Abstract
To tackle the clinical challenge of noninvasively assessing immunotherapy efficacy in patients, here we used positron emission tomography (PET) with 68Ga-grazytracer, which targets granzyme B, a crucial effector molecule secreted by activated CD8+ T cells. In this phase 1/2 clinical trial (NCT05000372) involving a diverse cohort of 24 patients with solid tumors and lymphomas who received immunotherapies, including immune checkpoint inhibitors (either alone or with chemotherapies) and chimeric antigen receptor-T cell therapy, we examined the in vivo behaviors of 68Ga-grazytracer. Primary endpoints were safety, biodistribution, granzyme B specificity, and the predictive utility of 68Ga-grazytracer, while secondary endpoint was the relationship between 68Ga-grazytracer uptake and tumor immune phenotype. 68Ga-grazytracer exhibited a safe profile and specifically targeted granzyme B in patients. 68Ga-grazytracer PET showed superior predictive value for short-term prognosis and progression-free survival than those of conventional assessment criteria, including RECIST 1.1 and PERCIST. Moreover, the uptake of 68Ga-grazytracer in tumors was significantly higher in those with a “non-desert” immune phenotype than those with an immune “desert” phenotype, thereby meeting the primary and secondary endpoints of this trial. Collectively, we successfully visualized CD8+ T cell effector function in humans using 68Ga-grazytracer PET, offering insights for enhancing immunotherapy assessment, patient stratification and treatment planning.
Similar content being viewed by others
Introduction
Immunotherapies such as immune checkpoint inhibition and chimeric antigen receptor (CAR)-T cell therapy have revolutionized tumor therapy. However, only a subgroup of patients respond to immunotherapy. In contrast to biopsy-based methods such as immunohistochemistry (IHC) staining of programmed death-ligand 1 (PD-L1) and measurement of tumor mutation burden1 and immunophenotypes2, imaging tools enable a systemic evaluation of tumor responses to immunotherapy in a noninvasive and longitudinal manner. The Response Evaluation Criteria in Solid Tumors (RECIST) guidelines3 and lymphoma-adapted RECIST4 based on computed tomography (CT) have been widely used to assess the efficacy of immunotherapy in treating solid tumors and lymphomas. However, these anatomical image-based evaluation criteria rely solely on changes in tumor lesion size and cannot discern abnormal progression patterns, especially pseudoprogression, during immunotherapy5,6. Although immune RECIST (iRECIST)7, immune-related RECIST (irRECIST)8, and the refined Lymphoma Response to Immunomodulatory Therapy Criteria (LYRIC)9 optimize the timing of decision-making by including follow-up scans, these wait-and-see strategies may delay the optimal timing for adjusting treatment strategies in patients.
Considering that metabolic changes in tumors precede anatomical size alterations10, there is an increasing clinical reliance on 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) as the current standard for evaluating immunotherapy effectiveness in treating solid tumors, following the PET-Response Criteria in Solid Tumors (PERCIST)11 guidelines12. For lymphomas, the Lugano classification also includes 18F-FDG-based evaluation methods13. However, 18F-FDG uptake is not tumor-specific, and immunotherapy-induced tumor-infiltrating immune cells exhibit heightened glucose metabolism, potentially resulting in pseudoprogression on early 18F-FDG PET14,15.
PET imaging of crucial immune checkpoints, such as programmed cell death protein 1 (PD-1) and its ligand PD-L1, through the use of radiolabeled antibodies or peptides, has been utilized to evaluate the effectiveness of immunotherapy in patients with non-small cell lung cancer and melanoma16,17,18. However, the predictive utility of PD-1/PD-L1 is confined predominantly to anti-PD-1/PD-L1 therapies and offers limited insight into the assessment of other immunotherapeutic approaches19,20. Radiotracers targeting other immune checkpoints, such as T cell immunoreceptor with Ig and ITIM domains (TIGIT)21 and lymphocyte-activation gene 3 (LAG-3)22 have been employed to evaluate patient responses to anti-TIGIT and anti-LAG-3 therapies. However, the clinical application of antibodies against TIGIT and LAG-3 is still in its infancy, which limits the widespread clinical utilization of the corresponding radiotracers23. Owing to the critical role of CD8+ T cells in immunotherapy, CD8-targeted PET imaging has been used to predict immune responses. Tumor uptake of CD8-specific radiotracers before treatment positively correlated with patient prognosis in clinical trials24,25. However, PET of CD8+ T cells has two limitations for monitoring immunotherapy. First, only a fraction of CD8+ T cells (tumor-associated antigen-specific populations) within the tumor microenvironment can recognize and eliminate tumor cells, making it challenging for CD8-targeted PET to identify antigen-specific CD8+ T cells. Second, CD8-targeted PET cannot distinguish between effector and naïve CD8+ T cell subsets26. A retrospective analysis based on the Cancer Genome Atlas also indicated that the overall expression levels of CD8 could not predict immune responses in epithelioid mesothelioma27.
To overcome the limitations associated with CD8-targeted PET imaging, radiotracers for the PET of CD8+ T cell effector function have been extensively investigated. For instance, radiotracers that target granzyme B, a crucial molecule secreted by activated T cells for executing effector functions, have been shown to predict early tumor responses to immunotherapy in preclinical studies28,29. We recently developed a peptidomimetic-based PET radiotracer named 68Ga-grazytracer, which demonstrated favorable stability in vivo, thus enabling PET imaging of granzyme B expression in patients30.
In this work, we conduct a phase 1/2 investigator-initiated clinical trial (NCT05000372) to further assess the clinical utility of 68Ga-grazytracer PET imaging. We perform 68Ga-grazytracer PET imaging on patients with solid tumors after receiving 2–3 cycles of immunotherapies and on patients with lymphomas one month post CAR-T cell therapy. We evaluate the safety and pharmacokinetic properties of 68Ga-grazytracer, as well as the association between 68Ga-grazytracer tumor uptake and patient clinical outcomes.
