Abstract
This study (NCT04728035) aimed to explore the safety and efficacy of liposomal irinotecan (HE072) in patients with metastatic triple-negative breast cancer (mTNBC). This study consisted of two parts. In part 1, the 3 + 3 design was used to investigate three dose levels of HE072 (50, 70 and 90 mg/m2). In part 2, patients were enrolled in two cohorts (mTNBC and HER2-negative breast cancer brain metastasis [BCBM]), and received HE072 70 mg/m2 every two weeks (Q2W). The primary endpoints were maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D), and treatment emergent adverse events (TEAEs). The secondary endpoints were pharmacokinetic profiles and efficacy including objective response rate (ORR) and disease control rate (DCR) (all patients) and Central Nervous System ORR and clinical benefit rate (CBR, for patients with HER2-negative BCBM), duration of response, progression free survival (PFS), overall survival (OS). A total of 119 patients were enrolled, including 101 mTNBC and 18 HER2-negative BCBM. One dose limiting toxicity (grade 3 nausea and vomiting) occurred at 70 mg/m2, and the MTD was not reached. The most common ≥ grade 3 TEAEs related to HE072 included neutropenia (21.0%), leukopenia (18.5%), diarrhea (10.1%). Among 87 evaluable patients with mTNBC, 22 patients (25.3%) achieved overall response. The DCR was 67.8% (59/87). The median PFS and OS were 4.8 months and 14.1 months, respectively. The RP2D was 70 mg/m2 Q2W. Promising antitumor activity in heavily pre-treated patients with mTNBC was observed, which warrants further validation.
Similar content being viewed by others
Introduction
Triple negative breast cancer (TNBC) is an aggressive subtype of breast cancer associated with poor prognosis, which accounts for 12–17% of all breast cancers1. About 45% of patients diagnosed with advanced TNBC will develop distant metastasis to the brain and/or visceral organs, with a median overall survival (OS) of 13.5–15.2 months2,3. Compared to other types of breast cancer, TNBC can’t benefit from endocrine therapy and human epidermal growth factor receptor 2 (HER2) targeting therapy and has limited treatment options. Sacituzumab govitecan (SG) has been approved for treatment of unresectable locally advanced or metastatic TNBC who have received at least two systemic therapies in China in June 2022. Despite the development of various targeted therapies in recent years, cytotoxic chemotherapy remains the backbone of treatment for TNBC.
The incidence of breast cancer brain metastasis (BCBM) is increasing with the longer survival of patients with breast cancer4. The prognosis of BCBM is poor5,6, with a median OS of 4.4–18.9 months. The limited ability to cross the blood-brain barrier (BBB) for the antitumor agents represents a great challenge for patients with BCBM. There is a highly unmet medical need for novel agents which can cross BBB and work in the brain lesions.
Liposomal irinotecan (HE072, CSPC Ouyi Pharmaceutical Co, Ltd), a generic form of Onivyde, has been recently approved for treatment of metastatic pancreatic adenocarcinoma after disease progression following gemcitabine-based therapy by National Medical Products Administration (NMPA). HE072 used pegylated liposome encapsulating an irinotecan sucroseoctasulfate salt, with vesicle size of 110 nm, which could prevent rapid elimination by mononuclear phagocyte system, thus increasing the circulation longevity of the drug and allowing more irinotecan accumulating into tumor zone7. Together with enhanced permeability and retention effect in cancer, HE072 exhibited improved efficacy profiles. Preclinical trials showed that compared with Onivyde, HE072 exhibited a similar or greater antitumor effect in the pancreatic cancer, small lung cancer and breast cancer xenografts model. Bioequivalence between Onivyde and HE072 was established for total irinotecan, free irinotecan and SN-38, and there were no significant differences between HE072 and Onivyde in patients with advanced pancreatic cancer (NCT04482257).
To explore whether HE072 can work in metastatic TNBC and HER2-negative BCBM and to find the optimal dose, we carried out this phase I trial to evaluate the safety, tolerability, efficacy, pharmacokinetics and determination of the recommended phase 2 dose (RP2D) of HE072 in patients with metastatic TNBC after ≥2 prior lines of chemotherapy.
