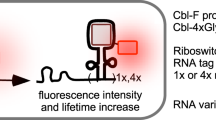
Abstract
Fluorogenic RNA aptamers have various applications, including use as fluorescent tags for imaging RNA trafficking and as indicators of RNA-based sensors that exhibit fluorescence upon binding small-molecule fluorophores in living cells. Current fluorogenic RNA:fluorophore complexes typically emit visible fluorescence. However, it is challenging to develop fluorogenic RNA with near-infrared (NIR) fluorescence for in vivo imaging and sensing studies. To address this issue, we identify and modulate red fluorescent protein-like fluorophores to bind Squash, a highly folded fluorogenic RNA. One of these fluorophores, DFQL-1T, exhibits photostable NIR fluorescence when bound to Squash, enabling RNA visualization in living mammalian cells and mice. With Squash:DFQL-1T complexes, we generate RNA-based sensors for detecting non-coding RNAs and small molecule targets in living mammalian cells and in mice. These studies reveal a fluorogenic RNA:fluorophore complex that can be readily developed into NIR fluorescent RNA tags for in vivo imaging and sensing.
Similar content being viewed by others
Introduction
Fluorogenic RNA aptamers are RNA sequences that bind otherwise non-fluorescent small-molecule fluorophores and switch them to a fluorescent state1. By tagging an RNA of interest with a fluorogenic RNA aptamer, one can visualize cellular RNA and the biological processes it involves, including RNA localization, RNA transcription rates, and RNA translocation kinetics2,3,4,5. Additionally, fluorogenic RNA aptamers have been developed as genetically encoded biosensors for real-time monitoring of ions, small-molecule metabolites, endogenous RNA, and proteins in living cells6,7,8,9,10,11,12,13,14,15,16,17.
A major limitation is that current fluorogenic RNA-based imaging and sensing tools have hardly been used in the near-infrared (NIR) range (>650 nm) for in vivo applications. In an NIR optical window, high tissue auto-fluorescence from indigenous pigments does not interfere with NIR fluorescence18. Moreover, NIR photons may penetrate relatively deeply into tissues and cause less damage to living cells19. Although there are reports on the development of NIR fluorogenic RNA aptamer for bacterial RNA imaging20, it is challenging to construct NIR fluorogenic aptamer for in vivo RNA imaging and sensing study. Therefore, the development of NIR fluorogenic RNA aptamer for RNA imaging and RNA-based sensing studies in vivo is highly desirable.
Here, we describe an NIR fluorogenic RNA aptamer:fluorophores complexes, allowing for in vivo RNA imaging and RNA-based sensing of various targets. We first determined that Squash21,22, a recently reported highly folded fluorogenic RNA aptamer, can accommodate red fluorescent protein (RFP) chromophores with π-conjugation extension. We then structurally modulated a series of fluorophores that resemble RFP chromophores, resulting in red-shifted emission spectra. Subsequently, we incorporated a trifluoroethyl group at the N2 position of these fluorophores, leading to the development of DFQL-1T, a fluorophore that emits photostable NIR fluorescence (Em=654 nm) upon binding to Squash with high affinity. With Squash:DFQL-1T complexes, we developed NIR fluorescent tags for RNA imaging in mammalian cells and living mice.
With Squash:DFQL-1T system, we engineered the NIR sensors for non-coding RNAs (ncRNAs) and small molecule detection. We first constructed several ncRNA sensors for the detection of microRNA and PIWI-interacting RNA (piRNA) in living mammalian cells and living mice. In addition, we developed NIR sensors for in vivo sensing of small molecules, including endogenous S-adenosylmethionine (SAM) and exogenous tetracycline. To the best of our knowledge, this is the first time that fluorogenic RNA-based sensors have been used for sensing targets in vivo. Overall, the Squash:DFQL-1T system provides a robust and versatile NIR fluorescent platform for in vivo RNA-based imaging and sensing.
Results
Identification of red fluorescent protein-like fluorophores to light up Squash RNA
To create NIR fluorescent RNA tags and RNA-based sensors, we chose the newly reported Squash as the fluorogenic RNA aptamer21,22. Squash is a highly folded RNA and binds DFHBI (3,5-difluoro-4-hydroxybenzylidene imidazolinone), a fluorophore that resembles the chromophore in green fluorescent protein (GFP) (Fig. 1a, b). Therefore, the Squash:DFHBI complex is an RNA mimic of GFP with green fluorescence (Ex = 428 nm, Em = 493 nm) (Table 1)21.
a, b Squash exhibits the solvent-accessible opening at the C2 position of DFHBI (indicated as red arrow) in relative to Broccoli. To determine if Squash is able to accommodate derivatives of DFHBI with C2 extension, we analyzed the crystal structure of Squash:DFHBI complex22 and Spinach/Broccoli:DFHBI26. The C2 position of DFHBI in Squash faces a solvent-accessible opening. In contrast, Spinach or Broccoli cannot accommodate extended π-conjugation at the C2 position of DFHBI. c Chemical structure of DFHBI and DFHO. DFHBI and DFHO are fluorophore mimics of eGFP and RFP, respectively. DFHO contains an extended π-conjugation at the C2 position, inducing a red-shift emission compared to DFHBI. d Squash can light up both DFHO and DFHBI, but Broccoli lights up DFHBI, rather than DFHO. Squash can bind DFHO and DFHBI to activate fluorescence, indicating that Squash can accommodate extended π-conjugation at C2 position of DFHBI. Broccoli or Squash fluorescence was measured in the presence of DFHBI (Broccoli: Ex = 470 nm, Em = 501 nm; Squash: Ex = 428 nm, Em = 493 nm) or DFHO (Broccoli: Ex = 513 nm, Em = 572 nm; Squash: Ex = 495 nm, Em = 562 nm). Data represent mean values ± s.d. for n = 3 independent experiments.
In addition, Squash binds DFHO (3,5-difluoro-4-hydroxybenzylideneimidazolinone-2-oxime) (Fig. 1b, Table 1)4,21, a fluorophore that resembles the fluorophore in DsRed red fluorescent protein (RFP)23. Compared to DFHBI, DFHO contains an N-hydroxyl imine (i.e., oxime) at the C2 position, which extends the π-electron conjugation (Fig. 1c). Therefore, Squash:DFHO complexes exhibit red-shift fluorescence (Ex = 495 nm, Em = 562 nm) compared to Squash:DFHBI (Table 1). This contrasts with Broccoli3,24,25,26, a well-studied fluorogenic RNA aptamer that binds DFHBI rather than DFHO, indicating that the fluorophore-binding pocket in Broccoli cannot accommodate DFHBI with bulky substituents at the C2 position (Fig. 1c, d; Supplementary Fig 1). These data suggest that the fluorophore-binding pocket in Squash can accommodate DFHBI derivatives with π-conjugation extension at the C2 position.
We considered modifying the DFHBI core at the C2 position to extend its π-conjugation for NIR fluorescence emission. Squash crystal structure showed that the hydroxybenzylidene imidazolinone (HBI) core in DFHBI and DFHO are responsible for Squash binding22. Moreover, the HBI core in DFHBI derivatives is responsible for low cell-induced background. In addition, the C2 position of DFHBI faces a solvent-accessible opening in the Squash aptamer (Fig. 1a)22,26. Based on this, we reasoned that DFHBI derivatives containing modifications at the C2 position would still fit into Squash with a low cellular background. Furthermore, the extended π-conjugation at the C2 position of DFHBI causes a red-shift in fluorescence4,27. We therefore sought to create NIR fluorophores by adding substituents at the C2 position of DFHBI to further extend its π-conjugation.
We first reasoned if the fluorophores found in RFPs can bind Squash and exhibit NIR fluorescence. Most RFPs utilize a fluorophore that is similar to hydroxybenzylidene imidazolinone in GFP, the difference being that the RFP fluorophores are modified with an imidazole substituent on the C2 position, which extends their π-conjugation (Fig. 2a–d). Since DFHO with oxime substituent at C2 position resembles the fluorophore in DsRed, it only exhibits orange fluorescence (Fig. 2b). We therefore considered the other fluorophores found in RFPs.
a Structures of eGFP fluorophore (green; top row) and the mimicking synthetic compounds DFHBI (middle row). Excitation and emission spectra were measured using 20 μM small-molecule fluorophores. DFHBI exhibits an excitation maximum of 423 nm and an emission maximum of 489 nm (bottom row). b Structure of DsRed fluorophore (red; top row) and synthetics DFHO and DFAME (middle row). The C2 methyl group in DFHBI was replaced with hydroxamic acid (DFHO) or methyl acrylate (DFAME), conferring a more extended π-electron conjugation system. Compared to DFHBI, DFHO exhibits a 72 nm (Ex = 473 nm, Em = 561 nm) and DFAME exhibits a 148 nm (Ex = 499 nm, Em = 637 nm) redshift in their emission spectra, respectively. c Structure of Kaede fluorophore (violet; top row) and its mimics DFIM and DFQL, respectively (middle row). We introduced an ethenylimidazole or ethenylquinoline substitution at position C2 to synthesize DFIM (Ex = 475 nm, Em = 568 nm) and DFQL (Ex = 513 nm, Em = 658 nm).
