Abstract
The activation of C−C bond of benzocyclobutenones under mild reaction conditions remains a challenge. We herein report a photoinduced catalyst-free regio-specific C1−C8 bond cleavage of benzocyclobutenones, enabling the generation of versatile ortho-quinoid ketene methides for aza-[4 + 2]-cycloaddition with imines, which offers a facile route to isoquinolinone derivatives, including seven family members of protoberberine alkaloids, gusanlung A, B, D, 8-oxotetrahydroplamatine, tetrahydrothalifendine, tetrahydropalmatine, and xylopinine. Furthermore, the catalytic enantioselective version of this strategy is also realized by merging synergistic photocatalysis and chiral Lewis acid catalysis. Mechanistic studies provide compelling evidence to rationalize the photoisomerization/cycloaddition cascade process.
Similar content being viewed by others
Introduction
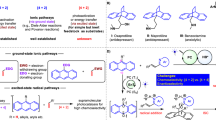
C−C bond activation streamlines synthetic routes of complex compounds and serves as a crucial methodology for molecular skeleton editing1,2,3,4,5,6,7. Benzocyclobutenones (BCBs)8,9,10,11,12, have been widely applied to organic synthesis enabled by transition metal-catalyzed activation of proximal C1−C2 or distal C1−C8 bonds, generating two types of reactive five-membered metallacycle intermediates. DFT calculations revealed that the aryl-metallacycle intermediate is more thermodynamically stable than benzyl-metallacycle intermediate13,14,15,16,17,18. Thus, BCBs normally prefer to undergo regio-selective C1−C2 bond cleavage followed by diverse migratory insertion reactions to construct bridged and fused ring compounds (Fig. 1a, left), especially for the synthesis of complex natural products and bioactive molecules. Even more, several elegant catalytic enantioselective transformations of BCBs mediated by chiral precious metal Rh(I) and Ru(0) complexes have also been accomplished. Dong19,20,21,22, Krische23,24, and Xu25,26 have made significant contributions to this area in the past ten years. Despite the significant advances, high temperatures are generally required due to the intrinsic inertness of the C−C bonds of BCBs, which was oftentimes incompatible with more functionalized and sensitive backbones. Meanwhile, notwithstanding the work of Krische23,24,27, Wei28 and Shi29, current reports were primarily restricted to intramolecular reactions of C3-substituted BCBs19,20,21,22,25,26,30,31,32,33,34,35,36,37,38,39,40,41, by contrast, the C−C bond activation-triggered intermolecular transformations of C3-unsubstituted BCBs are uncommon42,43,44,45,46 and of high challenge.
Photocatalysis has attracted much attention due to its unique advantages in realizing cleavage and recombination of inert chemical bonds under mild reactions47,48,49,50,51,52,53,54,55,56,57,58,59. Among them, Norrish type I reaction of ketones provides an efficient and facile tool for the homolysis of C−C bond at the α-position of carbonyl group60,61,62. To the best of our knowledge, the seminal work on photoinduced C−C bond cleavage of BCBs was reported by Cava and Spangler as early as 196763. Because of the lower bond dissociation energy of C1(sp2)−C8(sp3) bond than C1(sp2)−C2(sp2) bond and the higher stability of generated 1,4-diradical intermediate (Fig. 1a, right)64,65,66, BCBs prone to experience regio-specific C1−C8 bond cleavage and the subsequent intersystem crossing (ISC) to form the effective ortho-quinoid ketene methides, which could also be generated through thermal conditions67,68,69, and couple with nucleophiles or serve as a versatile 4 C synthon to participate in [4+n] cycloadditions, such as activated carbonyl and dienophiles. Needless to say, this photocatalysis strategy is complementary to the aforementioned thermocatalytically dominated C1−C2 bond cleavage, and may broaden the substrate scope and reaction type of BCBs. However, the related studies were grossly overlooked in the past five decades, and the synthetic application using this strategy was almost blank70,71,72,73,74,75.
