Abstract
Several international pharmacovigilance agencies have issued warnings regarding the potential risk of myasthenia gravis (MG) following statin therapy. Our study investigated this association using population-based electronic health records in Hong Kong. We conducted a sequence of target trial emulation (TTE) for interpersonal comparison and a self-controlled case series (SCCS) study for intrapersonal comparison. In the TTE for MG onset, the incidence rates (per 100,000 person-years) and adjusted HRs were 51.91(31.80, 84.74)[HR:6.11 (3.73, 10.01)] in month 1, 16.27(9.81, 26.99)[HR:1.92(1.15, 3.19) in months 2-4, and 15.27(9.05, 25.79)[HR:1.80(1.06, 3.04)] in months 5–7. For risk of exacerbation, the adjusted HRs were 10.69(5.48, 20.84) in month 1, 1.50(0.55, 4.06) in months 2–4, and 2.79(1.33, 5.84) in months 5–7. No increased risks were found during the subsequent 18 months. A similar pattern was observed in SCCS analysis. Our findings recommend a minimum monitoring period of approximately six months for MG symptoms for patients starting using statin.
Similar content being viewed by others
Introduction
Statins are widely recognized as one of the most common types of cholesterol-lowering drugs and have long been utilized to mitigate the risk of cardiovascular conditions1,2,3,4. However, in 2023, several international pharmacovigilance organizations, including those in Europe, the UK, Australia, Japan and Hong Kong, issued a warning about the risk of new-onset or worsening of pre-existing myasthenia gravis (MG) following statin therapy5,6,7,8,9. MG is a rare neuromuscular disorder that can be potentially fatal, particularly in severe cases where extreme muscle weakness leads to respiratory failure requiring intubation and mechanical ventilation10,11. A population-based study conducted in China reported a total mortality rate of 1.47% among MG patients, with a significantly higher rate of 10.7% among elderly12. While the warning issued by these agencies, there are limited studies examining the relationship between MG and statins, underscoring the urgent need for further investigation as emphasized by the agencies5,6,7. Given the widespread use of statins worldwide, it is important to analyse the potential association between statins and MG to ensure patient safety.
There have been case reports suggesting that statins might lead to the onset or exacerbation of myasthenia gravis (MG)13,14,15,16,17,18 within a few weeks or months following treatment. Moreover, only one study, based on a disproportionality analysis using the data from the World Health Organization pharmacovigilance system, has indicated a possible signal for the risk of MG after statin treatment19. However, the analysis relying solely on data from the pharmacovigilance system may introduce inherent reporting bias, as cases may be underreported and lack confirmation by physicians. Consequently, these observations, which were at the lower level of the evidence hierarchy, reflect the lack of a reliable conclusion from a real-world large-population study.
To support informed clinical decisions and provide specific guidance, our study aimed to apply two study designs for higher-level evidence to investigate the risk of new MG onset and exacerbation following statin initiation, using a sequence of nested target trials emulation (TTE) for interpersonal comparison and self-control cases series (SCCS) for intrapersonal comparison based on the real-world population-based electronic health records.
Results
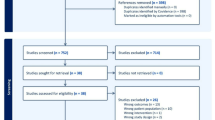
Table 1 presented the baseline characteristics of the statin initiators and non-initiators in our TTE study, where 369,850 initiators without pre-existing MG and 112 initiators with pre-existing MG were included in the analysis (Fig. 1). The average age was approximately 63 years across the groups. The baseline characteristics of the eligible person-trials stratified by the age groups and sex were presented in the Supplementary Table 1a, b. Following the SCCS design, we identified 187 cases with MG onset (Female: 104, Male: 83) and 86 cases (Female: 47, Male: 39) with first MG exacerbation for analysis (Supplementary Fig. 1).
