Abstract
Adjunctive rosuvastatin for rifampicin-susceptible pulmonary tuberculosis (rs-PTB) shows no effect on microbiological or radiological outcomes in a phase IIb randomised, controlled trial (NCT04504851). We explore the impact of adjunctive rosuvastatin on 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) imaging in a sub-study of 24 participants. Changes in standardised uptake value (SUVmax, SUVmean), Total Metabolic Volume, (TMV), Total Lesion Glycolysis (TLG), cavity diameter and volume, between week 0 and week 8 post-randomisation, are evaluated. Here we show no evidence of difference in the reduction in TLG [median 65.8% for the rosuvastatin group (Q1, Q3 38.6, 94.5) vs 64.3% for standard tuberculosis treatment group (Q1, Q3 −20.0, 81.7), P = 0.32], reduction in cavity volume on CT [median 3.2 cm3 (IQR 11.1, 0.5) for rosuvastatin, 2.2 cm3 (IQR 4.6, 0.7) for control (p = 0.72)], or any other PET-CT parameter measured. We show that the first 8-weeks of standard tuberculosis treatment results in a reduction in the volumetric indices (TLG and TMV), but had little change in SUVmax or SUVmean. Change in TLG and TMV holds promise as biomarkers of tuberculosis treatment response: future PET-CT studies should evaluate their role in predicting relapse-free cure, and the overall role of 18F-FDG-PET-CT as a tool for early-phase tuberculosis clinical trials.
Similar content being viewed by others
Introduction
Host-directed therapies (HDT), where drugs work on host targets rather than directly on mycobacteria, are being considered as therapeutic options for tuberculosis1. Statin drugs are a candidate HDT agent against tuberculosis, as shown by animal models and epidemiological studies2,3,4,5,6. We recently conducted a randomised, controlled Phase IIb clinical trial (ROSETTA) which investigated the impact of adjunctive 10 mg rosuvastatin for the first eight weeks of treatment in people with rifampicin-susceptible pulmonary tuberculosis (rs-TB). The primary outcomes of the clinical trial have been published7; we found that adjunctive 10 mg rosuvastatin used for the first eight weeks of treatment did not produce substantive benefit on culture conversion, change in the proportion of lung affected by chest x-ray or on spirometry.
In human studies, sputum based predictive biomarkers, such as time to culture conversion (TTCC), rate of change to time to positivity (TTP) on liquid culture, and colony forming units (CFU) on solid culture have been traditionally used to quantify response to treatments. However, they have been shown to only modestly predict relapse-free cure8. There is growing interest in alternative biomarkers of treatment response. Non-human primate models of tuberculosis9,10,11,12,13,14 and several human cohort studies15,16,17,18 have shown that treatment of tuberculosis results in metabolic and structural changes in the lung which can be captured on PET-CT, and can be quantified. These quantifiable changes that occur on PET-CT have the capacity to discriminate between the efficacy of various drug regimens in animal models of tuberculosis10,19,20. Thus there is interest in exploring whether PET-CT can be used as a research tool within early phase tuberculosis clinical trials21. This tool may be especially relevant for HDT experiments where therapeutic benefits may extend beyond bactericidal activity to modulation of immune activation, which can be measured as metabolic changes on PET imaging.
Here we report the findings from a PET-CT sub-study embedded within the ROSETTA trial which explored the impact of adjunctive rosuvastatin, used during the intensive phase of tuberculosis treatment, on the structural and metabolic changes within the lung. Other clinical trials have evaluated tuberculosis treatment using PET-CT; one measured the effect of the first 14 days of treatment20, two trials measured PET-CT outcomes after the intensive phase of treatment with various adjunctive HDT agents22,23, and others have focused on drug-resistant disease11,24. This study is the first to report the PET-CT outcomes from the use of adjunctive statin therapy in the intensive phase of TB treatment. We quantify several PET-CT outcome measures and find that the volumetric indices (TLG and TMV) hold promise and should be evaluated further for their potential as surrogate biomarkers of treatment efficacy for use in clinical trials. We also investigate if the analysis software influences the parameters measured from the images, and in a cohort of participants, explore inter-reader variability by having a proportion of scans analysed by a second reader.
Results
Study population and metrics analysed
Between September 2020 to January 2021, 56 ROSETTA trial participants from the Philippines and Vietnam were screened for the PET-CT sub-study (Fig. 1). Twenty-six of the screened participants met the study’s eligibility criteria, provided informed consent, and were enrolled into the PET-CT sub-study (Fig. 1). Two enrolled participants, one who did not complete the week 8 scan and another who did not have microbiologically confirmed Mycobacterium tuberculosis (Mtb) infection, were excluded from the scan analysis. The remaining 24 participants, 12 in the rosuvastatin and control groups, respectively, completed both scans and had data included in the analysis (Fig. 1).
The PET-CT sub-study recruited 26 participants with microbiological evidence of pulmonary tuberculosis from the main ROSETTA trial cohort of 137 participants by convenience sampling. Data from two scans, performed at week 0 and 8 of treatment, from 24 participants were analysed. Scans were analysed by a single reader using 2 software programmes, with a selection of scans read by a second reader using the OsiriX MD software. Source data are provided as a Source Data file.
