Abstract
Optical properties of cholesteric liquid crystal elastomers (CLCEs) can be tuned by an external field, however, it will spontaneously restore to the original state after the field is removed. Here, we introduce diselenide dynamic covalent bonds (DCBs) into CLCEs, whose optical properties can be reversibly and precisely tuned under the combined action of force and light. The tuned optical properties will be written into and remembered by the CLCEs, thus a programming effect is achieved. The prepared dynamical diselenide bonded CLCE films have the typical reversibly mechanochromism property, and high-resolution colourful patterning can be programmed by adjusting exposure time and intensity of masked visible-light under different tensile or compressive strain states. The DCB-CLCEs combine the novel anisotropy of CLCEs and the dynamic chain exchangeable ability of DCBs, which endows the materials with reprogrammable optical properties. We demonstrate a simple strategy of writing naked-eye high-resolution colourful patterning into a film with mechanochromism property by thermal or visible-light, it shows great potential in display devices, anticounterfeiting labels, sensors, optical films and smart materials.
Similar content being viewed by others
Introduction
Structural coloration in nature is a fascinating phenomenon, observed in the iridescent feathers of birds such as peacocks, the colorful wings of butterflies, and the shimmering scales of beetles1. These colors are not produced by pigments but by light striking micro-structured surfaces, resulting in vibrant colors through Bragg reflection that changes with the viewing angle2. The capability of natural systems to dynamically manipulate light and color through micro-structures, as opposed to pigments, has spurred the development of advanced materials such as cholesteric liquid crystals (CLCs)3,4,5, cellulose nanocrystals6,7, photonic glasses8, and other photonic crystals9,10,11.
CLCs have a special ability to regulate light due to their spontaneously formed one-dimensional periodic helical structure. It reflects a certain wavelength range of circularly polarized light, whose wavelength accords with Bragg’s formula: λ = npcosθ, where λ is the central reflected wavelength of the structural color, n is the average refraction index of CLCs, p is the helical pitch of the CLCs, and θ is the angle between the incident light and the helical axis of the cholesteric phase12. Therefore, the structural color can be tuned by changing the periodic parameter p through external stimuli such as temperature, light, mechanical stress, and applied voltage. CLC elastomers (CLCEs) are elastomers with the optical properties of CLCs, whose structural colors can be dynamically tuned through various extra stimuli13,14,15,16. Due to their special stimuli-responsive optical properties, they have been widely used in information display17, biological simulation18, light regulation19, and other optical film fields20.
However, the further practical applications of CLCEs are limited by the inability to modify the network once it is formed. Introducing dynamic covalent bonds (DCBs) into crosslinked networks is believed to be an effective improving approach to research21, including ester exchange22, Diels–Alder reaction23, boronic ester exchange24, disulfide bond exchange25, and diselenide bond exchange26. Recently, White et al. have employed radical-mediated addition-fragmentation chain transfer techniques in CLCEs, enabling thermally reversible programming of shape and color19. Wang et al. developed mechanically color-changing, shape-programmable, and self-healing CLCEs by incorporating dynamic covalent boronic ester bonds into the main chain27. Nevertheless, activating exchanges of some dynamic bonds necessitates rigorous conditions, encompassing high temperature or high-intensity ultraviolet light activation, which decreases the accessibility of practical applications based on DCB–CLCEs.
Notably, harmless visible-light responsiveness stands out among various activation mechanisms, providing substantial benefits attributed to its non-invasive characteristics and precise control capabilities. Diselenide bonds share analogous chemical properties with disulfide bonds but possess lower bond energy, rendering them activatable by visible light. Consequently, this attribute has garnered significant attention and facilitated its incorporation into polymer networks. Xu’s team incorporated diselenide bonds into polyurethane, resulting in patterns that are observable under a polarizer through the modulation of wavelength and exposure duration of visible light26. Li et al. integrated diselenide-containing chain extenders into LCEs, achieving reprogrammable LCE actuators capable of intricate deformations28.
In this study, we investigated the bond energies of various molecules containing diselenide bonds, then designed and synthesized a liquid crystalline diselenide derivative with a lower bond energy, and incorporated it into CLCEs. The prepared dynamical diselenide bonded CLCE films have the typical reversibly mechanochromism property, and naked-eye high-resolution colorful patterning can be programmed by adjusting exposure time and intensity of masked visible light under different tensile or compressive strain state. This strategy demonstrates road application potential in fields such as display devices, anticounterfeiting labels, sensors, optical films, and smart materials.