Results
Patient characteristics
Between September 2021 and March 2023, 24 participants (4 women and 20 men; median age, 64 years; interquartile range, 57–70 years) with malignant tumors (21 patients with solid tumors and 3 with lymphomas) who were scheduled to receive immunotherapy were enrolled (see Supplementary Fig. 1 for the consort diagram) in this prospective study (NCT05000372). The primary endpoints of this trial included safety, biodistribution, granzyme B specificity, and the predictive value of 68Ga-grazytracer, while the secondary endpoint focused on the correlation between the uptake of 68Ga-grazytracer and the tumor immune phenotype. One participant received durvalumab alone, twenty received anti-PD-1/anti-PD-L1 antibodies with chemotherapy, and three received CAR-T cell therapy (Table 1). All participants underwent pre-treatment 18F-FDG PET/CT, post-treatment 18F-FDG PET/CT, and 68Ga-grazytracer PET/CT after 2–3 cycles of immunotherapies or 1 month of CAR-T cell therapy for prognostic analysis. The median follow-up time for the cohort was 23 months (15–27 months). At the one-year follow-up, 14 patients experienced disease progression, resulting in a one-year progression-free survival (PFS) rate of 41.7%. At the end of the follow-up, none of the patients had died. The median PFS for all patients was 9 months (4–21 months).
Safety and pharmacokinetics of 68Ga-grazytracer
All patients underwent 68Ga-grazytracer PET/CT without complications, and no drug-related side effects or other adverse physiological responses were observed. The patients did not report any abnormal symptoms, demonstrating an overall tolerance to the procedures.
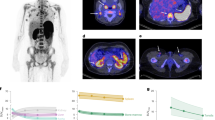
We assessed the in vivo metabolic kinetics of 68Ga-grazytracer using dynamic PET/CT (Fig. 1a). In the early phases of imaging, the peak physiological uptake of the radiotracer was evident in the kidneys, accompanied by discernible but moderate uptake in the heart, spleen, and liver. Subsequently, a significant temporal reduction in 68Ga-grazytracer uptake within these organs was observed (Fig. 1b, c), indicating rapid renal clearance of the radiotracer. This phenomenon aligns with efficient renal metabolism and the elimination of 68Ga-grazytracer from systemic circulation.
a Representative PET images of 68Ga-grazytracer at 5, 10, 20, 40, and 90 min post-injection. b, c Quantified mean standard uptake value (SUVmean) of 68Ga-grazytracer in healthy tissues at 5, 10, 20, 40, and 90 min post-injection (n = 4, collected from 4 different patients). The curves were fitted and depicted using a quadratic (second-degree polynomial) model. Data points represent the median SUVmean values, with error bars representing the interquartile range. Source data are provided as a Source Data file.
Tumor uptake of 68Ga-grazytracer
We analyzed the temporal uptake dynamics of 68Ga-grazytracer in tumors and healthy organs within 40 min of intravenous administration (Fig. 2a; Supplementary Fig. 2a–d). General additive mixed models were utilized to describe the biodistribution of 68Ga-grazytracer in healthy organs (Supplementary Table 1). Owing to the rapid clearance of 68Ga-grazytracer, its tumor uptake steadily decreased over time (Fig. 2b). However, the tumor-to-liver (Fig. 2c) and tumor-to-blood (Supplementary Fig. 2e) uptake ratios exhibited an upward trend, peaking at approximately 30 min post-injection. In the PET images of patients, diverse individual patterns of 68Ga-grazytracer uptake were noted, with a subset exhibiting tumor-rim uptake (Fig. 2d). Additionally, a significant uptake of 68Ga-grazytracer was observed in some metastatic lesions (Fig. 2e), suggesting its potential to assess whole-body granzyme B expression.
a Representative PET images of 68Ga-grazytracer at 2, 5, 10, 20, 30, and 40 min post-injection. b Quantified maximum standardized uptake value (SUVmax) of 68Ga-grazytracer uptake in tumors at 2, 5, 10, 20, 30, and 40 min post-injection (n = 4, collected from 4 different patients). Data points represent the median SUVmax values, with error bars representing the interquartile range. c Calculated mean SUVmax of the tumor-to-liver ratio at different time points (n = 4, collected from 4 different patients). Data points represent the median calculated tumor-to-liver ratio values, with error bars representing the interquartile range. d Representative PET/CT images of 68Ga-grazytracer depicting the tumor uptake in the rim. e Representative PET/CT images of 68Ga-grazytracer uptake in a metastatic lesion. (i) Uptake in a brain metastasis (SUVmax of 1.9) of a patient with right lung adenocarcinoma. (ii) Uptake in a pleural metastasis (SUVmax of 3.7) of a patient with left lung adenocarcinoma. f Immunohistochemical staining of granzyme B (GrzmB) in the tumor tissues harvested from three patients with different 68Ga-grazytracer uptake (SUVmax values of 2.0, 2.7, and 4.1). Experiments were conducted independently three times with similar results. Scale, 50 μm. g Correlation between the tumor uptake of 68Ga-grazytracer and the quantified GrzmB levels (n = 7, r = 0.7701, P = 0.0094). h SUVmax of 68Ga-grazytracer in tumor lesions with “desert” (n = 11) and “non-desert” immune phenotypes (n = 7). Data are presented as median with interquartile range. Tumors are indicated by arrows in the PET and PET/CT images. Two-sided P value was calculated using Pearson’s correlation analysis (g) and Mann‒Whitney U test (h). Source data are provided as a Source Data file.
To further elucidate the in vivo specificity of 68Ga-grazytracer, we performed IHC staining for granzyme B on surgical specimens from 7 patients who were eligible for surgery following immunotherapy (Fig. 2f). The results revealed a significant correlation between tumor uptake of 68Ga-grazytracer and the expression levels of granzyme B in tumor tissues (Pearson’s r = 0.7701, P = 0.0094; Fig. 2g). Using the CD8 staining results from tumor tissue samples collected from patients prior to immunotherapy (Supplementary Fig. 3a, b), we categorized the tumor immune phenotypes into immune “desert” (low CD8 expression) and immune “non-desert” (high CD8 expression). The maximum standardized uptake value (SUVmax) of 68Ga-grazytracer was significantly higher in the “non-desert” immune phenotype of tumors than in the immune “desert” tumors (P = 0.0017; Fig. 2h), suggesting the role of 68Ga-grazytracer PET in noninvasively distinguishing the immune phenotypes of tumors in patients.