The results from the TTE and SCCS were presented in Table 2 and Table 3, respectively. In TTE, the incidence rate of MG onset for the statin initiators is 51.91 per 100,000 person-years (95% CI: [31.80, 84.74]) in month 1, 16.27(9.81, 26.99) in months 2-4 and 15.27 (9.05, 25.79) in months 5-7. The incidence rates of MG onset among the statin initiators during the subsequent 18 months were comparable to those who did not initiate statin therapy ( < 10 per 100,000 person-years). The adjusted hazard ratios for the MG onset in ITT analysis were 6.11 (3.73, 10.01) in Month 1, 1.92 (1.15, 3.19) in months 2–4 and 1.80 (1.06, 3.04) in months 5–7. No significantly increased risk was found during the following 18 months. Similar results were found in the SCCS analysis, where the IRRs (95% CI) of MG onset was 7.68 (4.49, 13.13) in the first months of statin initiation, 2.42 (1.39, 4.20) in months 2–4 and 2.33 (1.32, 4.12) in months 5–7.
Among the patients with pre-existing MG, the incidence rates of exacerbation (per 1000 person-months) were also higher in month 1 (89.29[48.04, 165.94]), months 2–4 (13.38[5.02, 35.64]), and months 5–7 (24.48[11.67, 51.34]) (Table 2). The adjusted HRs (95% CI) for the exacerbation in ITT analysis were 10.69 (5.48, 20.84) in month 1, which was 1.50 (0.55, 4.06) in months 2–4, and 2.79 (1.33, 5.84) in months 5-7. No observable increased risk for exacerbation was found in the following 18 months. In the SCCS analysis, increased incidence rates (per 100 person-years, 95% CI) for the exacerbation were also observed in the month 1 (139.53[75.08, 259.33]), months 2–4 (18.82[7.06, 50.15]) and months 5–7 (34.15[16.28, 71.63]), compared to that in the non-exposure period (16.93[13.03, 22.00]). The discrepancy in model estimates between TTE and SCCS may attributed to the limited number of events in months 2–7 in SCCS analysis, resulting in varying estimates and a lack of statistical power. Despite this, the overall patterns of risk were similar in both approaches.
Consistent results for the risk of MG onset and exacerbation were observed in the per-protocol analysis in both TTE and SCCS design. The standardized cumulative incidence curves for MG onset and exacerbation were presented in Supplementary Fig. 2. Significantly increased risks for the MG onset and exacerbation were observed in most patient subgroups in the initial 7 months after statin initiation but not for the subsequent 18 months (TTE: Fig. 2 and Supplementary Table 2; SCCS: Supplementary Table 3). However, for the patients aged less than 60 years old, the HR for MG onset (ITT analysis: 1.27 [0.66, 2.45]) in the initial 7 months was not as apparent as those aged over 60 years (3.24 [2.33, 4.52], p interaction = 0.012) in the initial 7 months. All sensitivity analyses yielded consistent results to the main analysis (Supplementary Table 4–10).
The analysis was adjusted for demographic characteristics (sex and age), low-density lipoprotein cholesterol (LDL-C) level, Charlson comorbidity index (CCI), hypertension, diabetes, and Rheumatic/connective tissue disease, and drug history within 1 year before baseline (β-blocker, calcium channel blockers, Aminoglycosides, and Fluoroquinolones). Statistical significance was defined as a two-tailed p-value.
Discussion
To our knowledge, our population-based study is the first to provide evidence of an increased risk of MG onset in the first months and subsequent six months following statin initiation, as demonstrated through both interpersonal and intrapersonal comparisons. For the patients with pre-existing MG, an increased risk for MG exacerbation was also observed during the same period. Notably, the risk for MG onset in patients aged over 60 years was more apparent compared to their counterparts. These findings suggest the importance of monitoring MG symptoms for approximately six months after initiating statin therapy, particularly among the elderly population.
Our study, based on real-world evidence, confirms the signal identified in a previous study that employed a disproportionality analysis. However, unlike the disproportionality analysis, our study evaluated different risk periods after statin initiation, demonstrating the increased risk of MG within approximately six months after statin initiation, but no increased risk of newly diagnosed MG subsequently. Furthermore, our study also demonstrated the potential risk of MG exacerbation after the initiation of statin therapy among the MG patients in stable status. While there is no comparative study in the literature, a previous case-series study on 170 MG patients showed 6 out of 54 patients on statin (11%) experienced worsening or relapse of any MG symptoms within 4 months following statin treatment20, which is similar to the standardized cumulative incidence 8.62% (3.69%, 13.55%) in the analysis of target trial emulation in our study. Further, we utilized two advanced study designs for interpersonal and intrapersonal comparisons, which helped mitigate the limitations associated with each approach, to obtain robust evidence.
