Abstract
Efficiently mixed conduction between ionic and electronic charges stands to revolutionize the studies in organic electrochemical transistors (OECTs). However, inefficient ion transport due to the long-range injection and migration process in the bulk film presents challenges for enhancing the steady and transient performance of OECTs. In this work, we proposed a lateral intercalation-assisted ion transport strategy to assist volumetric ion charging, by introducing a striped microstructure in the conductive channel. By precisely adjusting the ratio of lateral area (RoL), the electrical performance, indicated by the maximum transconductance versus response time (Gm,max/τ), increases progressively by over 600%. We further unveiled the mechanism for the enhanced doping uniformity and increased volume capacitance at the lateral area. Based on the universality investigation, we uncovered the effects of molecular stacking on ionic lateral intercalation transport, contributing to the high-performance OECTs and the bio-applications in the recording of dynamic electrocardiography (ECG) signals with distinct features.
Similar content being viewed by others
Introduction
Mixed ionic-electronic conduction in organic semiconductors (OSCs) holds a great potential in technologies merging electronics with bio-interface1,2, enabling its spanning applications from signals translating3, stimulus delivery4, and artificial intelligence5,6. Organic electrochemical transistors (OECTs), which function by modulating channel conductivity of OSCs through gate-bias driven ion migration into the entire volume of the semiconductor film, have emerged as highly effective device platforms7,8,9, featuring high transconductance (up to mS), low operation voltage (<1 V) and biocompatibility. Over the past few years, OECTs have received increasing attention from the perspectives of molecular design10, device geometry evolution11, and logic circuitry integrations12, making OECT efficient switches and powerful amplifiers. However, the primary challenge with OECTs is the trade-off between gain and bandwidth, which arises from the mismatch in ionic and electronic carrier transport. The sluggish ion transport in OECTs leads to a relatively low switching speed and compromised amplification, sacrificing the signal fidelity at ionic-electronic signal transduction interfaces.
To unleash the full potential of ion transport, considerable efforts to manipulate ion penetration and to create ion transport pathways in the bulk channel have been reported. For example, Bischak et al. utilized a blended strategy between regiorandom and regioregular P3HT to reveal that balancing the distribution of amorphous and crystalline regions can enhance ion injection kinetics13. Ginger et al. then reported an OECT incorporating an ion exchange gel between the active layer and the aqueous electrolyte enabling a faster transient response of 3.1 s for P3HT14. Although manipulating the ion transport barrier in the vertical direction bridges the gap between ionic and electronic transport, they may fall short in providing the necessary consideration for the bulky migration after ion injection, which significantly impacts the operation speed of OECTs. To expand efficient ion injection and migration process, there is an urgent need to exploit microstructures with tunable lateral ionic transport pathways. Microstructure semiconductor films such as porous and wrinkles naturally exhibit lateral ion transport pathways, which may reduce disparities in ionic transport between vertical and lateral directions. However, effectively incorporating lateral pathways into OECTs remains a challenge, primarily due to the absence of precisely adjusting the lateral diffusion distance to leverage with the vertical pathways, from mechanisms to operations.
Here, we demonstrated a controllable lateral intercalation-assisted ion injection for transient behaviors and transconductance of OECTs, by adopting striped polymer thin films. Using a hydrophobic poly(3-hexylthiophene) (P3HT) as a model compound, the electrical performance of transistors with 2~100 μm-width strip structure has been investigated via photolithography (Supplementary Fig. 1). To render the ion dynamics of the bulk channel, we proposed a ratio of lateral (RoL) dependent electrical performance of P3HT based OECT. Using a combination of in-situ electrochemical and electrical characterization, along with ion distribution and molecular stacking analysis, we indicated a lateral intercalation-assisted chemical redox and lower ion penetration barrier for uniform ion diffusion in the bulk film. Incorporating the universality investigation of the lateral intercalation-assisted ion dynamics in varies polymeric semiconductor, we also demonstrated the effect of the molecular orientation of the conjugated backbone on the ionic mobility and transient behavior governed by RoL-dependent ion injection. With the well-designed RoL, the striped P3HT OECT exhibited a high Gm,max/τ of 16.2 mS s−1, enabling stable signal amplification for electrocardiogram (ECG) monitoring under a wide range of tensional strains. Our results elaborate on an effective pattern strategy to increase the RoL for balanced ionic-electronic transport through the utilization of lateral intercalation-assisted ionic transport, opening possibilities for high-performance organic mixed ionic-electronic devices.
Results and Discussion
OECTs have gained significant attention for a variety of bioelectronic applications, as they possess bulk ionic and carrier transport circuits. Compared to field-effect modulation mode, where the electric double layer (EDL) can form at the gate/electrolyte interface restricts ion permeability, bulk electrochemical doping allows ions to permeate through the EDL into the channel, facilitating the volumetric charge transport modulation15. For traditional OSC materials, the hydrophobic nature makes it extremely difficult for the channel to undergo an efficient and reversible electrochemical oxidation or reduction process, resulting in a relatively slow transient response (Fig. 1a-I). The problem arises from the fact that the disparity of transport kinetics between ions and charge carriers, which is further amplified by the isolated long-range ion diffusion. Inspired by electrochemically intercalating ions into layered materials relying on the natural lateral pathways, we recognized that similar modulation of ion injection directionality can be achieved by creating controllable microstructures to shorten the distance between two adjacent laterals for efficient lateral ion exchange, namely lateral intercalation-assisted (LI-assisted) ionic transport (Fig. 1a-II). Accordingly, the ions from the electrolyte can be injected into the channel from both vertical and lateral directions along the shortened lateral diffusion length to achieve high-performance OECTs with fast switching and uniform ion distribution.