Nitrogen heterocycles are prevalent in pharmaceuticals, agrochemicals, dyes, and natural products76,77,78,79. We envisioned that the cycloaddition of ortho-quinoid ketene methides with imines would offer an intriguing protocol to construct nitrogen-containing cyclic compounds. Herein, we describe the photoinduced catalyst-free [4+2]-cycloaddition of BCBs with imines through regio-selective C1–C8 bond cleavage under mild reaction conditions, affording an array of polycyclic 3,4-dihydroisoquinolinones alkaloids. Furthermore, as a continuity of our interest in synergistic photocatalysis and chiral Lewis acid catalysis80,81,82,83,84,85, the highly catalytic asymmetric version was also accomplished using an inexpensive chiral nickel(II) Lewis acid catalyst (Fig. 1b).
Results
Investigation of reaction conditions
Initial experiment of aza-[4+2]-cycloaddition was performed between 3,4-dihydroisoquinoline B1 and benzocyclobutenone A1. The mixture in Et2O was irradiated under 365 nm light at 10 °C (Tables S1–5 in Supplementary Information), directly delivering the product tetrahydroisoquinolinone C1 with nearly quantitative yield (99%). BCB derivatives bearing substituents at C3, C4, C5-positions and 8,8-difluoro substitutions, or cyclobuta[a]naphthalenone underwent cyclization upon light irradiation, forming the corresponding products C2-C8 in 75–99% yields (Fig. 2). Variation of 3,4-dihydroisoquinolines (C9-C17) also proved effective in this catalyst-free system, especially 7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinoline, which enabled one-step formation of protoberberine alkaloids86,87 gusanlung B, D, and oxotetrahydroplamatine (C11-C12 and C16, 62–99% yields). Moreover, several other alkaloids, such as gusanlung A, tetrahydropalmatine, tetrahydrothalifendine, and xylopinine, could be precisely synthesized upon straightforward deprotection or reduction of the photoinduced cyclization products C13, C15-C17.
Encouraged by the success of this photoactivation strategy, we turned attention to the asymmetric photo-driven cycloaddition between benzocyclobutenone A1 and benzosulfonimide D1 using chiral N,N’-dioxide/metal complexes88,89,90,91,92,93,94 as Lewis acid catalysts to control the enantioselectivity (Table 1). The racemic product E1 could be observed under irradiation (365 nm) in CH2Cl2 with a yield of 76% without any catalyst (Table 1, entry 1), indicating a strong background reaction and a high challenge for enantiocontrol. Delightedly, N,N’-dioxides, a type of promising chiral ligands, exhibited a pronounced impact on enantioselectivity upon coordination with Ni(OTf)2 (entries 2–6). The aromatic amine-derived N,N’-dioxide L3-PiMe2Br displayed lower enantioselectivity compared to the aliphatic counterparts (entry 2 vs entries 3–6). L3-PisEPh derived from S-pipecolic acid and S-phenylethylamine, provided a moderate yield and the highest enantioselectivity (entry 6, 62% yield, 91% ee). Other metal ions, including LaIII, MgII, and ZnII, yielded E1 with no more than 5% ee (entries 7–9). Further exploration of wavelength revealed UV light dependence in the C−C bond cleavage of BCB. Specifically, a slightly higher yield (74%) was obtained with 90% ee under 385 nm light (entry 10). However, visible light (400 nm) resulted in sharply diminished reactivity, providing only 11% yield and 90% ee (entry 11). When the counter ion of nickel salt was changed to NTf2−, higher enantioselectivity was achieved with a lower yield (entry 12, 37% yield, 92% ee). Delightfully, a slightly excessive Ni(NTf2)2 enabled up to 96% yield with enhanced enantioselectivity (93% ee, entry 13), which is likely to accelerate the dissociation of chiral catalyst from the product. Given the existence of photo-mediated hydrolysis and reduction of the imine95,96,97, the inclusion of 3 Å molecular sieve (MS) in this system, along with adjustment of concentration and temperature, is expected to further improve enantioselectivity to 95% ee with 95% yield (entry 14). The absolute configuration of E1 was determined to be S through X-ray crystallographic analysis98.
Subsequently, we conducted a comprehensive examination of benzosulfonimides (Fig. 3). Modifying the ester substituent of D led to efficient yields and enantioselectivities (E1-E4, 90–96% yields, 94–95% ee). Regardless of the position and electronic nature of substituents on the phenyl ring, benzosulfonimides underwent the desired cycloaddition to afford the products E5-E16 with yields ranging from moderate to excellent (40–93%) and high enantioselectivities (84–96% ee). The lower yield of some substrates was primarily due to the photohydrolysis and photoreduction of imines95,96,97. Generally, imines bearing an electron-donating group exhibited lower reactivity and higher enantioselectivities than those with electron-deficient imines. An imine with a fused naphthyl could also be transformed into the desired product (E17), albeit with 55% yield and 86% ee.