The mechanism of the association between statin and MG remains unclear. For MG onset, statins may cause mitochondrial dysfunction by depleting coenzyme Q10, and statins may induce the production of cytokines, which are involved in the synthesis of autoantibodies against acetylcholine receptors2. Statins may upregulate Th2 cells, which increase the activity of antibody-mediated humoral immunity1,18. The hypothesis that autoantibodies play a significant role may be supported by the fact that the circulating autoantibodies against acetylcholine receptors disappear after statin withdrawal in some cases3,19. Regarding MG exacerbation, statins may interfere with MG treatments, such as rituximab, as statins may cause conformational changes in CD20 and thereby reduce the effect of rituximab1. Moreover, the direct myotoxicity of statins may worsen the muscle weakness of MG patients2. Last but not least, due to the direct impact of cholesterol on presynaptic or postsynaptic membrane proteins, cholesterol depletion at the neuromuscular junctions (NMJ) following statin therapy might worsen synaptic transmission of the NMJ in the patients with pre-existing MG, leading to the exacerbation of MG symptoms10. It can be inferred that the aforementioned NMJ defects following statin therapy may also play a role in unmasking latent forms of MG21. Further research is needed to investigate the specific mechanisms.
Nevertheless, it is important to note that MG is a rare condition, and the estimated absolute risk difference for MG onset in our study was 0.00425% (95% CI: [0.00133%, 0.00717%]) in the subsequent two years following statin initiation in the population with indication for statins. This implied that there might be only one additional case of MG onset out of about 24,000 individuals initiating statins (number needed to harm [95% CI]: 23,529 [13,946, 75,211]). Therefore, while our study highlighted the increased relative risk of newly diagnosed MG following statin initiation, it also provided reassurance for the physician and patients regarding its overall low risk. The higher risk of MG onset in individuals aged over 60 years, may be attributed to their medical complexity and reduced tolerance to medications22, or the underlying neuromuscular diseases, which emphasizes the need for enhanced attention towards this age group in monitoring for MG symptoms and conducting further diagnostic evaluations when they experience muscle symptoms after taking statins. The risks of exacerbation of pre-existing MG were comparable between the two age groups during the initial 7 months, while a statistically significant interaction effect with age was observed during the subsequent 18 months. However, it is important to note that the estimations may be susceptible to the bias derived from the small number of events. Further studies with larger sample sizes are necessary to verify the interaction effect with age for the risk of MG exacerbation.
One of the major strengths of this study is the use of population-based electronic health records collected from real-life routine practice, which enhanced the generalizability of our study findings. Additionally, our method for identifying eligible patients with statin indication for analysis closely mirrored real-life clinical practice, which enhanced comparability between the statin initiators and non-initiators in the analysis and improved the applicability of the study’s conclusion to clinical practice. Furthermore, compared to the previous studies, the adoption of the two distinct advanced methods for interpersonal and intrapersonal comparison enhanced the precision of our estimates and cross-verified the risk of events following statin therapy.