To mimic DsRed fluorophore with further red-shifted spectra, we synthesized DFAME (3,5-difluoro-4-hydroxybenzylidene imidazolinone-2-acrylatemethyl), in which the C2 methyl group in DFHBI was replaced with methyl acrylate, conferring a more extended π-electron conjugation system27. As expected, DFAME exhibited substantially red-shifted emission spectra (Ex = 499 nm, Em = 637 nm) compared to DFHBI (Ex = 424 nm, Em = 497 nm) and DFHO (Ex = 473 nm, Em = 561 nm) (Fig. 2b, Table 1).
We then examined various fluorophores found in Kaede28 RFPs, which have red-shifted fluorescence emission (Fig. 2b, c). We therefore synthesized various fluorophores, termed RFP-mimics, resembling the naturally occurring fluorophores in these RFPs. We introduced an ethenylimidazole or ethenylquinoline substitution at position C2 to resemble the fluorophore of Kaede, named DFIM and DFQL (Fig. 2c, Table 1, Supplementary Figs. 16–17, 22–25). Compared to DFHBI, DFIM (Ex = 475 nm, Em = 568 nm) and DFQL (Ex = 513 nm, Em = 658 nm) exhibited a 71 nm and 161 nm red-shifted emission, respectively. Notably, DFQL shows the NIR emissive fluorescence.
To test if these RFP-mimics can bind to Squash, we measured the dissociation constants (KD) of Squash with these RFP-mimics fluorophores. Squash binds DFAME (KD = 104 nM), DFIM (KD = 32 nM), and DFQL (KD = 125 nM) with nanomolar affinity, similar to the affinity of Squash:DFHBI (KD = 150 nM) (Table 1). These results indicate that these RFP-mimics fluorophores can bind Squash with high affinity as DFHBI.
We next examined whether these fluorophores emit NIR fluorescence upon binding to Squash. Squash can modulate the spectral properties of its bound fluorophores29. We therefore measured the fluorescence spectra of various Squash:RFP-mimics complexes. When bound to Squash, the emission of DFAME (Em = 621 nm), DFIM (Em = 553 nm) and DFQL (Em = 630 nm) were blue-shifted (Supplementary Fig. 2). The spectral shift in Squash might be due to hydrogen bonds formation or π-stacking interactions between the fluorophores and nucleotides within Squash29.
Squash:DFQL-1T exhibits NIR fluorescence
We further red-shifted the emission of the Squash:RFP-mimics into the NIR region by modifying the RFP-mimics fluorophores. A previous study reported that replacing the N1 methyl substituent of the fluorophore with a trifluoroethyl substituent caused red-shifted emission spectra (Fig. 3a)30. For example, DFHBI-1T exhibits a 6 nm red-shift in the emission compared to DFHBI (Table 1). Furthermore, Squash:DFHBI-1T complex exhibited a ~10 nm red-shift in the emission peak compared to Squash:DFHBI (Table 1). In addition, Squash:DFHBI-1T exhibited a 1.39-fold fluorescent increase compared to Squash:DFHBI (Table 1). We therefore synthesized the above RFP-mimics with trifluoroethyl substituent at N1 position (RFP-mimics-1T), including DFHO-1T, DFAME-1T, DFIM-1T, and DFQL-1T (Fig. 3a, b, Supplementary Figs. 18–21, 26–33).
a Schematic drawing of Squash-binding fluorophores and their derivatives with trifluoroethyl substitutions at the N1 position. To enhance the brightness and red-shift the fluorescence spectra of Squash-binding fluorophores (left), we synthesized these fluorophore derivatives with trifluoroethyl at the N1 position (RFP-mimics-1T, right), including DFHBI-1T, DFHO-1T, DFIM-1T, DFAME-1T, DFQL-1T. b Excitation and emission spectra of Squash bound to RFP-mimics-1T. Excitation (Ex, dotted line) and emission (Em, solid line) spectra were measured using in vitro transcribed Squash RNA (10 μM) and RFP-mimics-1T (1 μM) in buffer. In particular, Squash:DFQL-1T complex exhibits NIR fluorescence (Ex=516 nm, Em= 654 nm). c RFP-mimics-1T exhibit improved fluorescence compared to RFP-mimics when binding to Squash. We incubated Squash (1 μM) with different Squash-binding fluorophores (RFP-mimics or RFP-mimics-1T, 10 μM) and measured the fluorescence. The RFP-mimics-1T exhibited increased fluorescence compared to the original RFP-mimics. Data represent mean values ± s.d. for n = 3 independent experiments. d RFP-mimics-1T exhibit increased Squash fluorescence in living cells. HEK293T cells expressing circular Squash RNA were imaged in the presence of 2 μM RFP-mimics or RFP-mimics-1T. RFP-mimics-1T exhibit increased Squash fluorescence in living cells compared to RFP-mimics. Exposure time: 500 ms for RFP-mimics or RFP-mimics-1T and 100 ms for Hoechst. Imaging filters: DFHBI-1T/DFHBI (Ex = 488 nm, Em = 525 ± 25 nm), DFHO-1T/DFHO/DFIM-1T/DFIM (Ex = 514 nm, Em = 575 ± 20 nm), DFAME-1T/DFAME (Ex = 561 nm, Em = 617 ± 32.5 nm), DFQL-1T/DFQL (Ex = 514 nm, Em = 685 ± 20 nm). Scale bar, 20 µm. e Quantification of average brightness of Squash-expressing cells incubated with different fluorophores. Background fluorescence was measured in control cells with fluorophores alone (10 μM). Data represent mean values ± s.d. for n = 100 cells per condition. Statistical significances were determined by a two-tailed Student’s t test. ****p-values: 4.8 × 10−36, 3.9 × 10−82, 1.8 × 10−71, 4.1 × 10−95, 1.0 × 10−95, in left-to-right order. f, g Imaging of transplanted mice using Squash:DFQL-1T system. The results showed that mice incubated with Squash-expressing cells exhibited obvious NIR fluorescence signal (top row), while mice incubated with control cells exhibited minimal fluorescence in the presence of DFQL-1T. Image acquisition time, 500 ms. Data represent mean values ± s.d. for n = 3 independent experiments.
Compared to Squash:RFP-mimics, Squash:RFP-mimics-1T including DFHO-1T, DFAME-1T, DFIM-1T, and DFQL-1T showed a 7 nm, 7 nm, 17 nm, 24 nm red-shifted emission, respectively (Supplementary Fig. 3).
In particular, the emission peak of Squash:DFQL-1T complex reaches the NIR region (Ex = 516 nm, Em = 654 nm) (Fig. 3b and Supplementary Fig. 3). In addition, the Squash:DFQL-1T complex exhibited the largest Stokes shift (138 nm) compared to all other reported fluorogenic RNA system (Supplementary Fig. 4 and Supplementary Table 1)31.
We next asked if these Squash:RFP-mimics-1T complexes exhibit higher fluorescence brightness than Squash:RFP-mimics complexes in vitro. We measured the fluorescence intensity of different RFP-mimics or RFP-mimics-1T with the addition of in vitro Squash RNA. Compared to the original Squash:RFP-mimics complexes, the fluorescence of Squash:RFP-mimics-1T is increased by 1.3- to 1.5-fold (Fig. 3c, Table 1). This increase in brightness is mainly due to the increased fluorescence quantum yield (Φ) (Table 1). In particular, the NIR fluorescent Squash:DFQL-1T complex exhibits a quantum yield of 0.14 (Fig. 3c, Table 1), which is fairly similar to or higher than other reported NIR fluorescent proteins, including iRFP670 (Φ=0.12), iRFP682 (Φ=0.11), iRFP713 (Φ=0.063)32. These results show that replacing the N1 methyl substituent with a trifluoroethyl substituent leads to an increase in fluorescence brightness.
To determine whether RFP-mimics-1T fluorophores light up the cellular Squash brightness. We expressed Squash in HEK293T cells using the Tornado expressing system, which resulted in high-level expression of Squash as a circular RNA9. One hour before imaging, we replaced the culture medium with a phenol red-free medium containing each fluorophore (2 μM). These RFP-mimics-1T or RFP-mimics fluorophores exhibit low cellular background in control HEK293T cells, and high fluorescence in Squash-expressing HEK293T cells (Fig. 3d). In addition, RFP-mimics-1T showed increased Squash-induced fluorescence compared to the cognate RFP-mimics (Fig. 3d, e). These RFP-mimics-1T also exhibited high fluorescence in BL21 Escherichia coli (E. coli) cells expressing Squash (Supplementary Fig. 5). In particular, we found that DFQL-1T alone shows low background in control cells, and high NIR fluorescence compared to DFQL in circular Squash-expressing cells (Fig. 3d, e). This is because Squash:DFQL-1T shows higher absolute brightness (Table 1) rather than higher thermostability (Supplementary Fig. 6) compared to Squash:DFQL.