A detailed examination of the BCB scope was also undertaken. A series of substituted BCBs reacted smoothly with D1 to afford the corresponding products E18-E25 with 54–99% yields and efficient enantioselectivities (92–97% ee) except for those bearing an electron-donating group at the C5 position (E22–E23, 31–78% yields, 73–85% ee). BCB with a fused ring was tolerated well in this reaction, giving the target product E26 with a 94% yield and 93% ee. The 8-methyl or 8-cyclopropyl substituted BCBs also underwent exclusively C1–C8 bond cleavage using this photoinduced strategy, giving the product E27–E28 in 86–98% yields and 90–98% ee, albeit with lower diastereoselectivity (1:1.6–3:1 dr). 8,8-Disubstituted BCBs were also compatible with this reaction system. For instance, a dimethyl substituted substrate exhibited moderate enantioselectivity probably due to its larger steric hindrance (E29, 64% yield, 73% ee), while the BCB containing a spiro framework generated E30 with 99% yield and 87% ee. The 8,8-difluoro substituted BCB provided an efficient result as well (E31, 92% yield, 96% ee).
Gram-scale experiment
To demonstrate the synthetic potential of the current protocol, the synthesis of E1 was conducted on a gram-scale under slightly modified optimal conditions. Given the difference in the reaction vessel, six 15 W LEDs (λmax = 365 nm) were utilized (Fig. S3 in Supplementary Information). As depicted in Fig. 4a, the selective C−C bond activation of A1 was achieved and reacted smoothly with D1 to produce E1 (1.30 g) with maintained yield and enantioselectivity. Upon treatment E1 with NaBH4 and AlCl3, it was converted into tetrahydroisoquinoline derivative F1 in 96% yield and 96% ee (Fig. 4b). When LiAlH4 was employed as a reducing agent, the C−S bond was further hydrolyzed to yield phenylsulfonic acid derivative F2 (70% yield, 96% ee).
Mechanistic studies
To elucidate the mechanism of C−C bond activation of BCBs, radical capture experiments were performed to verify the homolysis of the C−C bond of BCBs. When TEMPO was used as a radical scavenger, the coupling product G1 was isolated with 54% yield in the absence of D1 through C1−C8 bond cleavage (Fig. 5a). This compelling evidence strongly supports the occurrence of C1−C8 bond homolysis. However, when TEMPO (3.0 equiv.) was added to the standard reaction, the desired cycloaddition product E1 could be obtained with a maintained yield (95%), and G1 was detected with only 1% yield. The negligible influence of TEMPO on this reaction may be attributed to the quick ISC of 1,4-diradical species Int-1. To further validate the intermediary of ortho-quinoid ketene methide, two sets of in situ preparation experiments were carried out (Fig. 5b). Remarkably, heating led to the successful production of ortho-quinoid ketene methide, yielding the target product with a 64% yield and 27% ee, emphasizing the superiority of the photoactivation strategy. Additionally, a mixture of Aa1 with CsF for desilylation99 to generate the ortho-quinoid ketene methide was also feasible, which reacted with D1 to give racemic E1 with 63% yield. Moreover, the reaction could not proceed when the ester group was switched to a phenyl group, or the sulfonyl group was switched to O, S, or NMe, indicating the crucial role of the ester and N-sulfonyl moieties of imine in this reaction (Fig. S4 in Supplementary Information).