Our study has several limitations. Firstly, the data sources used in this study were only restricted to the electronic health records in the public health systems, and we were unable to investigate patients presenting MG to private hospitals. Nevertheless, the public sector is the main provider of specialist care in Hong Kong, and thus the majority of the MG cases should be included in our analysis23. Secondly, the identification of outcomes events in this study depends on the diagnosis coding of ICPC-2 and ICD-9-CM in the EHRs, which may cause misclassification bias. However, the database used for data extraction has been validated with high coding accuracy in prior studies24. Thirdly, there might exist residual unmeasured confounders in the TTE analysis. However, we concurrently conducted the SCCS analysis to control for the unmeasured time-invariant confounders that vary among individuals, such as genetic factors and socioeconomic status, limiting the potential influence of these confounders and improving the overall robustness of our results. Fourthly, it needs to be acknowledged that some of the newly diagnosed MG patients after baseline might represent the pre-existing latent forms of MG that were unmasked following statin therapy. Due to the nature of real-world data analysis based on electronic health records, we cannot exclude the cases with pre-existing unrecognized MG, and further research is needed to confirm the risk among patients with and without latent forms of MG. Fifthly, it is noteworthy that only monthly data for the territory-wide electronic health records were available for the present study. This might introduce potential bias for the risk estimation in the first month, where more granular data (e.g., on a daily or weekly basis) is necessary to verify the timing of statin initiation prior to the outcome incidence in the first month, so as the potential discontinuation of statin therapy. However, the current findings regarding the consistent trend of increased risk in the subsequent six months remain supportive of the primary conclusion regarding the monitoring for MG symptoms following statin therapy. Lastly, given the potential discrepancy in the diagnosis criteria of MG across different countries, evidence from different nations is essential to confirm the findings to inform global clinical practice in the future.
In conclusion, significantly increased risks of MG onset and the exacerbation of pre-existing MG were observed in the first months and subsequent six months following statin initiation, suggesting a minimum monitoring period of approximately 6 months for MG symptoms in patients starting using statins, irrespective of whether MG is pre-existing or not. The risk for MG onset in patients aged over 60 years was more apparent compared to the younger adults, highlighting the importance of enhanced attention towards this age group. Nevertheless, the benefits of the statin far outweigh the risk of this rare adverse event, and our study could also provide reassurance to patients regarding its overall low risk.
Methods
The study was approved by the Institutional Review Board of The University of Hong Kong—the HA Hong Kong West Cluster (reference number UW 24-457). Since all data used in this study were anonymized and obtained from the electronic health records from the Hong Kong Hospital Authority (HA), the patients’ consent to participate was not required.
Overview of study design
Our study employed two study designs to investigate the association between statin use and MG. For interpersonal comparison, we emulated a sequence of nested target trials25,26 to compare the risk of events between the patients who initiated statin therapy and those who did not. For intrapersonal comparison, we applied the self-control cases series (SCCS)27 method to estimate the risk difference within the same individuals before and after statin initiation. The risk of events was also estimated in different time intervals following statin therapy, in order to provide information on the appropriate monitoring period to inform the clinical practice.
Data source
This study was conducted using the territory-wide electronic health records from the HA. The database is an integrated clinical workstation that provides access to all available electronic clinical information (including prescription data, diagnosis codes, and clinical parameters) to support the real-time routine clinical practice. The diagnostic data has been validated with high coding accuracy28. The database was widely applied in pharmacovigilance and pharmacoepidemiologic studies29,30,31.
Exposure and outcomes
Statin therapy was defined as the treatment with simvastatin, atorvastatin, luvastatin, rosuvastatin, lovastatin, pitavastatin, and/or pravastatin. In Hong Kong, the health care for MG patients is mainly provided by public hospitals, where the inpatient department generally handles the MG patients presenting with more severe symptoms, while those with mild symptoms or in remission receive regular follow-up care at the Specialist Out-patient Clinics (SOPCs) of the hospitals. The diagnosis of MG is based on the following criteria23: (1) symptoms and signs compatible with MG; (2) at least one of the following three supportive diagnostic tests: presence of acetylcholine receptor antibody, significant decremental response on repetitive nerve stimulation, or objective clinical response to edrophonium; and (3) exclusion of other disease that mimic MG, such as myopathies or peripheral neuropathy. Patients with any attendance records related to MG at hospitals or SOPCs before baseline were classified as having pre-existing MG history. The incidence of MG onset was determined by considering patients who were newly diagnosed with MG (ICD-9-CM: 358.0) after baseline. Exacerbation of pre-existing MG was determined as the occurrence of hospital admissions in MG patients who initially had mild symptoms or were in a state of remission, but subsequently experienced a deterioration or MG crisis that led to the admission. The patients without any MG-related hospitalizations for a minimum period of one year were included in the analysis32. Our study only investigated the incidence of first exacerbation.