In addition, we compared Squash:DFQL-1T system with a previously developed NIR fluorogenic RNA imaging system, DIR2s:DIR-pro33. DIR2s RNA aptamer binds and activates the otherwise non-fluorescent small molecules DIR-pro (Em = 658 nm)33. We expressed and imaged DIR2s or Squash RNA in HEK293T cells in the presence of their cognate fluorophore DIR-pro or DFQL-1T (2 μM), respectively. However, although DIR2s:DIR-pro was reported with high in vitro brightness (ε = 164000 M–1 cm–1, Φ=0.33)33, we could not image intracellular DIR2s (Supplementary Fig. 4), which is in agreement with the previous reports33. In contrast, the intracellular Squash can be readily imaged with DFQL-1T (Supplementary Fig. 4). In addition, DFQL-1T exhibits a low cellular background but DIR-Pro shows obvious non-specific cellular background fluorescence (Supplementary Fig. 4). Therefore, Squash:DFQL-1T system exhibits low cellular background and readily detectable RNA fluorescence compared to the reported NIR emissive DIR2s:DIR-pro system in living mammalian cells.
Next, we asked whether Squash:DFQL-1T system can be used for visualizing linear RNAs in living cells. In contrast to Tornado-expressed circular RNA, which accumulates in high concentrations in cells9, linear RNAs typically reach physiological concentrations of nanomolar levels34. Since the DFQL-1T can detect Squash-fused RNA with high sensitivity (Supplementary Fig. 7), we reasoned that Squash:DFQL-1T system may be able to image linear RNA in cells. We then fused Squash to U6 and 7SK RNA and expressed them as linear RNA in HEK293T cells. Squash-fused U6 and 7SK RNA were readily detected as a punctate NIR signal in DFQL-1T-treated cells. Typically, U6 RNA and 7SK RNA are reported to localize to Cajal bodies35 and nuclear speckles36, respectively. To further validate the puncta localization, we labeled Cajal bodies and nuclear speckles with co-transfected SART335 and SC35 protein36. As expected, we found that the U6 RNA puncta co-localized with SART3 and 7SK RNA puncta co-localized with SC35, respectively (Supplementary Fig. 8). These data indicated that Squash:DFQL-1T system can NIR fluorescently image linear RNAs and their subcellular RNA localization in living cells.
To image Squash:DFQL-1T with an NIR excitation wavelength, we used two-photon microscopy to image Squash:DFQL-1T. The RFP-like fluorophore can be excited by simultaneously absorbing two photons with about twice the usual excitation wavelength37,38. We imaged Squash-expressing cells with excitation wavelengths ranging from 980 nm to 1140 nm. Notably, we observed obvious NIR fluorescence in Squash-expressing cells at 1100 nm excitation (Supplementary Fig. 9). These results showed that Squash:DFQL-1T complex can be effectively excited with NIR wavelength excitation using two-photon microscopy.
We then wondered if Squash:DFQL-1T complex can image in vivo RNA. To test this, we first transplanted the engineered or controlled HEK293T cells (~108 cells) subcutaneously into the right buttocks of mice. Next, we injected DFQL-1T (10 μM) in situ at the transplantation site, and then observed marked NIR signals in mice transplanted with Squash-expressing HEK293T cells (Fig. 3f). No significant fluorescence was observed in control mice injected with untransfected HEK293T cells (Fig. 3g). Overall, these data show that Squash:DFQL-1T complex with NIR fluorescence enables RNA imaging in living mice.
Squash:DFQL-1T shows photostable fluorescence
We next examined the photostability of Squash:DFQL-1T complexes in vitro. As a control we used Squash:DFHO, which was reported to exhibit high photostability4. We incubated DFQL-1T or DFHO (1 μM) with Squash RNA (10 μM) in a cuvette. Then, the samples were continuously irradiated for 10 min. The Squash:DFHO complex showed obvious photobleaching, with >50% of the fluorescence lost after 10 min. In contrast, Squash:DFQL-1T exhibited remarkable photostability, showing minimal loss of fluorescence after 10 min of irradiation (Fig. 4a).
a Squash:DFQL-1T complex exhibits significantly high photostability compared to Squash:DFHO complex in vitro. Fluorescence was measured in a solution containing Squash RNA (10 μM) with DFQL-1T or DFHO (1 μM). The Squash:DFHO complex showed a ~60% loss of fluorescence in 10 min. In contrast, the Squash:DFQL-1T complex exhibited no significant loss of fluorescence. b Quantification of cellular photostability. After 60 s of continuous light exposure, the Squash:DFQL-1T complex exhibited minimal cellular fluorescence loss. As a control, the Squash:DFHO complex lost 40% of cellular fluorescence. Squash:DFQL-1T and Squash:DFHO were both irradiated with identical light sources (514 nm laser, 20 mW/cm2). Error bars indicate mean values ± s.d. for n = 10 cells per condition. c DFQL-1T exhibits markedly improved Squash photostability in living HEK293T cells compared to DFHO. Cellular photostability of Squash:fluorophore (DFQL-1T or DFHO, 2 μM) was assessed by continuous imaging of circular Squash-expressing cells for 60 s. The acquisition time for each frame is 200 ms. Scale bar, 20 µm.
We also measured the photostability in Squash-expressing HEK293T cells in the presence of DFQL-1T or DFHO during continuous irradiation with identical light sources (514 nm laser, 20 mW/cm2). DFHO-treated cells showed a 40% loss of fluorescence in 60 s. In contrast, photobleaching was considerably less in DFQL-1T-treated cells, showing little fluorescence loss in 60 s (Fig. 4b, c). These results show that the Squash:DFQL-1T exhibits significantly high photostability both in vitro and in cells.
Squash-based NIR fluorescent sensors for imaging non-coding RNAs
We next converted the Squash:DFQL-1T complex into an NIR fluorescent sensor. RNA-based sensors have been extensively designed for various target detection. However, current fluorogenic RNA-based sensors are limited to green to red fluorescence. RNA-based sensors with NIR fluorescence are highly desirable for in vivo sensing studies and have not been developed before. Thus, we sought to design NIR fluorescent sensors based on the Squash:DFQL-1T complex.
We then designed a sensor with Squash:DFQL-1T complex for non-coding RNAs (ncRNAs). ncRNAs play essential roles in regulating various biological processes39,40,41. Therefore, real-time imaging of the abundance and subcellular distribution of ncRNAs can help us to understand their biological functions. Previous fluorogenic RNA-based sensors for ncRNA imaging typically used linear RNAs (Supplementary Fig. 10)2,42,43,44. However, linear RNAs can be rapidly degraded and do not accumulate to levels that generate a sensitive fluorescence signal in mammalian cells14,45. In contrast, circular RNA and RNA-based sensors are more stable in cells9,46. Thus, we sought to design a circular ncRNA sensor.
To design circular ncRNA sensors, we used the “three-way junctions” approach (Fig. 5a)47. In this approach, the ncRNA-targeting sequence is inserted into one stem of a three-way junction and Squash is inserted into another stem. When the targeted ncRNA hybridizes to its binding sequence, it triggers the folding of Squash in the other stem, leading to fluorescence output (Fig. 5a). In our design, the 5’ and 3’ ends of the sensor can be engineered with the Tornado system and circularized in cells.
a Design of the Squash-based ncRNA sensors. The ncRNAs probe (green), stem ___domain, and Squash ___domain (beige) are depicted. The stem ___domain is composed of Squash aptamer and target sequence that form a short helix. Target ncRNA binding to the antisense probe would form a longer and stabler duplex than the original stem, thus inducing the folding of Squash aptamers and NIR fluorescence of DFQL-1T. b, c Optimization of the stems for the ncRNA sensors. We generated the microRNA-21 and piRNA-36026 sensors with different stems by in vitro transcription, respectively. Sensors with different stems (5 µM) were incubated with DFQL-1T (10 µM) in the presence or absence of targets (10 µM). The optimal microRNA-21 sensor containing stem 3 and piRNA-36026 sensor containing stem 4 showed a 6-fold and 5-fold increase in fluorescence upon target incubation, respectively. The optimal stem is indicated in the black box. Data represent mean values ± s.d. for n = 3 independent experiments. d, e Imaging endogenous ncRNAs with Squash-based ncRNA sensors. To determine if the Squash-based ncRNA sensors can image ncRNAs in living cells, we expressed Squash in LO2 and MCF-7. Expression levels of miRNA-21 and piRNA 36026 in MCF-7 are significantly higher compared to LO2. Cell culture media was then replaced with phenol red-free DMEM containing DFQL-1T (10 μM) for 1 h before imaging. NIR fluorescence can be readily observed in MCF-7 cells, and the corresponding ncRNA inhibitors can reduce the fluorescence to a minimum level. Image acquisition time, 500 ms. Scale bar, 20 µm. Data represent mean values ± s.d. for n = 3 independent experiments. f, g Squash-based NIR fluorescent sensor for ncRNAs imaging in vivo. Cells expressing ncRNA sensors (108 cells) were transplanted into mice, and then injected with DFQL-1T (10 µM) in situ for imaging. Sensor-expressing MCF-7 cells exhibit significantly higher NIR fluorescence (bottom row) compared to LO2 cells (top row). Image acquisition time, 500 ms. Data represent mean values ± s.d. for n = 3 independent experiments.