a Schematic of device configuration and molecular structure of P3HT. I Conventional Ionic Transport: Ions penetrate into the channel manly dominated by vertical pathway. II LI-assisted Ionic Transport: Ion transports through a synergistic effect of the lateral and vertical pathways. b AFM images and (c) corresponding transfer curves (black line) and transconductance (red line) of the patterned P3HT films with stripe width of 100, 50, 30, 10 and 2 μm, respectively. The grey dash line in panel c represents corresponding gate current (IGS). Electrical performance in d ION/IOFF, e VTh, and f Hysteresis width of OECTs based on P3HT stripe films. Error bars represent standard deviations from the mean (n = 5).
Following this concept, we fabricated OECTs decorated with striped films as shown in Fig. 1a, using the P3HT polymer as channel material. While applying a gate bias (VGS), the anion in electrolyte (1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, EMIM+ TFSI−) is driven to transport vertically into the active layer and induce with hole carrier. Compared with conventional ion transport, the LI-assisted ionic transport based on lateral channels delivered by the striped films, which not only allows ions to penetrate vertically but also laterally. Figure 1b exhibits the AFM images of P3HT thin films with different stripe structures obtained after photolithography, with stripe widths precisely controlled to 2 μm. The device geometry and the stripped structure in the channel were shown in Supplementary Fig. 2. Notably, for the 100 µm-patterned film, the stripe width equals the device width, resulting in ion penetration from both edges; while other patterned are within the device channel. In this research, the stripe spacing is fixed at 2 μm and the thickness of the films is set at approximately 1 μm for P3HT. A thick OSC layer is adopted first to reveal the volume-dominated trade-off between transconductance and response time. This is achieved by modulating ionic transport directions to emphasize the effect of lateral injection16.
We first investigate the effect of the striped structure on the electrical properties of P3HT-based OECTs. The transfer and output curves show standard p-type device performance working in an accumulation mode for stripe widths ranging from 100 μm to 2 μm (Fig. 1c and Supplementary Fig. 3). Note that the saturated drain current (IDS) of OECTs based on P3HT film increases by approximately 50% when the stripe width is reduced from 100 μm to 2 μm. At the same time, the transconductance (Gm) provided along with the transfer curve is a vital parameter to evaluate the gain of the OECTs, which is formulated below:
where W, L, and d represent the channel width, channel length, and thickness of the OSC layer, μ is charge carrier mobility, C* is volumetric capacitance, and VGS and VTh represent the gate voltage and threshold voltage, respectively. In our study, a maximum transconductance (Gm,max) increase from 7.4 (100 μm-patterned P3HT film) to 11.8 mS (2 μm-patterned P3HT film) was achieved for OECT with the same channel thickness and device dimensions. Interestingly, the increasing device performance in OECTs based on 2 μm-patterned P3HT film is accompanied by a half reduction in film volume, which is detrimental for OECTs performance due to its volume doping mechanism. Therefore, we speculate that the improved performance might be attributed to elevated doping homogeneity caused by lateral ion intercalation. The intermediate fluctuations of saturated IDS in the stripe widths ranging from 50 μm to 10 μm could also stem from a competitive balance between the cons of volume reduction and the pros of lateral ion intercalation. Moreover, the ION/IOFF ratio is summarized and depicted in Fig. 1d, and it rises by approximately 40-fold from 3.3 × 104 to 1.3 × 106 with the decrease of stripe widths. The threshold voltage of the OECTs patterned with different stripe widths ranges from −0.8 to −0.7 V (Fig. 1e), which means that OECTs can be modulated towards a lower operation voltage via lateral ion-assisted intercalation. Besides, another significant change that cannot be ignored is the voltage hysteresis extracted from the difference of VTh between the forward and reverse sweeping curves. The hysteresis width significantly decreases from 0.65 V to 0.21 V, indicating more balanced ion movement dynamics into and out of the channel, primarily due to the faster transient response time of OECTs with striped channel structure17.
In order to reveal the role of the lateral intercalation-assisted ion transport on the transient OECT performance, we characterized the switching behavior with various stripe widths. As shown in Fig. 2a, the transient response of striped OECTs was evaluated by fitting the IDS current change using an exponential function. The results show a significant difference in the transient response of IDS under the same square voltage step (VGS = –1.2 V). At the initial stage of applying VGS, the IDS of OECTs based on 2 μm-patterned P3HT increased immediately compared to devices with 100 μm-patterned film. For a quantitative comparison of this phenomenon, we extrapolated the fitted IDS response curve and obtained the intersection with the x-axis as the diffusion time (tDiff) from the source to drain electrode as depicted in Fig. 2a. The tDiff for OECTs based on 100 and 2 μm-patterned films were estimated to be 4.7 and 0.2 s, respectively, indicating that the lateral area for ion transport is effective in aiding horizontal doping front propagation18. A similar trend is shown in OECTs based on P3HT films patterned with various stripe widths (Supplementary Fig. 4), where the diffusion time decreases as the stripe widths narrow from 50 to 10 μm. When the doping front reaches the opposite drain electrode, the channel becomes conductive, followed by a rapid increase in IDS current, which can be explicitly described by the response time (τ) from the previous fitted results. The τ for OECTs patterned with 100 and 2 μm striped P3HT films was estimated to be 21.3 and 1.9 s, respectively, demonstrating that accelerated ion kinetics due to the introduction of lateral channels play a vital role even in the doping enhancement stage, incorporating both lateral and vertical mixed ion transport. Similarly, the off time (τoff) as summarized in Supplementary Fig. 5 also follows the same trend, decreasing with the introduction of lateral ion transport channels, further highlighting the impact of lateral intercalation on enhancing ion kinetics. It is worth noting that GIWAXS measurements reveal the TFSI− anions intercalate laterally without disturbing the orientation of P3HT as shown in Supplementary Figs. 13, 14. This intercalation results in a decreased distance of π–π stacking and an increased lamellar packing distance, suggesting that the dopant primarily resides among the side-chains19.