Results
The synthetic route for diselenide bonded molecule adhered to Supplementary Fig. 1, with comprehensive synthesis and characterization data available in Supplementary Note 1 and Supplementary Figs. 2–6. The diselenide bond-exchange process can occur between any two molecules containing diselenide bonds, in order to obtain the direct chemical evidence, here a bond-exchange process between two different chemical structures was conducted. The bond-exchange process is illustrated in Supplementary Fig. 7. To systematically study the bond-exchange capability of our synthesized Se–LC and Se–OH (synthetic intermediate of Se–LC), we simulated and characterized the bond-exchange behavior between these two molecules. Molecular structure simulations reveal that the diselenide bond length in Se–LC is the longest, measuring 2.43385 Å (Fig. 1b). The bond lengths of the other two molecules, Se–OH is 2.42779 Å and the exchanged product is 2.42775 Å, are quite similar, suggesting that the diselenide bond in Se–LC is more susceptible to cleavage compared to Se–OH. Additionally, calculations indicated that the diselenide bond dissociation energy in Se–LC is the lowest, at 122.4 kJ/mol, compared to 132.4 kJ/mol for Se–OH and 131.1 kJ/mol for the exchanged product. It can be speculated that the exchange reaction between Se–LC and Se–OH may occur via a free radical mechanism, whereby each molecule generates radicals and subsequently recombines into new molecules, as illustrated in Fig. 1c. Simulations determined that at 25 °C, the activation energy (Ea) of this reaction is 60.90 kJ/mol, with a Gibbs free energy change (ΔG) of −1.76 kJ/mol. According to the laws of thermodynamics, the formula for Gibbs free energy is given as follows:
where R is the universal gas constant, T is the thermodynamic temperature at the time of the reaction, and K is the reaction equilibrium constant. We define Ca, Cb, and Cc as the equilibrium concentrations of Se–LC, Se–OH, and the exchanged product at a specific temperature, respectively. Since Se–LC and Se–OH react in equimolar proportions, their concentrations remain equal, allowing Eq. (1) to be obtained as follows:
a Free radical-induced bond-exchange reaction occurs between Se–LC and Se–OH. b Simulated structures of Se–LC, Se–OH, and exchange products. c Free energy changes in the bond-exchange reaction in (a). d EPR of Se–LC and Se–OH after exposure to a 405 nm light source. e 77Se NMR spectra of Se–LC, Se–OH and mix the two in equal molar ratios. f 1H NMR spectra after adding Se–OH to Se–LC in different ratios. g The DSC curves of the Se–LC upon different thermal histories. h Dynamic XRD pattern upon the variable temperature of Se–LC.
When Se–LC and Se–OH react in equimolar amounts at 25 °C, the expected theoretical conversion rate reaches 68.5%.
After exposure to a 405 nm light source, Se–LC and Se–OH underwent testing by Electron Paramagnetic Resonance (EPR). Figure 1d demonstrates that both molecules exhibited signals indicative of unpaired electrons under a specific magnetic field. This suggests that visible light at 405 nm is sufficient to excite the molecules to a high-energy state, subsequently resulting in the dissociation of electron pairs into free radicals. After mixing Se–LC and Se–OH in equimolar proportions, the 77Se nuclear magnetic resonance (NMR) spectra revealed obvious changes even without light exposure (Fig. 1e). In the 77Se NMR spectra, Se–LC exhibited a selenium peak at 320 ppm, while Se–OH exhibited one at 316 ppm. The mixture revealed two new selenium peaks at 311 ppm and 328 ppm, indicative of the formation of new molecules with asymmetric diselenide bonds resulting from a bond-exchange reaction. It demonstrates that the lower bond energy of the diselenide bond enables two molecules to spontaneously undergo a bond-exchange reaction in solution without light exposure.
To further verify the kinetics of this reaction, we introduced 0.25, 0.5, 0.75, and 1 equivalent of Se–OH into a solution containing 1 equivalent of Se–LC and conducted a 1H NMR experiment (Supplementary Fig. 8). As demonstrated in Fig. 1f, with increasing proportions of Se–OH, the conversion rate of Se–LC gradually increases. Upon reaching a 1:1 molar ratio of the two molecules, the conversion rate of Se–OH increases from 20% (with the initial addition of 0.25 equivalents) to 50%. Theoretical calculations suggest that the bond energy of the diselenide bond in Se–OH and the mixed product are nearly equivalent, thus increasing the concentration of Se–OH in the solution favors the forward progression of the reaction.
As shown in Supplementary Fig. 9, polarized optical microscopy (POM) observation of Se–LC revealed that the molecule exhibits a smectic focal conic texture at around 68–70 °C. Differential scanning calorimetry (DSC) analysis demonstrates that Se–LC exhibits an unusual exothermic peak between 15 °C and 25 °C during heating (Fig. 1g). During the cooling process, annealing treatments at various temperatures were applied. Annealing at 65 °C did not affect the anomalous exothermic peak, whereas annealing at 55 °C resulted in its disappearance.
Furthermore, a mild exothermic peak emerged between 35 °C and 55 °C during the cooling phase. Remarkably, after annealing at 45 °C, the exothermic peaks observed during both heating and cooling disappear. Since Se–LC contains diselenide bonds, it is speculated that this may be related to dynamic metathesis of diselenide bonds.
