy mounts and polished before analyses. These basalt fragments are composed of primary clinopyroxene (6.13% ~ 81.53%), plagioclase (0.24% ~ 45.96%), olivine (0% ~ 81.16%), and ilmenite (0.54% ~ 13.98%) (Supplementary Table 1), as well as minor amounts of silica, spinel, apatite, whitlockite, baddeleyite, zirconolite, and tranquillityite. Despite exhibiting various petrographic textures and modal abundances of minerals, their mineral chemistry remains highly similar and follows the same evolutionary path (Supplementary Figs. 1–4), consistent with those previously reported8,10,11. The differences in textures and mineralogical abundances of these basalts are likely due to the different cooling rates within different parts of a single lava flow and small sample sizes (<3 mm) of the basalt fragments, respectively10. Recent reports have investigated the petrology, geochemistry, and volatile contents of the CE-5 basalt8,10,11,26,32,50,51 and the bulk compositions of lunar soils7,28,29,52 in detail. The results suggest that the CE-5 basalts are more evolved than the Apollo and Luna samples. The olivine in these basalts with grain sizes ranging from 35–300 μm occurs as phenocrysts with Fo from 60 to 16. These olivine phenocrysts are one of the earliest crystallizing phases in the CE-5 basalt and thus may have been in equilibrium with the original basaltic melt based on their Fo and the whole-rock Mg# (ref. 11), which provides the basis for using olivine V oxybarometers20. The spinel of these basalts, with small grain sizes from 10–50 μm, exists as Cr- and Ti-spinel. The Cr-spinel crystallized at the early stage of the “low-Ti” lunar mare basalts10. The equilibrium between Cr-spinel and basaltic fragments provides the basis for using spinel V oxybarometers23. Olivine and spinel co-crystallized during the cooling of the CE-5 basaltic magma. In the early stage of crystallization, olivine and spinel are enriched in Mg and Cr, respectively, and they gradually enriched in Fe and Ti in the late stage of crystallization, respectively (Fig. 1). The equilibrium between olivine and spinel provides the basis for using olivine-spinel V oxybarometers20.
Scanning electron microscope imaging
Back-scattered electron (BSE) images were obtained with a Thermo scientific Apreo scanning electron microscopy (SEM) using an FEI Apreo equipped with an energy dispersive x-ray spectrometer (EDS) at the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS) in Beijing. The accelerating voltage was 15.0 kV, and the probe current was 6.4 nA. The EDS data were used to identify the textures and mineral characteristics of the CE-5 basalts.
Electron microprobe analysis
The major element concentrations of primary minerals in the samples were measured using a JEOL JXA8100 electron probe at IGGCAS. The experiment utilized an accelerating voltage of 15 kV, a beam current of 12 nA, a beam diameter smaller than 5 μm, and a counting period ranging from 10–30 s. Elemental data was calibrated using a series of natural minerals and synthetic oxides. The precision for major elements is better than 1.5%, as shown by the study of analysis of internal laboratory standards.
High-precision electron probe microanalysis
Spinel in the CE-5 basalts was identified and measured using the high-precision electron probe microanalysis method (HP-EPMA) on the CAMECA SXFive electron microscope at IGGCAS. Analytical procedures referred to ref. 53. The acceleration voltage was set to 25 kV. The beam currents for trace elements (Ti, V, Mn, Co, Ni, and Zn) and major elements (Cr, Al, Mg, and Fe) were 200 nA and 60 nA, respectively, with a beam diameter of 1 μm. The natural minerals and synthetic oxides were used for high-precision (elemental) analyses: rhodonite (Si and Mn), apatite (Ca), rutile (Ti), FeS2 (Fe), ZnS (Zn), Cr2O3 (Cr), CoO (Co), NiO (Ni), MgO (Mg), and V2O5 (V). Peak count times were as follows: 10 s for Al, Mg, and Fe; 20 s for Cr; 80 s for Ca, Ti, V, Co, and Zn; 120 s for Mn and Ni; and 240 s for P and Si. The LBS13-04 spinel standard was analyzed two times per 10 measurements to monitor machine drift. Average detection limits (3σ) for Ti, V, Mn, Co, Ni, Zn, Ca, and Si are 17, 18, 33, 20, 55, 48, 16, and 17 µg/g, respectively.