SUVmax, SUVmean, total metabolic volume (TMV) and total lesion glycolysis (TLG) from both lungs were analysed and recorded from 48 PET-CT scans (Fig. 2). The number of cavities, aggregate cavity diameter and volume were analysed on CT (Fig. 2). Eighteen PET-CT scans (from nine randomly sampled participants) were analysed by a second reader to assess the reproducibility of the method of analysis. Percentage of lung affected, largest cavity size and aggregate cavity diameter assessed on chest x-rays performed at trial entry (baseline, week 0) and eight weeks post-randomisation (week eight) are also reported here.
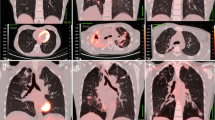
A Coronal section of thoracic cavity on a baseline PET-CT scan. Red outlined area is the region of interest (ROI) where all pixels have standardised uptake value (SUV) of 2.0 or greater. Maximum standardised uptake value (SUVmax) is defined as the hottest pixel within the ROI. SUVmean is defined as the average SUV of all pixels within the ROI. Dark blue outlined area represents a cavity within consolidated lung tissue. Cavity diameter (cm) was the widest measurement of the cavity on CT imaging. B 3-D of a volume of interest (VOI, cm3) of a single lesion where the SUV of voxels is 2.0 or greater. Total Metabolic Volume (TMV, cm3) is defined as the summation of all VOIs within a single scan. Total lesion glycolysis (TLG) is the product of metabolic volume in cm3 and SUVmean and is expressed without units.
Baseline characteristics of participants are comparable across groups
The median age of participants was 32 years; 19/24 (79%) were male, 13 were from Vietnam and 11 were from Philippines. There were no differences in age, sex, country, smoking status, weight, body mass index, proportion of lung affected, cavity size on chest x-ray, smear grade or Xpert semi-quantitative bacterial burden between rosuvastatin and control groups ((P > 0.05) (Table 1). The median duration of rosuvastatin received was 3.5 days (Q1 = 2, Q3 = 12), and participants received between two to 13 days of tuberculosis treatment prior to week 0 scan. There were no differences between groups in the baseline radiological metrics (Table S2).
Imaging metrics did not show a difference between rosuvastatin and control groups
In the rosuvastatin group, 10/12 (83%) participants culture converted within eight weeks, one remained culture positive and the remaining participant was culture negative at entry and for the duration of the trial. 9/12 (75%) culture converted in the control group; no difference was observed between the proportions who culture converted [Fishers exact test, P = 0.37].
There was no evidence of a difference between the rosuvastatin and control groups in TLG change or in any other PET-CT parameters measured after 8 weeks of treatment (Fig. 3, Table S3). The median change in TLG was −258.7 (Q1 = −478.5, Q3 = −91.1) and −141.2 (Q1 = −1062 Q3 = 53.5) (P = 0.84) (Fig. 3C, Table S3) for the rosuvastatin and control groups, respectively. This represents a median percentage reduction of 65.8% (Q1 = 38.6, Q3 = 94.5) in TLG for rosuvastatin and a reduction of 64.3% (IQR 20.0% increase, 81.7% decrease) (P = 0.32 in TLG for the control (Table S3). Findings did not change with the exclusion of the single participant with negative sputum culture at baseline (Table S4)
Blue dots represent Control group, Red dots represent Rosuvastatin group. Open dots represent Week 0 timepoint, coloured in dots represent Week 8 timepoint. A SUVmax [Week 0 median = 10.3 (IQR 6.4, 13.4), Week 8 median = 7.9 (IQR 5.8, 9.9) for Control, Week 0 median = 8.3 (IQR 5.1, 10.2), Week 8 median = 6.8 (IQR 3.9, 9.2) for Rosuvastatin], B TMV (cm3) [Week 0 median = 223.5 (68.8, 393.6), Week 8 median = 102.1 (IQR 32.1, 229.4) for control, Week 0 median =114.2 (IQR 37.4, 297.5), Week 8 median 27.3 (IQR 0.8, 135.0) for Rosuvastatin], C TLG [Week 0 median = 804.7 (IQR 183.1, 1358.7), Week 8 median = 308.5 (IQR 92.4, 771.4) for control, Week 0 median = 328.2 (IQR 1031, 987.4), Week 8 median = 79.8 (IQR 2.0, 443.3) for rosuvastatin], D Cavity diameter on CT (cm) [Week 0 median = 5.0 (IQR 2.7, 7.4), Week 8 median = 3.4 (IQR 1.4, 6.2) for Control, Week 0 median = 4.8 (IQR 1.2, 11.3), Week 8 median = 1.8 (IQR 0.6, 7.0) for Rosuvastatin], E Cavity volume on CT (cm3) [Week 0 median = 4.4 (IQR 1.7, 10.1), Week 8 median = 1.3 (IQR 0.3, 3.0) for Control, Week 0 median = 3.9 (IQR 1.0, 20.5), Week 8 median = 1.0 (IQR 0.3, 5.3) for Rosuvastatin], F Percentage of lung affected (%) [Week 0 median = 30 (IQR 20, 45), Week 8 median = 20 (IQR 15, 40) for Control, Week 0 median = 27.5 (IQR 17.5, 52.5), Week 8 median = 20 (IQR 5, 52.4) for Rosuvastatin]. Source data are provided as a Source Data file.