a Transient response measurement of drain-current response at a constant VDS of –0.3 V and a gate voltage pulse for OECT devices with a 100 (black circle) and 2 μm (red triangle) stripe-patterned structure. The pulse VGS is –1.2 V and the inset graphs highlights the initial diffusion times, tdiff. The dots and solid line represent corresponding experimental data and fitting line, respectively. b the ratio of maximum transconductance and transient response time as a function of the RoL. Error bars represent standard deviations from the mean (n = 5). c Comparison of normalized transient response time for the OECTs with LI-assisted ionic migration (P3HT films patterned with diverse RoL) as well as the previously reported OECTs based on P3HT films20,38,39,40,41. Shading in this panel labels the results obtained in this work. d The relationship between amplitude and the RoL at different sin wave stimulation (VDS = –0.3 V). Square, triangle, and circle symbols represent the data points for 100 Hz, 10 Hz, and 1 Hz respectively. The data are fitted with an exponential decay function for each frequency. Dashed lines indicate the 90% threshold of the maximum amplitude, above which signals are considered to be distinguished. The channel dimension of W/L = 100 μm/10 μm and thickness d = 1 μm.
It is noteworthy that a combination of fast operation response and high transconductance in OECTs is of importance in diverse applications encompassing neural link for electrophysiological signal monitoring and biochemical sensors and highly related with the ion kinetic behaviors20,21. To evaluate the impact of the lateral area on ion injection, we define the ratio of lateral (RoL) by the equation below:
where AL and AT represent the area at the lateral and top regions respectively, as schematically indicated by pink and blue colors in the inset of Fig. 2b. We characterized the trade-off relationship based on the ratio of transconductance and response time (Gm,max/τ) with increasing RoL. The values of Gm,max/τ gradually increase from 2.2 to 16.2 mS s−1, indicating a growth of more than 600%, accompanying the RoL reaching to 50%, with a film thickness of 1 µm. The simultaneous improvement of Gm,max and τ might be attributed to the enlarged lateral area, allowing a large number of ions to intercalate the P3HT lattice laterally, complementary to vertical penetration. This not only enhances the homogeneity of doping but also accelerates the doping process via lateral channels. Additionally, the RoL variation is closely related to the film thickness (d). As shown in Supplementary Figs. 6–9, there is a general trend of increasing Gm,max/τ with rising RoL across different film thicknesses. Tailoring the channel geometry, specifically by optimizing the RoL based on film thickness, can significantly improve the overall performance of OECT devices by balancing transconductance and response time through enhanced lateral ion transport. Moreover, LI-assisted ionic transport is broadly applicable in various ion sizes and demonstrates efficacy in both accumulation-mode and depletion-mode OECTs (Supplementary Figs. 10, 11), ensuring optimized performance across diverse material systems.
To confirm a fair comparison of τ, the response time is normalized by the device architecture based on the relationship \(\tau \propto d\sqrt{{WL}}\), as summarized in Fig. 2c. The results show that the required time for doping per unit channel area exponentially decreases as the RoL increases, meaning that the doping process becomes more efficient with the assist of latera intercalation ion transport. The normalized rise time for OECTs patterned with 2 μm striped film is 30.3 s μm−2, which is three times lower than that of previous results based on P3HT (125.4 s μm−2).
The substantial improvement in transient response observed in P3HT-based OECTs with striped patterns underscores how lateral ion intercalation enhances the device’s ability to respond to higher-frequency signals. To evaluate the effect of response time changes caused by lateral ion intercalation on signal acquisition, we characterize the IDS amplitude under VGS driven by a sine wave at different frequencies (Supplementary Fig. 15). As summarized in Fig. 2d, the amplitude rises with the increasing RoL, consistent with the observed transient response changes based on RoL variations. When the RoL approaching 50%, devices are able to perceive the sin wave of 100 Hz with an amplitude of 11.9 μA, suitable for recording electrophysiological signals such as ECG and EEG at low frequency (<100 Hz)22. Furthermore, the dashed lines indicate the 90% threshold of the maximum amplitude, implying the voltage input from a sine wave is challenging to be effectively converted to a recognizable current signal without the introduction of additional lateral channels (RoL < 3%). Our results demonstrate that the increasing RoL accelerates ion transport speed due to lateral ion intercalation, leading to faster transient response for promising applications in signal acquisition with high signal-to-noise (SNR)23.