Results
Patients
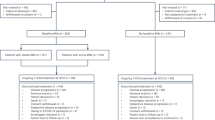
Between May 2021 and December 2022, 178 patients were screened and 119 patients (including 101 metastatic TNBC and 18 HER2-negative BCBM) were enrolled, with 27 in the dose-escalation and expansion part and 92 in the expansion cohort part (Fig. 1). In total, 12 patients received HE072 at 50 mg/m2 dose level, and 107 patients received HE072 at 70 mg/m2 dose level. The demographics and baseline characteristics of the study patients were summarized in Table 1. TNBC patients had received a median 3.0 (2~8) lines of chemotherapy prior to enrollment. Of all TNBC patients, 20.8% had received PD-1/PD-L1 inhibitors, and 23.8% had more than four metastatic sites. Five of 18 patients with HER2-negative BCBM had previously received endocrine therapy.
A total of 119 patients were enrolled, including 101 patients with metastatic TNBC and 18 patients with HER2-negative BCBM. At the cutoff date of June 5, 2023, all 119 patients discontinued the treatment due to disease progression, withdrawn from the study, intolerable toxicity, and others. BCBM breast cancer brain metastasis, EAS efficacy analysis set, FAS full analysis set, HER2 human epidermal growth factor receptor, PKCS pharmacokinetics concentration set, PKPS pharmacokinetics parameter set, TNBC triple negative breast cancer.
Primary endpoints
Safety
At 70 mg/m2 dose level (n = 107), a total of 104 patients (97.2%) had one or more treatment emergent adverse events (TEAEs) of any grade, and 50 patients (46.7%) had ≥ grade 3 TEAEs (Supplementary Table 1). Treatment related adverse events (TRAEs) occurred in 103 patients (96.3%, Table 2). The most common TRAEs included diarrhea (72/107, 67.3%), leukopenia (64/107, 59.8%), nausea (61/107, 57.0%), anemia (59/107, 55.1%), neutropenia (57/107, 53.3%), vomiting (54/107, 50.5%), hypokalemia (37/107, 34.6%), fatigue (35/107, 32.7%),weight loss (30/107, 28.0%), lymphocytopenia (27/107, 25.2%), γ-glutamyl transpeptidase elevation (26/107, 24.3%), decreased appetite (25/107, 23.4%), hyponatremia (23/107, 21.5%), increased ALT (22/107, 20.6%), and abdominal pain (22/107, 20.6%)(Table 2). The safety of HE072 in patients with HER2-negative BCBM was shown in Supplementary Table 2.
Serious TEAEs were reported in 25 patients (23.4%), of whom 20 patients (18.7%) experienced serious TEAEs related to HE072 (Supplementary Table 3). TRAEs leading to permanent treatment discontinuation were reported in five patients (4.2%), including diarrhea (n = 3), upper abdominal pain (n = 1) and coma (n = 1). One patient had a TEAE leading to death, which was not related to HE072 treatment. She was found to develop brain and lung metastases after 5th dosing of HE072, and died two weeks later.
The median exposure to HE072 among all patients was 80.0 (range 14-436) days, and the mean (±SD) relative dose intensity was 85.7 ± 17.1%. Forty-seven patients (39.5%) experienced dose delay, reduction or interruption; of these, 37 patients had a dose delay, 26 patients had a dose reduction, and one patient had a dose interruption (Supplementary Table 4). A total of 119 patients discontinued the treatment at the cutoff date of June 5, 2023. Reasons for discontinuation treatment were disease progression (n = 83), withdrawal from the study (n = 21), intolerable toxicity (n = 6), and others (n = 9) (Fig. 1).
Determination of the RP2D
Only one dose limiting toxicity (DLT) (grade 3 nausea and grade 3 vomiting) was observed at 70 mg/m2 dose level in dose escalation part (n = 9), and the maximum toxicity dose (MTD) was not reached. Subsequently, the 50 mg/m2 and 70 mg/m2 every two weeks (Q2W) were expanded with additional 18 patients enrolled. Three serious adverse events (SAEs) occurred in 50 mg/m2 group in contrast to one SAE in 70 mg/m2 group. Based on the comprehensive review, the safety and tolerability were similar between 50 mg/m2 and 70 mg/m2. Together with pharmacokinetic data which showed that exposure (Cmax, AUC0-t and AUC0-∞) of free irinotecan and SN-38 in 70 mg/m2 group was greater than that in 50 mg/m2 group, 70 mg/m2 Q2W was determined as the RP2D. Thus, a dose level of 90 mg/m2 was not explored.