Based on the aforementioned experimental results, the absolute configuration of E1 and our previous work82, a possible reaction pathway was proposed (Fig. 5c). Upon excitation of A1 with UV light, it reaches the excited singlet state S1 and subsequently undergoes ISC to reach the excited triplet state T1, enabling the homolysis of the C1−C8 bond and generating the diradical Int-1. The following fast ISC yields the ortho-quinoid ketene methide Int-2, which engages in a [4+2] cycloaddition with the Ni(II)/L3-PisEPh complex-activated D1. To explain the origin of stereoinduction, DFT calculations were performed, revealing that Int-2 prefers to approach D1 from its Re face (TS-S) to generate the S-configured product E1, because the energy barrier for forming the R-configured product (TS-R) is 3.1 kcal/mol higher. Weak interaction analyses indicate that this difference is primarily due to the C-H∙∙∙π interactions between Int-2 and the amide moiety of L3-PisEPh in TS-S. Additionally, the pathway through direct radical addition of Int-1 to D1 was also calculated and revealed a significantly higher activation energy requirement (Fig. S6 in Supplementary Information and Source data). Combined with the control experiments, the radical addition mechanism may not be involved in this reaction.
Discussion
We achieved a type of photo-driven aza-[4+2] cycloaddition of BCBs through C1−C8 bond homolysis to generate ortho-quinoid ketene methide in situ, offering a straightforward methodology for synthesizing 3,4-dihydroisoquinolinones and protoberberine alkaloids under mild conditions. A catalytic asymmetric version between BCBs and benzosulfonimides was also realized by merging chiral N,N’-dioxide/Ni(II) complex catalysis, producing a series of optically active nitrogen heterocyclic ring compounds containing a tetrasubstituted stereocenter. Control experiments and computational studies have confirmed the plausible mechanism. Further studies on photoinduced C1−C8 bond activation to extend the potential applications in preparing other optically pure valuable compounds are ongoing.
Methods
General procedures for photoinduced aza-[4 + 2]-cycloaddition between benzocyclobutenones and 3,4-dihydroisoquinolines
To a 20 mL dry quartz tube was added benzocyclobutenones A (0.1 mmol), 3,4-dihydroisoquinoline B (0.1 mmol), and Et2O (3.0 mL) under N2 atmosphere, the resulting mixture was stirred under UV LED (5 W, λmax = 365 nm) irradiation at 10 °C. After the starting material was fully consumed (detected by TLC), the mixture was purified by column chromatography (petroleum ether:ethyl acetate = 5:1) on silica gel to afford product C.
General procedures for photoinduced asymmetric aza-[4+2]-cycloaddition between benzocyclobutenones and benzosulfonimides
To a 20 mL dry quartz tube was added L3-PisEPh (10 mol%), Ni(NTf2)2 (12 mol%), 3 Å MS (25 mg), benzocyclobutenones A (0.2 mmol), benzosulfonimides D (0.1 mmol) and CH2Cl2 (3.0 mL), the mixture was stirred at 35 °C for 30 minutes and then was stirred under UV LED (20 W, λmax = 365 nm) irradiation at 10 °C. After the benzosulfonimide was fully consumed (detected by TLC), the solvent was removed in vacuo, and the residue was purified by column chromatography (petroleum ether/ethyl acetate = 2:1) on silica gel to afford the product E.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 2293174 (E1). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via https://www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon request. Source data are provided with this paper.
References
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Murakami, M. & Ishida, N. Potential of metal-catalyzed C–C single bond cleavage for organic synthesis. J. Am. Chem. Soc. 138, 13759–13769 (2016).
Fumagalli, G., Stanton, S. & Bower, J. F. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 117, 9404–9432 (2017).
Kim, D.-S., Park, W.-J. & Jun, C.-H. Metal–organic cooperative catalysis in C–H and C–C bond activation. Chem. Rev. 117, 8977–9015 (2017).
Song, F., Gou, T., Wang, B.-Q. & Shi, Z.-J. Catalytic activations of unstrained C–C bond involving organometallic intermediates. Chem. Soc. Rev. 47, 7078–7115 (2018).
Xu, T. Synthetic applications of C−C bond activation reactions. Compr. Organomet. Chem. 12, 332–346 (2022).
Flores-Gaspar, A. & Martin, R. Recent advances in the synthesis and application of benzocyclobutenones and related compounds. Synthesis 45, 563–580 (2013).
Chen, P.-H. & Dong, G. Cyclobutenones and benzocyclobutenones: versatile synthons in organic synthesis. Chem. Eur. J. 22, 18290–18315 (2016).
Deng, L. & Dong, G. Carbon‒carbon bond activation of ketones. Trends Chem. 2, 183–198 (2020).