The intensive phase of tuberculosis treatment results in a reduction in TLG and TMV
Table 2 shows chest x-ray and PET-CT metrics at week 0 and week eight for all 24 participants. Twenty-three out of 24 participants had multiple pulmonary lesions with avid uptake on the initial PET-CT scan. The median SUVmax for all participants in the sub-study was 9.3 (Q1 = 5.5, Q3 = 11.4) with no significant change in SUVmax [median reduction of 1.07 (Q1 = −3.8, Q3 = 1.8) p = 0.15] (Table 2, Fig S2B), at the 8-week scan. Seven participants [5/12 (42%) in the control arm, 2/12 (17%) in the rosuvastatin arm) had increased SUVmax after eight weeks of treatment (Fig S3A and Fig S4). There was little change in SUVmean [median reduction of 0.03 (Q1 = −0.2, Q3 = 0.2), p = 0.86] (Table 2, Fig S2A) after the intensive phase of therapy. By contrast, median TMV and TLG at week 0 was 167.3 cm3 (IQR 57.9, 323.9) and 544.2 (Q1 = 158.5, Q3 = 1021.7), respectively, with very strong evidence (p < 0.001) of change in both after eight weeks of treatment (Table 2, Fig S2C, D); median reduction of 69.6 cm3 (Q1 = −191.4, Q3 = −7.4) for TMV and 202.0 (Q1 = −664.4, Q3 = −13.9) for TLG. This represents a 56.1% (IQR 20.7, 88.0) and 64.3% (Q1 = 9.0, Q3 = 88.7) reduction in TMV and TLG, respectively, after eight weeks of tuberculosis treatment (Table 2).
Fewer participants had cavities detected on baseline chest x-ray compared to CT (16 vs. 22, respectively). Cavity diameter on chest x-ray had fair correlation with CT (Spearman’s r = 0.42, p = 0.03, Fig S1A), however Bland-Altman analysis revealed evidence of clinically significant bias (−1.4 cm) [95% Limit of Agreement (LoA) −7.3, 4.5], with evidence of increasing underestimation of cavity size by chest x-ray as the size of the cavity increases (Fig S1B). On CT, cavity diameter reduced by a median of 2.0 cm (Q1 = −3.7, Q3 = 0, p = 0.011) and cavity volume reduced by a median of 2.9 cm3 (IQR −6.0, −0.5, p < 0.0001) at the week eight scan (Table 2). The percentage of lung affected on chest x-ray decreased from a median of 30% (Q1 = 25, Q3 = 53) to 20% (Q1 = 10, Q3 = 40), a change of −7.5% (Q1 = −15, Q3 = 0, p = 0.0002) after eight weeks of treatment (Table 2).
After the intensive phase of TB treatment, changes were detectable on PET-CT scan by with the volumetric metabolic indices (TMV and TLG), in cavity size, but not by SUVmax and SUVmean.
Baseline TLG varied widely and increased in some participants with lung cavitation
Amongst the 24 participants in the sub-study, there was a wide range of TLG (0 to 3499.4) (Figs S2D and S3B) at the week 0 scan. Most participants (20/24) showed a reduction in TLG, although three had an increased TLG (Fig S3B) and a single participant, who was culture negative throughout the trial, had no avidity detectable at week 0 and minimal uptake at week eight (TLG = 0.14). The three participants with increased TLG at week eight are profiled in Table S5; they also had increases in SUVmax (Fig S4). All three had aggregate cavities diameters of > 4 cm that had persisted or developed over the eight- week study period and had minimal or no change in the percentage of lung affected on chest x-ray. One out of three did not sputum culture convert by week eight, despite all three participants recording 100% treatment adherence (as measured by Directly Observed Therapy) and all had susceptibility confirmed to first line drugs used. All 24 participants in the sub-study culture converted by end of tuberculosis treatment (six months) and were deemed to have been cured. No data was collected on relapse in the trial or this sub-study.
Linear regression shows that TLG is related to other markers of disease burden
In a simple linear regression model, semi-quantitative Mtb burden on Xpert, sputum smear and the proportion of lung affected at trial entry was related to TLG at week 0 (Table S6). In a multiple linear regression model, only semi-quantitative burden on Xpert was significant; week 0 TLG increased by 218 (95% CI −346.8 782.7) and 1128.1 (547.3, 1708.9) when Xpert semiquantitative value was Medium or High, respectively, compared to when it was Low (P = 0.0002) (Table S6). Semiquantitative Xpert value was also associated with TLG at week eight in a simple regression model, along with the number of cavities and cavity diameter on CT, and the percentage of lung affected on chest x-ray (Table 3). In a multiple linear regression model, a positive culture at week eight was associated with increased week eight TLG, by an estimated 438.5 (95% CI 111.1, 765.9, p = 0.012) and a high Xpert Mtb burden at trial entry was associated with increased week eight TLG by an estimated 703.7 (95% CI 376.3, 1031.2, p = 0.0001) (Table 3).
Consistency in PET-CT findings by two independent readers
Serial PET-CT scans of nine randomly selected participants (18 scans) were analysed by a second reader, blinded to the results of the first reader, to assess inter-reader variability of the analysis method. The two-reader measurements of SUVmax, TMV and TLG showed strong correlation (r > 0.9, p ≤ 0.0001) (Fig. 4A, C, E). On Bland-Altman plots there was excellent agreement in SUVmax with minimal bias (−0.12, 95% LoA −1.17 – 0.93, Fig. 4B), a bias of 10.7 for TLG (95% LoA: −49.9 – 71.21, Fig. 4F) and a bias of 5.8 cm3 for TMV (95% LoA: −26.4 – 38.0, Fig. 4D). The correlation in the number of PET-CT avid lesions recorded by the two readers was fair (r = 0.72, p = 0.0008, Fig. 4G), although the Bland-Altman analysis revealed disagreement as the number of lesions increased (Fig. 4H).