Secondary endpoints
Pharmacokinetics profile
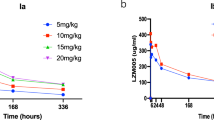
The mean plasma concentration-time curve and the detailed pharmacokinetic parameters of total irinotecan, free irinotecan, and SN-38 at HE072 50 mg/m2 and 70 mg/m2 were shown in Fig. 2 and Table 3, respectively. Over the dose range of 50 to 70 mg/m2, the Cmax and AUC of total irinotecan, free irinotecan, and SN-38 increases with dose. The mean Cmax and AUC0-∞ of total irinotecan and SN-38 with 70 mg/m2 were 45.0 μg/mL and 1706.2 h*μg/mL, 5.6 ng/mL and 427.9 h*ng/mL, respectively. The mean volume of distribution of total irinotecan with 70 mg/m2 was 3.6 L. Following a 90-min infusion, a low concentration of free irinotecan was recorded during the whole cycle, indicating that HE072 mainly presented in an encapsulated form in plasma.
Mean (SD) plasma concentration-time curve of free irinotecan (a), total irinotecan (b) and SN-38 (c) after the treatment with HE072 50 mg/m2 (n = 12) and 70 mg/m2 (n = 107). Over the dose range of 50–70 mg/m2, the Cmax and AUC of total irinotecan, free irinotecan, and SN-38 increases with dose (n = 119). Source data are provided as a Source Data file.
Efficacy
As of June 5 2023, 12 patients were not evaluable for response due to adverse events (n = 6), severe adverse events (n = 3), or withdrawal of consent (n = 3). Two patients with estrogen receptor (ER) > 10% had been excluded from efficacy evaluable set. Therefore, 87 (88.1%) of the 101 patients with TNBC were included in efficacy evaluable set. After a median follow-up of 10.1 months (range 8.8–10.8), 22 of 87 patients (25.3%, 95% confidence interval [CI] 16.6–35.8) had a partial response (PR), with four responses occurred at 50 mg/m2 Q2W and 18 at 70 mg/m2 Q2W. The median duration of response was 5.5 months (95% CI 4.3–9.4). Fifty-nine patients (67.8%, 95% CI 56.9–77.4) achieved disease control (22 PRs and 37 stable diseases [SDs]). The waterfall plot also showed very nice tumor shrinkage in 53 (59.6%) of 87 patients with measurable lesions (Fig. 3). In all patients with TNBC, the median progression-free survival (PFS) was 4.8 months (95% CI 3.2–5.8), the median OS was 14.1 months (95% CI 11.2-not reached), while 12-month and 18-month OS rate were 59.3% (95% CI 47.7–69.1) and 47.3% (95% CI 34.8–58.9), respectively (Table 4, Fig. 4).
Kaplan–Meier progression-free survival (a) and overall survival (b) curves in the patients with TNBC. a The median PFS was 4.8 months (95% CI 3.2–5.8). b The median OS was 14.1 months (95% CI 11.2-not reached), the OS rate was 59.3% (95% CI 47.7–69.1) at 12 months and 47.3% (95% CI 34.8–58.9) at 18 months. TNBC triple negative breast cancer. Source data are provided as a Source Data file.
Activity of HE072 was observed across multiple TNBC patient subgroups. The prespecified subgroup analysis showed a consistent response in patients with ECOG performance status (PS) of 1 (17/73, 23.3%), metastatic organs ≥4 (7/24, 29.2%) and presence of CNS metastasis (2/8, 25.0%; Supplementary Fig. 2).
Given the suboptimal response (objective response rate [ORR] 7.1%, 1/18) in 18 patients with HER2-negative BCBM treated with HE072, the recruitment of this cohort had been pre-terminated. All 18 patients had progression after prior radiotherapy. As of June 5, 2023, of 18 patients with HER2-negative BCBM, 1 patient (7.1%, 95% CI 0.2–33.9) achieved PR and 9 patients (64.3%, 95% CI 35.1–87.2) achieved disease control. The median PFS was 5.6 months (95% CI 1.4-NA), the median OS was not reached (95% CI 4.5-NA) and the 12-month OS rate was 55.5% (95% CI 27.4–76.5).