Sequential trial emulation
The specification and details of the TTE can be found in Supplementary Table 11. The analysis included all adult patients fulfilling the indication criteria for statin therapy in each calendar month from Jan 2009 to Dec 2016. The pre-defined indications for statin eligibility were derived from local clinical guidelines to reflect real-world statin prescription practices, similar to the 2019 ESC/EAS (Society of Cardiology/European Atherosclerosis Society) Guidelines for the Management of Dyslipidaemias33. Additional details are presented in Supplementary Table 12. Case definition was based on the International Classification of Primary Care, 2nd Edition (ICPC-2) and International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), or relevant clinical parameters (Supplementary Table 13). Further details on the eligible criteria are presented in Fig. 1. The eligible patients were extracted from the database on a rolling basis in each calendar month from January 2009 to December 2016 (Supplementary Table 14), and followed up until the outcome of interest, death, or the end of the 24th month after baseline, whichever occurred first.
The effect of statin initiation on MG onset or exacerbation was analysed in both intention-to-treat (ITT) and per-protocol approaches. A time-discrete dataset was constructed by month for each eligible person-trial, where the marginal structural model (MSM) was adopted to estimate the causal effect that accounts for the time-varying information34. As the typical statistical model in MSM, the pooled logistic model was employed34,35 to estimate the association between statin therapy and the incidence of MG onset or exacerbation. Because the outcome of the models is rare at all times, the odds ratio from the pooled logistic model approximates the hazard ratio (HR)36. The ITT analysis compared the risk of MG onset or exacerbation between the statin initiators and non-initiators defined by their treatment strategy at baseline. A separate hazard ratio (HR) was estimated within each time interval (i.e., month 1, months 2–4, months 5–7, months 8–13, months 14–19, and months 20–25) by fitting a pooled logistic model for the outcome incidence with the interaction term between the treatment indicator and factor variable of the pre-defined time intervals as mentioned above. The length of the reviewed risk period and the cut-off of examined time intervals were chosen based on the previous case reports16,18,20. The analysis was adjusted for the covariates of demographic characteristics (sex and age), low-density lipoprotein cholesterol (LDL-C) level, comorbidities (Charlson comorbidity index (CCI), hypertension, diabetes, and Rheumatic/connective tissue disease), and drug history within 1 year before baseline (β-blocker, calcium channel blockers, aminoglycosides, and fluoroquinolones). In the per-protocol analysis for continuous statin use, the person-trials were artificially censored when they deviated from their designated strategy. Each person-trial was weighted by the inverse probability of receiving their designated treatment strategy at each time point, to adjust for potential selection bias introduced by the artificial censoring. A pooled logistic model was fitted to predict the probability of receiving statin therapy (conditional on the baseline and time-varying covariates) at each time point. To obtain the stabilized weight, the inverse probability weights were multiplied by the subject’s probability of having received the designated treatment strategy conditional on the baseline covariates only. The absolute risk differences were estimated in the fitted model, where the estimated incidence rate of MG onset and exacerbation was standardized to the empirical distribution of the confounders at baseline in the whole study population (Supplementary Method 1).
Subgroup analysis was conducted regarding the sex, age ( < 60/ ≥ 60) and CCI ( < 4/ ≥ 4) at baseline, where the HRs of MG onset and exacerbation for the months 1–7 and months 8–25 were generated, respectively. Due to variations in medical complexity and medication tolerances between the younger and old patients, we examined the differences in the risk patterns across age groups using the age of 60 years as the threshold22,37,38. Several sensitivity analyses were conducted, including (1) excluding the patients with pre-existing thymoma39,40; (2) adopting propensity score matching to improve the balance in baseline confounders; (3) truncating the weights at their 0.5th and 99.5th percentile in per-protocol analysis; (4) additionally adjusting for other common comorbidities; (5) additionally adjusting for other clinical parameters in lipid profile, including high-density lipoprotein, total cholesterol, triglyceride.