Primarily speaking, the electrical performance in an OECT is manipulated via the speed of ion injection at OSC/electrolyte interface, ion movement, and ion-electron coupling transport in the semiconductor aggregates24,25,26. To elaborate on the mechanism of lateral intercalation-assisted ionic transport on device performance, we propose the corresponding equivalent circuits to connect ion kinetics at the electrolyte-semiconductor interface and electronic circuit components in Fig. 3a. Here, we utilize a classic resistor-capacitor circuit to describe the movement of ions from the electrolyte to the bulk semiconductor film, where the resistor and capacitor connect in parallel representing ion flow and storage respectively9. In the circuit without lateral area, ions penetrate the film solely through the vertical pathway, with the vertical symbol-marked resistor contributing the whole channel resistor (Rch). In contrast, the model with lateral channels comprises two resistors in parallel, indicating different ion transport directions, vertical and lateral, co-exist with different flow resistance caused by the molecular stacking mode including the conjugated backbone and film crystallinity27,28,29. The introduction of lateral channels significantly impacts ion transport by providing alternative pathways that reduce the overall resistance. Without lateral channels, vertical ion transport dominates, facing higher resistance due to the presence of highly crystalline regions that act as barriers to ion movement. When lateral channels are introduced, the total resistance is substantially reduced, as lateral channels enable ions to bypass the high-resistance crystalline domains. Here, we conduct EIS measurements to validate the circuit model and analyze differences in ion transport directions and fit the results using a modified Randles circuit as illustrated in Fig. 3a and Supplementary Fig. 16, where RE represents the electrolyte resistor, the channel capacitor (Cch) is replaced using constant phase element (CPE), and Rch is the channel resistor. We observed a significant decrease in Rch from 1330 (W/O channels) to 408 Ω (W/ channels, 2 μm striped patterns) with similar RE, indicating a reduced flow resistance facilitated by lateral ion intercalation (Fig. 3b and Table S1).
a Schematic illustration of ion injection and interaction with molecular stacking structures, and the corresponding equivalent circuit model for striped OSC films. The CCh, RE and RCh represents channel capacitance, electrolyte resistance and channel resistance, respectively. b Comparison of total resistance including RE and RCh. c ToF‐SIMS depth profiles showing the concentration of TFSI− anion and normalized Au− across the doped P3HT films. d In-situ monitoring of electrochemical UV-Vis absorption under a 1.2 V bias for P3HT films without and with stripe patterns. The absorptions are normalized in arbitrary units (arb. unites). e τ value of OECT devices fabricated with striped PBTTT, P3HT and p(g2T-TT) films with the stripe width of 100 μm (grey column) and 2 μm (red column). All the semiconductor layers were controlled with the film thickness of 400 nm. Error bars represent standard deviations from the mean (n = 5). f Comparison of τ100/τ2 (red point), Lc (blue column) and g factor (green column) obtained from PBTTT, P3HT and p(g2T-TT) films and corresponding OECT devices.
Following the above process, LI-assisted transport refers to ions intercalating laterally into the channel, distributing more evenly across the entire volume, which reduces localized high-resistance areas and provides a more uniform ion distribution, thereby lowering overall resistance. It is corroborated by the diffusion coefficient calculated from the parameter of Warburg components. The diffusion coefficients are 9.9 × 10−11 and 2.7 × 10−10 m2 s−1 for films without and with lateral channels (2 μm striped patterns) respectively, consistent with previous results of device response time, indicating that the introduction of lateral channels significantly enhances ion mobility due to increased surface area and reduced diffusion path length. With ultralow channel resistance, an optimal trade-off between fast responses and capacitance can be achieved according to the time constant equation in RC circuits (\(\tau={RC}\))30. The result shows that the volume capacitance, highly related to device transconductance, elevates as the time constant shortens in correspondence with previously measured transient device response. We speculate that lateral ion intercalation not only renders bulk doping more uniform, leading to an increase in capacitance, but also achieves faster oxidation speed.
To validate the capacitance difference as a result of the spatial distribution of ions, we employ the TOF-SIMS depth profiling on the P3HT films without and with 2 μm stripe patterns under the same doping process. The TOF-SIMS detects signals for TFSI− and Au− while sputtering through the film using an ion beam. Initially, the TFSI− doped P3HT film without striped patterns is characterized and TFSI− and Au− signals are observed in the TOF-SIMS depth profile (Fig. 3c). At approximately 1 μm into the film, a sharp rise of the Au− signal becomes prominent indicating the ion beam has reached the underlying Au layer. However, the TFSI− signal gradually declines at about 546 nm depth, indicating that anions only dope into the top layer of the whole P3HT film. As a comparison, the 2 μm-patterned P3HT film achieves a homogeneous volume doping as the TFSI− signal exhibits a similar downward trend occurring at nearly 1 μm, accompanied by an increase in Au− intensity. Taking the anion content into account to get a detailed comparison, the films without and with 2 μm stripe patterns show an average TFSI− ratio of 7.5% and 17.4% respectively. The higher TFSI− ratio in the 2 μm-patterned P3HT film is consistent with its higher transconductance, indicating that the lateral pathway allows ions to penetrate deeper into the striped film, achieving a higher degree of doping.
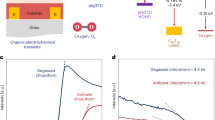
To evaluate the kinetics of electrochemically driven ion oxidation and doping, we use electrochemical UV-Vis spectroscopy to measure transitions in absorption via applying a voltage step. A series of absorption spectra monitoring under a VGS step of −1.2 V (versus Ag/AgCl) to the P3HT films without and with 2 μm stripe patterns is depicted in Fig. 3d. These spectra show a substantial decrease in the main absorption peak at 515 nm attributed to the ground-state bleaching and an increase in the absorption located at roughly 800 nm due to the formation of polarons31. We extract the decreasing absorption at 515 nm as a function of time and fit with exponential decay (Supplementary Fig. 17). The time constant decreases from 21 to 1.8 s upon introducing lateral area for ion intercalation, representing a ~ 11× increase in oxidized rate. Based on a combination result of TOF-SIMS and electrochemical absorption spectrum, we consider that ions penetrating laterally contribute to homogeneous doping and elevated redox speed, providing an excellent trader-off between transconductance and response time.