Exploratory endpoints
The frequencies of UGT1A1 homozygosity/compound heterozygosity, single heterozygous, and wild type were 10.9% (13/119), 26.9% (32/119) and 46.2% (55/119), respectively. More patients with homozygosity/compound heterozygosity had an incidence of grade ≥3 neutropenia than patients with wild type (75.0% vs. 31.4%; p = 0.042) and those with single heterozygous (75.0% vs. 41.2%; p = 0.20). No significant differences were observed in the incidence of grade ≥3 diarrhea, with 22.2% in patients with homozygosity/compound heterozygosity, 18.2% in those with single heterozygous and 13.6% in those with wild type (all p > 0.05) (Supplementary Fig. 3).
In total 119 patients, 26 were not available for Topo I expression, and samples from four patients were inappropriate for assessing Topo I expression. Topo I positivity and negativity were found in 11 (9.2%) and 78 patients (65.5%), respectively. No significant difference in PFS or OS was observed between patients with Topo I negative expressions and those with the Topo I positive expressions (both p > 0.05) (Supplementary Fig. 4).
Discussion
The prognosis of metastatic TNBC is rather poor, with an OS of around 20 months even after treatment8,9. For the first-line treatment of metastatic TNBC, the median PFS is only 9–10 months even with addition of an immune checkpoint inhibitor9,10. For patients after first-line therapy, few treatment options are limited and generally have disappointing efficacy. Chemotherapeutic agents11,12,13,14,15, such as nab-paclitaxel, capecitabine, vinorelbine or gemcitabine had been evaluated with a median PFS of 1.7–3.7 months and a median OS of 6.7–13.4 months.
Given the unmet medical needs for metastatic TNBC, novel therapies have been explored. A pooled analysis of two phase 3 studies16 (EMBRACE and Study 301) included 352 patients with TNBC, of whom, 199 patients received eribulin. The results showed a 2.8-month of median PFS and 12.4-month of median OS with eribulin. Meanwhile, novel formulations of traditional cytotoxic agents have been developed and exhibited promising efficacy for later-line treatment of TNBC17. In ASCENT trial13, the antibody-drug conjugate SG significantly improved PFS and OS in metastatic TNBC patients previously treated with at least 2 lines of chemotherapy compared with conventional chemotherapy, with median PFS and OS increased from 1.7 month to 5.6 months (HR 0.41, p < 0.001) and from 6.7 months to 12.1 months (HR 0.48, p < 0.001), respectively. Based on this trial, SG has been approved in metastatic TNBC patients treated with ≥2 lines of chemotherapy.
Liposomal irinotecan (Onivyde) had been tested previously in a phase I study in metastatic breast cancer18. Thirty patients were enrolled including 10 with ER+ and/or progesterone receptor (PR)+/HER2− breast cancer, 10 with metastatic TNBC and 10 with metastatic breast cancer with active brain metastasis. The median number of prior cytotoxic anticancer regimens was 3.0 (range: 0–6). The results showed that for non-CNS disease, the median PFS was 3.2 (range 1.8–8.4) months and the ORR was 34.5% (10/29) in advanced breast cancer. In total, nine patients with metastatic TNBC were evaluable for efficacy, and three (33.3%) achieved partial response. The median PFS was 4.3 (range 1.0–9.4) months and no median OS was reported.
The current trial is the trial investigating the efficacy of a liposomal irinotecan specifically in metastatic TNBC. Patients in our trial, compared with ASCENT trial13, possessed similar baseline characteristics. The median number of previous lines of systemic therapy was 3.0 for both SG and our study. It is inspiring to see that the median PFS of HE072 (4.8 months) in this study was comparable to or even better than previous studies13,16,19. The OS was also superior13,16,19 (14.1 months vs 12.1 months). Interestingly, no matter whether it is SG, Onivyde or HE072, Topoisomerase I inhibitor acts as the chemotherapy backbone. The consistent results suggested that Topoisomerase I inhibitor, when modified into new form, was a reasonable choice for metastatic TNBC and competent candidate for future drug development.
The prognosis of patients with BCBM is poor and no effective drug has been identified, especially for HER2-negative BCBM. Previous study showed that the median OS was 7.1 months for HER2−/HR+BCBM patients and 4.4 months for triple negative BCBM patients5. Recently, apatinib-based chemotherapy was evaluated in this type of patients in a real-world study, with a CNS ORR of 10.2% and a CNS PFS of 6.4 months20. In the present study, although the ORR was not ideal, the median PFS was 4.5 months and the median OS was 11.0 months, which suggested that HE072 might be a potential agent for treatment of HER2-negative BCBM, and warranting further exploration.