We chose microRNA-21, an ncRNA overexpressed in most cancer cells48, as a target to validate this “three-way junction” sensor design approach. We designed the RNA-probing module as an antisense sequence of microRNA-21 (Fig. 5b). To optimize the microRNA-21 sensor, we tested different stem domains between microRNA-21 antisense and Squash. Each stem has a different number of nucleotides complementary to Squash, resulting in different levels of impact on Squash folding. Among these sensors, microRNA-21 sensor containing stem 3 showed a 6-fold fluorescence activation in the presence of microRNA-21 (Fig. 5b).
We next generated other ncRNA sensors using the “three-way junction” sensor design approach. PIWI-interacting small non-coding RNA (piRNA)-36026 is a cancer biomarker candidate because of its high expression level in various cancer types and its role in the control of tumor suppressor genes49. We set the antisense of piRNA-36026 as the RNA-probing module to generate piRNA-36026 sensors. We designed various piRNA-36026 sensors with different stems and generated a sensor that exhibited a 5-fold fluorescence activation upon binding to piRNA-36026 (Fig. 5c). Each of the sensors was only activated by its cognate ncRNA, rather than by the control RNAs (Supplementary Fig. 11). These data suggest that Squash-based sensors with the “three-way junction” approach can be applied to detect various ncRNAs in vitro.
To determine the sensitivity of ncRNA sensors, we incubated the sensors with different concentrations of target RNA. The sensors exhibited increased fluorescence at a target RNA concentration of 0.1 nM–5 μM, with a linear detection range of 1–100 nM (Supplementary Fig. 11). In addition, the detection limits of the miRNA-21 sensor and piRNA-36026 sensor were estimated to be 0.2 nM and 0.3 nM, respectively (Supplementary Fig. 11).
We then imaged endogenous ncRNAs in living cancer cells. The endogenous microRNA-21 and piRNA-36026 are highly expressed in MCF-7 cancer cells, but lowly expressed in human fetal hepatocyte LO2 cells50. We expressed microRNA-21 sensor or piRNA-36026 sensor in both MCF-7 and LO2 cells9. To locate the transfected cells, these ncRNA sensors were co-expressed with a blue fluorescent protein (BFP) encoded on the same plasmid. Although ncRNA sensors were expressed in MCF-7 or LO2 cells at a similar level, sensor-expressing MCF-7 cells, rather than LO7 cells, exhibit readily detectable NIR fluorescence (Fig. 5d, e and Supplementary Fig. 12). No significant NIR fluorescence was detected in MCF-7 or LO2 cells transfected with a mock plasmid (Fig. 5d, e).
To determine whether the NIR fluorescence in sensor-expressing MCF-7 cancer cells is due to high levels of microRNA-21 or piRNA-36026, we treated the cells with two distinct ncRNA inhibitors. These cells exhibited significantly reduced NIR fluorescence after inhibitor treatment (Fig. 5d, e). Additionally, we performed qPCR experiments to quantify the levels of these two ncRNAs. Our results confirmed that microRNA-21 and piRNA-36026 are expressed at high levels in MCF-7 cells and at low levels in LO2 cells (Supplementary Fig. 12). These results showed that ncRNA sensors enable the detection of endogenous ncRNAs in living cancer cells in the NIR channel.
To determine if Squash-based sensors can image ncRNAs in living mice, we then transplanted the sensor-expressing cells to mice. We found that mice with the engineered MCF-7 cells (~108 cells) exhibited high NIR fluorescence, whereas mice with the engineered LO2 cells showed limited NIR fluorescence (Fig. 5f, g). Therefore, the results showed that these sensors can detect ncRNAs in vivo.
NIR imaging of intracellular small molecules in living mammalian cells and in vivo
We next designed NIR fluorescent sensors for small molecules imaging with Squash:DFQL-1T complex. To do so, we converted Squash into a tetracycline sensor and a SAM sensor, respectively. These sensors comprise a tetracycline-binding aptamer51 or a SAM-III riboswitch51,52 fused to Squash via a transducer ___domain. The transducer ___domain is a thermodynamically unstable helix, and therefore stays unfolded in the absence of the target small molecule. However, upon target binding, the transducer ___domain properly folds, which allosterically induces the folding of Squash (Fig. 6a).
a Modular design of the Squash-based NIR fluorescent sensor for small molecule targets. The target binding aptamer (blue) was fused to Squash (beige) through a transducer module (black). Target binding induces the allosteric conformation of tetracycline aptamer, then stabilizes the helix structure of the transducer ___domain, thus enabling Squash to bind NIR fluorophore. b, c Optimization of the transducer for small molecules sensor. The optimal tetracycline sensor (b) containing transducer 2 ___domain (black box), or SAM sensor (c) was chosen with the highest signal-to-background of 14-fold or 5-fold, respectively. Data represent mean values ± s.d. for n = 3 independent experiments. d Using Squash-based NIR fluorescent sensors to image tetracycline in living cells. We expressed the tetracycline sensor in HEK293T cells. Cells treated with tetracycline (0.1 mM) showed increased NIR fluorescence. Image acquisition time, 500 ms. Scale bar, 20 µm. Data represent mean values ± s.d. for n = 3 independent experiments. e Squash-based NIR fluorescent sensor for tetracycline imaging in vivo. We incubated sensor-expressing cells (108 cells) in the presence or absence of tetracycline (0.1 mM). Then, we transplanted these cells to mice by subcutaneous injection. After 2 h, DFQL-1T (10 µM) was injected in situ for imaging. Mice transplanted with tetracycline-treated cells exhibit higher NIR fluorescence (bottom row). Image acquisition time, 500 ms. Data represent mean values ± s.d. for n = 3 independent experiments. f Imaging SAM in live cells in the NIR channel. We readily detected NIR fluorescence in cells expressing SAM sensors. After being treated with cycloleucine (30 mM), NIR fluorescence decreased significantly. Scale bar, 20 µm. Data represent mean values ± s.d. for n = 3 independent experiments. g Monitoring SAM metabolites in vivo. Engineered HEK 293T cells expressing SAM sensor (108 cells) were incubated with cycloleucine (30 mM) for 1 h. Cells were then transplanted into mice by subcutaneous injection. About 2 h after transplant, DFQL-1T (10 µM) was injected in situ for imaging. Sensor-expressing cells display pronounced skin-permeable NIR fluorescence in mice. Cycloleucine treatment induced a significant decrease in fluorescence. Image acquisition time, 500 ms. Data represent mean values ± s.d. for n = 3 independent experiments.
We then determined whether Squash-based NIR fluorescent sensors could detect SAM and tetracycline in vitro, respectively. In a previous study, Squash-based SAM sensor with transducer 1 was reported to detect SAM with a 5-fold fluorescence increase (Fig. 6c)21. To further optimize the SAM sensor, we designed several transducer domains, each with a different thermodynamic stability. However, the previously reported SAM sensor with transducer 121 still gave the optimal signal-to-noise ratio (Fig. 6c). Next, we focused on the optimization of the Squash-based tetracycline sensor (Fig. 6b, c). The optimal tetracycline sensor exhibited a 14-fold increase in fluorescence upon binding to tetracycline (Fig. 6b). In addition, the Squash-based SAM sensor and tetracycline sensor both exhibit high specificity (Supplementary Fig. 11).
To measure the sensitivity of these small molecule sensors, we incubated SAM or tetracycline sensors with different concentrations of SAM or tetracycline, respectively. Both sensors exhibited fluorescence responses to the cognate targets in a dose-dependent manner with a linear detection range of 1–100 μM (Supplementary Fig. 11). The detection limit of SAM sensor and tetracycline sensor was estimated to be 40 nM and 5.4 nM, respectively (Supplementary Fig. 11).
We next expressed Squash-based sensors to image their target molecules in HEK293T cells. After incubation with DFQL-1T, cells expressing tetracycline sensor exhibit minimal fluorescence in the absence of the tetracycline (Fig. 6d). When incubated with a medium containing tetracycline, HEK293T cells expressing tetracycline sensors showed dose-dependent fluorescence enhancement in the range of 25–250 μM (Fig. 6d and Supplementary Fig. 13), which is in agreement with the previous reports53. In cells expressing SAM sensor, we observed strong NIR fluorescence, indicating that Squash-based SAM sensor is capable of imaging endogenous SAM in living cells. After incubation with cycloleucine (30 mM), an inhibitor of SAM synthesis, NIR fluorescence decreased to a minimum in cells expressing SAM sensor (Fig. 6f). Cellular fluorescence was recovered within 3 h after cycloleucine removal (Supplementary Fig. 14). In addition, we also imaged tetracycline and monitored SAM biosynthesis in bacterial cells using the Squash-based NIR sensors (Supplementary Fig. 15). Together, these results confirmed that NIR fluorescent sensors based on Squash:DFQL-1T can image small molecules in living cells.