Correlation (Left column) and Bland-Altman (Right column). Dashed lines in Bland-Altman plots represent Limits of Agreement (LoA). A two-sided test was applied to all tests of statistical significance. TLG (A) Spearman’s r > 0.99, P < 0.0001, and (B) Bias = 10.7, 95% LoA: −49.9, 71.21; SUVmax (C) Spearman’s r = 0.98, P < 0.0001, and (D) Bias = −0.12, 95% LoA: −1.17, 0.93; TMV in cm3 (E) Spearman’s r = 0.99, P < 0.0001), and (F) Bias = 5.80, 95% LoA: −26.4, 38.0; Number of PET-CT avid lesions (G) Spearman’s r = 0.72, P = 0.0008, and (H) Bias = −1.67, 95% LoA: −10.61, 7.3. Single outlier of disagreement in Bland-Altman plots of TMV (3D) and TLG (3 F) corresponds to the highest readings of TMV and TLG by both readers (means of 850.8 cm3 and 3436.1 respectively). Source data are provided as a Source Data file.
Poor agreement between manual and semi-automated analysis methods
In addition to the primary software used (OsiriX MD, Pixmeo, Switzerland), all 48 PET-CT scans were analysed using a secondary method using a more time-efficient, semi-automated software syngo.via MM oncology (Siemens Healthineers, Erlangen, Germany). Whilst there was strong correlation for TLG (Spearman’s r = 0.96, P < 0.0001, Supplementary Fig. S5E), a Bland-Altman analysis revealed a large bias of 227.3 (95% LoA −289.8, 744.3). Analysis of the plots showed that syngo.via underestimated TLG, with the difference between the methods increasing as higher values were recorded (Supplementary Fig. S5F). The differences in the readings of TLG was driven by the bias in the readings of TMV (Supplementary Fig. S5D), since there was minimal bias in SUVmax (0.19, 95% LoA −1.75, 2.12, Supplementary Fig. S5B). There was also evidence of systematic error with syngo.via underestimating the number of FDG-avid lesions as the number of lesions increased (Supplementary Fig. S5).
Discussion
The ROSETTA trial was the first clinical trial published which explored efficacy and safety of an adjunctive statin in the treatment of tuberculosis7, and one of a handful of tuberculosis trials to include PET-CT data as a biomarker11,20,22,24. Previous PET-CT studies in humans and animals have shown that with effective use of anti-mycobacterial agents, avidity can be expected to fall over time. Several animal models have shown that metabolic activity on PET-CT correlates with mycobacterial burden in lung-tissue11,13,25,26,27. However, since FDG is taken up primarily by metabolically active immune cells, PET-CT avidity reflects the immunological response to bacterial burden. When considering the use of HDT agents like rosuvastatin which has the potential to activate clearance of mycobacteria through increased immune cell activity, and tamp down hyperimmune activation that drives tissue destruction28, the expected net effect on the host immunological response, as measured by FDG uptake, is less clear. Consistent with findings of the main study7, this sub-study did not find any impact of adjunctive rosuvastatin on PET-CT metrics versus standard treatment alone. This finding supports the null hypothesis that adjunctive rosuvastatin does not appear to have any additive antimycobacterial and anti-inflammatory effects over standard tuberculosis treatment.
The absence of any effect of rosuvastatin seen in this PET-CT sub-study may be because the adjunctive drug does not have any effect on immune control of Mtb and subsequent bacterial burden. Most of the strong evidence for adjunctive statins in TB is based on mouse models which show that the treatment adjunct reduces bacterial burden2,3,4,29,30, but in the absence of these studies incorporating PET-CT scans, we are unclear whether the impact of adjunctive statins can be detected by PET-CT even in mouse-models. Alternatively, it could be that in this sub-study of 24 participants, a small effect from the adjunctive drug was missed, or that 18F-FDG PET-CT lacks the resolution to detect small changes against a background of highly effective standard TB treatment. Several alternative tracers to 18F-FDG have been shown to have better signal-to-noise ratios compared to FDG-PET, have specificity for specific immune-cell subtypes (macrophages in particular), whilst being able to discriminate between the bactericidal efficacy of different TB regimens21. Epidemiological studies conducted in moderate to high TB burden countries, suggest that statins may protect against progression from infection to active disease5,6. It may be that statins can have an impact at the subclinical stage of infection when bacterial burdens are low and largely intracellular, by successfully enhancing intracellular immune pathways (e.g., phagolysosomal fusion)2,31. In active, high-burden disease, where a large proportions of bacteria are extracellular and where alternative immune pathways are more important for control32, statins may be ineffective. Given that 18F-FDG PET-CT has the capacity to detect subclinical disease and predict progression to active (high-burden) disease33,34, future studies could explore the role of statins in preventing progression to active disease amongst those with subclinical infection.
Overall, PET-CT could detect a response to tuberculosis treatment after the eight-week intensive phase of treatment, if volumetric indices such as TLG and TMV were used, but not by SUVmax and SUVmean. Whilst it may be that the study’s sample size limited discovery of a detectable change in SUVmax, since this metric has shown a response to treatment previously35,36,37,38,39,40,41, large, prospective studies of PET-CT in tuberculosis have similarly shown that volumetric indices, in particular TLG, are better surrogates of treatment response compared to several other metrics11,15,16,24,42.