Our findings indicated that HE072 had an acceptable safety profile with no new safety signal. A total of 43% of patients had ≥grade 3 TRAEs, which was comparable to the incidence observed with Onivyde (41.4%)18. In the present study, the most common ≥grade 3 TRAEs included neutropenia (21.0%), leukopenia (18.5%), diarrhea (10.1%), anemia (9.2%) and hypokalemia (8.4%). While in NAPOLI-1 study21, patients with metastatic pancreatic cancer in the Onivyde monotherapy group experienced the most frequent ≥grade 3 TRAEs including diarrhea (21.0%), decreased appetite (19.0%), neutropenia (15.0%), vomiting (14.0%), hypokalemia (12.0%), anemia (11.0%), fatigue (9.0%), and nausea (8.0%). The safety profiles were actually quite similar, and the small differences might be explained by different disease and ethical differences in drug metabolism. Asian patients had a higher Cmax of SN-38 and a lower Cmax of total irinotecan than Caucasian patients, which was associated with an increased incidence of grade 3 or 4 neutropenia and a decreased incidence of grade 3 or 4 diarrhea in Asian versus Caucasian patients22.
In this trial, the homozygosity/compound heterozygosity group included patients with UGT1A1*6/*6, UGT1A1*28/*28 or UGT1A1*6/*28; the single heterozygous group included patients with UGT1A1*1/*6; and the wild type group included patients with UGT1A1*1. Since evidence showed that the UGT1A1*28 gene polymorphism alone is not predictive of irinotecan related toxicities in Asian patients and need to be analyzed in combination with UGT1A1*623, only patients with UGT1A1 *1/*6 were included in the single heterozygous group. The exploratory analysis showed that patients with homozygosity/compound heterozygosity had a numerically higher frequency of grade ≥3 neutropenia than those with single heterozygosity or wild type, while no significant differences were found in the frequency of grade ≥3 diarrhea across three groups. Previous studies implied that hematological toxicity may be more related to the UGT1A1*6 mutation, while diarrhea is more associated with the UGT1A1*28 mutation24, which might partly attribute to our results.
The current study had some limitations. First, this was a single-arm study with no control group, the results needed to be interpreted with caution, and future randomized controlled trials are warranted to confirm our findings. Secondly, few patients (20.8%) had previously received treatment with checkpoint inhibitors and no patient received treatment with SG (the reason was that all the patients of this study were enrolled between May 2021 and December 2022, while SG was approved in China for treatment of locally advanced and metastatic TNBC in June 2022), thus further investigations are needed to explore the efficacy of HE072 in these patients. Other limitations included patient recruitment solely in China, our findings extrapolation beyond Asian to other patient populations needed to be determined.
In summary, this study demonstrates that HE072 has encouraging antitumor activity with an acceptable safety profile in patients with heavily pretreated metastatic TNBC or HER2-negative BCBM, supporting further exploration of HE072 in this setting.
Methods
Study design and eligibility
This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. The study protocol and informed consent were approved by the independent ethic committee of all the study sites (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College; Affiliated Hospital of Chengde Medical University; Cangzhou Central Hospital; Guangxi Medical University Cancer Hospital & Guangxi Cancer Institute; Harbin Medical University Cancer Hospital; The Fourth Hospital of Hebei Medical University; Affiliated Hospital of Hebei University; The First Affiliated Hospital of Henan University of Science and Technology; Henan Cancer Hospital; Yuncheng Central Hospital; Chinese People’s Liberation Army General Hospital; The First Affiliated Hospital of China Medical University; Sun Yat Sen Memorial Hospital of Sun Yat Sen University; West China Hospital; Tianjin Medical University Cancer Institute & Hospital; Beijing Hospital; Jiangsu Provincial People’s Hospital; The First Affiliated Hospital of Chongqing Medical University; Xuzhou Central Hospital; The First Affiliated Hospital of Bengbu Medical University; Liaoning Cancer Hospital; The First Affiliated Hospital of USTC; The Fifth Affiliated Hospital of Guangzhou Medical University; Linyi Cancer Hospital; The First Affiliated Hospital of Guangxi Medical University; The Third Xiangya Hospital of Central South University; The Second People Hospital of Yibin; The First Affiliated Hospital of Hainan Medical University; Zhejiang University Hospital of Medicine SIR Run Run Shaw Hospital; The First Affiliated Hospital of Xi’an Jiaotong University; Fudan University Huashan Hospital; The Second People Hospital of Neijiang; Shandong Cancer Hospital) and registered at ClinicalTrial.gov (NCT04728035, date of registration: January 28 2021). Written informed consents had been obtained from all the patients before the enrollment.