Predictive values of 68Ga-grazytracer PET uptake for immunotherapy outcomes
Using CT data from patient lesions 6 months post-treatment, we evaluated the overall response using the RECIST 1.1 and Lugano criteria. Complete response (CR) and partial response (PR) were observed in 45.8% (11/24) of the patients, whereas progressive disease (PD) and stable disease (SD) were observed in 54.2% (13/24) of the patients. We further analyzed and compared the predictive accuracy of pre-treatment PD-L1 IHC with three imaging parameters: the SUVmax of 68Ga-grazytracer after immunotherapies, changes in the Sum of the Longest Diameter of CT after therapies compared to baseline (ΔSLD), and changes in SUVmax (ΔSUVmax) of 18F-FDG after therapies compared with baseline. Discordance between PD-L1 IHC results and patient prognosis (PD or SD vs. PR or CR) was observed (Fig. 3a), suggesting the limited predictive value of traditional biopsy-based pathological assessment for immunotherapy. In patients who achieved PR or CR according to RECIST 1.1 or Lugano criteria, the SUVmax of 68Ga-grazytracer was significantly higher than that in the patients with PD or SD (P = 0.0004; Fig. 3b). By contrast, the ΔSLD of CT scan (P = 0.28) and ΔSUVmax of 18F-FDG (P = 0.13) showed a tendency to differ between these two groups, but these differences did not reach statistical significance (Fig. 3c, d). Supplementary Fig. 4a–c further illustrates the differences in the aforementioned imaging parameters among patients with varying overall responses. Receiver operating characteristic (ROC) curve analysis further confirmed the superior predictive performance of the SUVmax of 68Ga-grazytracer (area under the ROC curve [AUC] = 0.899; 95% confidence interval [CI], 0.77–1.00; P = 0.0010) than that of the ΔSLD of CT scan (AUC = 0.636; 95% CI, 0.41–0.86; P = 0.26) and ΔSUVmax of 18F-FDG (AUC = 0.685; 95% CI, 0.46–0.91; P = 0.12) (Fig. 3e).
Comparison of the positive PD-L1 proportion (a), SUVmax of 68Ga-grazytracer (b), ΔSLD of CT scan (c), and ΔSUVmax of 18F-FDG (d) in patients categorized as PD/SD (n = 13) and PR/CR (n = 11) according to the overall response. e Receiver operating characteristic (ROC) curves for the SUVmax of 68Ga-grazytracer, ΔSLD of CT scan, and ΔSUVmax of 18F-FDG (n = 24 for each group). The area under the curve (AUC) was calculated for each ROC curve, with 95% confidence intervals derived using the Wilson/Brown method. Statistical differences in AUCs were evaluated using DeLong’s tests, resulting in two-sided P values. To account for the false discovery rate in multiple comparisons, the Bonferroni correction was utilized to adjust the P values. Two-sided P value was determined by the Chi-square and Fisher’s exact test (a) and Mann–Whitney U test (b–d). SUVmax the maximum standardized uptake value, ΔSLD changes in the sum of the longest diameter, ΔSUVmax changes in the maximum standardized uptake value, PD progressive disease, SD stable disease, PR partial response, CR complete response. Source data are provided as a Source Data file.
To validate the clinical utility of 68Ga-grazytracer in assessing immunotherapy efficacy, patients were stratified based on the median SUVmax of 68Ga-grazytracer, and its predictive accuracy was compared with that of the existing clinical criteria, including RECIST 1.1, PERCIST, and Lugano. The highest prediction value for the overall response 6 months post-treatment was observed in the group assessed with 68Ga-grazytracer PET (SUVmax cutoff of 2.3) (P = 0.0022; Fig. 4a) than those with RECIST 1.1/Lugano (based on ΔSLD of CT scan) (P = 0.10; Fig. 4b) and PERCIST/Lugano (based on the changes in peak SUV corrected for lean body mass of 18F-FDG [ΔSULpeak] or Deauville score of the SUVmax of 18F-FDG) (P = 0.12; Fig. 4c).
a–c The Sankey diagram compares the concordance between early assessment results based on 68Ga-grazytracer uptake (SUVmax cutoff of 2.3) (a), ΔSLD of CT scan (RECIST 1.1 and Lugano) (b), and ΔSUVmax of 18F-FDG (PERCIST and Lugano) (c) and the overall response based on RECIST 1.1 or Lugano 6 months post-treatment. Two-sided P value was determined by Chi-square and Fisher’s exact tests (a–c). d–f Progression-free survival (PFS) curves estimated using the Kaplan–Meier method according to the early assessment results based on 68Ga-grazytracer uptake (d), ΔSLD of CT scan (RECIST 1.1 and Lugano) (e), and ΔSUVmax of 18F-FDG (PERCIST and Lugano) (f). The log-rank test was used to compare PFS curves across different groups, and Cox regression models were employed to estimate the hazard ratios. In this context, “time 0” refers to the commencement of treatment, serving as the starting point for measuring the duration of PFS (d–f). SUVmax the maximum standardized uptake value, RECIST Response Evaluation Criteria in Solid Tumors, PERCIST PET-Response Criteria in Solid Tumors, PR partial response, CR complete response, PD progressive disease, SD stable disease, PMR partial metabolic response, CMR complete metabolic response, PMD progressive metabolic disease, SMD stable metabolic disease. Source data are provided as a Source Data file.
In the follow-up studies, patients with an SUVmax of 68Ga-grazytracer >2.3 displayed significantly higher PFS than did patients with an SUVmax of 68Ga-grazytracer ≤2.3 (P = 0.0009; hazard ratio, 7.3; 95% CI, 2.7–20.2; Fig. 4d). In contrast, the defined responders (PR and CR) by RECIST 1.1/Lugano showed no superior PFS compared with that of defined nonresponders (P = 0.80; hazard ratio, 1.1; 95% CI, 0.4–3.5; Fig. 4e). Patients assessed as the responders (partial metabolic response [PMR] and complete metabolic response [CMR]) using PERCIST/Lugano showed a trend towards improved PFS but no significant differences were observed (P = 0.11; hazard ratio, 2.2; 95% CI, 0.8–6.6; Fig. 4f). Proportional hazard assumptions were met for the Cox model and demonstrated that the impact of 68Ga-grazytracer uptake on the risk of disease progression did not significantly vary over time (Supplementary Table 2). Cox regression analysis also revealed that 68Ga-grazytracer uptake was significantly associated with PFS after adjusting the confounding factors (Supplementary Table 3). Therefore, the criteria using SUVmax cutoff of 2.3 for 68Ga-grazytracer PET demonstrated superior accuracy in predicting the responsiveness to immunotherapy compared to RECIST 1.1/Lugano and PERCIST/Lugano. Additionally, we performed a PFS analysis in patients with solid tumors alone and found similar results (Supplementary Fig. 5).