SCCS design
Patients who initiated statin therapy from Jan 2009 to Dec 2016 were identified from the electronic health record database of HA. The study design and the timeline for a single hypothetical participant are illustrated in Supplementary Fig. 3. Similar to the design in TTE, we divided the patient-time into the aforementioned six risk windows and one baseline period (i.e., the non-exposure period), and conducted the analysis in ITT and per-protocol approach. Details of the SCCS assumptions relevant to the current study are available in Supplementary Method 2, and two sensitivity analyses were conducted to test the validity and robustness of study findings: (1) adding a 12-month pre-exposure period; (2) excluding the patients with mortality during the observation period. Subgroup analysis was conducted regarding the sex.
The observation period for the cases of MG onset in the SCCS spanned from Jan 2009 until the end of the second year after statin initiation. The patients with MG onset during the observation period were included for analysis. Further details in the eligible criteria for the study participants were presented in Supplementary Fig. 1, which were consistent with those in the TTE. The incidence rate ratios (IRRs) for MG onset were estimated by conditional Poisson regression.
The observation period for the cases of MG exacerbation spanned from the later of January 2009 or the date of the first MG diagnosis to the end of the second year after statin initiation. The patients with MG history before statin initiation and experiencing the first MG exacerbation during the observation period were included for analysis (details in Supplementary Fig. 1). The MG-related admission in the patients with remission status (i.e., without another MG-related admission occurred within the preceding 12 months) was considered as MG exacerbation, for which the IRR was evaluated in the similar model as specified above.
All analyses were performed in Stata/MP 17.0 and R version 4.1.1. Statistical significance was defined as a two-tailed p-value < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data underlying this article were provided by the Hospital Authority of Hong Kong. The data contains confidential information and therefore cannot be shared with the public due to restrictions imposed by the third-party usage agreements. Local academic institutions, government departments, or non-governmental organizations may apply for the access to data through the Hospital Authority’s data-sharing portal (https://www3.ha.org.hk/data). The investigators are responsible for the archiving and safekeeping of the personal and study data during and after the study. The study data will be kept for 5 years after the completion of the study.
Code availability
The codes used for this study are available online in Github (https://github.com/w01092021/statin_mg.git) and Zenodo (https://doi.org/10.5281/zenodo.13928836).
References
Attardo, S., Musumeci, O., Velardo, D. & Toscano, A. Statins Neuromuscular Adverse Effects. Int J. Mol. Sci. 23, 8364 (2022).
Andronie-Cioara, F. L., Jurcau, A., Jurcau, M. C., Nistor-Cseppento, D. C. & Simion, A. Cholesterol Management in Neurology: Time for Revised Strategies? J. Pers. Med 12, 1981 (2022).
Loh, W. J. & Watts, G. F. The Management of Hypercholesterolemia in Patients with Neuromuscular Disorder. Curr. Atheroscler. Rep. 25, 43–53 (2023).
Guadamuz, J. S., Shooshtari, A. & Qato, D. M. Global, regional and national trends in statin utilisation in high-income and low/middle-income countries, 2015-2020. BMJ Open 12, e061350 (2022).
PRAC recommendations on signals: Adopted at the 9-12 January 2023 PRAC meeting). European Medicine Agency (2023).
Medicines and Healthcare products Regulatory Agency. Statins: very infrequent reports of myasthenia gravis) (2023).
Drug Information Pharmacovigilance Division. Statins: very infrequent reports of myathenia gravis). Department of Health (2023).
Department of Health and Aged Care. Product Information safety updates - November 2023) (2023).
Pharmaceuticals and Medical Devices Agency. Summary of Investigation Results: Preparations containing HMG-CoA reductase inhibitor) (2023).
Paz, M. L. & Barrantes, F. J. Cholesterol in myasthenia gravis. Arch. Biochem Biophys. 701, 108788 (2021).
Wendell, L. C. & Levine, J. M. Myasthenic crisis. Neurohospitalist 1, 16–22 (2011).