In situ trace-element analysis
The trace element analyses for olivine in the CE-5 basalts were carried out using a GeolasHD 193 nm ArF excimer LA system (Coherent; Göttingen, Germany) coupled to an Element XR sector field (SF)-ICP-MS (Thermo Fisher Scientific; Bremen, Germany) at the State Key Laboratory of Lithospheric Evolution, IGGCAS. The laser diameter is 24 µm with a repetition rate of 5 Hz. The laser energy density is ca. 3 J/cm2. Helium was employed as the ablation gas to improve the transporting efficiency of ablated aerosols. All trace element determinations were performed using time-resolved analysis in the fast, peak jumping mode. The signal intensities of 7Li, 23Na, 25Mg, 27Al, 29Si, 31P, 43Ca, 45Sc, 49Ti, 51V,53Cr, 55Mn, 57Fe, 59Co, 60Ni, 63Cu, 67Zn, 69Ga, and 89Y were monitored during each analysis. The GOR132-G olivine standard54,55 was used to correct for the instrument- and time-dependent fractionations of Fe/Mg ratios and all trace elements. The MongOLSh11-2 and XEN olivine standards56,57 were employed for quantity control. The GOR132-G, MongOLSh11-2, and XEN olivine standards were analyzed each time per 10 measurements. Average detection limits (3σ) for most elements are better than 0.1 µg/g. The accuracies of the major elements (Mg, Fe, and Si) and trace elements (2 RSD) are better than 1.5% and 10%, respectively. The analytical uncertainty of Fo (2 RSD) is ±0.17% for the long-term analysis. More details of the analytical procedures and data acquisition are provided in ref. 58.
V oxybarometer
In this study, we used the vanadium (V) in olivine oxybarometer (Dvolivine/melt = 0.316×e-0.356×ΔIW; ref. 20) and spinel V oxybarometer (log (Dvspinel/melt) = −1.09 −0.186 × ΔFMQ + 2477/T + 0.004 ×Cr#; ref. 23) to constrain the fO2 of the CE-5 basalt. The olivine V oxybarometer was developed by studying the distribution of V between synthetic olivine and melt at a constant temperature (1,200 °C) under varying oxygen fugacity conditions. The spinel V oxybarometer was developed by systematically assessing the impact of various factors (including fO2, T, P, and mineral or melt composition) on the Dvspinel/melt ratio. In the above equations, T represents the temperature in Kelvin; Cr#=Cr/(Al + Cr)(%); ΔIW and ΔFMQ are the fO2 difference between experimental fO2 and IW and FMQ buffer, respectively. The compositions in our investigation were obtained from two CE-5 basalt fragments29. The eruption temperature estimated for CE-5 basalts is around 1150–1230 °C (ref. 11), so the average temperature of 1200 °C was adopted in this study. According to the IW and FMQ buffer formulations59,60 at a given temperature of 1200 °C, the ΔIW is approximately 3.5 log units below ΔFMQ (ref. 61).
We employed the spinel-olivine V oxybarometer20 (Dvolivine/spinel = 29.42×ΔIW + 89.41) to quantify the changes in oxygen fugacity in the CE-5 basalts during crystallization. First, a relationship (Fo = -0.7581× Ti#+82.466; R2 = 0.99) between the Ti# of spinel and Fo of olivine was established using representative samples in which spinel and olivine coexist (Fig. 1). Then, the V contents in olivine co-crystallized with spinel were calculated based on the positive correlation between V contents and Fo in olivine (Vcontent = 0.8853 × Fo-10.459; R2 = 0.84). It should be noted that the average Dvspinel/olivine value of our samples ( ~ 122) is slightly higher than the calibration formulas previously reported (~60-120; ref. 20). The main reason should be that the spinel-olivine V oxybarometer was established by homogeneous synthesized Mg and Al-spinel (MgAl2O4) and Mg-olivine20. However, the late-stage crystallization minerals in the CE-5 basalt are Fe-olivine and Ti-spinel10,11. Therefore, the accuracy of the estimated fO2 during late-stage crystallization still needs to be verified by future experimental studies. Nevertheless, these results of the late-stage crystallization do not affect the fO2 estimation of the original melt of the CE-5 basalt.
Mantle potential temperature calculation
The mantle potential temperature (Tp) of the CE-5 basaltic fragments was calculated using a spreadsheet provided by ref. 31. The average bulk compositions of low-Ti lunar basalts, meteorites, and CE-5 basalts were collected from the literature26,62,63. The pressures of ca. 4.4–3.0 Ga lunar samples and ca. 2.0 Ga CE-5 basalts were assumed to be 3.5 GPa and 2.0 GPa, respectively, as suggested by ref. 64. Detailed results are listed in Supplementary Data 7.