In a representative case of tumor pseudoprogression, a patient with lung adenocarcinoma, identified by a 5.2 × 3.3 cm right lung mass, had a SUVmax of 7.6 on baseline 18F-FDG PET (Fig. 5a). After two cycles of immunotherapy and chemotherapy, no changes in tumor size were observed; however, the tumor showed a flare-up on 18F-FDG PET, with the SUVmax increasing to 11.3 (Fig. 5b). 68Ga-grazytracer PET showed high tumor uptake with a SUVmax of 5.2 (Fig. 5c), suggesting CD8+ T cell infiltration and activation within the tumor. A concurrent chest CT scan performed alongside the 68Ga-grazytracer PET showed no significant change in tumor size (Fig. 5d). However, the CT results obtained 19 days later (Fig. 5e) revealed a notable decrease in tumor size. This not only indicates the therapeutic efficacy in this patient but also highlights the predictive power of 68Ga-grazytracer PET in assessing immunotherapy responses earlier than conventional CT scanning.
a The baseline 18F-FDG PET/CT revealed a mass (5.2 × 3.3 cm in size) with intense 18F-FDG uptake (SUVmax of 7.6) in the upper lobe of the right lung. b 18F-FDG PET/CT after two cycles of treatment (immunotherapy and chemotherapy) showed no changes in tumor size, while the 18F-FDG uptake was increased compared with baseline (SUVmax of 11.3 vs. 7.6). c 68Ga-grazytracer PET/CT after two cycles of treatment showed a mass in the upper lobe of the right lung with inhomogeneous increased activity (arrows; SUVmax of 5.2). d Chest CT after 3 days of 68Ga-grazytracer PET/CT revealed no change in the tumor size. e Chest CT after 19 days of 68Ga-grazytracer PET/CT indicated a significant reduction in tumor size. Arrows indicate tumors in the PET, PET/CT, and CT images.
Two cases showing a correlation between 68Ga-grazytracer PET and long-term prognosis in patients with lymphomas who received CAR-T cell therapy are shown in Fig. 6. Patient 1 was diagnosed with follicular lymphoma (a left abdominal mass of 12.1 × 8.7 cm) with an SUVmax of 13.9 on baseline 18F-FDG PET (Fig. 6a). After 1 month of CAR-T cell therapy, 68Ga-grazytracer PET revealed high uptake in the lymphoma (SUVmax, 5.0; Fig. 6b), suggesting a favorable outcome. Meanwhile, 18F-FDG PET/CT revealed low lymphoma uptake (SUVmax of 2.2) and reduced tumor size (7.0 × 3.1 cm) (Fig. 6c). After 14 months of CAR-T cell therapy, the patient exhibited a complete metabolic response, as determined via 18F-FDG PET (Fig. 6d). Patient 2 was diagnosed with B-cell lymphoma at the left side of the neck who had an intense activity on baseline 18F-FDG PET/CT (SUVmax of 15.9; Fig. 6e). After 1 month of CAR-T cell therapy, 68Ga-grazytracer PET revealed no activity in the neck mass (Fig. 6f), suggesting a poor prognosis. Although 18F-FDG PET/CT revealed a reduced tumor size (2.8 × 2.4 cm) and decreased activity (SUVmax of 4.3) (Fig. 6g), the patient experienced overall disease progression after 3 months (Fig. 6h).
a–d A patient with follicular lymphoma. a The baseline 18F-FDG PET demonstrated intense 18F-FDG-avid mass (SUVmax of 13.9; 12.1 × 8.7 cm in size) in the left abdominal cavity. b 68Ga-grazytracer PET after 1 month of CAR-T cell therapy revealed increased lesion uptake (SUVmax of 5.0). c 18F-FDG PET after 1 month of CAR-T cell therapy showed that the tumor size decreased (7.0 × 3.1 cm vs. 12.1 × 8.7 cm) and SUVmax of 18F-FDG also decreased (SUVmax of 2.2 vs. 13.9) compared with the baseline 18F-FDG PET. d 18F-FDG PET after 14 months of CAR-T cell therapy revealed a complete metabolic response in this patient. e–h A patient with B-cell lymphoma. e The baseline 18F-FDG PET showed a mass with intense 18F-FDG uptake in the left side of the neck (SUVmax of 15.9; 4.1 × 3.4 cm in size). f 68Ga-grazytracer PET after 1 month of CAR-T cell therapy showed no radiotracer uptake in the mass at the left side of the neck. g 18F-FDG PET after 1 month of CAR-T cell therapy revealed that the tumor size decreased (2.8 × 2.4 cm vs. 4.1 × 3.4 cm) and SUVmax of 18F-FDG also decreased (SUVmax of 4.3 vs. 15.9) compared with the baseline 18F-FDG PET. h 18F-FDG PET after 3 months of CAR-T cell therapy revealed overall disease progression in this patient. Arrows indicate tumors in the PET images.
Discussion
Although CD8+ T cells play a pivotal role in immunotherapy, only a small subset of CD8+ T cells within the tumor possesses the effector function of recognizing and eliminating cancer cells31. Therefore, visualizing effector CD8+ T cells in vivo is crucial in accurately assessing the efficacy of immunotherapy. In this phase 1/2 clinical trial, we used 68Ga-grazytracer to assess granzyme B expression levels within tumor lesions, thereby characterizing the functional status of CD8+ T cells post-immunotherapy. We evaluated the pharmacokinetics of 68Ga-grazytracer in vivo, demonstrating its significant uptake in the blood pool and kidneys owing to its rapid clearance as a small molecule32. The radiotracer exhibited concentrated uptake at the tumor periphery, consistent with the tumor rim pattern of T-cell infiltration33. Moreover, the IHC results for granzyme B in tumor specimens correlated with the tumor uptake of 68Ga-grazytracer, confirming its specificity for granzyme B. Compared with conventional clinical assessments, such as CT, 18F-FDG PET, and PD-L1 IHC, 68Ga-grazytracer PET more effectively predicted short-term prognosis and PFS in patients undergoing immunotherapy.