TLG decreased by 64% after eight weeks of standard treatment, a value that is comparable to previous studies. A recent study showed an ~40% reduction in TLG occurs as early as after 2 weeks of standard treatment20, and anywhere between 15–60% reduction by four weeks occurs in those who go on to have favourable outcomes15,16. In one study, a reduction of less than 5% TLG at one month of treatment carried a 2.24 (95% CI 1.05–4.78, p = 0.07) relative risk of an unfavourable outcome (failure or disease recurrence), and less than 80% reduction in TLG at treatment completion carried a 6.97 (95% CI 2.53–19.22, p < 0.0001) relative risk of an unfavourable outcome15. These data, supported by the current study, suggest that proportional change in TLG can be used as a biomarker to predict treatment response. A proportional rather than absolute change in TLG is necessary given the significant variability in TLG prior to treatment, which, as shown in this study, is a function of tuberculosis disease burden. Measuring proportional change in TLG at eight weeks into treatment allows sufficient time for volumetric and metabolic changes to occur and be early enough to act as a predictive tool. More data are needed to determine the specific size of proportional change in TLG that would be predictive of relapse-free cure.
The finding that those with a poor TLG response also had poorly responsive cavitatory disease, and that cavity number and diameter is related to week eight TLG is interesting given the accepted dictum that the presence of large cavities increases the risk of treatment failure, relapse, drug resistance, and warrants treatment extension43. In a rabbit model of tuberculosis, increased 18F-FDG uptake on PET-CT predicted the development of lung cavitation44. A previous PET-CT study found that the persistence of large cavity volume despite treatment came second only to TLG in predicting an increased risk of unfavourable outcome15. Future studies should investigate whether a high pre-treatment TLG or poor TLG response to treatment is driven by cavitatory disease.
The main limitation of this study is that it was a small study with limited power to detect differences between the rosuvastatin and control groups. Setting an SUV cut-off of ≥ 2, which has been used in other studies27, may have missed areas of inflammation with low-intensity that were representative tuberculosis lesions. The protocol incorporated a permissive window for PET-CT scans to occur, with participants receiving a median of nine days of TB treatment prior to baseline scans. A recently published trial has shown that up to 40% TLG can occur within the first 14 days of standard TB treatment20, thus the prior treatment received in our cohort of patients would have affected baseline values, may have reduced the magnitude of changes seen as well as any between-group differences, especially if effects of rosuvastatin occur early. This sub-study did not measure CT-based markers of lung disease such as radiodensity and lung volume. Changes in PET indices have been shown to occur before structural CT indices in several studies1,13. Nevertheless it is possible that statins could have had a specific impact on lung structure which may have been captured by measuring changes in lung density or volume, which is a limitation of this study.
Our finding that there was no substantive difference between two readers, one of whom is a trained radiologist, suggests that segmentation in a PET-CT study such as this can be precise when protocolised. However, the manual segmentation needed to determine the volumetric indices on PET-CT is time-consuming and labour intensive. It involves creating a CT lung mask where all non-lung parenchymal tissues such as mediastinal structure and large bronchi are removed. This CT lung mask is then overlaid on the PET images and all avid lesions within the lung mask are manual drawn out on each CT slice, which finally allows the software to create a volume of interest (VOI). Each VOI represents the metabolic volume of an individual tuberculous lung lesion, of which there can be > 20 in a patient who has extensive disease. The analysis of each scan could take an operator up to two hours to complete, depending on the disease burden present. The use of the alternative semi-automated software, used in oncology to provide metabolic tumour volumes and total tumour glycolysis, was more time efficient. However, the finding in this study, that the software used underestimated tuberculosis disease burden, highlights the need for the development of disease-specific artificial intelligence algorithms within such software.
Adjunctive rosuvastatin did not have an impact on PET-CT parameters over that of TB treatment alone. The substantial reduction in TLG and TMV from the first eight weeks of tuberculosis treatment seen in this study calls for future studies to determine whether this biomarker is predictive of relapse-free cure. As tuberculosis treatment regimens are improved upon to be made safer, shorter in duration and more efficacious, there will also be increased focus on addressing the impact and sequelae of lung inflammation from tuberculosis, through the use of HDT agents, early identification of disease and early treatment. The benefit of TLG as a metric in PET-CT is that it provides a quantitative marker of inflammation at the site of the disease and should therefore continue to be investigated as a biomarker in future studies that address the mitigation of lung inflammation. Should PET-CT prove to be a helpful surrogate biomarker of tuberculosis treatment outcomes in clinical trials, there remains a need to develop disease-specific software that would allow the tool to be used in a time-efficient manner, without compromising precision.
Methods
Study design
Ethical approval from the institutional review boards of each participating hospital and from the national regulatory agency of each country where this study was conducted was obtained (Table S1). A randomised controlled Phase 2b trial was conducted in the Philippines, Vietnam, and Uganda from 2020–20217. In total, 137 adult participants with rifampicin-susceptible pulmonary tuberculosis were enrolled and received standard treatment for 24 weeks, with or without adjunctive rosuvastatin for eight weeks. Sputum culture conversion was assessed weekly for the first eight weeks and then monthly until end of treatment at week 24. This trial is registered with ClinicalTrials.gov (NCT04504851). Full trial protocol can be accessed from the supplement.