We also determined whether the sensors can image small molecules in living mice. Engineered HEK293T cells expressing tetracycline sensor or SAM sensor were treated with the cognate target. We then transplanted these cells to the living mice. As expected, mice transplanted with tetracycline-treated cells exhibited detectable NIR fluorescence, while vehicle-treated mice exhibited minimal NIR fluorescence. In mice transplanted with SAM sensor-expressing cells, we readily detected NIR fluorescence of Squash. We observed decreased NIR fluorescence in mice transplanted with cycloleucine-treated cells (Fig. 6e, g). Therefore, these data suggest that Squash-based NIR sensors can be broadly used as small molecule imaging platforms in vivo.
Discussion
Although there are a lot of fluorogenic RNA aptamers, there is little development of NIR fluorogenic RNA for imaging and sensing study. In this study, we converted green fluorescent Squash to a red and NIR fluorescent RNA tag by rationally designing Squash-binding fluorophores. Based on Squash crystal structure with the chemical structure of RFP chromophores, we developed DFQL-1T, a Kaede RFP-like fluorophore, to light up Squash with a low background, and obvious NIR fluorescence. With DFQL-1T, we readily imaged RNA in vivo in the NIR channel. Furthermore, we constructed various Squash-based NIR fluorescent sensors for ncRNAs and small molecules in living mice.
To design Squash-binding NIR fluorophores, we resembled the naturally occurring chromophores in DsRed and other RFPs. We synthesized a series of RFP-like fluorophores, including DFHO, DFAME, DFIM and DFQL. Compared to GFP-like DFHBI fluorophores, these RFP-like fluorophores have additional diverse substituents on the C2 position of imidazolinone that extend the π-conjugation system, resulting in red-shifted fluorescence emissions.
To further redshift the emission of these fluorophores, we introduced a trifluoroethyl substituent to the N1 position of the imidazolinone ring. The trifluoroethyl substituent has been previously shown to red-shift the fluorescence of DFHBI30. In our study, we found that the trifluoroethyl substituent effectively red-shifted the fluorescence of DFHBI derivatives. When introducing trifluoroethyl substituent to DFQL, we created DFQL-1T, which exhibits NIR spectra when bound to Squash.
In addition to red-shifting the fluorophore emission, the trifluoroethyl substituent can increase the brightness of the fluorophore. Squash:DFQL-1T complex shows a 1.3-fold increase in fluorescence compared to Squash:DFQL. Other RFP-like fluorophores with trifluoroethyl substituent also exhibited fluorescence enhancement, which reflects an increase in the extinction coefficient and the quantum yield (Table 1). The quantum yield of Squash:DFQL-1T is fairly similar to or higher than other reported NIR fluorescent proteins, including iRFP670 (Φ = 0.12), iRFP682 (Φ = 0.11), iRFP713 (Φ = 0.063)32 and is close to widely used Cy5 NIR fluorophores(Φ = 0.2)54. However, the relatively moderate extinction coefficient (ε = 18,300 M-1cm-1) resulted in a lower overall brightness compared to Cy5 (ε = 250,000 M−1cm−1). Therefore, the extinction coefficient of Squash-binding fluorophores still needs to be improved to increase the overall RNA:fluorophores brightness.
Moreover, Squash:DFQL-1T exhibits significantly high photostability. The increased photostability of Squash:DFQL-1T makes it particularly advantageous for long-term imaging applications. This is in contrast to other RNA mimics of fluorescent protein, such as Spinach/Broccoli1,24, whose fluorescence was rapidly photobleached under constant irradiation3. Therefore, our findings demonstrated that RNA mimics of fluorescent proteins can be highly photostable with optimized aptamer:fluorophores.
In addition, the Squash:DFQL-1T complex exhibits a large Stokes shift of 138 nm. This significant difference between the absorption and emission wavelengths offers various advantages. Fluorophores with large Stokes shift exhibit reduced reabsorption of emitted light and enhanced imaging accuracy and specificity. Moreover, these fluorophores can be simultaneously excited with other fluorophores at a single wavelength, thus enabling multiplexed imaging with reduced cross-talk55. Importantly, the Squash:DFQL-1T complex can be readily imaged with NIR excitation light (Ex =1100 nm) using two-photon microscopy. These characteristics make Squash:DFQL-1T complex well-suited for various fluorescence-based applications.
Additionally, we converted Squash:DFQL-1T complex into RNA-based sensors for ncRNAs and small molecular targets in living cells. To our knowledge, this is the first time RNA-based sensors have been developed to reach the NIR region. NIR fluorescence provides advantages such as deep tissue penetration, reduced background noise, and compatibility with existing imaging systems. Squash-based NIR sensors enable non-invasive imaging and sensing, offering valuable insights into molecular processes in vivo. With Squash-based NIR sensors, we can detect cellular targets in their intact and native physiological state in vivo.
The Squash-based sensor exhibits detection sensitivity in a low nanomolar range toward ncRNAs, which is consistent with previously reported RNA-based sensors2. However, the Squash-based sensors without signal amplification might not readily detect lowly-abundant RNA (mRNA et al.) in living cells. To overcome this limitation, it may be effective to combine the Squash:DFQL-1T system with signal amplification techniques, such as hybridization chain reaction (HCR)56 or rolling circle amplification (RCA)57 to further enhance the detection sensitivity for lowly-abundant RNA detection.
A few NIR fluorogenic RNA:fluorophore complexes have been previously developed for RNA imaging, including BeCA:BC620, SiRA:SiR-PEG3-NH258,59, DIR2s:DIR-pro33 and biSiRA:SiR260. However, these NIR fluorogenic RNAs have only been reported to be imaged in E. coli bacteria or mammalian cells, rather than in vivo. Different from these systems, we here developed an NIR fluorogenic RNA:fluorophore complexes for RNA imaging and sensor development in living mammalian cells and in vivo. The DFQL-1T fluorophore shows low molecule weight (MW = 459 Da) compared to the large NIR emissive fluorophores like SiR2 (MW = 1902 Da)60, potentially showing efficient pharmacokinetics and biodistribution, resulting in minimal perturbation61. Therefore, we expected that the Squash:DFQL-1T system would readily facilitate the use of RNA-based imaging tags or sensors in vivo and mammalian cells.
In addition, the ability to modulate the spectral properties of Squash by using different fluorophores provides a versatility that is not seen with fluorescent proteins, which typically have fixed fluorescence properties. With these fluorophores, Squash exhibited a multicolor spectral range from cyan (Em = 498 nm) to NIR (Em = 654 nm). This flexibility will provide great convenience for RNA-based imaging and sensing. For example, the ratiometric sensors can be readily designed with Squash and other orthogonal fluorogenic RNA aptamers, which are spectrally separated from each other11,21. Moreover, the Squash-binding fluorophores also enable imaging of the same target in different colors by simple washing and re-staining.
Furthermore, we showed that NIR fluorogenic RNA-based tools can track RNA in vivo. By real-time monitoring of RNA in living organisms, we can unravel the intricacies of gene expression regulation and diagnose diseases through abnormal RNA expression patterns. Our study presents the crucial application of fluorogenic RNA as an in vivo imaging agent, offering novel perspectives for in vivo RNA imaging and sensing.
Methods
Reagents and equipment
For in vivo imaging, all procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Institute of Zoology (IOZ), Chinese Academy of Sciences (CAS). All primers used in this experiment were ordered from Tsingke Biotechnology (Beijing, China). In vitro fluorescence experiments were measured on FluoroMax+ spectrofluorometer (Horiba Scientific) using FluorEssence software (v.3.9). In-cell fluorescence imaging experiments were obtained on Olympus SpinSR10 microscope (Olympus). The living mice fluorescence imaging was carried out using IVIS Spectrum in vivo imaging system (PerkinElmer). The data were plotted using Origin 2021 and GraphPad Prism 9 software.
Cell culture and transfection
HEK293T (ATCC-CRL-11268), Hela (ATCC-CCL-2), MCF-7 (ATCC-HTB-22), LO2 (ATCC-HL-7702) cell lines were obtained from American Type Culture Collection (ATCC). Cells were grown in DMEM (Invitrogen C11995500BT) supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin under 37 °C with 5% CO2. Cells were detached using TrypLE Express (Gibco 25200056) according to the manufacturer’s instructions. Plasmid transfection was conducted using Lipofectamine 3000 (Invitrogen L3000008) according to the manufacturer’s instructions. Cells were seeded onto 24-well plates (Nest 801006) 1 day prior to transfection.
In vitro synthesis of RNA
For Squash, Broccoli, and all Squash-based sensors RNA, double-stranded DNA templates were designed to contain a 5’ T7 promoter to be used for in vitro transcription. Double-stranded DNA templates were prepared from single-stranded DNA oligos. DNA templates were amplified by PCR using Taq DNA polymerase (Vazyme P112) and purified with the PCR purification kit (Axygen AP-PCR-250).
In vitro transcription reactions using the T7 High Yield RNA Transcription Kit (Vazyme D4201) were carried out at 37 °C at least 4 h. Transcription reactions were terminated by treating with DNase I at 37 °C for at least 15 min. After termination of the reaction, 80 µL of RNase-free water was added, followed by an equal volume of water-saturated phenol chloroform, and centrifuged at 4 °C for 15 min at 13,000g. The supernatant was aspirated and one-tenth of the volume of sodium acetate and 2 times the volume of anhydrous ethanol was added and settled at -20 °C for at least 30 min. Subsequently, the precipitate was washed with 75% ethanol prepared with RNase-free water, centrifuged for 5 min, and then dissolved with RNase-free water.