The precision of patient categorization using 68Ga-grazytracer PET/CT during early immunotherapy significantly outperformed current clinical evaluations based on CT and 18F-FDG PET/CT (such as RECIST 1.1, PERCIST, and the 2014 Lugano criteria) in predicting short-term outcomes and overall prognosis for immunotherapy. 68Ga-grazytracer PET/CT superiority stems from the foundational mechanism of immunotherapy, which involves reinstating potent cytotoxic T-cell responses against tumors, distinct from conventional chemotherapy and targeted therapy modalities34. Using anatomical criteria (RECIST 1.1) for assessing the tumor response to immunotherapy is challenging, as alterations in tumor size may not promptly or reliably signify the tumor response to immunotherapy, with metabolic shifts and necrosis often preceding changes in tumor size35. In addition, an increase in tumor size and the appearance of new lesions do not invariably indicate disease progression but could reflect immune cell infiltration36. The underlying principle of 18F-FDG PET/CT-based assessments for evaluating immunotherapy efficacy relies on the close association between 18F-FDG uptake and cancer cell abundance. However, distinguishing tumors cells from infiltrating T lymphocytes is challenging because both express high-affinity glucose transporter-1 that facilitate glycolysis37.
68Ga-grazytracer PET/CT holds considerable promise across various clinical applications for evaluating the efficacy and prognosis of immunotherapies in patients with cancer. Its exceptional utility in gauging immunotherapy efficacy encompasses several dimensions. First, 68Ga-grazytracer PET/CT facilitates the specific recognition of activated CD8+ T cells, aiding in discriminating pseudoprogression. This capability is instrumental for accurately evaluating immunotherapy efficacy and guiding timely therapeutic interventions. Timely recognition and continuation of therapy are pivotal in managing pseudoprogression38. Second, 68Ga-grazytracer PET/CT has emerged as a valuable tool for predicting immunotherapy efficacy during the initial treatment phases, thereby facilitating the optimization of strategy refinement. Given the heterogeneous responses observed among patients, early differentiation between responders and nonresponders is important39, as it enables the timely adjustment of treatment strategies for nonresponders. Third, 68Ga-grazytracer may have prognostic significance in patients treated with CAR-T cell therapy. Patients with high 68Ga-grazytracer uptake in lesions may have a better long-term prognosis after CAR-T cell therapy, suggesting that 68Ga-grazytracer PET/CT holds promise for addressing the limitations of 18F-FDG PET/CT in the early monitoring of CAR-T cell therapy. Early 18F-FDG PET/CT assessments a month post CAR-T cell therapy often fail to predict long-term responses40. Therefore, 68Ga-grazytracer PET/CT offers a promising approach for assessing the efficacy of CAR-T cell therapy at an early stage.
In this clinical trial, we did not perform baseline 68Ga-grazytracer PET imaging to directly observe the changes in granzyme B levels following immunotherapy. Our previous animal studies have shown that the tumor uptake of 68Ga-grazytracer in the absence of immunotherapy was consistently low (usually less than 1%ID/g) with negligible variability between individuals30. This is because the activation of CD8+ T cells and secretion of granzyme B are minimal before immunotherapy. It could be inferred that the granzyme B levels in patients prior to immunotherapy are at background levels. Therefore, the SUVmax of 68Ga-grazytracer post-immunotherapy as we used in this study could serve as an indicator of granzyme B expression induced by immunotherapy.
Due to the variety of tumor types encountered in this study, the small number of patients with specific tumor types may preclude the definition of T-cell functional status within patient subgroups. In certain patients, we observed significant therapeutic outcomes despite the presence of low 68Ga-grazytracer uptake in the tumor area. In these cases, it is possible that granzyme B may have already penetrated tumor cells, a scenario that cannot be directly detectable by 68Ga-grazytracer PET, which primarily detects granzyme B in the tumor microenvironment rather than within the tumor cells41. We chose to conduct granzyme B PET imaging after 2–3 cycles of immunotherapy in patients with solid tumors and one month following CAR-T cell therapy for lymphoma patients, based on our pilot study that indicated consistent 68Ga-grazytracer uptake at one, three, and four cycles of immunotherapy. Nevertheless, dynamic consecutive imaging in patients to establish the most suitable timing for granzyme B PET imaging would be appropriate. This method has been successfully utilized in our previous animal studies30 and could facilitate a more precise capture of the peak secretion phase of granzyme B.
The participants in this study include patients who received a combination of immunotherapy and chemotherapy. While studying a more homogenous group of patients undergoing immunotherapy alone could lead to stronger clinical conclusions about the utility of 68Ga-grazytracer PET in monitoring the efficacy of immunotherapy, in clinical practice, the combination therapy is more commonly used due to several practical factors. In our previous preclinical study30, we performed an extensive evaluation of 68Ga-grazytracer in mouse models that were treated with immunotherapy alone, which encompassed both immune checkpoint inhibitors and adoptive cell transfer therapies. These studies highlighted the potential of 68Ga-grazytracer PET for the early prediction of immunotherapy responses and for differentiating between pseudoprogression and true tumor progression. Furthermore, there is a growing body of evidence suggesting that chemotherapy can augment the effectiveness of immunotherapy via diverse mechanisms42,43. Nonetheless, the secretion of granzyme B represents a terminal event in various antitumor immune pathways and serves as a direct measure of the cytotoxic capacity of T cells to eradicate tumor cells. This is true whether the treatment approach consists of immunotherapy by itself or in conjunction with other therapeutic modalities. As such, 68Ga-grazytracer PET offers the potential to visualize the terminal antitumor effects in patients who have received a combination of chemotherapy and immunotherapy.
In conclusion, this study demonstrated that 68Ga-grazytracer PET can specifically identify granzyme B expression in humans, offering a noninvasive method for monitoring the effector function of CD8+ T cells. Compared to conventional imaging techniques such as 18F-FDG PET and CT, 68Ga-grazytracer PET could represent an alternative tool for predicting early-stage responses of patients with tumors to immunotherapy.