The proposed mechanism for LI-assisted ionic transport provides a foundation for exploring the universality of the striped pattern strategy in enhancing OECT device performance. However, the molecular orientation, particularly π–π stacking and lamellar stacking, plays a crucial role in determining the transport resistance. In edge-on π–π stacking, the densely packed layers hinder ion mobility along the lateral direction, while face-on stacking allows for more efficient ion transport due to the increased spacing between layers (Fig. 3a). The packing-induced film morphology paves the way for optimizing ion movement dynamic process in OECTs32. To evaluate this influence, we delicately selected three organic semiconductors including poly[2,5-bis(3-tetradecylthiophen-2-yl) thieno[3,2-b]thiophene] (PBTTT), P3HT and poly(2-(3,3’-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-[2,2’-bithiophen]−5-yl)thieno[3,2-b]thiophene) (p(g2T-TT), to characterize the transient response of corresponding OECT devices. Based on GIWAXS analysis (Supplementary Fig. 18), PBTTT films demonstrate a tightly packed π–π crystalline stacking with an edge-on orientation, while P3HT displays a slightly disordered face-on crystalline aggregation29,33. Meanwhile, p(g2T-TT) shows low crystallinity, predominantly exhibiting an edge-on texture34,35. Interestingly, the LI-assisted dynamic performance changes shown in τ vary were obtained from OECTs fabricated on different striped semiconductors (Fig. 3e, Supplementary Figs. 19, 21). We found that devices based on PBTTT was less affected by an increased lateral area for ion intercalation, exhibiting a τ changing from 74.6 s (100 μm striped patterns) to 9.6 s (2 μm striped patterns), compared to the devices based on P3HT showing a decreased response time from 4.6 s to 0.23 s. Notably, p(g2T-TT) demonstrated a significant alteration in τ due to lateral ion intercalation, decreasing from 3.5 s to 0.028 s as the stripe width was reduced from 100 μm to 2 μm.
For quantitative comparison, we defined τ100/τ2, calculated as the ratio of the τ value of OECTs based on 100 μm-patterned films to that based on 2 μm-patterned films. As shown in Fig. 3f, the values of τ100/τ2 are 7.8, 19.8, and 124.9 for PBTTT, P3HT, and p(g2T-TT) respectively, indicating that p(g2T-TT) enables more feasible ion intercalation via the lateral area and achieve a longer diffusion at the same time scale compared with PBTTT. Surprisingly, the variation in τ100/τ2 reveals a pronounced correlation with molecular packing parameters, evidenced by an increase in the τ100/τ2 as the corresponding material shows gradually decreased crystal coherence length (Lc) and increased para-crystalline disorder (g factor) (Fig. 3f and Table S2). These results indicate that the film with high crystallinity and edge-on packing mode (PBTTT) would hinder the ion transport in a lateral pathway, whereas a face-on (P3HT) or disordered film (p(g2T-TT)) is prone to leverage the effects of lateral intercalation36,37. To deliver a more thorough analysis of molecular stacking effects on lateral ion movement, we conducted moving front experiments to enhance our investigation. As shown in Supplementary Fig. 22, p(g2T-TT) exhibits the highest ion mobility (157.2 μm2 V−1 s−1) due to its glycol side chains and low crystallinity, while P3HT and PBTTT demonstrates ion mobility at 8.9 and 4.8 μm2 V−1 s−1, respectively. When introducing additional lateral area in the bulk channel, the increased ion mobility results in pronounced changes along the lateral pathways, yielding faster ion injection into the bulk layer and faster τ. In this case, the densely packed edge-on backbone of PBTTT hinders direct ion injection and transport at the lateral OSC/electrolyte interface, and ions primarily undergo segmental transport through the amorphous regions formed by the alkyl chains or the polymer backbone, resulting in a smaller τ100/τ2 enhancement, compared to the face-on stacking in a disordered or amorphous aggregate (as shown in Fig. 3f)24,28,37. For polymers with hydrophilic side chains, which predominantly exhibit more amorphous stacking and provide intrinsic ion transport, the addition of more ion-injecting compounds at the lateral area significantly enhances the transient response. Herein, further optimization of the molecular stacking mode will contribute to the fine-tuning of the LI-assisted ionic transport and electrical performance of OECT.