Figure 1h shows the dynamic X-ray diffraction (XRD) patterns of Se–LC at different temperatures. As the temperature increases to 55 °C, the q value of the diffraction peaks in the small q range decreases, the peaks become sharper, and disappear when the temperature reaches the clearing point of Se–LC. This suggests that Se–LC undergoes tighter packing with enhanced crystallinity near 55 °C. This phenomenon may be due to increased molecular mobility following the cleavage of diselenide bonds to form radicals. Variable-temperature EPR tests (Supplementary Fig. 10) in dark show that the radical signals of Se–LC significantly intensified at nearly 55 °C, indicating an increase in radicals formed from diselenide bonds above this temperature, thus altering the stacking state. Therefore, we believe that during the cooling process, a large number of radicals in Se–LC recombine to form diselenium bonds around 45–55 °C, releasing heat. However, since the breaking and formation of DCBs is a continuous process, it may exhibit some lag in the DSC curve. After holding at 55 °C for 30 min, most of the radicals reached a relatively uniform thermodynamic state, but some radicals had not yet recombined. As a result, a more gradual exothermic peak appeared during further cooling, and the anomalous exothermic peak during heating disappeared. After holding at 45 °C for 30 min, the vast majority of radicals had recombined, hence no exothermic peaks were observed during subsequent cooling below 45 °C or during heating. Consequently, the exothermic peak in the DSC curve can be attributed to the energy changes from radical recombination and interference from the supercooling effect.
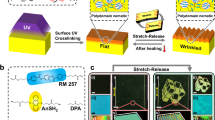
To construct a DCB–CLCE enabling mechanochromism with a wide color-changing range, we well-designed the material composition of the polymerization precursor to obtain a CLCE matrix with desired mechanical properties. The precursor composition is a completely polymerized system containing liquid crystalline monomers (C6M, C3M, and C4V) and two thiols (EDDET and C4S) as well as a chiral agent DK756 (Fig. 2a). They synergistically self-assembly to an ordered helical nanostructure enabling to reflect specific wavelengths. As shown in Fig. 2b, the preparation process involves vortex ultrasonication of all components, followed by pouring the precursor solution onto a glass substrate and controlling the thickness using the bar-coating method. Subsequently, the mixture is subjected to thermal and photopolymerization steps, resulting in the formation of CLCE. During the preparation process of the DCB–CLCE, varying concentrations of Se–LC molecules (0–20%) were added into the system, designating the samples as Se-5%, Se-10%, Se-15%, and Se-20%. The shear alignment during blade-coating caused the liquid crystal molecules to align parallel to the glass substrate. The liquid crystal molecules underwent self-assembly induced by the chiral dopant, resulting in a well-aligned, multi-___domain planar-oriented CLC. POM observations of CLCs with different Se–LC concentrations, before photopolymerization, revealed well-aligned, multi-___domain state cholesteric oily streak textures (Supplementary Fig. 11). Details of the preparation process and composition of the DCB–CLCE films are provided in Methods and Supplementary Table 1.
Stress–strain testing on DCB–CLCE films with varying Se–LC contents revealed that as Se–LC content increased, the elongation at break for the films increased from 108% for Sample Se-0% to 232% for Sample Se-20%, while the maximum tensile stress decreased from 7 to 1.74 MPa (Fig. 2c). The Se–LC molecules possess a dimeric structure, with a molecular core composed of diselenium bonds and flexible alkyl chains. Compared with C6M, C3M, and C4V used in the experiment, the flexible core structure makes it more mobile in the polymer network than other liquid crystal monomers, and the rotation and movement of the flexible core can enhance the deformation capability of the network. Thus, by increasing the concentration of Se–LC while keeping the chain extender constant, the fabricated CLCEs exhibited larger tensile strain as the Se–LC concentration increased. The modulus of the DCB–CLCE films also decreased, permitting more significant deformation under lower stress conditions. DSC analysis in Fig. 2d showed that with increasing Se–LC content, the glass transition temperature of the films decreased from −3.8 °C to −10.25 °C. The lower glass transition temperature facilitates bond-exchange reactions by enhancing the mobility of the polymer chains. This increased mobility promotes the recombination of free radicals formed following the cleavage of diselenide bonds, thereby enhancing the material’s programmability. The variations in mechanical properties and glass transition temperatures (Tg) are likely linked to the structural characteristics of Se–LC molecules. We evaluated the mechanochromic properties of the films, and the results show that an increase in Se–LC content significantly enlarges the blueshift range of the films. From Se-0% to Se-20% (Supplementary Fig. 12 and Fig. 2e), the reflective wavelength range of the films expanded from 100 to 200 nm. The central reflective wavelength of the Se-20% sample spans red, green, and blue (Fig. 2f and Supplementary Video 1), providing a basis for color programming across the whole visible spectrum range. Unless otherwise specified, all subsequent tests in this manuscript will utilize the Se-20% film. After modeling the relationship between strain and central reflective wavelength for the Se-20% CLCE film, we derived the following equation:
where Δλ represents the change in wavelength blueshift, n represents the refractive index of the film, \({{{{\rm{\nu }}}}}_{{zx}}\) represents the film’s transverse Poisson’s ratio, and N represents the number of periods of the helical structure in the thickness direction of the film (a detailed derivation is provided in Supplementary Note 2).