PET-CT scans
Informed consent was obtained from participants from trial sites in Vietnam and Philippines for the PET-CT sub-study. These participants were sequentially screened for eligibility for the PET-CT sub-study, with a target of 38 participants. Participants were eligible if they had had no more than 14 days of tuberculosis treatment prior to the first scan, were able to tolerate lying flat for 15 min, did not have poorly controlled diabetes mellitus (based on site investigator), or have an occupation or a history of medical procedures that exposed them to > 10 mSv of radiation in the previous 12 months. [18 F]-FDG-PET-CT scans were performed at week 0 and at week eight after randomisation into the main trial, within a 14-day window period for the scans.
Scans were performed using a standardised protocol. Briefly, scans were only performed if a blood glucose level was <11 mmol/L. Participants were administered 3.5 mCi (129.5MBq) of [18 F] fluoro-2-deoxy-2-d-glucose an hour prior to the scan and kept warm and at rest prior to the scan. Acquisition parameters for the PET-CT scan were dependent on the specifications of the machine at the site (Siemens Biograph 64 slice in Philippines and Philips Gemini in Viet Nam). The scanning sequence was a CT topogram followed by a low-dose CT scan and then PET acquisition, with the scanner positioned 1 cm above the shoulder to mid liver with the participant positioned with their arms up. Scans were done on a single machine at a central scan facility in each country.
Lung segmentation and manual lesion ROI delineation using OsiriX MD
Scans were analysed centrally at the Clinical Imaging Research Centre (CIRC) in Singapore by GBC. The protocol for segmentation, manual delineation of regions of interest (ROIs) and calculation of SUV, TLG and TMV, adapted from previously published work is provided in the supplement27. Briefly, DICOM files were imported into OsiriX MD (Pixmeo, Switzerland). Slices on the CT images that did not contain any lung tissue were removed. A lung mask was developed using the in-built segmentation tool using thresholds −1023 to −200 HU. Segmentation of the lung mask was checked manually to ensure only the lung fields were highlighted, with errors in the lung mask generation corrected for across all scans performed In each slice, non-lung parenchyma including trachea, major bronchi, oesophagus, and other non-lung tissue were removed to generate a CT-derived lung mask. The CT lung mask was then transposed to the PET images, after which a second visual check was performed, and where needed, manual adjustment of the mask to ensure that all lung parenchymal fields were captured. All images were manually verified to be free from large mis-registration artifacts between PET and CT modalities. Avid lesions inside the lung mask were isolated by an in-built ROI selection tool with the threshold set to SUV of >2.0 and grown to isolate a volume of interest (3-D) based on the 2-D selected region (Fig. 2). Metabolic volume, SUVmean and SUVmax were recorded for each avid lesion. Total lesion glycolysis (TLG) was calculated from the product of metabolic volume in cm3 and SUVmean. TLG is expressed without units. Cavities were isolated visually on CT-pulmonary windows and distinguished from bullae or airways. A visual assessment was made by the reader to determine where the cavity diameter was the largest on CT imaging. The in-built ROI selection tool on OsiriX (threshold set to Hounsfield units of −1000 to −800) was used to identify cavities within the lung-mask. Manual correction was made of the iso-contour of 2-D selection region to ensure cavities were adequately captured. This was then grown to isolate a volume of interest (3-D) based on the 2-D selected region. All parameters were obtained using combined data from both lungs, even if the disease was unilateral.
Lung segmentation and generating VOI using syngo.via MM Oncology
A sub-study of this work aimed to investigate if the choice of image analysis software influenced the study results. For this investigation, we utilised second commercial system (Syngo.Via MM Oncology, Siemens Healthineers, Erlangen, Germany). All scan data was processed and analysed on both software platforms centrally at the Clinical Imaging Research Centre (CIRC) in Singapore by a single reader (GBC). Semi-automated measurements were performed using Syngo.via MM Oncology. Lung mask segmentation was performed using in-built automatic segmentation for lung tissue. Volumes of interest (VOI) within the lung masks were generated using the isocontour tool with the same SUV threshold as in the OsiriX analysis (SUV > 2.0). Using visual assessment, manual adjustment of the VOIs was performed if needed. The software produced the SUVmax, metabolic volume and TLG for each VOI.
Second reader Validation
We further aimed to investigate the effects of potential reader bias in a reproducibility study of 9 randomly selected participants (18 scans) that were analysed by a second independent reader (CWY) using only the software platform (OsiriX MD).
Blinding
Both readers (GBC and CWY) who analysed the scans were blinded to the treatment group allocation. Participants and trial site investigators were not blinded to the treatment group allocation. Treatment group allocation was revealed after scans were analysed and the database was locked, to enable one of the investigators to complete the statistical analysis.