Excitation and emission spectra measurement of Squash: fluorophore complexes
In vitro purified Squash RNA (10 µM) was mixed with cognate fluorophores (1 µM) in the buffer (40 mM HEPES, pH 7.4, 100 mM KCl and 0.5 mM MgCl2). After 1 h of incubation, the fluorescence was measured at 37 °C using FluoroMax+ spectrofluorometer with 5 nm slit widths and 0.1 s integration time.
Quantum yield and extinction coefficient measurements
All quantum yields were determined by comparing the integral of the corrected emission spectra for each fluorophore or RNA: fluorophore complex with the corresponding integral obtained from a solution of DFHO or Squash-DFHO1. The integrals at different concentrations were then compared to the absorbance obtained at the wavelength corresponding to the excitation wavelength, and the slope of the curve was compared to the slope of the curve for the reference fluorophore. All measurements on RNA-fluorophore complexes were performed in the presence of excess RNA (10 µM) to avoid interference from unbound fluorophores. The extinction coefficient was calculated based on the absorbance spectrum and the Beer–Lambert–Bouguer law.
Affinity measurements
Dissociation constants (KD) for the Squash-fluorophore complexes were determined by measuring the increase in fluorescence as a function of increasing fluorophore concentration in the presence of a fixed concentration of Squash2. In brief, the KD was determined by measuring at a fixed RNA aptamer concentration (50 nM) with increasing fluorophore concentration, and the resulting increase in fluorescence was recorded. For each concentration of fluorophore measured, a background signal for fluorophore only was also measured separately and subtracted from the signal measured for RNA-fluorophore together.
Curves were determined using nonlinear regression analysis in Origin software and matched by least-squares fitting to a dose-response stimulation model for log (agonist) vs. normalized response–Variable slope. The data points refer to the mean of three measurements (n = 3).
Thermostability measurements
To determine the thermostability of Squash: fluorophore, RNA (1 μM) was mixed with 10 μM DFQL-1T or DFHO in the buffer for 10 min. Then we recorded the Squash fluorescence in 2 °C increments from 20 °C to 80 °C using a FluoroMax+ spectrofluorometer. For each temperature, the cuvette undergoes a 5-min incubation to allow for equilibration. Fluorescence measurements were performed using a FluoroMax+ spectrofluorometer. Data was quantified and normalized to the intensity at 20 °C in Origin software.
In vitro photostability measurement of Squash: DFQL-1T
To measure Squash: fluorophore photostability in vitro, DFHO or DFQL-1T (50 nM) was mixed with excess Squash (1 µM) in buffer in a cuvette (100 µl) for 10 min. Fluorescence measurements were performed using a FluoroMax+ spectrofluorometer (Horiba Scientific) at room temperature. The slit widths of excitation were set to maximum (29.1 nm) to make sure the entire solution volume (100 µl) was maximally illuminated. The fluorescence intensities at different time points were quantified and normalized to the intensity at t = 0 s in Origin software.
Photostability measurement in living mammalian cells
DFHO or DFQL-1T fluorophores were added to the circular Squash-expressing HEK293T cells. Images were acquired at a rate of 5 frames per second and exposure time of 200 ms. The brightness was computed using ImageJ by measuring the signal in 10 cells’ area and subtracting background based on the average signal of control cells. Imaging conditions were maintained at 37 °C and 5% CO2. The brightness at different time points was quantified in Origin software and normalized to the intensity at time = 0 s.
Imaging living cell with NIR wavelengths excitation
Squash-expressing HEK293T cells were plated in a 6 cm cell culture dish. Thirty minutes before imaging, the medium was changed to phenol red-free DMEM (Biological Industries 06-1052-04-1ACS) containing DFQL-1T (2 µM). Images were acquired through a 20x water immersion lens mounted on a Nikon two-photon upright microscope (AX R MP) and analyzed with NIS-Elements software. Cells were imaged by modulating the titanium sapphire laser in a range of 980 nm-1140 nm in steps of 20 nm, with an emission filter that transmits light at 692 ± 37 nm. Acquisition time is 500 ms. Total cell fluorescence was computed using ImageJ by measuring the total signal in a cell’s area and subtracting the background. The cellular fluorescence values were analyzed and plotted using Origin 2021 software.
In vitro characterization of the Squash-based sensors
For Squash-based non-coding RNA sensors, sensor RNA (5 µM) was mixed with DFQL-1T (10 µM) in the absence or presence of non-coding RNA (10 µM) in the buffer. All samples were annealed by first holding at 65 °C for 10 min and then cooling to 25 °C at a rate of 0.5 °C /30 s. The incubation step was performed with a Thermal Cycler Applied Biosystems. Fluorescence signal of each sample was measured at room temperature using a FluoroMax+ spectrofluorometer with 516 nm excitation and 654 nm emission, 5 nm slit widths, and 0.1 s integration time. The buffer containing DFQL-1T (10 µM) was measured as a background signal. The background signal was subtracted from the signal obtained from each RNA sample measurement.
To test the ability of each indicated transducer sequence to mediate Tetracycline or SAM-induced fluorescence, in vitro purified RNA (5 µM) was mixed with DFQL-1T (10 µM) in the absence or presence of tetracycline (0.1 mM, BBI A600504-0025) or SAM (0.1 mM, Sigma-Aldrich A7007) in the buffer. After 1 h of incubation, the fluorescence of each sample was measured at 37 °C using a spectrofluorometer with 516 nm excitation and 654 nm emission, 5 nm slit widths, and 0.1 s integration time. The buffer containing DFQL-1T (10 µM) was measured as a background signal and was subtracted from the signal obtained from each measurement.
The sequences of all Squash-based sensors used in this study are shown in Supplementary Table 2.
Selectivity measurement of Squash-based sensor
To test the selectivity of the Squash-based non-coding RNA sensors. Non-coding RNA sensors (5 µM) were mixed with DFQL-1T (10 µM) in the presence of the target RNA (10 µM) or its analog RNAs (100 µM) in a buffer containing 40 mM HEPES-KOH (pH 7.4), 100 mM KCl and 0.5 mM MgCl2. All samples were annealed by first holding at 65 °C for 10 min and then cooling to 25 °C at a rate of 0.5 °C /30 s. The fluorescence signal of each sample was measured at room temperature using a FluoroMax+ spectrofluorometer with 516 nm excitation and 654 nm emission, 5 nm slit widths, and 0.1 s integration time.
For the selectivity of Squash-based small molecule sensors. Tetracycline or SAM sensors RNA (5 µM) was mixed with DFQL-1T (10 µM) in the presence of targets (0.1 mM) or its analogs (1 mM) in buffer. Analogs of tetracyclines used in selective experiments include neomycin (Solarbio N8090), tobramycin (Solarbio T8810), gentamycin (Solarbio G8170), ampicillin (Solarbio A1170) and kanamycin (Solarbio K1030). Analogues of SAM include SAH (Aladdin S139501), adenosine (Coolaber CA1241) or methionine (Coolaber CM7211).
Sensitivity range and detection limit measurement of Squash-based sensor
The Squash-based sensors (5 µM) were incubated with a range of concentrations of the corresponding target. The lower concentration range is fitted to a linear equation (R2 > 0.99). The detection limit (D) of Squash-based sensor can be determined using the following formula:
where δ is the standard deviation of the blank signal and S is the slope of the linear calibration plot3.
Detection of non-coding RNA expression by real-time PCR
Total RNA of all samples was isolated by TRIzol (Invitrogen™) following the manufacturer’s instructions. The quality and quantity of RNA were assessed using Nanodrop (Thermo Fisher). The cDNAs were reverse-transcribed from the purified RNA with specific primers (Supplementary Table 3) for the microRNA using the miRNA 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd MR101).
Real-time PCR analysis was conducted using Power SYBR Green PCR Master Mix (Applied Biosystems, 4309155) and carried on a LightCycler 480 Real-Time PCR System (Roche Applied Science). Primers used for real-time PCR were given in Supplementary Table 3.
The amplification protocol included an initial denaturation step at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing and extension at 60 °C for 15 s. A melt curve analysis was performed to verify the specificity of the amplification products.
Gene expression levels were calculated using 2(-ΔΔCt) method4, where the Ct values of the target gene were normalized to the Ct values of GAPDH and compared to a calibrator sample. Statistical analysis was performed using Origin 2021 software.