Figure 3a shows the schematic diagram of stretching-mode programming of CLCEs with diselenium bonds. Initially, the film is in large-pitch multi-___domain planar orientation, where the mesogenic units self-assemble into a cholesteric phase (with pitch p1) in the direction perpendicular to the film, while presenting a multi-___domain state in the direction parallel to the film. Upon applying external force, the molecular chains shift to small-pitch single-___domain planar orientation. In the film plane, the multi-___domain structure aligns along the stretch direction to become a single ___domain, while in the perpendicular pitch direction, the chains become more tightly packed, reducing the pitch to p2. Under light or thermal stimulation, diselenium bonds break, releasing selenium radicals. These radicals migrate with the polymer chains, promoting the reformation of diselenium bonds, thereby relieving stress. Once the external force is removed, the rearranged polymer chains tend to maintain the network structure formed under stress, instead of reverting to the initial state. During this process, the CLCE film macroscopically elongates in the stretching direction, becomes narrower and thinner, and its reflection wavelength blue-shifts.
a Schematic diagram of stretching-mode programming of CLCEs with diselenium bonds (p1 > p2). b Stress relaxation curves of CLCEs irradiated for 1 h under different light intensities. c Central reflection wavelength of CLCEs after irradiation for different durations at different light intensities. The data are presented as the mean of three measurements ± SD. d Reflectance spectra of CLCEs after 1 h of irradiation under different light intensities. e Tg values of CLCEs after exposure to 405 nm light at an intensity of 100 mW/cm2 for different times. f Azimuthal intensity distribution of CLCEs after exposure to 405 nm light at an intensity of 20 mW/cm2 for different times. The corresponding 2D-WAXD patterns (g) and POM images (h) of CLCEs undergo 0 min (i) and 30 min (ii) irradiating in (f).
To systematically investigate the effect of light on changes in the stress during stretching, Sample Se-20% film was stretched to 5% elongation and exposed to 405 nm light. During different intensities ranging from 10 to 150 mW/cm2 exposure for 1 h, stress relaxation test was conducted. As shown in Fig. 3b, the exchange of diselenide bonds facilitated stress relaxation in the film with exposure time extending, and higher light intensity further accelerated this process. By controlling the exposure duration and intensity, we adjusted the helical pitch through stress relaxation, achieving changes in the structural color of the CLCE films under various stress conditions. We stretched the Se-20% films to 200% of their original length and exposed them to 405 nm light, with intensities ranging from 10 to 150 mW/cm2 and durations ranging from 10 min to 2 h. Figure 3c illustrates the extent of changes in the central reflected wavelength of the films after relaxation, influenced by varying light intensities and exposure durations. The results indicate that the central reflected wavelength of the CLCE films exhibited a blueshift under all light intensities with exposure time extending, and the magnitude of this shift increases with higher light intensity. This suggests that at higher intensities, diselenide bonds break and reform more rapidly, leading to faster network reorganization and a more pronounced blueshift in the reflected wavelength. At lower light intensities, such as 10 mW/cm2, the lesser bond exchange occurs, resulting in only about 100 nm blueshift after 2 h of exposure. In contrast, at a higher intensity of 150 mW/cm2, the same 100 nm blueshift can be achieved with just 20 min of exposure. After prolonged exposure, as the bond exchange continues, the reorganization of the network structure leads to reduced entanglement of the chain segments, thereby gradually reducing the stress within the film. Once the stress decreases to a low enough level, subsequent DCB fracture and reconstruction reach an equilibrium state. Macroscopically, this phenomenon manifests as a deceleration or cessation of the blueshift in the reflected wavelength after prolonged exposure to high-intensity light, indicating that the stress has been fully released. At a light intensity of 150 mW/cm2, the extent of blueshift begins to decrease markedly after 60 min of exposure. The radicals generated from the cleavage of diselenide bonds are highly reactive, and if they fail to recombine swiftly, they may form byproducts that could potentially degrade the film’s properties. All subsequent tests, unless otherwise specified, will employ a 405 nm light source at an intensity of 100 mW/cm2.
Furthermore, because bond exchange leads to the reorganization of the polymer network structure, we discovered that increasing the exposure time of CLCE while it is in a stretched state can improve its modulus. After stretching the CLCE film to 200%, we exposed it to a 405 nm light source with an intensity of 100 mW/cm2 for different durations. According to the stress–strain tests (Supplementary Fig. 14), with increasing exposure time, the maximum strain of the CLCE film decreases from 232% to 69%, while the maximum breaking stress rises from 1.7 to 10.8 MPa. As a result, the modulus of the CLCE film increases with longer exposure times during programming. Figure 3d displays the central wavelength reflection spectra of the Se-20% film after 1 h of exposure across a range of light intensities. The spectra reveal that the central reflected wavelength shifted from 640 to 460 nm, demonstrating that within 1 h of light exposure, adjusting the light intensity can enable any desired color change in the CLCEs structural color from red to blue. To confirm changes in the internal polymer network structure of the films, we conducted DSC tests on films treated with light for various durations. As shown in Fig. 3e, after 90 min of exposure to light at an intensity of 100 mW/cm2, the Tg value of the film increased from −10.25 °C to −6.53 °C, which indicates the degree of order of the polymer network has increased. After conducting 2D-WAXS tests, we found that the ultimate orientation state was achieved within 30 min, with no significant orientation changes observed after extra 30 and 60 min (Supplementary Figs. 15 and 16). To elucidate the changes in orientation within the polymer network, 2D-WAXS tests were performed on films under light intensity of 20 mW/cm2 exposure. As shown in Fig. 3f, g and Supplementary Fig. 17, when 405 nm light is applied to the stretched CLCE, 2D-WAXS demonstrates that starting from a multi-___domain state, gradually orienting in the stretching direction. The 2D pattern changes from a ring to a pair of bright arcs, indicating that the degree of orientation in the stretching direction of the film gradually increases due to light exposure, with the order parameter rising from 0.02 to 0.45. The order parameter is calculated using Herman’s orientation function based on 2D-WAXD data29. Additionally, we used POM to observe the texture of CLCE before and after programming (Fig. 3h). Initially, the oily streak texture is not oriented, but with bond exchange under 405 nm light, the orientation gradually forms and strengthens along the stretching direction. The reflection color of the CLCE film also shifts from red to green as well. This is consistent with the changes in CLCE orientation under stretching mode that we discussed (Supplementary Note 3 and Supplementary Fig. 18).