Mantle fO2 data for Earth, Mars, and the Moon
The Earth mantle fO2 constraints from two available studies show a similar fO2 trend1,2, but each dataset was determined using a different oxybarometer. The fO2 derived from different oxybarometers shows a systematic offset. Ref. 3 corrected these two datasets using the current canonical modern MORB value of ΔFMQ + 0.2 ± 0.3. Upon correction, the two mantle oxidation trends overlap within error, thus supporting the mantle oxidation concept. The emendated data suggest a notably elevated fO2 of Earth’s mantle compared to the values (~ΔIW -2) during the final stages of core formation33. The oxygen fugacity of Mars may be directly estimated by analyzing Martian meteorites such as shergottites, nakhlites, and chassignites. The fO2 of shergottites based on the V olivine oxybarometer yields an average fO2 of ΔIW + 1.4 ± 1.8 (ref. 5). In contrast, chassignites and nakhlites are relatively oxidized (ΔIW + 5.6 ± 0.4; ΔIW + 2.7 to ΔIW + 3.4; refs. 5,65). The elevated fO2 observed in chassignites and nakhlites is thought to result from metasomatism5,6,66. Hence, the average fO2 of shergottite is considered the most accurate approximation for bulk silicate Mars5. According to models of early Mars differentiation, it is concluded that the Martian mantle reached equilibrium with core-forming metal under reduced circumstances (~ΔIW -1.5; ref. 34). This result suggests that the fO2 of the Martian mantle must have oxidized since the formation of its core. The fO2 estimations for Apollo materials, including basalts and pyroclastic glasses of various crystallization ages (3.6–3.0 Ga) were obtained from two earlier studies18,19. The fO2 values used in this study were derived only using the V oxybarometer, thus avoiding any potential systematic bias among different oxybarometers.
Data availability
All data generated or analyzed during this study are included in this published article and supplementary information files. For the data policy, all of the data above for this paper are also available in Figshare (https://figshare.com/articles/dataset/Supplementary_Data/26550187). Source data are provided with this paper.
References
Aulbach, S. & Stagno, V. Evidence for a reducing Archean ambient mantle and its effects on the carbon cycle. Geology 44, 751–754 (2016).
Nicklas, R. W. et al. Secular mantle oxidation across the Archean-Proterozoic boundary: Evidence from V partitioning in komatiites and picrites. Geochim. Cosmochim. Acta 250, 49–75 (2019).
Kadoya, S., Catling, D. C., Nicklas, R. W., Puchtel, I. S. & Anbar, A. D. Mantle data imply a decline of oxidizable volcanic gases could have triggered the great oxidation. Nat. Comm. 11, 2774 (2020).
Lin, Y., Westrenen, W. V. & Mao, H. K. Oxygen controls on magmatism in rocky exoplanets. Proc. Natl. Acad. Sci. USA 118, 45 e2110427118 (2021).
Nicklas, R. W. et al. Uniform oxygen fugacity of shergottite mantle sources and an oxidized Martian lithosphere. Earth Planet. Sc. Lett. 564, 116876 (2021).
Wadhwa, M. Redox Conditions on Small Bodies, the Moon and Mars. Rev. Mineral. Geochem. 68, 493–510 (2008).
Li, C. et al. Characteristics of the lunar samples returned by the Chang’E-5 mission. Natl Sci. Rev. 9, nwab188 (2022).
Che, X. C. et al. Age and composition of young basalts on the Moon, measured from samples returned by Chang’e-5. Science 374, 887–890 (2021).
Li, Q. L. et al. Two-billion-year-old volcanism on the Moon from Chang’e-5 basalts. Nature 600, 54–58 (2021).
Tian, H. C. et al. Non-KREEP origin for Chang’e-5 basalts in the Procellarum KREEP Terrane. Nature 600, 59–63 (2021).
He, Q. et al. Detailed petrogenesis of the unsampled Oceanus Procellarum: The case of the Chang’E-5 mare basalts. Icarus 383, 115082 (2022).
Sato, M., Hickling, N. L. & McLane, J. E. Oxygen fugacity values of Apollo 12, 14, and 15 lunar samples and reduced state of lunar magmas. Proceedings of the 4th Lunar Science Conference 1061, 1079 (1973).