We subsequently investigate the impact of the variations in operation speed and transconductance on the integrity of electrophysiological signals like ECG recorded by OECTs. We fabricated the device based on P3HT films with the 2 μm and 100 μm striped patterns for ECG record considering the potential signal distortion caused by different ion shuttle rates. The simulated ECG signals (77 bpm) were input to the OECT device through an Ag/AgCl gate electrode, driving ion penetration to modulate the conductivity of semiconductor film, which was reflected in IDS changes as shown in Fig. 4b. For a fair comparison, the device was biased using a VGS at its peak transconductance and a VDS of −0.3 V before the ECG monitoring. It is obvious that the OECTs based on the 100 μm-patterned P3HT films exhibit attenuated ECG signals (Ipeak-to-peak = 1.9 μA) compared to the device with the 100 μm-patterned P3HT films (Ipeak-to-peak = 3.1 μA), as summarized in Fig. 4c. Owing to a combination of broader bandwidth and higher gain value achieved by latera ion intercalation, the SNR of the recorded ECG is positively associated with the Gm,max/τ value of OECTs, with SNR rising from 14.5 to 19.6 as the stripe width decreases from 100 to 2 μm. Finally, to match actual scenarios of ECG signal monitoring with skin strain stretching less than 20%, we analyzed the device stability and the ratio of T wave to R wave amplitude (T/R ratio) of ECG signals under a strain as depicted in Fig. 4d. The P3HT films with 2 μm striped patterns exhibited steady normalized transconductance under a maximum strain of 50%, attributed to stress dissipation rendered by the spacing between stripes, implying that the striped strategy also enables devices with robust tolerance to deformation. In this scenario, devices based on striped films show comparable signal quality under different strains with the T/R ratio distributed in a narrow range. As a result, OECTs employing ion intercalation are well-suited to acquire high-quality and stable ECG signals, while also exhibiting great potential for future applications in enhancing the accuracy of ECG pattern recognition for pathological diagnosis.
a Schematic illustration of ECG recording set-up. b The simulated ECG acquired from the OECT operated at VDS = − 0.3 V and VGS = − 1 V with the Ag/AgCl gate electrode. c, Compare the influence of LI-assisted ionic migration (with lateral channels or not) on the quality of acquired ECG signal (peak-to-peak current and SNR). d, The maximum transconductance ratio Gm,max/Gm,max,0 (under strain to initial) and T/R ratio of ECG signals under different strains. Error bars represent standard deviations from the mean (n = 5).
In summary, the lateral ion intercalation effect was successfully employed in this study to accelerate long-range horizontal diffusion and enhance the device performance of OECTs enabled by stripe microstructures. By adjusting the RoL in the bulk conductive channel, we were able to create a fine-tuned ion transport physical process along the semiconductor aggregates, establishing a pathway for homogeneous doping and oxidized rate for faster ion dynamics. This strategy significantly enhances the Gm,max/τ of organic semiconductors in OECTs, achieving an improvement of over 600%, with a maximum value of 16.2 mS s−1. Notably, this phenomenon is independent of the electrolyte, material types or device geometry. Additionally, the observation of faster transient behavior and a noticeably reduced horizontal diffusion time, aided by the assistance of lateral intercalation, suggests that molecular stacking-enabled ion injection and transport pathways would predominantly control the lateral ion intercalation effect. This strategy provides abundant opportunities to control lateral ion transport and attain high electrochemical performance in organic semiconductors, playing a critical role in advancing OECT technologies. Moreover, the broad bandwidth enabled by the lateral intercalation strategy provides an avenue to achieve high-quality and stable signals in ECG monitoring, greatly broadening the potential for future applications in signal recognition and pathological diagnosis.
Methods
Materials
1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIM-TFSI), sodium chloride (NaCl), poly(3-hexylthiophene-2,5-diyl) (P3HT, average Mw 50,000-100,000), poly[2,5-bis(3-tetradecylthiophen-2-yl)thieno[3,2-b]thiophene] (PBTTT, Mw >50,000) were purchased from Sigma Aldrich and were used without further purification. PEDOT:PSS aqueous solution (Clevios PH1000) was purchased from Heraeus and stored at 4 °C before use.
OECTs Fabrications
Glass substrates were cleaned by sonication in deionized water, ethanol and acetone for 10 min respectively and blow-dried. Source/drain electrodes (Cr/Au: 5 nm/50 nm) were obtained by thermally evaporated onto the precleaned substrates. P3HT films with varying thicknesses were spin-coated from chloroform solutions with controlled concentrations and speeds. Films with 1000 nm, 800 nm, 400 nm, 200 nm, 100 nm, and 50 nm were obtained by spin-coating at 3000 r.p.m. from a 60 mg mL–1 solution, 4500 r.p.m. from a 60 mg mL–1 solution, 2000 r.p.m. from a 30 mg mL–1 solution, 6500 r.p.m. from a 30 mg mL–1 solution, 2000 r.p.m. from a 15 mg mL–1 solution, and 3000 r.p.m. from a 6 mg mL–1 solution, respectively, followed by annealing at 180 °C for 30 minutes. PBTTT films (400 nm) were spin-coated at 2000 r.p.m. from a 50 mg mL–1 1,2-dichlorobenzene solution and annealed at 180 °C for 30 minutes. p(g2T-TT) films (400 nm) were spin-coated at 1500 r.p.m. from a 20 mg mL–1 chloroform solution and annealed at 65 °C for 30 minutes. PEDOT:PSS films (65 nm) were prepared by spin-coating an aqueous dispersion containing 5 vol% ethylene glycol at 3,000 r.p.m., followed by annealing at 120 °C for 30 minutes. Following the deposition of organic semiconductors (OSCs), Cytop films were fabricated by spin-coating the Cytop solution (CTX-809SP2 and CT-SOLV180 mixed at a mass ratio of 1:3) onto glass substrates coated with a P3HT semiconductor layer at a spin speed of 3000 r.p.m. for 60 s and annealed at 180 °C for 15 s. After plasma treatment under 50 W for 30 s, a third layer of photoresist (AZ 1500) was spin-coated at 1000 r.p.m. for 60 s and exposed to ultraviolet light using a maskless aligner (μMLA, Heidelberg Instruments). The photoresist striped patterns were generated with an AZ 400 K developer, followed by plasma at 120 W for 8 min and a lift-off process using 3 M™ polyimide film tape to create striped semiconductor films. The device was completed by applying the ionic liquid electrolyte (EMIM-TFSI) and Ag/AgCl electrodes.