Figure 4a illustrates the process of stretching-mode programming and erasing structural color. Initially, red films become green after stretching and light exposure. Subsequently, the films are heated on a hotplate at 100 °C, which allows the structural color to revert to red due to the thermal recovery property of CLCEs. To demonstrate the contrast between color-restored and non-restored areas, half of the film was covered with a light mask while the other half was exposed to 405 nm light at 100 mW/cm2 on a hotplate for 1 h. The image (Fig. 4b) reveals that the exposed area’s structural color returned to red under the natural state, while the unexposed area remained green. A detailed video of the “write-erase” process is provided in Supplementary Video 2. Supplementary Fig. 19 provides a schematic illustrating the erasing mechanism after stretching-mode programming of the CLCE, in which the programmed CLCE is placed on a hot stage. Due to the shape memory effect of the CLCE, the programmed CLCE reverts to its initial state when heated, shortening along the original stretching direction and elongating in the other two directions. Therefore, the small-pitch single-___domain orientation formed during programming reverts to the initial large-pitch multi-___domain orientation. Additional light exposure can induce diselenium bond cleavage, generating radicals. These radicals migrate with the thermal motion of the polymer chains, leading to dynamic bond exchange and the formation of new diselenium bonds, thus preserving the large-pitch multi-___domain orientation. During this process, the CLCE film macroscopically shortens in the original stretching direction, becomes wider and thicker, and the pitch recovers from p2 (programmed state) to p1 (initial state), with a red shift in reflection wavelength.
Scheme (a) and corresponding images (b) of the stretching-mode CLCEs color “write-erase” mechanism. c Photographs of the CLCE stretched after self-healing. d Stress–strain curves of the original CLCE and the self-healing CLCEs after thermal pressing for different times. e Cross-section SEM of the interface at the bonding site of CLCEs with Se–LC after 12 h of hot pressing.
We conducted five cycles of the “write-erase” experiment on the film, and the results of the reflection wavelength are shown in Supplementary Fig. 20. The CLCE film’s reflection wavelength can reversibly change between 670 and 520 nm during the process, verifying that the state reprogramming of the film can be well achieved. We systematically investigated initial state, programmed state, and recovered state during the first cycle of repeatability tests using POM, reflectance spectrometer, 2D-WAXS, and scanning electron microscope (SEM). Supplementary Fig. 21 shows that POM and 2D-WAXS images reveal CLCE transitioning from a multi-___domain state to a single-___domain state after programming, and reverting to the initial multi-___domain state after erasing. SEM images show that the pitch of CLCE decreased from 409 to 335 nm after programming and increased to 401 nm after erasing process. Corresponding POM images and reflection spectra indicate that the reflection color of CLCE blue shifted from red to green after programming and reverted to red after erasing. These characterizations of the “write-erase” mechanism also confirm our explanation of the “write-erase” mechanism presented in Fig. 3a and Supplementary Fig. 19. Additionally, we tested the self-healing properties of the CLCE films. Overlaying two sections of red-reflective CLCE films and placing them on a 70 °C hot plate with a 500 g loading weight on the overlap resulted in a self-healed CLCE film after some time. Figure 4c shows the actual image of the CLCE film self-healing and the film stretching after self-healing. As shown in Fig. 4d, we compared the mechanical properties of films after different self-healing times with the original films and found that the mechanical properties of films self-healed for 12 h were the same as the initial state. We also conducted self-healing experiments on Se-0% films and captured SEM images of the cross-sections post self-healing, comparing with the film containing Se–LC (Fig. 4e and Supplementary Fig. 22). We observed clear gap in the films without diselenide bonds, whereas those with diselenide bonds merged at the overlapping interfaces, successfully achieving self-healing.