Chen, J. et al. Incidence, mortality, and economic burden of myasthenia gravis in China: A nationwide population-based study. Lancet Reg. Health West Pac. 5, 100063 (2020).
Keogh, M. J., Findlay, J. M., Leach, S. & Bowen, J. Statin-associated weakness in myasthenia gravis: a case report. J. Med Case Rep. 4, 61 (2010).
Cartwright, M. S., Jeffery, D. R., Nuss, G. R. & Donofrio, P. D. Statin-associated exacerbation of myasthenia gravis. Neurology 63, 2188 (2004).
Purvin, V., Kawasaki, A., Smith, K. H. & Kesler, A. Statin-associated myasthenia gravis: report of 4 cases and review of the literature. Med. (Baltim.) 85, 82–85 (2006).
Khalid, R., Ibad, A. & Thompson, P. D. Statins and Myasthenia Gravis. Muscle Nerve 54, 509 (2016).
Frasson, E. et al. Statin-associated necrotizing autoimmune myopathy with concurrent myasthenia gravis. Clin. Case Rep. 9, e03925 (2021).
Gale, J. & Danesh-Meyer, H. V. Statins can induce myasthenia gravis. J. Clin. Neurosci. 21, 195–197 (2014).
Gras-Champel, V., Batteux, B., Masmoudi, K. & Liabeuf, S. Statin-induced myasthenia: A disproportionality analysis of the WHO’s VigiBase pharmacovigilance database. Muscle Nerve. 60, 382–386 (2019).
Oh, S. J. et al. Statins may aggravate myasthenia gravis. Muscle Nerve. 38, 1101–1107 (2008).
Tsivgoulis, G. et al. Presymptomatic neuromuscular disorders disclosed following statin treatment. Arch. Intern Med. 166, 1519–1524 (2006).
McMillan, G. J. & Hubbard, R. E. Frailty in older inpatients: what physicians need to know. QJM 105, 1059–1065 (2012).
Lee, C. Y. et al. Clinical outcome of generalized myasthenia gravis in Hong Kong Chinese. J. Neuroimmunol. 289, 177–181 (2015).
Wong, A. Y. et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 352, h6926 (2016).
Hernan, M. A., Wang, W. & Leaf, D. E. Target Trial Emulation A Framework for Causal Inference From Observational Data. Jama-J. Am. Med Assoc. 328, 2446–2447 (2022).
Danaei, G., Rodriguez, L. A., Cantero, O. F., Logan, R. & Hernan, M. A. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat. Methods Med Res. 22, 70–96 (2013).
Petersen, I., Douglas, I. & Whitaker, H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 354, i4515 (2016).
Wan, E. Y. et al. Association of Visit-to-Visit Variability of Systolic Blood Pressure With Cardiovascular Disease and Mortality in Primary Care Chinese Patients With Type 2 Diabetes-A Retrospective Population-Based Cohort Study. Diabetes Care 40, 270–279 (2017).
Wan, E. Y. F. et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect. Dis. 22, 64–72 (2022).
Wan, E. Y. F. et al. Effectiveness of Molnupiravir and Nirmatrelvir–Ritonavir in Hospitalized Patients With COVID-19: A Target Trial Emulation Study. Ann. Intern. Med. 176, 505–514 (2023).
Lau, W. C. et al. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: a population-based cohort study. Ann. Intern. Med. 173, 1–9 (2020).
Jaretzki, A. 3rd et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 55, 16–23 (2000).
Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188 (2020).
Fewell, Z. et al. Controlling for time-dependent confounding using marginal structural models. Stata J. 4, 402–420 (2004).
Murray, E. J., Caniglia, E. C. & Petito, L. C. Causal survival analysis: a guide to estimating intention-to-treat and per-protocol effects from randomized clinical trials with non-adherence. Res. Methods Med. Health Sci. 2, 39–49 (2021).
Ngwa, J. S. et al. A comparison of time dependent Cox regression, pooled logistic regression and cross sectional pooling with simulations and an application to the Framingham Heart Study. Bmc Med Res Methodol. 16, 148 (2016).