Sato, M. Oxygen fugacity and other thermochemical parameters of Apollo 17 high-Ti basalts and their implications on the reduction mechanism. Proceedings of the 7th Lunar Science Conference 1323, 1344 (1976).
El Goresy, A. Oxide minerals in lunar rocks. Rev. Mineral. 3, EG1–EG43 (1976).
Jones, J. Redox conditions on small bodies. Oxygen in the Terrestrial Planets Workshop, paper 3013 (2004).
Drake, M. J. The oxidation state of europium as an indicator of oxygen fugacity. Geochim. Cosmochim. Acta 39, 55–64 (1975).
Sutton, S. R. et al. Vanadium K edge XANES of synthetic and natural basaltic glasses and application to microscale oxygen barometry. Geochim. Cosmochim. Acta 69, 2333–2348 (2005).
Shearer, C. K., McKay, G., Papike, J. J. & Karner, J. M. Valence state partitioning of vanadium between olivine-liquid: Estimates of oxygen fugacity of Y980459 and application to other olivine-phyric martian basalts. Am. Mineral. 91, 1657–1663 (2006).
Karner, J. M. et al. Application of a new vanadium valence oxybarometer to basaltic glasses from the Earth, Moon, and Mars. Am. Mineral. 91, 270–277 (2006).
Papike, J. J. et al. Developing vanadium valence state oxybarometers (spinel-melt, olivine-melt, spinel-olivine) and V/(Cr+Al) partitioning (spinel-melt) for Martian olivine-phyric basalts. Am. Mineral. 98, 2193–2196 (2013).
Simon, S. B. & Sutton, S. R. Valences of Ti, Cr, and V in Apollo 17 high-Ti and very low-Ti basalts and implications for their formation. Meteorit. Planet. Sci. 53, 2138–2154 (2018).
Canil, D. Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, 842–845 (1997).
Wang, J. et al. Oxidation state of arc mantle revealed by partitioning of V, Sc, and Ti between mantle minerals and basaltic melts. J. Geophys. Res. Solid Earth 124, 4617–4638 (2019).
Canil, D. Vanadium in peridotites, mantle redox, and tectonic environments: Archean to present. Earth Planet. Sc. Lett. 195, 75–90 (2002).
Mallmann, G. & O’Neill, H. S. C. Calibration of an empirical thermometer and oxybarometer based on the partitioning of Sc, Y and V between olivine and silicate melt. J. Petrol. 54, 933–949 (2013).
Yang, W. et al. Petrological and geochemical characteristics of Chang’E-5 basalt and discussions on its origin. Bulletin of Mineralogy. Petrology and Geochemistry 42, 442–461 (2023). (In Chinse with English abstract).
Su, B. et al. Low Ni and Co olivine in Chang’E-5 basalts reveals the origin of the young volcanism on the Moon. Sci. Bull. 68, 1918–1927 (2023).
Yang, W. et al. Geochemistry of impact glasses in the Chang’e-5 regolith: Constraints on impact melting and the petrogenesis of local basalt. Geochim. Cosmochim. Acta 335, 183–196 (2022).
Jiang, Y. et al. Fe and Mg Isotope compositions indicate a hybrid mantle source for young Chang’E-5 mare basalts. Astrophys. J. Lett. 2302, 10507 (2023).
Roeder, P. L. & Emslie, R. Olivine-liquid equilibrium. Contrib. Mineral. Petrol. 29, 275–289 (1970).
Putirka Rates and styles of planetary cooling on Earth, Moon, Mars, and Vesta, using new models for oxygen fugacity, ferric-ferrous ratios, olivine-liquid Fe-Mg exchange, and mantle potential temperature. Am. Mineral. 101, 819–840 (2016).
Hu, S. et al. A dry lunar mantle reservoir for young mare basalts of Chang’e-5. Nature 600, 49–53 (2021).
Frost, D. J., Mann, U., Asahara, Y. & Rubie, D. C. The redox state of the mantle during and just after core formation. Phil. Trans. R. Soc. A 366, 4315–4337 (2008).
Righter, K., Yang, H., Costin, G. & Downs, R. T. Oxygen fugacity in the Martian mantle controlled by carbon: new constraints from the nakhlite mil 03346. Meteorit. Planet. Sci. 43, 1709–1723 (2008).
Armstrong, K., Frost, D. J., McCammon, C. A., Rubie, D. C. & Boffa Ballaran, T. Deep magma ocean formation set the oxidation state of Earth’s mantle. Science 365, 903–906 (2019).