The photopatterning process is shown in Fig. 5a, the stretched film is covered by a photomask. Areas covered by the opaque parts of the mask retain their original structural color, while the exposed areas undergo a blueshift of structural color. And different parts of the pattern can be controlled by adjusting the exposure duration and intensity of the light. Similar to stretching, compressing can also reduce the pitch of the film, resulting in a blue shift of the reflection color, as shown in the mechanism diagram of compressing-mode programming of CLCE in Supplementary Fig. 23. Applying pressure to the film compresses it in the thickness (pitch) direction, reducing the pitch from the initial state p1 to p2, causing a blue shift in reflection color. Pressure perpendicular to the pitch direction does not affect the orientation of the polymer chains parallel to the film, preserving the initial multi-___domain state in the film plane (Supplementary Note 3 and Supplementary Fig. 18). Under thermal stimulation, the structure of the film in its compressed state is preserved, achieving color programming through control of the thermal imprinting time. Therefore, we explored thermal imprinting programming by applying 10 MPa pressure to the film while maintaining it at 90 °C on a heating stage, and controlling the duration to regulate the extent of the blue shift in reflection color (Supplementary Fig. 24). To compare with the orientation of stretch-programmed CLCE, we selected CLCE that underwent hot pressing for 40 min, as it showed a similar degree of blue shift in reflection wavelength. 2D-WAXS and POM images (Supplementary Fig. 25) indicate that hot pressing does not alter the film plane orientation (The order parameter is both 0.02 before and after hot pressing). The oily streak texture of the cholesteric phase remains in a multi-___domain state, with only the reflected color changing from red to green. It is consistent with the explanation in Supplementary Note 3 regarding the effects of compression mode on CLCE structural changes. Figure 5b shows a schematic of the thermal-pressing process. The film is placed on a heating plate, and a patterned stamp is then pressed onto the film. The pressure causes the film to thin, resulting in a blue shift of the reflection color. The heat stimulates the exchange of dynamic bonds, causing the structural color with a blue shift in the stamped area to be preserved, achieving precise color patterns.
Scheme of patterned film fabrication via photopatterning (a) and thermal pressing (b). c Images of CLCEs featuring photopatterning text under various elongations. d The text in the frame of (c) under various elongations and laser confocal images of the text on the green background CLCE from c-ii. e Images of photopatterning colorful Tai Chi patterns under various elongations. f Images of thermal-patterning QR code being stretched and released. g and h show images of thermos-patterning designs.
Figure 5c-i displays the patterned film obtained using a photomask with ancient Chinese text (Supplementary Fig. 26a), where the red background and blue text are distinctly visible. Additionally, stretching the patterned film (Fig. 5c-ii, iii and Supplementary Video 3) reveals significant differences in the degree of blueshift between the patterned areas and the background. The patterned area color exhibits minimal blueshift, whereas the background color transitions from red to blue. This is attributed to the increased modulus in the bond-exchange areas described in Supplementary Fig. 14; parts of the film with a higher modulus (the patterned areas) experience a smaller deformation (Δx) under the same loading force as the background. As previously derived, the change in wavelength (Δλ) is proportionally related to deformation (Δx), causing the central reflected wavelength of the patterned part to undergo a smaller blueshift. We used a laser confocal microscope (Fig. 5d) to investigate the thickness variations of the film (Fig. 5c-ii) and found that the patterned area of the film (blue) exhibits a greater degree of indentation compared to the background area (green), with clear boundaries. Thus, the photo-imprinted pattern has high resolution. Consequently, while manipulating the structural colors, we perform photolithography on the film surfaces. The variation in film thickness offers potential for our patterning strategy to be utilized in Braille printing.
Furthermore, we can control the exposure time of different sections of a photomask pattern to input color programming. A mask composed of transparent and semi-transparent patterns in a Tai Chi shape (Supplementary Fig. 26b) was placed on a film, firstly only exposing the upper half to the light for 20 min, and then both the upper and lower half were exposed for another more 20 min. A color pattern with multi-level color gradients is shown in Fig. 5e-i. This method enables arbitrary input of red to purple patterns on the film. Due to the different moduli of the sections, after stretching, areas with longer reflection wavelengths exhibit a faster blueshift than those with shorter wavelengths. We can conceal the input color information by stretching, as shown in Fig. 5e-ii, iii. Furthermore, we found that the color change of the stretched multi-level color patterns almost no difference from the initial state after 4 months of preparation, indicating high environmental stability (Supplementary Video 4).
By reducing the concentration of chiral dopants, we tuned the initial reflection wavelength of the film to the infrared region. The QR code stamp (Supplementary Fig. 27a) was hot-pressed at 90 °C with a pressure of 10 MPa for 15 min. Reflectance spectra (Supplementary Fig. 28) indicate a blue shift in the patterned area’s reflection wavelength, reducing from 858 nm in the background to 806 nm, yet still within the infrared range. When the film was stretched, both the patterned and background regions exhibited a blue shift in their reflective colors. During the stretching phase, the patterned area transitioned into the visible spectrum, appearing red (731 nm) when stretched to 15% of its original length, while the background remained in the infrared at 804 nm. When the film is stretched, red QR code will appear and the information can be identified by mobile phone scanning code. By applying and releasing tension on the film, the QR code can switch between appearing and disappearing (Fig. 5f and Supplementary Video 5), demonstrating significant potential in the field of information encryption.
Additionally, by using patterning stamps (Supplementary Fig. 27b, c) to emboss on a hotplate, we created complex shapes, as depicted in Fig. 5g, h. Complex designs and a variety of structural colors can be achieved in CLCEs through light or thermal embossing, demonstrating the vast potential for applications in anticounterfeiting and data encryption. The bond-exchange mechanism, controlled by external stimuli, enables effective adjustment of the film’s structural color, offering an innovative approach to developing smart optical materials. This technological advancement not only enhances the functionality of these materials but also paves the way for novel optical applications in the future.