OECTs measurements
OECTs were characterized on a probe station using Keysight B2902A. Stretchable OECT device characterization was performed under both strained and relaxed states. For measuring the OECT device under strain, devices were maintained under a strain by using a small tensile stage, and 10 μL EMIM-TFSI electrolyte was directly dropped onto the channel area. A home-made Ag/AgCl electrode was connected to a probe station and immersed in the electrolyte as the gate electrode. Another two probes were contacted with the Au source–drain electrodes, respectively.
Electrochemical Impedance Spectroscopy (EIS)
EIS were carried out to evaluate the electrolyte resistance (RE) and channel resistance (Rch) when operated in EMIM-TFSI electrolyte, using CHI 660E (CH Instruments). The measurement setup was based on a three-electrode system, using a home-made Ag/AgCl wire as the reference electrode and a Pt wire as the counter electrode. The P3HT film was fabricated on the Au-coated glass under the same conditions as used for OECT fabrication. EIS was performed over a frequency range from 105 to 10 Hz, with a 10 mV sinusoidal signal at an offset bias of 1 V.
TOF-SIMS characterization
TOF-SIMS-M6 (ION-TOF, Germany) analysis with Bi3+ (30 keV) as the primary ion source was used to determine the doping profile, where negative secondary ions from an area of 20 × 20 μm2 were detected. Sputter etching was performed over an area of 300 × 300 μm2 using an Ar gas cluster ion beam with an acceleration voltage of 5 keV. The currents of the primary ion beam and Ar gas cluster ion beam was 1.0 pA and 1.0 nA, respectively, for P3HT films.
Optical spectroscopy
Optical Spectroscopy UV-Vis spectroscopy and spectro-electrochemistry were carried out using an Agilent Cary 60 UV-vis spectrometer. Thin films used for UV-Vis characterization were prepared with a controlled film thickness of 400 nm, by spin-coating on an ITO-coated glass.
Grazing-Incidence Wide-Angle X-ray Scattering (GIWAXS)
GIWAXS measurements were conducted on the Anton Paar SAXS point 5.0 system with an X-ray wavelength of 1.5418 Å and 0.2° as an incidence angle. The diffraction intensity was detected with a 2D HPC Detector (PILATUS3 R 1 M), while the sample-to-detector distance was adjusted to be 80 mm. All X-ray measurements were performed in a vacuum environment to minimize air scattering and beam damage to samples. Coherence length (Lc), paracrystalline disorder (g factor) and relative degree of crystallinity (rDoC) were calculated based on the (010) or (100) peak following the equations:
where q0 is the peak position of the diffraction peak, ∆q is the FWMH of the diffraction peak, χ represents the polar angle in the range of 0~π/2 obtained from GIXRD, and I(χ) is the peak intensity.
Data availability
The data generated in this study are provided in the Supplementary Information and Source Data file. Source Data are provided with this paper. Other related raw data are available from the corresponding authors on request. Source data are provided with this paper.
References
Saleh, A. et al. Bioelectronic interfaces of organic electrochemical transistors. Nat. Rev. Bioeng. 2, 559–574 (2024).
Gkoupidenis, P. et al. Organic mixed conductors for bioinspired electronics. Nat. Rev. Mater. 9, 134–149 (2024).
Wang, S. et al. An organic electrochemical transistor for multi-modal sensing, memory and processing. Nat. Electron. 6, 281–291 (2023).
Liu, S. et al. Conjugated polymer for voltage-controlled release of molecules. Adv. Mater. 29, 1701733 (2017).
Sarkar, T. et al. An organic artificial spiking neuron for in situ neuromorphic sensing and biointerfacing. Nat. Electron. 5, 774–783 (2022).
Zhipeng, X. et al. A hybrid ambipolar synaptic transistor emulating multiplexed neurotransmission for motivation control and experience-dependent learning. Chin. Chem. Lett. 34, 107292 (2023).
Bernards, D. A. & Malliaras, G. G. Steady-state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 17, 3538–3544 (2007).
Keene, S. T. et al. Hole-limited electrochemical doping in conjugated polymers. Nat. Mater. 22, 1121–1127 (2023).
Ohayon, D., Druet, V. & Inal, S. A guide for the characterization of organic electrochemical transistors and channel materials. Chem. Soc. Rev. 52, 1001–1023 (2023).
Tang, H. et al. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature 611, 271–277 (2022).
Huang, W. et al. Vertical organic electrochemical transistors for complementary circuits. Nature 613, 496–502 (2023).
Andersson Ersman, P. et al. All-printed large-scale integrated circuits based on organic electrochemical transistors. Nat. Commun. 10, 5053 (2019).
Jackson, S. R., Kingsford, R. L., Collins, G. W. & Bischak, C. G. Crystallinity determines ion injection kinetics and local ion density in organic mixed conductors. Chem. Mater. 35, 5392–5400 (2023).
Bischak, C. G., Flagg, L. Q. & Ginger, D. S. Ion exchange gels allow organic electrochemical transistor operation with hydrophobic polymers in aqueous solution. Adv. Mater. 32, 2002610 (2020).
Kim, J. H., Kim, S.-M., Kim, G. & Yoon, M.-H. Designing polymeric mixed conductors and their application to electrochemical-transistor-based biosensors. Macromol. Biosci. 20, 2000211 (2020).