Microscopy and image processing of mammalian and bacterial cells
To image Squash in living mammalian cells, we coated confocal dishes (Nest Biotechnology 806001) with poly-D-lysine (Sangon E607014) for at least 4 h and rinsed once in water. HEK293T cells were transfected with plasmids encoding Squash RNA. One day after transfection, the cells were subcultured onto pre-treated confocal dishes. One hour before imaging, the medium was changed to phenol red-free DMEM (Biological Industries 06-1052-04-1ACS) supplemented with 10% FBS and cognate fluorophores (2 µM). Live cell fluorescence images were acquired through a 20× air objective (NA 0.75) mounted on the Olympus SpinSR10 confocal microscope and analyzed with the CellSens software. The imaging settings for each fluorophore are as follows: DFHBI-1T or DFHBI-treated cells were imaged with 488 nm laser excitation, and a bandpass emission filter that transmits 525 ± 25 nm light. DFHO-1T, DFIM-1T, DFHO or DFIM-treated cells were imaged with a 514 nm laser excitation and a modified YFP emission filter that transmits light at 575 ± 20 nm. DFAME-1T, DFAME-treated cells were imaged with a 561 nm laser excitation and 617 ± 32.5 nm emission filter. DFQL-1T, DFQL-treated cells were imaged with a 514 nm laser excitation, and NIR emission filter that transmits light at 685 ± 20 nm. DIR-Pro treated cells were imaged with a 640 nm laser excitation, and NIR emission filter that transmits light at 685 ± 20 nm. Hoechst-stained nuclei were imaged with 405 nm laser excitation filter, and 460 ± 25 nm emission filter. Exposure times: 200–500 ms for Squash fluorescence,100 ms for BFP or Hoechst. Cell fluorescence was computed using ImageJ by measuring the cell area signal and subtracting background based on the average signal of control cells. During live cell imaging acquisition, conditions were maintained at 37 °C and 5% CO2.
To image Squash in E. coli cells, we selected single colonies of target plasmids in LB media at 37 °C for 14-16 h. One day after E. coli cells multiplication, the BL21 (DE3) cells were grown in LB media at 37 °C until the optical density at OD600 reached 0.4, and then 1 mM IPTG was added for 4 h induction. We incubated IPTG-induced E. coli with M9 minimal medium (Gibco A1374401) containing fluorophore (10 µM) on poly-L-lysine (Sangon Biotech A600751) pretreated confocal discs for 1 h. Live cell fluorescence images were acquired through a 100× oil objective. Images were analyzed using CellSens and ImageJ software. During E. coli cell imaging acquisition, conditions were maintained at 37 °C and 5% CO2.
Sensing of ncRNAs or small molecules using Squash-based sensors in living mammalian cells
To image ncRNAs using Squash-based non-coding RNA sensors, the plasmids encoding non-coding RNA sensors were transfected into MCF-7 and LO2 cells, respectively. One day after transfection, the cells were subcultured onto PDL-coated confocal dishes. One hour before imaging, we replaced the cell culture medium with phenol red-free DMEM containing DFQL-1T (10 μM). Cells were imaged using the live cell imaging conditions described above.
For tetracycline imaging, HEK293T cells were transfected with plasmids encoding tetracycline sensor and subcultured onto PDL-coated confocal dishes the next day. One hour before imaging, we replaced the cell culture medium with phenol red-free DMEM containing DFQL-1T (10 μM) and tetracycline (100 μM, BBI A600504) and incubated for 1 h at 37 °C. The cells were then imaged using the live cell imaging conditions described above.
For SAM sensor imaging, HEK293T cells were transfected with plasmids encoding Squash-based SAM sensors or plasmids encoding Squash aptamer. After one day of transfection, the cells were subcultured onto coated glass-bottomed plates. After 36-48 h of transfection, we replaced the cell culture medium with phenol red-free DMEM containing DFQL-1T (10 μM) and incubated for 1 h at 37 °C. After adding cycloleucine (30 mM, Ark Pharm AK-29341), we imaged the cells for 2 h at 15-min intervals. We then withdrew the cycloleucine by changing the cell medium to a fresh medium and continued to image the cells at 15-min intervals for 3 h.
In vivo imaging and sensing using Squash: DFQL-1T complex
BALB/c mice (female, 6–8 weeks of age) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd and kept under specific pathogen-free conditions. The mice were housed in a conventional facility (12 h light/dark cycles at 22 °C), fed with standard food, and provided with water ad libitum.
Plasmids encoding Squash or Squash-based non-coding RNA sensor were transfected to cells. 36 h after transfection, we resuspended the cells (1 × 108) and transplanted them into the right buttocks of mice by subcutaneous injection. DFQL-1T (10 μM) was then injected in situ at the transplant site 2 h post-transplantation. Then, the mice were anesthetized with isoflurane and imaged using the IVIS Spectrum in vivo imaging system (Caliper Life Sciences). The excitation filter was 530 ± 20 nm, and the emission filter was 660 ± 20 nm. Exposure times: 1.0 s.
For Squash-based small molecules sensors, sensor-expressing HEK293T cells were incubated with the cognate targets (tetracycline, 0.1 mM) or inhibitor (cycloleucine, 30 mM) for 1 h and transplanted into mice. DFQL-1T (10 μM) was then injected in situ for imaging. Images were analyzed using ImageJ software.
Statistics and reproducibility
All in vitro experiments were performed in three independent repeats. The data are represented as the mean ± standard deviation (SD) of three independent repeats in the plots (n = 3). Statistical significances were determined by a two-tailed Student’s t test. No statistical method was used to predetermine sample size. No data were excluded from the analyses. The experiments were not randomized. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors affirm that all data generated or analyzed during this study are accessible through the corresponding author upon request. There is no restriction to accessing the source data, and the requests will be fulfilled within 4 weeks. Source data for the figures and Supplementary Figs. are available as a Source Data file accompanying this publication. Source data are provided with this paper.
References
Paige, J. S., Wu, K. Y. & Jaffrey, S. R. RNA mimics of green fluorescent protein. Science 333, 642–646 (2011).
Ying, Z. M., Wu, Z., Tu, B., Tan, W. & Jiang, J. H. Genetically encoded fluorescent RNA sensor for ratiometric imaging of MicroRNA in living tumor cells. J. Am. Chem. Soc. 139, 9779–9782 (2017).
Li, X., Kim, H., Litke, J. L., Wu, J. & Jaffrey, S. R. Fluorophore-promoted RNA folding and photostability enables imaging of single Broccoli-Tagged mRNAs in live mammalian cells. Angew. Chem. Int. Ed. Engl. 59, 4511–4518 (2020).
Song, W. et al. Imaging RNA polymerase III transcription using a photostable RNA-fluorophore complex. Nat. Chem. Biol. 13, 1187–1194 (2017).
Chen, W., Zhao, X., Yang, N. & Li, X. Single mRNA imaging with fluorogenic RNA aptamers and small-molecule fluorophores. Angew. Chem. Int. Ed. Engl. 62, e202209813 (2023).
Paige, J. S., Nguyen-Duc, T., Song, W. & Jaffrey, S. R. Fluorescence imaging of cellular metabolites with RNA. Science 335, 1194 (2012).
Song, W., Strack, R. L. & Jaffrey, S. R. Imaging bacterial protein expression using genetically encoded RNA sensors. Nat. Methods 10, 873–875 (2013).
You, M., Litke, J. L. & Jaffrey, S. R. Imaging metabolite dynamics in living cells using a Spinach-based riboswitch. Proc. Natl Acad. Sci. USA 112, E2756–E2765 (2015).
Litke, J. L. & Jaffrey, S. R. Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts. Nat. Biotechnol. 37, 667–675 (2019).
Li, X. et al. Imaging intracellular S-adenosyl methionine dynamics in live mammalian cells with a genetically encoded red fluorescent RNA-based sensor. J. Am. Chem. Soc. 142, 14117–14124 (2020).
Chen, Z. et al. Genetically encoded RNA-based sensors with Pepper fluorogenic aptamer. Nucleic Acids Res. 51, 8322–8336 (2023).
Fang, M. et al. Imaging intracellular metabolite and protein changes in live mammalian cells with bright fluorescent RNA-based genetically encoded sensors. Biosens. Bioelectron. 235, 115411 (2023).
Zhang, Z. et al. Fluorogenic CRISPR for genomic DNA imaging. Nat. Commun. 15, 934 (2024).
Zhang, Y. P. et al. In Situ Self-Assembly of Fluorogenic RNA Nanozipper Enables Real-Time Imaging of Single Viral mRNA Translation. Angew. Chem. Int. Ed. Engl. 62, e202217230 (2023).
Ma, K., Li, X., Xu, B. & Tian, W. Label-free bioassay with graphene oxide-based fluorescent aptasensors: A review. Anal. Chim. Acta. 1188, 338859 (2021).
Modell, A. E., Siriwardena, S. U., Shoba, V. M., Li, X. & Choudhary, A. Chemical and optical control of CRISPR-associated nucleases. Curr. Opin. Chem. Biol. 60, 113–121 (2021).
Li, X., Zamora-Olivares, D., Diehl, K. L., Tian, W. & Anslyn, E. V. Differential sensing of oils by conjugates of serum albumins and 9,10-distyrylanthracene probes: a cautionary tale. Supramol. Chem. 29, 308–314 (2016).
Rao, J., Dragulescu-Andrasi, A. & Yao, H. Fluorescence imaging in vivo:recent advances. Curr. Opin. Biotechnol. 18, 17–25 (2007).
Weissleder, R. & Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 9, 123–128 (2003).