Methods
Study participants
This prospective investigator-initiated trial (NCT05000372) was approved by the Clinical Research Ethics Committee of the Peking University Cancer Hospital (no. 2021KT86) in August, 2021. The clinical study was conducted in accordance with the criteria set by the Declaration of Helsinki and all pertinent regulations concerning the involvement of human subjects. Eligibility for enrollment was open to both male and female participants, with gender not being a consideration in the study’s design. Participants’ sex was determined based on self-reporting. The participants were recruited between September 2021 and March 2023 at our institute. The enrolled participants provided written informed consent. The inclusion criteria were as follows: 1) participants who were diagnosed with malignant tumors, 2) participants who were scheduled to receive immunotherapy based on a decision made by a multidisciplinary team, 3) participants who had no prior immunotherapy, 4) participants who had no regional therapy within 3 months, and 5) participants aged ≥18 years. The exclusion criteria were as follows: 1) participants with a concurrent disease that would impede the treatment regimen and 2) participants who were unable or unwilling to provide written informed consent.
Treatment
Based on initial histological findings and staging, the treatment options were as follows: For checkpoint inhibitors alone or with chemotherapy, patients were treated for 4–6 cycles every 3 weeks until disease progression or intolerable adverse reactions occurred. For CAR-T cell therapy, following leukapheresis and CD19 CAR-T cell (Relma-cel) production, patients were subjected to a low-dose conditioning chemotherapy regimen consisting of fludarabine (40 mg/m2 of body surface area per day) and cyclophosphamide (400 mg/m2 per day) on days −5, −4, and −3 and then received an intravenous infusion of Relma-cel on day 0.
PET/CT protocols
Baseline 18F-FDG PET/CT was performed before treatment. Interim 18F-FDG PET/CT and 68Ga-grazytracer PET/CT were performed after 2–3 cycles of immune checkpoint blockade therapy or 1 month post-CD19 CAR-T cell therapy. The interval between the interim 18F-FDG PET/CT and 68Ga-grazytracer PET/CT was within 2 weeks. Interim 18F-FDG PET/CT and 68Ga-grazytracer PET/CT were performed separately. The minimum time between scans was >24 h.
18F-FDG was synthesized with 18F and produced using an HM-20 medical cyclotron (Sumitomo Corporation, Japan), with a radiochemical purity of >95%. 68Ga-grazytracer was synthesized using a previously described method30. Briefly, 30 nmol of the labeling precursor was dissolved in 300 μL of 0.1 M sodium acetate buffer (pH 5.5) and mixed with 555 MBq of 68GaCl3. This mixture was subjected to a reaction at 99 °C for 10 min and subsequently purified with Sep-Pak C18 cartridges. The final radiotracer was filtered through 0.22-μm filters, and the radiochemical purity was assessed through analytical radio-high-performance liquid chromatography (radio-HPLC). The 18F-FDG and 68Ga-grazytracer doses were 5.55 MBq/kg and 3.70 MBq/kg, respectively. Before the 18F-FDG PET/CT scan, the participants fasted for ≥6 h to ensure their blood glucose levels were <11.0 mmol/L. No special preparation was required for 68Ga-grazytracer PET/CT.
The static 18F-FDG PET/CT and 68Ga-grazytracer PET/CT were performed 1 h and 40 ± 10 min post-injection, respectively. The same scanning parameters were used for the PET/CT. CT was performed using the following parameters: tube voltage, 120 kV; current, 146 mA; and section thickness, 3 mm. PET was acquired in the continuous bed motion mode at 1.5 mm/s from the top of the skull to the upper thigh of each participant. PET data were reconstructed using the ordered subset expectation maximization (OSEM) algorithm with 2 iterations and 11 subsets, using CT data for attenuation correction. Static PET/CT with 68Ga-grazytracer and 18F-FDG was performed using a Biograph mCT Flow 64 scanner (Siemens Healthcare).
For dynamic imaging of 68Ga-grazytracer, a low-dose CT (120 kV, 46.13 mAs on average, matrix 512 × 512) was performed to correct attenuation. Subsequently, a dynamic whole-body PET was performed for 40 min post-intravenous 68Ga-grazytracer injection through a peripheral vein in the wrist. A total-body PET/CT μEXPLORER scanner (UNITED IMAGING, Shanghai, China) with a maximized PET field of view (FOV) of 194 cm was used. The reconstruction protocols included an OSEM algorithm, 3 iterations, 20 subsets, a 192 × 192 image matrix, 600 mm FOV, 2.886 mm slice thickness, and a 4.5 mm Gaussian post-processing filter. Raw PET data were reconstructed into 95 frames (15 frames × 2 s, 30 frames × 5 s, 30 frames × 14 s, and 20 frames × 90 s). In four patients, a static whole-body PET/CT examination was performed 90 min post-injection for approximately 20 min.
Image analysis
18F-FDG and 68Ga-grazytracer PET/CT studies were reviewed in consensus by ≥2 nuclear medicine physicians (≥10 years of experience) blinded to the clinical data and results of other imaging studies. To decrease bias, all images were re-evaluated by each physician at monthly intervals. The static PET/CT images were co-registered and displayed using a Siemens workstation (Syngo.via VB20, MM Oncology). For quantitative PET/CT analysis, circular regions of interest were manually drawn around the tumor lesions on transaxial slices of the PET images, and the SUVmax was automatically calculated to evaluate tracer uptake in the tumors semi-quantitatively. The SUVpeak was calculated using a 1.2 cm region of interest positioned at the tumor’s hottest site and subsequently normalized to the SUV corrected for lean body mass (SULpeak) (calculated as SUVpeak × [lean body mass]/[total body mass]).
The dynamic PET/CT images were processed on a uWS-MI R002.20.0.1386284 workstation, where they were meticulously registered and fused with the CT images to ensure precise anatomical alignment. A series of 95 PET frames were combined into a dynamic sequence to facilitate a quantitative analysis of the 68Ga-grazytracer uptake kinetics. Nine specific organs (including the brain, heart, lungs, spleen, liver, kidneys, bone marrow, muscle, and skin) were chosen for detailed examination. The volumes of interest (VOIs) were carefully outlined within these organs and tumors on the reconstructed dynamic PET images, with precise adjustments made by referencing the corresponding CT images. Following this process, time-activity curves were derived for each of the specified regions.