In summary, we prepared DCB–CLCEs by introducing diselenide DCBs into CLCEs, which can achieve naked-eye high-resolution colorful patterning programming by a simple visible-light writing strategy. The prepared films have the typical reversibly mechanochromism property, and the multi-level naked-eye high-resolution colorful patterning can be programmed by adjusting exposure time and intensity of masked visible light under different tensile or compressive strain states. The realization of the programming effect is based on the typical mechanical properties of LCE and the internal stress relief capacity of the dynamic bonded polymer chain segment. The outstanding optical and machinal properties as well as programable ability endow the DCB–LCE with great potential in display devices, anticounterfeiting labels, sensors, optical films, and smart materials.
Methods
Materials
2-Methyl-1,4-phenylene bis(4-((6-(acryloyloxy)hexyl)oxy)benzoate) (C6M), 2-Methyl-1,4-phenylene bis(4-(3-(acryloyloxy)propoxy)benzoate) (C3M) Purchased from Jiangsu Hecheng Display Technology Co., Ltd., 1,4:3,6-dianhydro-, 2,5-bis[4-[[4-[3-[(1-oxo-2-propen-1-yl)oxy]propoxy]benzoyl]oxy]benzoate] (DK756), 3,6-dioxy-1,8-octyldithiol (EDDET) Purchased from Bide Pharmaceutical, acryloyl chloride, 1,2-Diphenyl-2,2-dimethoxyethanone (Irg651), dipropylamine (DPA), triethylamine (TEA), selenium, 4-(2-Bromoethyl)phenol, thionyl chloride (SOCl2), 4-Dimethylaminopyridine (DMAP), sodium sulfite (Na2SO3), sodium sulfate (Na2SO4), 4-hydroxybenzoic acid, 6-bromo-1-hexanol and potassium hydroxide (KOH) purchased from Aladdin, and all reagents used were purchased from Tongguang Reagents. All purchased drugs and reagents do not require purification and can be used directly. C4S and C4V were synthesized in the laboratory.
Measurements
1H NMR and 77Se NMR spectra were recorded using a Bruker 500 MHz spectrometer. Absorption spectra were obtained using a Perkin Elmer Lambda 950 spectrophotometer. Optical textures were assessed using a Carl Zeiss polarizing optical microscope (AxioVisionSE64). Tensile tests were conducted on a universal testing machine (CMT6103, MTS Systems, China). DSC tests were performed using a PerkinElmer DSC 8000, under nitrogen purge, at a scanning rate of 10 °C/min. The stress relaxation experiment was carried out on a DMAQ800TA dynamometer using a CLCE specimen of 40 × 10 × 0.15 mm. A constant strain of 5% was applied to the CLCE film at 25 °C. During the experiment, the film was irradiated with a 405 nm light source of different light intensities. Reflectance spectra were measured with an Avaspec-ULS2048 fiber optic spectrometer. Fracture analysis of the CLCEs was conducted using a HITACHI S-4800 SEM. XRD measurements were performed using a GANSHA SaxsLab SAXS instrument. EPR tests were conducted using a Bruker EMXPlus (Karlsruhe, Germany) at room temperature.
Preparation process of the CLCE films
C6M, C3M, C4V, DK756, and Se–LC were added to a centrifuge tube according to the contents listed in Supplementary Table 1, and ultrasonically stirred at 80 °C for 10 min to ensure uniformity. EDDET, C4S, and Irg651 were then added to the precursor solution and ultrasonically stirred at 50 °C for 10 min. Finally, 0.02 g of DPA (diluted with DCM to a ratio of 1:10) as a thermal initiator was added to the mixture. The prepared mixture was coated onto a 35 °C glass substrate using a 150 μm gap coater at a speed of 5 mm/s, and the oligomer was obtained by maintaining it at 45 °C in a light-proof environment for 24 h. A self-supporting CLCE film was obtained by irradiating the sample with a 365 nm light source at 30 mW/cm2 for 15 min.
Data availability
The data that support the findings of this study have been included in the main text and Supplementary Information. Source data are provided with this paper.
References
Zhang, Z., Chen, Z., Shang, L. & Zhao, Y. Structural color materials from natural polymers. Adv. Funct. Technol. 6, 2100296 (2021).
Yu, K., Fan, T., Lou, S. & Zhang, D. Biomimetic optical materials: integration of nature’s design for manipulation of light. Prog. Mater. Sci. 58, 825–873 (2013).
Zheng, Z. G. et al. Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light. Nature 531, 352 (2016).
Nagai, H., Liang, X., Nishikawa, Y., Nakajima, K. & Urayama, K. Periodic surface undulation in cholesteric liquid crystal elastomers. Macromolecules 49, 9561–9567 (2016).
Park, S., Lee, S. S., Yang, S. & Kim, S.-H. Asymmetric pairing of cholesteric liquid crystal droplets for programmable photonic cross-communication. Small 19, 2303728 (2023).
Espinha, A. et al. Hydroxypropyl cellulose photonic architectures by soft nanoimprinting lithography. Nat. Photonics 12, 343–348 (2018).
Droguet, B. E. et al. Large-scale fabrication of structurally coloured cellulose nanocrystal films and effect pigments. Nat. Mater. 21, 352–358 (2022).
Llorens, J. S., Barbera, L., Demirörs, A. F. & Studart, A. R. Light-based 3D printing of complex-shaped photonic colloidal glasses. Adv. Mater. 35, 2302868 (2023).