This study consisted of two parts, dose escalation and expansion part, expansion cohort part. In expansion cohort part, there were two cohorts: cohort 1 for metastatic TNBC, cohort 2 for HER2-negative BCBM (Supplementary Fig. 1).
In the dose escalation and expansion part, 3 + 3 design was used to investigate three dose levels of HE072 (50 mg/m2, 70 mg/m2 and 90 mg/m2). The starting dose was selected based on a combination of pharmaceutical/preclinical data of the investigational agent and dose in clinical study of Onivyde (NCT03328884). DLTs were defined as grade ≥3 treatment-related nonhematologic adverse events or any grade ≥4 treatment-related hematologic adverse event during the 28-observation period. If two of the six enrolled patients in a dose level experienced DLTs, a lower dose level was determined as MTD. Once a dose level has been determined to have not exceeded the MTD, up to six to nine additional subjects may be enrolled for dose expansion to provide additional safety information on that dose level.
Eligible patients had histologically confirmed TNBC according to American Society of Clinical Oncology–College of American Pathologists (ASCO-CAP) guidelines25,26; pretreated with taxanes and anthracyclines; progressed after at least two prior lines of therapy or be intolerant to prior therapy; at least one measurable lesion at baseline as per RECIST v1.1; an ECOG PS of 0-2; a life expectancy of at least three months. Exclusion criteria were previous treatment with irinotecan, topotecan, or any other topoisomerase I inhibitors; previous treatment with nitrosoureas within six weeks before the first administration of HE072, cytotoxic chemotherapy or PD-1/PD-L1inhibitors within three weeks, small molecular targeted therapy, or endocrine therapy within two weeks or 5 half-lives of the agent (whichever is longer), radiation therapy within two weeks.
In the expansion cohort part, patients all received HE072 70 mg/m2 Q2W. The eligibility criteria for cohort 1 were same as that of the dose escalation and expansion part. In cohort 2, patients were required to have HER2-negative breast cancer, defined as HER2 immunohistochemistry (IHC) score 0-1 or FISH negative; had developed new lesions or progressed after radiation therapy and/or surgery for the treatment of intracranial lesion; with at least one measurable (≥10 mm) intracranial lesion. The complete lists of inclusion and exclusion criteria of this two-part study were provided in the study protocol (available in the Supplementary Information file).
Intervention
Patients were premedicated with antiemetics (e.g. tropisetron 5 mg or ramosetron 0.3 mg or palonosetron 0.25 mg) and dexamethasone 12 mg within 30 min prior to administration of HE072, then received HE072 (43 mg/10 ml) intravenously infused over 90 min on day 1 of each two-week cycle. Treatment continued until disease progression, intolerable toxicity, or loss to follow-up (whichever occurred first).
Treatment discontinuation would be applied if any treatment related grade 3 or 4 adverse effects occurred during the study. Patients could continue the treatment with dose reduction when the symptoms resolve to grade 1 or baseline level after the intervention. A maximum of two dose reduction was allowed. The study was completed in six months after the last patient received last dose of HE072.
Procedure
ER/PR status and HER2 status of all patients were evaluated and recorded from archival tumor tissue samples. Safety was evaluated at baseline and day 1, day 8, day 14 of each cycle, up to the end of the study. Safety assessment included vital signs, physical examination, ECG, and laboratory tests, and adverse events. Blood samples were collected at the following time point: 0.5 h before dosing and during the 90 min administration of HE072, 0.5, 1, 1.5, 2.5, 4.5, 10.5, 24, 48, 72, and 168 h postdose, as well as 0.5 h before dosing of the second cycle. Tumor assessment was carried out using enhanced CT scan (covering neck, thorax, and abdomen), bone ECT at baseline and every 6 weeks during the treatment and follow-up period for PFS. Patients were followed up for survival status every 12 weeks after disease progression or initiation of new anticancer therapy. Archival tumor tissue blocks (FFPE formalin-fixed, paraffin-embedded tumor tissue) at baseline were collected to examine Topo I expression by IHC assays (Topo-I antibody [1:200], SC-32736, SANTA, U.S.A) and blood samples were obtained for analysis of UGT1A1 gene polymorphism (Sanger sequencing) before the first administration of HE072.