Zhang, J., Wang, L., Jaschke, A. & Sunbul, M. A color-shifting near-infrared fluorescent aptamer-fluorophore module for live-cell RNA imaging. Angew. Chem. Int. Ed. Engl. 60, 21441–21448 (2021).
Dey, S. K. et al. Repurposing an adenine riboswitch into a fluorogenic imaging and sensing tag. Nat. Chem. Biol. 18, 180–190 (2022).
Truong, L. et al. The fluorescent aptamer Squash extensively repurposes the adenine riboswitch fold. Nat. Chem. Biol. 18, 191–198 (2022).
Gross, L. A., Baird, G. S., Hoffman, R. C., Baldridge, K. K. & Tsien, R. Y. The structure of the chromophore within DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci USA 97, 11990–11995 (2000).
Filonov, G. S., Moon, J. D., Svensen, N. & Jaffrey, S. R. Broccoli:rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J. Am. Chem. Soc. 136, 16299–16308 (2014).
Li, X., Wu, J. & Jaffrey, S. R. Engineering fluorophore recycling in a fluorogenic RNA aptamer. Angew. Chem. Int. Ed. Engl. 60, 24153–24161 (2021).
Warner, K. D. et al. Structural basis for activity of highly efficient RNA mimics of green fluorescent protein. Nat. Struct. Mol. Biol. 21, 658–663 (2014).
Wu, J. et al. Self-assembly of intracellular multivalent RNA complexes using dimeric corn and beetroot aptamers. J. Am. Chem. Soc. 144, 5471–5477 (2022).
Schnitzler, C. E. et al. Spectral diversity of fluorescent proteins from the anthozoan Corynactis californica. Mar. Biotechnol. (NY) 10, 328–342 (2008).
Filonov, G. S., Song, W. & Jaffrey, S. R. Spectral tuning by a single nucleotide controls the fluorescence properties of a fluorogenic aptamer. Biochemistry 58, 1560–1564 (2019).
Song, W., Strack, R. L., Svensen, N. & Jaffrey, S. R. Plug-and-play fluorophores extend the spectral properties of Spinach. J. Am. Chem. Soc. 136, 1198–1201 (2014).
Jiang, L. et al. Large Stokes shift fluorescent RNAs for dual-emission fluorescence and bioluminescence imaging in live cells. Nat. Methods https://doi.org/10.1038/s41592-023-01997-7 (2023).
Shcherbakova, D. M. et al. Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging. Nat Commun 7, 12405 (2016).
Tan, X. et al. Fluoromodules consisting of a promiscuous RNA aptamer and red or blue fluorogenic cyanine dyes:selection, characterization, and bioimaging. J. Am. Chem. Soc. 139, 9001–9009 (2017).
Filonov, G. S., Kam, C. W., Song, W. & Jaffrey, S. R. In-gel imaging of RNA processing using broccoli reveals optimal aptamer expression strategies. Chem. Biol. 22, 649–660 (2015).
Novotny, I. et al. SART3-dependent accumulation of incomplete spliceosomal snRNPs in Cajal bodies. Cell Rep 10, 429–440 (2015).
Prasanth, K. V. et al. Nuclear organization and dynamics of 7SK RNA in regulating gene expression. Mol. Biol Cell 21, 4184–4196 (2010).
So, P. T., Dong, C. Y., Masters, B. R. & Berland, K. M. Two-photon excitation fluorescence microscopy. Annu. Rev. Biomed. Eng. 2, 399–429 (2000).
Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat Commun 7, 11928 (2016).
Lee, J. T. Epigenetic regulation by long noncoding RNAs. Science 338, 1435–1439 (2012).
Cao, J. The functional role of long non-coding RNAs and epigenetics. Biol Proced Online 16, 11 (2014).
Dong, H. et al. MicroRNA:function, detection, and bioanalysis. Chem Rev 113, 6207–6233 (2013).
Wang, Z. et al. In situ spatial complementation of aptamer-mediated recognition enables live-cell imaging of native RNA transcripts in real time. Angew. Chem. Int. Ed. Engl. 57, 972–976 (2018).
Wang, Q. et al. Inert Pepper aptamer-mediated endogenous mRNA recognition and imaging in living cells. Nucleic Acids Res. 50, e84 (2022).
Gao, W., Chen, Z., Rong, X. & Li, X. Progress in RNA dynamic imaging technology in live cells. SCIENTIA SINICA Vitae 54, 651–667 (2024).
Chen, Z., Duan, H. & Li, X. In vivo single-molecule RNA structural profiling. Trends Biochem. Sci. 48, 211–212 (2023).
Unti, M. J. & Jaffrey, S. R. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem. Biol. 31, 163–176.e165 (2024).
Moon, J. D. et al. Naturally occurring three-way junctions can be repurposed as genetically encoded RNA-based sensors. Cell Chem Biol 28, 1569–1580.e1564 (2021).
Lagos-Quintana, M., Rauhut, R., Lendeckel, W. & Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 (2001).
Hashim, A. et al. RNA sequencing identifies specific PIWI-interacting small non-coding RNA expression patterns in breast cancer. Oncotarget 5, 9901–9910 (2014).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005).
Berens, C., Thain, A. & Schroeder, R. A tetracycline-binding RNA aptamer. Bioorg. Med. Chem. 9, 2549–2556 (2001).
Lu, C. et al. Crystal structures of the SAM-III/S(MK) riboswitch reveal the SAM-dependent translation inhibition mechanism. Nat. Struct. Mol. Biol. 15, 1076–1083 (2008).
Wu, R. et al. Genetically encoded ratiometric RNA-based sensors for quantitative imaging of small molecules in living cells. Angew. Chem. Int. Ed. Engl. 58, 18271–18275 (2019).
Mujumdar, R. B., Ernst, L. A., Mujumdar, S. R., Lewis, C. J. & Waggoner, A. S. Cyanine dye labeling reagents:sulfoindocyanine succinimidyl esters. Bioconjug. Chem. 4, 105–111 (1993).
Shcherbakova, D. M., Hink, M. A., Joosen, L., Gadella, T. W. & Verkhusha, V. V. An orange fluorescent protein with a large Stokes shift for single-excitation multicolor FCCS and FRET imaging. J. Am. Chem. Soc. 134, 7913–7923 (2012).
Evanko, D. Hybridization chain reaction. Nat. Methods 1, 186–186 (2004).
Garafutdinov, R. R., Sakhabutdinova, A. R., Gilvanov, A. R. & Chemeris, A. V. Rolling circle amplification as a universal method for the analysis of a wide range of biological targets. Russ J Bioorg Chem 47, 1172–1189 (2021).
Wirth, R., Gao, P., Nienhaus, G. U., Sunbul, M. & Jaschke, A. SiRA: a silicon rhodamine-binding aptamer for live-cell super-resolution RNA imaging. J. Am. Chem. Soc. 141, 7562–7571 (2019).
Zhang, Y. et al. Structural mechanisms for binding and activation of a contact-quenched fluorophore by RhoBAST. Nat. Commun. 15, 4206 (2024).
Buhler, B. et al. Avidity-based bright and photostable light-up aptamers for single-molecule mRNA imaging. Nat. Chem. Biol. 19, 478–487 (2023).
Yan, K. et al. Ultra-photostable small-molecule dyes facilitate near-infrared biophotonics. Nat Commun 15, 2593 (2024).
Acknowledgements
The authors thank Li lab members for their comments and suggestions. This work was supported by the National Key Research Program (No. 2023YFC2604300, X. Li), National Natural Science Foundation of China (No. 32271515, 32311530120, X. Li; No. 32494800, H. Song), High-level Talent Program for Innovate Talent, Hunan Province (No. 2024RC4015, X. Li), Hunan Provincial Natural Science Foundation of China (No. 2024JC0007, Y. Yin), Agricultural Biological Breeding Major Project (No. 2023ZD04046, Y. Yin) the start-up grant from UMass Amherst (J. Wu), Initiative Scientific Research Program, Institute of Zoology, Chinese Academy of Sciences (No. 2024IOZ0202, X. Li).
Author information
Authors and Affiliations
Contributions
X.L. conceived and designed the project. Z.C., W.C., and C.X. contributed equally to this work. C.X., H.J. and H.D. contributed compound synthesis. Z.C. and W.C. conducted imaging system construction. H.S. and X.J. contributed to the analysis of imaging. Z.C., W.C., Z.L., and W.G. contributed to the in vivo imaging experiment. Z.C. and W.C. and T.Y. contributed to in vitro fluorescence measurements. W.C. and Z.Z. contributed to the analysis of ncRNA level using RT-qPCR. L.H., Y.Y., N.Y., W.T., and J.W. gave technical support and conceptual advice. X.L., Z.C., W.C., and C.X. wrote the paper with help from all the authors.
Corresponding author
Ethics declarations
Competing interests
X. Li, W. Chen, and C. Xu are authors of a Chinese patent application related to the technology described in this manuscript. The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Murat Sunbul, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Z., Chen, W., Xu, C. et al. Near-infrared fluorogenic RNA for in vivo imaging and sensing. Nat Commun 16, 518 (2025). https://doi.org/10.1038/s41467-024-55093-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55093-1