Roller‐Wirnsberger, R., Thurner, B., Pucher, C., Lindner, S. & Wirnsberger, G. H. The clinical and therapeutic challenge of treating older patients in clinical practice. Br. J. Clin. Pharmacol. 86, 1904–1911 (2020).
World Health Organization. Decade of healthy ageing: baseline report (2021).
Kim, A. et al. Risk factors for developing post-thymectomy myasthenia gravis in patients with thymoma. Muscle Nerve 63, 531–537 (2021).
Xue, L. et al. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis. Eur. J. Cardio-Thorac. 52, 692–697 (2017).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) Excellent Young Scientists Fund (Hong Kong and Macau) (No. 82222902; E.Y.F.W.). The funders had no role in study design, data collection, data analysis, and data interpretation. The data analysis was performed using research computing facilities provided by Information Technology Service, The University of Hong Kong. We would like to thank them for their ongoing support in this project.
Author information
Authors and Affiliations
Contributions
E.Y.F.W had the original concept, obtained funding, approval and data. W.X. V.K.C.Y., and Z.Z analyzed the data. W.X., E.Y.F.W, and K.K.F drafted the manuscript. K.H.C., G.K.K.L., C.S.L.C., F.T.T.L., X.L., E.W.Y.C, and I.C.K.W. provided critical input to the analyses, design and discussion. All authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
E.Y.F.W. has received research grants from the Health Bureau of the Government of the Hong Kong SAR, the Hong Kong Research Grant Council, National Natural Science Foundation of China, outside the submitted work. I.C.K.W. reports research funding from Amgen, Bristol Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, the European Commission, and the National Health and Medical Research Council in Australia, consulting fees from IQVIA and World Health Organisation, payment for expert testimony for Appeal Court of Hong Kong, outside the submitted work; and is a non-executive director of Jacobson Medical in Hong Kong, Advance Data Analytics for Medical Science (ADAMS) Limited (HK), Asia Medicine Regulatory Affairs (AMERA) Services Limited and OCUS Innovation Limited (HK, Ireland and UK) a former director of Therakind in England, outside of the submitted work; I.C.K.W. reports role as member of Pharmacy and Poisons Board in Hong Kong, the Expert Committee on Clinical Events Assessment Following COVID-19 Immunisation, and the Advisory Panel on COVID-19 Vaccines of the Hong Kong Government, outside the submitted work. C.S.L.C. has received grants from the Health Bureau of the Hong Kong Government, the Hong Kong Research Grants Council, the Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen, and personal fees from Prime Vigilance, outside the submitted work. E.W.Y.C. reports honoraria from the Hospital Authority and grants from the Hong Kong Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Natural Science Fund of China, the Wellcome Trust, Bayer, Bristol Myers Squibb, Pfizer, Janssen, Amgen, Takeda, RGA Reinsurance Company, AstraZeneca, the Narcotics Division of the Security Bureau of Hong Kong Special Administrative Region, Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region, Novartis, National Health and Medical Research Council Australia, outside the submitted work. Francisco Tsz Tsun Lai are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by AIR@InnoHK administered by the Innovation and Technology Commission, outside the submitted work. X.L. received research grants from the Research Fund Secretariat of the Health Bureau, Health and Medical Research Fund (HMRF, HKSAR), Health and Medical Research Fund Fellowship Scheme (HMRF Fellowship, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Research Grants Council Research Impact Fund (RGC/RIF, HKSAR), Commission grants from Hospital Authority of Hong Kong; educational and investigator initiate research fund from Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Pfizer, Merck Sharp & Dohme, Open Health; she is also the former non-executive director of ADAMS Limited Hong Kong; all outside the submitted work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, W., Yan, V.K.C., Zhang, Z. et al. Myasthenia gravis following statin therapy: evidence from target trial emulation and self-controlled case series study. Nat Commun 15, 10317 (2024). https://doi.org/10.1038/s41467-024-54097-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54097-1
This article is cited by
-
Statin initiation increases risk of myasthenia gravis
Reactions Weekly (2024)