Outcome
The primary endpoints were to determine the MTD, RP2D, and profile of TEAEs graded according to CTCAE, version 5.0. All adverse events were coded by MedDRA (version 25.0). The secondary endpoints were pharmacokinetic profiles and efficacy including ORR and disease control rate (DCR) assessed by investigators as per RECIST v1.1(all patients) and CNS ORR and CNS clinical benefit rate (CBR) as per RANO-BM (patients with HER2-negative BCBM), DoR, PFS, OS. The prespecified exploratory endpoints were UGT1A1 gene polymorphism expressed as the frequencies of homozygosity/compound heterozygosity (UGT1A1*6/*6, UGT1A1*28/*28 or UGT1A1*6/*28), single heterozygous (UGT1A1*1/*6) and wild type and Topo I expression (negative/positive, based on the percentage of positive tumor cells, <30% interpreted as negative and ≥30% as positive27).
Statistical analysis
As a phase Ib trial, the sample size was not determined based on statistical assumption, and the maximum sample size for the dose escalation and expansion part and expansion cohort part were set to 36 and 100, respectively.
DLT was assessed in DLT evaluable set, which included patients enrolled in the DLT evaluation period and completed the DLT evaluation or withdrawn the study due to AEs in the DLT evaluation period. Safety was assessed in safety analysis set, which included all patients who received at least one dose of HE072 and have safety assessment result. Mean plasma concentration-time curve was generated based on the pharmacokinetic concentration set (PKCS), who have received at least one dose of HE072 and have at least one drug concentration result. The main pharmacokinetic parameter of total irinotecan, free irinotecan and SN-38 were estimated using noncompartmental model (Phoenix WinNonlin version 8.3.4) based on pharmacokinetic parameter set (PKPS), who have received at least one dose of HE072 and have at least one available pharmacokinetic parameter. Full analysis set (FAS) included patients receiving at least one dose of HE072 and efficacy evaluable analysis set (EAS), included patients receiving at least one dose of HE072 and having at least one available post-baseline tumor assessment. ORR/DCR and CNS ORR/CBR were calculated with 95% CI using Clopper-Pearson method. Kaplan-Meier method was used to estimate median PFS and OS and 95% CI. Prespecified subgroup analyses (age [<65 years, ≥65 years], number of previous systemic therapies [<5, ≥5], ECOG PS [0, 1, 2], metastatic organs [<4, ≥4], previous treatment with PD-1/PD-L1 inhibitor [yes, no], CNS metastasis [yes, no]) of best overall response rate in patients with metastatic TNBC was planned. Kaplan–Meier analysis was used to examine the relationship between Topo I expression categories (positive vs negative) and survival outcomes (PFS/OS). A chi-square test was used for comparison of the incidence of grade ≥3 neutropenia/diarrhea in patients with UGT1A1 homozygosity/compound heterozygosity, single heterozygous and wild type.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Individual clinical data cannot be made publicly available. The datasets (including de-identified individual data) generated during the current study are available from the corresponding author upon request by contacting [email protected], not for commercial use. All requests will be reviewed by the corresponding author and the sponsor, CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd within two weeks. A signed data access agreement with the sponsor is required before data sharing. The study protocol is available in the Supplementary Information file. Remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Pareja, F. et al. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer 2, 16036 (2016).
Lin, N. U. et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113, 2638–2645 (2008).
Galvin, A. et al. First-line real-world treatment patterns and survival outcomes in women younger or older than 40 years with metastatic breast cancer in the real-life multicenter French ESME cohort. Eur. J. Cancer 196, 113422 (2024).
Kuksis, M. et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 23, 894–904 (2021).
Darlix, A. et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br. J. Cancer 121, 991–1000 (2019).
Witzel, I. et al. Breast cancer brain metastasis: biology and new clinical perspectives. Breast Cancer Res. 18, 8 (2016).
Mohammad, A. S. et al. Liposomal Irinotecan Accumulates in Metastatic Lesions, Crosses the Blood-Tumor Barrier (BTB), and Prolongs Survival in an Experimental Model of Brain Metastases of Triple Negative Breast Cancer. Pharm. Res. 35, 31 (2018).