Statistical analysis
The sample size estimate assumed a 50% decrease in TLG in the standard of care arm over 8 weeks. Based on an α of 0.05 level of significance, a sample size of 15 participants per arm would provide a power of 80% to see a 66% difference between control and rosuvastatin groups (i.e., 50% drop vs. 83% drop in TLG at week 8 scan compared to baseline), with a standard deviation of 30%. The primary outcome was the change in TLG between baseline and week 8 scan. Secondary outcomes were the changes in TMV, SUVmax, SUVmean and cavity size on CT scan. Data which was not normally distributed was compared between Week 0 and Week eight using the Wilcoxon matched-pairs sign-rank test. Comparison of these outcomes between treatment groups (rosuvastatin vs. control) utilised the Wilcoxon rank sum test. Where data was normally distributed (percentage of lung affected on chest x-ray), a student’s t-test was used for comparison between groups. Spearman’s r was calculated for correlation coefficients. Bland-Altman plots of difference between readers’ readings versus the average reading; and reading taken by OsiriX MD and syngo.via versus the average reading from the two methods. All statistical tests were done with a 5% two-sided significance level in Stata Statistical Software: Release 18. Plots were graphed using GraphPad Prism version 10.0.0 for Windows.
Inclusion statement
Researchers from the study sites in Uganda, Vietnam and Philippines were integral to the design, data collection, and analysis phases of this study. Their knowledge and expertise ensured culturally relevant approaches were used in this study. Efforts were made to build capacity and ensure equitable recognition of their contributions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided with this paper.
Code availability
Code written for the study will be made available on request to the corresponding author from 6 months to 2 years after publication of this Article. An approved project proposal and a signed data access agreement will be required before the code can be shared.
References
Wallis, R. S. et al. Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial. Lancet Respir. Med. 9, 897–908 (2021).
Parihar, S. P. et al. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 209, 754–763 (2014).
Dutta, N. K. et al. Statin adjunctive therapy shortens the duration of TB treatment in mice. J. Antimicrob. Chemother. 71, 1570–1577 (2016).
Dutta, N. K. et al. Adjunctive host-directed therapy with statins improves tuberculosis-related outcomes in mice. J. Infect. Dis. 221, 1079–1087 (2020).
Lai, C. C. et al. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax 71, 646–651 (2016).
Su, V. Y. et al. Statin use is associated with a lower risk of TB. Chest 152, 598–606 (2017).
Cross, G. B. et al. Rosuvastatin adjunctive therapy for rifampicin-susceptible pulmonary tuberculosis: a phase 2b, randomised, open-label, multicentre trial. Lancet Infect. Dis. 23, 847–855 (2023).
Phillips, P. P. et al. Limited role of culture conversion for decision-making in individual patient care and for advancing novel regimens to confirmatory clinical trials. BMC Med. 14, 19 (2016).
Sharan, R. et al. Isoniazid and rifapentine treatment effectively reduces persistent M. tuberculosis infection in macaque lungs. J. Clin. Invest. 132, e161564 (2022).
Via, L. E. et al. A sterilizing tuberculosis treatment regimen is associated with faster clearance of bacteria in cavitary lesions in marmosets. Antimicrob. Agents Chemother. 59, 4181–4189 (2015).
Coleman, M. T. et al. PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci. Transl. Med. 6, 265ra167 (2014).
Lin, P. L. et al. Radiologic responses in cynomolgus macaques for assessing tuberculosis chemotherapy regimens. Antimicrob. Agents Chemother. 57, 4237–4244 (2013).
Via, L. E. et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [18F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob. Agents Chemother. 56, 4391–4402 (2012).
Davis, J. M. & Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 (2009).
Malherbe, S. T. et al. Quantitative 18F-FDG PET-CT scan characteristics correlate with tuberculosis treatment response. EJNMMI Res. 10, 8 (2020).
Malherbe, S. T. et al. A semi-automatic technique to quantify complex tuberculous lung lesions on (18)F-fluorodeoxyglucose positron emission tomography/computerised tomography images. EJNMMI Res. 8, 55 (2018).
Thompson, E. G. et al. Host blood RNA signatures predict the outcome of tuberculosis treatment. Tuberculosis (Edinb.) 107, 48–58 (2017).
Penn-Nicholson, A. et al. RISK6, a 6-gene transcriptomic signature of TB disease risk, diagnosis and treatment response. Sci. Rep. 10, 8629 (2020).
Davis, S. L. et al. Noninvasive pulmonary [18F]−2-fluoro-deoxy-D-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob. Agents Chemother. 53, 4879–4884 (2009).
Xie, Y. L. et al. Fourteen-day PET/CT imaging to monitor drug combination activity in treated individuals with tuberculosis. Sci. Transl. Med. 13, eabd7618 (2021).
Cross, G. B., J, O. D., Chang, C. C., Kelleher, A. D. & Paton, N. I. Does PET-CT have a role in the evaluation of tuberculosis treatment in phase 2 clinical trials? J. Infect. Dis. 229, 1229–1238 (2024).
Wallis, R. S. et al. Lung and blood early biomarkers for host-directed tuberculosis therapies: secondary outcome measures from a randomized controlled trial. PLoS One 17, e0252097 (2022).
Paton, N. I., et al. Adjunctive pascolizumab in rifampicin-susceptible pulmonary tuberculosis: proof-of-concept, partially-randomised, double-blind, placebo-controlled, dose-escalation trial. J. Infect. Dis. 230, 590–597 (2024).
Chen, R. Y. et al. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci. Transl. Med. 6, 265ra166 (2014).
Medrano, J. M. et al. Characterizing the spectrum of latent mycobacterium tuberculosis in the cynomolgus macaque model: clinical, immunologic, and imaging features of evolution. J. Infect. Dis. 227, 592–601 (2023).