For CT analysis, two board-certified radiologists (≥10 years of experience) reviewed the contrast-enhanced CT images and reached an agreement without knowledge of patients’ additional imaging or clinical data. Contrast-enhanced CT was performed at baseline and then every 3 months until disease progression, death, or withdrawal from the study.
Response evaluation
We performed 18F-FDG PET interpretation according to the 2014 Lugano44 (for lymphomas) and PERCIST criteria11 (for solid tumors). Efficacy was categorized as CMR, PMR, stable metabolic disease (SMD), or progressive metabolic disease (PMD). To facilitate comparison between the various criteria, 18F-FDG PET/CT findings were classified as either metabolic (CMR + PMR) or metabolic non-response (SMD + PMD) after primary interpretation. The interval between the interim contrast-enhanced CT and PET/CT was within 2 weeks. CT results were interpreted according to the 2014 Lugano criteria (for lymphomas) and RECIST 1.13 (for solid tumors). Efficacy was categorized into CR, PR, SD, and PD. Based on the treatment outcomes, the patients were further classified into response (CR + PR) and non-response groups (SD + PD). The overall response was determined based on CT results 6 months post-treatment and evaluated using the RECIST 1.1 or Lugano criteria.
Immunohistochemistry
Histological diagnosis was performed by two pathologists blinded to the clinical and PET findings. Patient tumor sections from biopsy samples before treatment were stained with the PD-L1 pharmDx kit (clone: 22C3; Agilent Technologies, Santa Clara, CA, USA) and CD8 antibody (1:1000; catalog#12068; Servicebio, Wuhan, China), respectively. For granzyme B staining, tumor sections from surgical samples were stained with an anti-human granzyme B antibody (1:200; catalog#17215; Cell Signaling Technology, Beverly, MA, USA). After incubation with horseradish peroxidase-conjugated secondary antibodies, all sections were visualized by incubating with a diaminobenzidine substrate. Immunohistochemical images were obtained using Slide viewer (version 2.7), and the data were analyzed with Image J software (version 1.52p).
Tumor sections were assessed, with positive PD-L1 expression being classified as a tumor proportion score of 1% or above. CD8+ T cell infiltration was characterized as either a “desert” or “non-desert” phenotype45, as defined by the criteria outlined as follows. Tumor phenotype was designated as “desert” if the frequency of CD8+ T cells was low and specifically fewer than 10 CD8+ T cells per high-power field (200× magnification); for larger specimens, this was determined by averaging counts from 10 randomly selected high-power fields. Conversely, tumors were labeled as “non-desert” when the number of CD8+ T cells in the field of view surpassed this threshold of 10.
The granzyme B positivity rate was determined by quantifying the percentage of stained cells positive for granzyme B out of the total number of cells counted. This involved assessing a minimum of 10 randomly chosen unit areas per sample. In each unit area, the number of cells positive for granzyme B was recorded. The positivity rate was then computed by summing the counts of granzyme B-positive cells across all unit areas and dividing by the cumulative total of cells counted within those fields.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) and R software (version 4.3.3; available at www.r-project.org). Descriptive statistics were employed to summarize the demographic data of patients. Non-linear approaches were utilized to compare the biodistribution of 68Ga-grazytracer among various healthy organs. Specifically, the General Additive Mixed Model (GAMM) was applied to examine variations in 68Ga-grazytracer uptake in different organs over time. Fixed effects in the model comprised time and organ (as categorical variables including brain, heart, kidney, liver, lung, muscle, skin, and spleen), while a random intercept was included for each patient to account for the within-subject variability due to repeated measurements. Furthermore, a quadratic (second-degree polynomial) model was used to illustrate the time-dependent biodistribution curves.
Pearson correlation analysis was used to assess the association between the uptake of 68Ga-grazytracer in tumors and the levels of granzyme B, as measured by IHC staining of the corresponding tumor tissue sections. The relationships between PD-L1 IHC findings and the overall response, as well as between different imaging assessment criteria and the overall response, were analyzed utilizing Chi-square test and Fisher’s exact test. ROC curves were generated to assess the specificity and sensitivity of 68Ga-grazytracer PET in predicting clinical outcomes, compared to CT and 18F-FDG PET; P values were determined using the Wilson/Brown method.
In the analysis of PFS, the time to event was defined as the duration from the start of the initial treatment (time zero) to the occurrence of tumor progression or death from any cause. The data of patients who did not experience disease progression by the final tumor assessment were censored. The log-rank test was utilized to assess the statistical significance of differences in PFS between groups. Additionally, the Cox regression model was used to estimate the hazard ratios. To investigate potential violations of the proportional hazards assumption, the Schoenfeld residual test was conducted.
Other comparisons were performed using the Mann–Whitney U test to calculate P values. All reported P values are two-tailed, with statistical significance established at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The study protocol for this clinical trial is available in the Supplementary Information file. To protect patient privacy, the clinical data are not publicly accessible. However, researchers with a scientifically sound proposal may request access from the corresponding author (Z.-F.L.). Data will be provided for a period of three years after the article’s publication, with all patient-related information being de-identified. The anticipated response time for data access requests is about four weeks, and the data will be available to the requester for a duration of one year. All remaining data can be found in the Article, Supplementary Information, and Source Data files. Source data are provided with this paper.
References
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Jimenez-Sanchez, A. et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170, 927–938.e20 (2017).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Assouline, S. et al. Development of adapted RECIST criteria to assess response in lymphoma and their comparison to the International Workshop Criteria. Leuk. Lymphoma 48, 513–520 (2007).
Chiou, V. L. & Burotto, M. Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol. 33, 3541–3543 (2015).
Turtle, C. J. et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 8, 355ra116 (2016).
Seymour, L. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18, e143–e152 (2017).
Le Lay, J., Jarraya, H., Lebellec, L. & Penel, N. irRECIST and iRECIST: the devil is in the details. Ann. Oncol. 28, 1676–1678 (2017).
Cheson, B. D. et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128, 2489–2496 (2016).
Mokrane, F. Z. et al. Performance of CT compared with 18F-FDG PET in predicting the efficacy of nivolumab in relapsed or refractory hodgkin lymphoma. Radiology 295, 651–661 (2020).
Wahl, R. L., Jacene, H., Kasamon, Y. & Lodge, M. A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50, 122s–150s (2009).