O’Neill, H. S. C. The origin of the moon and the early history of the earth—A chemical model. Part 2: The earth. Geochim. Cosmochim. Acta 55, 1159–1172 (1991).
Wood, B. J., Walter, M. J. & Wade, J. Accretion of the Earth and segregation of its core. Nature 441, 825–833 (2006).
Bottke, W. F., Walker, R. J., Day, J. M., Nesvorny, D. & Elkins-Tanton, L. Stochastic late accretion to Earth, the Moon, and Mars. Science 330, 1527–153 (2010).
Worsham, E. A. & Kleine, T. Late accretionary history of Earth and Moon preserved in lunar impactites. Sci. Adv. 7, eabh2837 (2021).
Hirschmann, M. M. The deep Earth oxygen cycle: Mass balance considerations on the origin and evolution of mantle and surface oxidative reservoirs. Earth Planet. Sc. Lett. 619, 118311 (2023).
Hamano, Y., Abe, Y. & Genda, H. Emergence of two types of terrestrial planet on solidification of magma ocean. Nature 497, 607–610 (2013).
Sharp, Z. D., Mccubbin, F. M. & Shearer, C. K. A hydrogen-based oxidation mechanism relevant to planetary formation. Earth Planet. Sc. Lett. 380, 88–97 (2013).
Renggli, C. J., King, P. L., Henley, R. W. & Norman, M. D. Volcanic gas composition, metal dispersion and deposition during explosive volcanic eruptions on the Moon. Geochim. Cosmochim. Acta 206, 296–311 (2017).
Tian et al. Potassium isotope composition of Mars reveals a mechanism of planetary volatile retention. Proc. Natl. Acad. Sci. USA. 118, e2101155118 (2021).
Gaillard, F. et al. The diverse planetary ingassing/outgassing paths produced over billions of years of magmatic activity. Space Sci. Rev. 217, 22 (2021).
Zhang, Y. & Ni, H. Diffusion of H, C, and O components in silicate melts. Rev. Mineral. Geochem. 72, 171–225 (2010).
Namur, O. et al. Sulfur solubility in reduced mafic silicate melts: Implications for the speciation and distribution of sulfur on Mercury. Earth Planet. Sc. Lett. 448, 102–114 (2016).
Liu, X. et al. Sulfur isotopic fractionation of the youngest chang’e‐5 Basalts: constraints on the magma degassing and geochemical features of the mantle source. Geophys. Res. Lett. 49, e2022GL099922 (2022).
Saal, A. E. et al. Volatile content of lunar volcanic glasses and the presence of water in the Moon’s interior. Nature 454, 192–195 (2008).
Tian, H. C. et al. Petrogenesis of Chang’E-5 mare basalts: Clues from the trace elements in plagioclase. Am. Mineral. 108, 1669–1677 (2023).
Zhang, D. et al. Titanium in olivine reveals low-Ti origin of the Chang’E-5 lunar basalts. Lithos 414–415, 106639 (2022).
Zong, K. et al. Bulk compositions of the Chang’E-5 lunar soil: Insights into chemical homogeneity, exotic addition, and origin of landing site basalts. Geochim. Cosmochim. Acta 335, 284–296 (2022).
Jia, L. H. et al. Simultaneous in-situ determination of major, trace elements and Fe3+/∑Fe in spinel using epma. At. Spectrosc 1, 43 (2022).
Jochum, K. P. et al. The preparation and preliminary characterization of eight geological MPI–DING reference glasses for In-Situ microanalysis. Geostand. Newsl. 24, 87–133 (2000).
Jochum, K. P. et al. MPI-DING reference glasses for in situ microanalysis: new reference values for element concentrations and isotope ratios. Geochem. Geophys. Geosyst. 7, 1–44 (2006).
Batanova, V. G., Sobolev, A. V. & Kuzmin, D. V. Trace element analysis of olivine: High precision analytical method for JEOL JXA-8230 electron probe microanalyzer. Chem. Geol. 419, 149–157 (2015).
Batanova, V. G. et al. New olivine reference material for in situ microanalysis. Geostand. Geoanalytical Res. 43, 453–473 (2019).
Wu, S. T. et al. Simultaneous quantification of forsterite content and minor-trace elements in olivine by LA–ICP–MS and geological applications in Emeishan large igneous province. Minerals 10, 634 (2020).
Frost, B. R. Introduction to oxygen fugacity and its petrologic importance. Rev. Mineral. Geochem. 25, 1–9 (1991).