Maiello, P. et al. Rhesus macaques are more susceptible to progressive tuberculosis than cynomolgus macaques: a quantitative comparison. Infect. Immun. 86, e00505–e00517 (2018).
White, A. G., et al. Analysis of 18FDG PET/CT imaging as a tool for studying mycobacterium tuberculosis infection and treatment in non-human primates. J. Vis. Exp. (2017).
Parihar, S. P., Guler, R. & Brombacher, F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat. Rev. Immunol. 19, 104–117 (2019).
Skerry, C. et al. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J. Antimicrob. Chemother. 69, 2453–2457 (2014).
Parihar, S. P. et al. Simvastatin enhances protection against Listeria monocytogenes infection in mice by counteracting Listeria-induced phagosomal escape. PLoS One 8, e75490 (2013).
Lobato, L. S. et al. Statins increase rifampin mycobactericidal effect. Antimicrob. Agents Chemother. 58, 5766–5774 (2014).
Chandra, P., Grigsby, S. J. & Philips, J. A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 20, 750–766 (2022).
Esmail, H., et al. High resolution imaging and five-year tuberculosis contact outcomes. Preprint medRxiv https://doi.org/10.1101/2023.07.03.23292111 (2023).
Yoon, S. H., Goo, J. M., Yim, J. J., Yoshiyama, T. & Flynn, J. L. CT and (18)F-FDG PET abnormalities in contacts with recent tuberculosis infections but negative chest X-ray. Insights Imaging 13, 112 (2022).
Demura, Y. et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur. J. Nucl. Med. Mol. Imaging 36, 632–639 (2009).
Martinez, V. et al. (18)F-FDG PET/CT in tuberculosis: an early non-invasive marker of therapeutic response. Int. J. Tuberc. Lung Dis. 16, 1180–1185 (2012).
Sathekge, M. et al. Tuberculous lymphadenitis: FDG PET and CT findings in responsive and nonresponsive disease. Eur. J. Nucl. Med. Mol. Imaging 39, 1184–1190 (2012).
Dureja, S., Sen, I. B. & Acharya, S. Potential role of F18 FDG PET-CT as an imaging biomarker for the noninvasive evaluation in uncomplicated skeletal tuberculosis: a prospective clinical observational study. Eur. Spine J. 23, 2449–2454 (2014).
Lefebvre, N. et al. Clinical usefulness of (18)F-FDG PET/CT for initial staging and assessment of treatment efficacy in patients with lymph node tuberculosis. Nucl. Med. Biol. 50, 17–24 (2017).
Martin, C. et al. Prospective serial FDG PET/CT during treatment of extrapulmonary tuberculosis in HIV-infected patients: an exploratory study. Clin. Nucl. Med. 43, 635–640 (2018).
Sood, A. et al. 18F-FDG PET/CT in tuberculosis: can interim PET/CT predict the clinical outcome of the patients? Clin. Nucl. Med. 45, 276–282 (2020).
Choi, J. Y., Jhun, B. W., Hyun, S. H., Chung, M. J. & Koh, W. J. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for assessing treatment response of pulmonary multidrug-resistant tuberculosis. J. Clin. Med 7, 559 (2018).
Nahid, P. et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 63, e147–e195 (2016).
Luna, B. et al. In vivo prediction of tuberculosis-associated cavity formation in rabbits. J. Infect. Dis. 211, 481–485 (2015).
Acknowledgements
We thank the participants of the PET-CT study and all the study team members across all trial sites for their contributions. Funding for this study was provided by the National Medical research Council, Singapore (CIRG16nov049, NIP & GBC). This research is part of the Singapore Programme of Research Investigating New Approaches to Treatment of Tuberculosis and was funded by the Singapore Ministry of Health National Medical Research Council (Grant NMRC/CIRG/1322/2012, NIP). GBC received support from the National University Health System Clinician Scientist Programme and is the recipient of the David Cooper Scholarship through the Kirby Institute, University of New South Wales.
Author information
Authors and Affiliations
Contributions
Conceptualisation: G.B.C. and N.I.P. Methodology: G.B.C., C.W.Y., N.I.P., J.O., S.M.B., C.C., A.D.K. Investigation: G.B.C., C.W.Y., I.P.S., H.N., Q.D., V.B.D., E.G., V.M.B., R.J.C. Scan analysis: G.B.C., C.W.Y. Data Visualisation: G.B.C., S.M.B., C.C. Funding acquisition: N.I.P., A.D.K., G.B.C. Project administration: G.B.C., I.P.S. Supervision: N.I.P., A.D.K., C.C. Writing—original draft: G.B.C. Writing—reviewing & editing: All authors. All authors had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: Dr. Jim O’Doherty is employed by Siemens Medical Solutions. However, Siemens Medical Solutions had no role in the conception, design, execution, data analysis, manuscript preparation, or the decision to submit this study for publication. The views expressed in this study are solely those of the authors and do not necessarily reflect those of Siemens Medical Solutions. All other authors declare no financial or non-financial competing interests.
Peer review
Peer review information
Nature Communications thanks Stephanus Malherbe, Clifton Barry, III and the other, anonymous, review(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cross, G.B., Sari, I.P., Burkill, S.M. et al. PET-CT outcomes from a randomised controlled trial of rosuvastatin as an adjunct to standard tuberculosis treatment. Nat Commun 15, 10475 (2024). https://doi.org/10.1038/s41467-024-54419-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54419-3