Kang, Y., Walish, J. J., Gorishnyy, T. & Thomas, E. L. Broad-wavelength-range chemically tunable block-copolymer photonic gels. Nat. Mater. 6, 957–960, (2007).
Ye, B. et al. Colorimetric logic response based on aptamer functionalized colloidal crystal hydrogels. Nanoscale 7, 7565–7568 (2015).
Lee, S. Y., Kim, S.-H., Hwang, H., Sim, J. Y. & Yang, S.-M. Controlled pixelation of inverse opaline structures towards reflection-mode displays. Adv. Mater. 26, 2391–2397, (2014).
Bisoyi, H. K. & Li, Q. Liquid crystals: versatile self-organized smart soft materials. Chem. Rev. 122, 4887–4926 (2022).
Nam, S., Jung, W., Shin, J. H. & Choi, S. S. Omnidirectional color wavelength tuning of stretchable chiral liquid crystal elastomers. Light Sci. Appl. 13, 114 (2024).
Ma, J. et al. Mechanochromic and ionic conductive cholesteric liquid crystal elastomers for biomechanical monitoring and human–machine interaction. Mater. Horiz. 11, 217–226 (2024).
Gao, J., He, Y., Cong, X., Yi, H. & Guo, J. Reconfigurable fluorescent liquid crystal elastomers for integrated visual and haptic information storage. ACS Appl. Mater. Interfaces 14, 53348–53358 (2022).
Li, X. et al. Bioinspired multi-stimuli responsive actuators with synergistic color- and morphing-change abilities. Sci. Adv. 8, 2101295 (2021).
Zhang, S. N. et al. Reversible information storage based on rhodamine derivative in mechanochromic cholesteric liquid crystalline elastomer. Adv. Funct. Mater. 33, 2305364 (2023).
Bi, R. et al. 3D-printed biomimetic structural colors. Small 20, 2306646 (2024).
Martinez, A. M., McBride, M. K., White, T. J. & Bowman, C. N. Reconfigurable and spatially programmable chameleon skin‐like material utilizing light responsive covalent adaptable cholesteric liquid crystal elastomers. Adv. Funct. Mater. 30, 2003150 (2020).
Park, H. et al. Mechanochromic palettes of cholesteric liquid crystal elastomers for visual signaling. Adv. Opt. Mater. 12, 2400266 (2024).
Mohand, O. S., Alexandra, G. & Eugene, M. T. Exchangeable liquid crystalline elastomers and their applications. Chem. Rev. 122, 4927–4945 (2021).
Yao, Y. et al. Enabling liquid crystal elastomers with tunable actuation temperature. Nat. Commun. 14, 3518 (2023).
Jiang, Z.-C., Xiao, Y.-Y., Yin, L., Han, L. & Zhao, Y. “Self-lockable” liquid crystalline Diels–Alder dynamic network actuators with room temperature programmability and solution reprocessability. Angew. Chem. Int. Ed. 59, 4925–4931 (2020).
Chen, Y. et al. Covalently cross-linked elastomers with self-healing and malleable abilities enabled by boronic ester bonds. ACS Appl. Mater. Interfaces 10, 24224–24231 (2018).
Li, Y., Zhang, Y., Rios, O., Keum, J. K. & Kessler, M. R. Photo-responsive liquid crystalline epoxy networks with exchangeable disulfide bonds. RSC Adv. 7, 37248–37254 (2017).
Liu, C., Fan, Z., Tan, Y., Fan, F. & Xu, H. Tunable structural color patterns based on the visible-light-responsive dynamic diselenide metathesis. Adv. Mater. 32, 1907569 (2020).
Ma, J. Z. et al. Mechanochromic, shape-programmable and self-healable cholesteric liquid crystal elastomers enabled by dynamic covalent boronic ester bonds. Angew. Chem. Int. Ed. 61, e202116219 (2022).
Chen, L. et al. Healable and rearrangeable networks of liquid crystal elastomers enabled by diselenide bonds. Angew. Chem. Int. Ed. 60, 16394–16398 (2021).
Fowler, H. E., Rothemund, P., Keplinger, C. & White, T. J. Liquid crystal elastomers with enhanced directional actuation to electric fields. Adv. Mater. 33, 2103806 (2021).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2023YFB3812800 (W.H.)) and the National Natural Science Foundation of China (Grant Nos. 52373260 (W.H.), 51921002 (H.Y.) and 51927806 (H.Y.)).
Author information
Authors and Affiliations
Contributions
W.H., H.Y., and J.L. conceived and supervised the study. J.L. performed the main experiments. S.Z. and X.X. assisted in the fabrication of the CLCE films. Z.W. performed the DFT calculations and analyzed the data. J.Z., Y.R., and J.C. assisted in the experiments and measurements. Y.Y., Y.X., B.Y., and W.X. analyzed and interpreted the results. J.L. wrote the manuscript. W.H. and H.Y. arranged the funding and infrastructure for the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks MinSu Kim, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Zhang, S., Wang, Z. et al. Visible-light-programmed patterning in dynamically bonded cholesteric liquid crystal elastomer. Nat Commun 15, 10367 (2024). https://doi.org/10.1038/s41467-024-54881-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54881-z
This article is cited by
-
Programmable optical encryption using thickness-controlled stretchable chiral liquid crystal elastomers
Light: Science & Applications (2025)