Kandathil, A., Kay, F. U., Butt, Y. M., Wachsmann, J. W. & Subramaniam, R. M. Role of FDG PET/CT in the eighth edition of TNM staging of non-small cell lung cancer. Radiographics 38, 2134–2149 (2018).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Tagliabue, L. & Del Sole, A. Appropriate use of positron emission tomography with [18F]fluorodeoxyglucose for staging of oncology patients. Eur. J. Intern. Med. 25, 6–11 (2014).
Bollineni, V. R., Kramer, G. M., Jansma, E. P., Liu, Y. & Oyen, W. J. A systematic review on [18F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer 55, 81–97 (2016).
Kok, I. C. et al. 89Zr-pembrolizumab imaging as a non-invasive approach to assess clinical response to PD-1 blockade in cancer. Ann. Oncol. 33, 80–88 (2022).
Bensch, F. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 24, 1852–1858 (2018).
Zhou, X. et al. First-in-humans evaluation of a PD-L1-binding peptide PET radiotracer in non-small cell lung cancer patients. J. Nucl. Med. 63, 536–542 (2022).
Aguiar, P. N. Jr., De Mello, R. A., Hall, P., Tadokoro, H. & Lima Lopes, G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy 9, 499–506 (2017).
Davis, A. A. & Patel, V. G. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 7, 278 (2019).
Wang, X. et al. Preclinical and exploratory human studies of novel 68Ga-labeled D-peptide antagonist for PET imaging of TIGIT expression in cancers. Eur. J. Nucl. Med. Mol. Imaging 49, 2584–2594 (2022).
Miedema, I. H. C. et al. 89Zr-immuno-PET using the anti-LAG-3 tracer [89Zr]Zr-BI 754111: demonstrating target specific binding in NSCLC and HNSCC. Eur. J. Nucl. Med. Mol. Imaging 50, 2068–2080 (2023).
Xin Yu, J., Hubbard-Lucey, V. M. & Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 18, 899–900 (2019).
Kist de Ruijter, L. et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: a phase 1/2 trial. Nat. Med. 28, 2601–2610 (2022).
Farwell, M. D. et al. CD8-targeted PET imaging of tumor-infiltrating T cells in patients with cancer: a phase I first-in-humans study of 89Zr-Df-IAB22M2C, a radiolabeled anti-CD8 minibody. J. Nucl. Med. 63, 720–726 (2022).
Oliveira, G. & Wu, C. J. Dynamics and specificities of T cells in cancer immunotherapy. Nat. Rev. Cancer 23, 295–316 (2023).
Lu, C. et al. G6PD functions as a metabolic checkpoint to regulate granzyme B expression in tumor-specific cytotoxic T lymphocytes. J. Immunother. Cancer 10, e003543 (2022).
Larimer, B. M. et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 77, 2318–2327 (2017).
Larimer, B. M. et al. The effectiveness of checkpoint inhibitor combinations and administration timing can be measured by granzyme B PET imaging. Clin. Cancer Res. 25, 1196–1205 (2019).
Zhou, H. et al. Noninvasive interrogation of CD8+ T cell effector function for monitoring early tumor responses to immunotherapy. J. Clin. Invest. 132, e161065 (2022).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Bhutani, P. et al. U.S. FDA approved drugs from 2015-June 2020: a perspective. J. Med. Chem. 64, 2339–2381 (2021).
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18, 197–218 (2019).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Avril, N., Sassen, S. & Roylance, R. Response to therapy in breast cancer. J. Nucl. Med. 50, 55s–63s (2009).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Shyer, J. A., Flavell, R. A. & Bailis, W. Metabolic signaling in T cells. Cell Res. 30, 649–659 (2020).
Dercle, L. et al. Emerging and evolving concepts in cancer immunotherapy imaging. Radiology 306, 32–46 (2023).
Das, S. & Johnson, D. B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 7, 306 (2019).
de Boer, J. W. et al. Inflammatory reactions mimic residual or recurrent lymphoma on [18F]FDG-PET/CT after CD19-directed CAR T-cell therapy. Blood Adv. 7, 6710–6716 (2023).
Thiery, J. et al. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 12, 770–777 (2011).
Zhou, X. A. et al. KLHL22 maintains PD-1 homeostasis and prevents excessive T cell suppression. Proc. Natl Acad. Sci. USA 117, 28239–28250 (2020).
Eskander, R. N. et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N. Engl. J. Med 388, 2159–2170 (2023).
Van Heertum, R. L. et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des. Devel Ther. 11, 1719–1728 (2017).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFC3404600 to Z.-F.L. and 2022YFC2409405 to N.L.), the National Natural Science Foundation of China (81920108020 and 82325028 to Z.-F.L.), the Beijing Natural Science Foundation (Z220011 and Z220014 to Z.-F.L.), and the Beijing Nova Program Interdisciplinary Cooperation Project (20220484182 to Z.-F.L.).
Author information
Authors and Affiliations
Contributions
Z.-F.L. and N.L. conceived the project. Z.-F.L., N.L., Z.Y., H.Z., and X.S. designed the study. H.Z., L.Z., and K.L. prepared the 68Ga-grazytracer. X.S., X.Z., R.G., and F.W. recuirted patients. Y.S. acquisited the PET/CT images. H.Z., X.S., and Z.-F.L. analyzed the data, prepared the figures and wrote the manuscript. H.Z., Z.-C.L., X.M., and W.L. performed the statistical analysis. Z.-F.L., N.L., and Z.Y. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
Z.-F.L. and H.Z. are inventors of a granted patent entitled “Granzyme B-targeting complex, radiopharmaceutical, preparation method therefor and use thereof” (patent number, ZL202111370138.7) related to 68Ga-grazytracer described in this study. Other authors declare no potential conflicts of interest.
Peer review
Peer review information
Nature Communications thanks Thomas Marron and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, X., Zhou, H., Zhou, X. et al. 68Ga-grazytracer PET for noninvasive assessment of response to immunotherapy in solid tumors and lymphomas: a phase 1/2 clinical trial. Nat Commun 15, 8791 (2024). https://doi.org/10.1038/s41467-024-53197-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53197-2
This article is cited by
-
Robust and generalizable artificial intelligence for multi-organ segmentation in ultra-low-dose total-body PET imaging: a multi-center and cross-tracer study
European Journal of Nuclear Medicine and Molecular Imaging (2025)