Huang, W. et al. A real-world study of the effectiveness and safety of apatinib-based regimens in metastatic triple-negative breast cancer. BMC Cancer 24, 39 (2024).
Schmid, P. et al. First-Line Ipatasertib, Atezolizumab, and Taxane Triplet for Metastatic Triple-Negative Breast Cancer: Clinical and Biomarker Results. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-23-2084 (2023).
Jiang, Z. et al. Toripalimab plus nab-paclitaxel in metastatic or recurrent triple-negative breast cancer: a randomized phase 3 trial. Nat. Med. https://doi.org/10.1038/s41591-023-02677-x (2024).
Cortes, J. O. et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 377, 914–923 (2011).
Yuan, P. et al. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: A randomised clinical trial. Eur. J. Cancer 112, 57–65 (2019).
Bardia, A. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384, 1529–1541 (2021).
Winer, E. P. et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 499–511 (2021).
Curigliano, G. et al. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. Breast 22, 650–656 (2013).
Pivot, X. et al. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann. Oncol. 27, 1525–1531 (2016).
Li, S., Bao, C., Huang, L. & Wei, J. Current therapeutic strategies for metastatic triple-negative breast cancer: from pharmacists’ perspective. J. Clin. Med. 11, 6021 (2022).
Sachdev, J. C. et al. Phase I study of liposomal irinotecan in patients with metastatic breast cancer: findings from the expansion phase. Breast Cancer Res Treat. 185, 759–771 (2021).
Kaufman, P. A. et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 33, 594–601 (2015).
Chen, X. et al. The anti-tumor efficiency of low-dose apatinib-based chemotherapy in pretreated HER2-negative breast cancer with brain metastases. Ann. Med. 55, 2218647 (2023).
Wang-Gillam, A. et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 108, 78–87 (2019).
Adiwijaya, B. S. et al. Population Pharmacokinetics of Liposomal Irinotecan in Patients With Cancer. Clin. Pharm. Ther. 102, 997–1005 (2017).
Ju, X. Y., Luo, X. M., Wang, Y. Q. & Ge, W. H. UGT1A1 genetic information combined with therapeutic drug monitoring in individualized treatment with irinotecan. Cent. South Pharm. 13, 1178–1182 (2015).
Peng, H., Duan, Z., Pan, D., Wen, J. & Wei, X. UGT1A1 gene polymorphism predicts irinotecan-induced severe neutropenia and diarrhea in Chinese cancer patients. Clin. Lab. 63, 1339–1346 (2017).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Robert, N. J. et al. Predictive value of topoisomerase 1 by immunohistochemistry (TOP1 IHC) in patients with metastatic breast cancer receiving irinotecan-based therapy. J. Clin. Oncol. 34, 1037 (2016).
Acknowledgements
We thank all the patients who participated in this study. This study was supported by CSPC Ouyi Pharmaceutical Co, Ltd, and CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-12M-1-014 & 2023-I2M-C&T-B-077 and Major project of Medical Oncology Key Foundation of Cancer Hospital Chinese Academy of Medical Sciences CICAMS-MOMP202203. The sponsor provided the investigated drugs and worked with investigators on the study design, data collection, data analysis, and results interpretation. We would like to thank Lei Wang for the medical writing assistance, who is an employee of the CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd.
Author information
Authors and Affiliations
Contributions
B.X. and Y.L. contributed to the conception and design of the study. Y.F., Q.Z., M.Y., X.Q., Y.Y., T.S., J.Y., Y.W., X.W., Z.N., X.S.W., S.S., W.Z. and X.Z. enrolled patients and contributed to data acquisition. M.N. was responsible for data analysis. Y.F. wrote the first draft of the manuscript. All authors had reviewed the data analyses, contributed to data interpretation, contributed to revise the manuscript, and all authors approved the manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
Y.L., M.N. and X.Z. are employees of CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd. B.X. receives consulting fees from AstraZeneca and Novartis. All other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Communications thanks Elizabeth Sakach, Xinjie Hu, Mahtab Rouhi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, Y., Zhang, Q., Yan, M. et al. Intravenous liposomal irinotecan in metastatic triple-negative breast cancer after ≥ 2 prior lines of chemotherapy: a phase Ib study. Nat Commun 16, 3 (2025). https://doi.org/10.1038/s41467-024-55090-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55090-4