Rivnay, J. et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 1, e1400251 (2015).
Bisquert, J. Hysteresis in organic electrochemical transistors: distinction of capacitive and inductive effects. J. Phys. Chem. Lett. 14, 10951–10958 (2023).
Guo, J. et al. Understanding asymmetric switching times in accumulation mode organic electrochemical transistors. Nat. Mater. 23, 656–663 (2024).
Jin, W. et al. Photocatalytic doping of organic semiconductors. Nature 630, 96–101 (2024).
Wu, X. et al. High performing solid-state organic electrochemical transistors enabled by glycolated polythiophene and ion-gel electrolyte with a wide operation temperature range from −50 to 110 °C. Adv. Funct. Mater. 33, 2209354 (2023).
Yang, A. et al. Wearable organic electrochemical transistor array for skin-surface electrocardiogram mapping above a human heart. Adv. Funct. Mater. 33, 2215037 (2023).
Cea, C. et al. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat. Mater. 19, 679–686 (2020).
Zhong, Y., et al. Ionic liquid gated organic electrochemical transistors with broadened bandwidth. ACS Appl. Mater. Interfaces, https://doi.org/10.1021/acsami.1023c11214 (2023).
Paulsen, B. D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 19, 13–26 (2020).
Dong, B. X. et al. Influence of side-chain chemistry on structure and ionic conduction characteristics of polythiophene derivatives: a computational and experimental study. Chem. Mater. 31, 1418–1429 (2019).
Keene, S. T., Rao, A. & Malliaras, G. G. The relationship between ionic-electronic coupling and transport in organic mixed conductors. Sci. Adv. 9, eadi3536 (2023).
Kim, J. H. et al. Peculiar transient behaviors of organic electrochemical transistors governed by ion injection directionality. Nat. Commun. 14, 7577 (2023).
Rivnay, J. et al. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 7, 11287 (2016).
Guardado, J. O. & Salleo, A. Structural effects of gating Poly(3-hexylthiophene) through an ionic liquid. Adv. Funct. Mater. 27, 1701791 (2017).
Hu, Y. et al. Ultralow-resistance electrochemical capacitor for integrable line filtering. Nature 624, 74–79 (2023).
Cavassin, P. et al. Electrochemical doping in ordered and disordered domains of organic mixed ionic-electronic conductors. Adv. Mater. 35, 2300308 (2023).
Onorato, J. W. & Luscombe, C. K. Morphological effects on polymeric mixed ionic/electronic conductors. Mol. Syst. Des. Eng. 4, 310–324 (2019).
Xiang, L. et al. Nanoscale doping of polymeric semiconductors with confined electrochemical ion implantation. Nat. Nanotechnol. 19, 1122–1129 (2024).
Quill, T. J. et al. Ion pair uptake in ion gel devices based on organic mixed ionic–electronic conductors. Adv. Funct. Mater. 31, 2104301 (2021).
Giovannitti, A. et al. Controlling the mode of operation of organic transistors through side-chain engineering. P. Natl Acad. Sci. 113, 12017–12022 (2016).
Wang, B. et al. Face-on orientation matches vertical organic electrochemical transistors for high transconductance and superior non-volatility. Adv. Funct. Mater. 33, 2312822 (2023).
Flagg, L. Q. et al. Polymer crystallinity controls water uptake in glycol side-chain polymer organic electrochemical transistors. J. Am. Chem. Soc. 141, 4345–4354 (2019).
Thiburce, Q., Giovannitti, A., McCulloch, I. & Campbell, A. J. Nanoscale ion-doped polymer transistors. Nano Lett. 19, 1712–1718 (2019).
Huang, L. et al. Porous semiconducting polymers enable high-performance electrochemical transistors. Adv. Mater. 33, 2007041 (2021).
Hsu, C.-H. et al. Enhancing the response speed of organic electrochemical transistors via ion liquid/metal–organic framework-embedded semiconducting polymers. Adv. Electron. Mater. 10, 2300645 (2023).
Zhang, X. et al. Breath figure–derived porous semiconducting films for organic electronics. Sci. Adv. 6, eaaz1042 (2020).
Acknowledgements
The authors acknowledge financial support from the Chinese Academy of Science (No. ZDBS-LY-SLH034, F. Z.), the National Natural Science Foundation (61971396, F. Z.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0520000, F. Z.), and the Fundamental Research Funds for the Central Universities (E2ET0309X2, F. Z.; E2E40305X2, L. X.). We also thank Yao Zhao (Institute of Chemistry, Chinese Academy of Sciences) for helping to perform the TOF-SIMS characterization.
Author information
Authors and Affiliations
Contributions
F. Z. conceived and led the study; C. Y. and F. Z. designed the experiments and mechanism discussion; C. Y. did the device fabrication, measurements, and data analysis; L. X. and Y. X. helped to carry out device fabrication; X. Z. contributed to the GIWAX measurements; and Z. J., B. Z., C. L., and S. D. helped with the concept modification. All the authors contributed to the manuscript organization, preparation, and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Simone Marasso, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, C., Xiang, L., Xiao, Y. et al. Lateral intercalation-assisted ionic transport towards high-performance organic electrochemical transistor. Nat Commun 15, 10118 (2024). https://doi.org/10.1038/s41467-024-54528-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54528-z
This article is cited by
-
Fibre-based organic electrochemical transistors: principle, evaluation, and application
npj Flexible Electronics (2025)