O’Neill, H. S. C. & Pownceby, M. I. Thermodynamic data from redox reactions at high temperatures. I. An experimental and theoretical assessment of the electrochemical method using stabilized zirconia electrolytes, with revised values for the Fe-“FeO”, Co-CoO, Ni-NiO and Cu-Cu2O oxygen buffers, and new data for the W-WO2 buffer. Contrib. Mineral. Petrol. 114, 296–314 (1993).
Righter K., Drake M. J. & Scott E. R. D. Compositional relationships between meteorites and terrestrial planets. In Meteorites and the Early Solar System II, edited by Lauretta D. S. and McSween H. Y. Jr Tucson: The University of Arizona Press, pp. 803–828 (2006).
Warren, P. H. & Taylor, G. J. The Moon. Treat. Geochem. 2, 213–250 (2014).
Cone, K. A., Palin, R. M. & Singha, K. Unsupervised machine learning with petrological database ApolloBasaltDB reveals complexity in lunar basalt major element oxide and mineral distribution patterns. Icarus 346, 113787 (2020).
Su, B. et al. Fusible mantle cumulates trigger young mare volcanism on the cooling Moon. Sci. Adv. 8, eabn2103 (2022).
Syzmanski, A., Brenker, F. E., Palme, H. & El Goresy, A. High oxidation state during formation of Martian nakhlites. Meteorit. Planet. Sci. 45, 21–31 (2010).
Day, J. M. D. et al. Martian magmatism from plume metasomatized mantle. Nat. Commun. 9, 4799 (2018).
Herd, C. D. K., Papike, J. J. & Brearley, A. J. Oxygen fugacity of martian basalts from electron microprobe oxygen and TEM-EELS analyses of Fe-Ti oxides. Am. Mineral. 86, 1015–1024 (2001).
Herd, C. D., Borg, L. E., Jones, J. H. & Papike, J. J. Oxygen fugacity and geochemical variations in the Martian basalts: Implications for Martian basalt petrogenesis and the oxidation state of the upper mantle of Mars. Geochim. Cosmochim. Acta 66, 2025–2036 (2002).
Herd, C. D. The oxygen fugacity of olivine‐phyric Martian basalts and the components within the mantle and crust of Mars. Meteorit. Planet. Sci. 38, 1793–1805 (2003).
Bryant, J. A., Yogodzinski, G. M. & Churikova, T. G. Melt‐mantle interactions beneath the Kamchatka arc: Evidence from ultramafic xenoliths from Shiveluch volcano. Geochem. Geophys. Geosyst. 8, Q04007 (2007).
Righter, K., Drake, M. J. & Scott, E. R. D. Compositional relationships between meteorites and terrestrial planets. Meteorites and the Early Solar System II. pp. 803–827 (2006).
Merle, R. E., et al. Pb-Pb ages and initial Pb isotopic composition of lunar meteorites: NWA 773 clan, NWA 4734, and Dhofar 287. Meteorit. Planet. Sci. 55 (2020).
Gaffney, A. M., Borg, L. E., Depaolo, D. J. & Irving, A. J. Age and isotope systematics of Northwest Africa 4898, a new type of highly-depleted Mare Basalt. Lunar and Planetary Science Conference 1877 (2008).
Acknowledgements
The Chang’e-5 lunar samples were provided by the China National Space Administration. We thank Yuyang He for discussion. This study was funded by the National Natural Science Foundation of China (42241103, 62227901 to Wei Yang) and the Key Research Program of the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS-202101, IGGCAS-202401 to Wei Yang).
Author information
Authors and Affiliations
Contributions
W.Y. and H.-J.Z. designed this research; W.Y., H.-J.Z., D.Z. (the third author), H.-C.T., and R.-H.R. prepared the sample and characterized the petrography and mineral chemistry of CE-5 basalts; H.-J.Z., D.Z. (the eleventh author), S.-T.W., L.-H. J., L.-X., G performed the EDS, EPMA and LA-ICP-MS experiments; W.Y. and H.-J.Z. wrote the manuscript; R.N.M., H.-C.T., S.H., Y.C., H.-J.H., Y.-H.L., Y.-T.L., X.-H.L. and F.-Y.W. proposed suggestions for the manuscript. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks James Karner and Laura Schaefer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Yang, W., Zhang, D. et al. Long-term reduced lunar mantle revealed by Chang’e-5 basalt. Nat Commun 15, 8328 (2024). https://doi.org/10.1038/s41467-024-52710-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52710-x