Murakami, M. & Ishida, N. Cleavage of carbon–carbon σ-bonds of four-membered rings. Chem. Rev. 121, 264–299 (2021).
Xue, Y. & Dong, G. Deconstructive synthesis of bridged and fused rings via transition-metal-catalyzed “cut-and-sew” reactions of benzocyclobutenones and cyclobutanones. Acc. Chem. Res. 55, 2341–2354 (2022).
Huffman, M. A., Liebeskind, L. S. & Pennington, W. T. Jr. Synthesis of metallacyclopentenones by insertion of rhodium into cyclobutenones. Organometallics 9, 2194–2196 (1990).
Lu, G., Fang, C., Xu, T., Dong, G. & Liu, P. Computational study of Rh-catalyzed carboacylation of olefins: ligand-promoted rhodacycle isomerization enables regioselective C–C bond functionalization of benzocyclobutenones. J. Am. Chem. Soc. 137, 8274–8283 (2015).
Lu, Q., Wang, B., Yu, H. & Fu, Y. Mechanistic study on ligand-controlled Rh(I)-catalyzed coupling reaction of alkene-benzocyclobutenone. ACS Catal. 5, 4881–4889 (2015).
Yang, S., Xu, Y. & Li, J. Theoretical study of nickel-catalyzed proximal C–C cleavage in benzocyclobutenones with insertion of 1,3-diene: origin of selectivity and role of ligand. Org. Lett. 18, 6244–6247 (2016).
Zou, H., Wang, Z.-L. & Huang, G. Mechanism and origins of the chemo- and regioselectivities in nickel-catalyzed intermolecular cycloadditions of benzocyclobutenones with 1,3-dienes. Chem. Eur. J. 23, 12593–12603 (2017).
Xu, Z.-Y. et al. Mechanism and origins of chemo- and regioselectivities of Pd-catalyzed intermolecular σ-bond exchange between benzocyclobutenones and silacyclobutanes: a computational study. Organometallics 37, 592–602 (2018).
Xu, T., Ko, H. M., Savage, N. A. & Dong, G. Highly enantioselective Rh-catalyzed carboacylation of olefins: efficient syntheses of chiral poly-fused rings. J. Am. Chem. Soc. 134, 20005–20008 (2012).
Deng, L., Xu, T., Li, H. & Dong, G. Enantioselective Rh-catalyzed carboacylation of C=N bonds via C–C activation of benzocyclobutenones. J. Am. Chem. Soc. 138, 369–374 (2016).
Deng, L., Chen, M. & Dong, G. Concise synthesis of (−)-cycloclavine and (−)-5-epi-cycloclavine via asymmetric C–C activation. J. Am. Chem. Soc. 140, 9652–9658 (2018).
Hou, S.-H., Prichina, A. Y. & Dong, G. Deconstructive asymmetric total synthesis of morphine-family alkaloid (−)-thebainonea. Angew. Chem. Int. Ed. 60, 13057–13064 (2021).
Ambler, B. R. et al. Enantioselective ruthenium-catalyzed benzocyclobutenone–ketol cycloaddition: merging C–C bond activation and transfer hydrogenative coupling for type II polyketide construction. J. Am. Chem. Soc. 140, 9091–9094 (2018).
Huynh, N. O., Hodík, T. & Krische, M. J. Enantioselective transfer hydrogenative cycloaddition unlocks the total synthesis of SF2446 B3: An aglycone of arenimycin and SF2446 type II polyketide antibiotics. J. Am. Chem. Soc. 145, 17461–17467 (2023).
Qiu, B. et al. Catalytic enantioselective synthesis of 3,4-polyfused oxindoles with quaternary all-carbon stereocenters: a Rh-catalyzed C–C activation approach. Org. Lett. 20, 7689–7693 (2018).
Li, X. et al. Divergent Rh catalysis: asymmetric dearomatization versus C–H activation initiated by C–C activation. ACS Catal. 13, 4873–4881 (2023).
Bender, M., Turnbull, B. W. H., Ambler, B. R. & Krische, M. J. Ruthenium-catalyzed insertion of adjacent diol carbon atoms into C–C bonds: entry to type II polyketides. Science 357, 779–781 (2017).
Lu, H. et al. Divergent coupling of benzocyclobutenones with indoles via C−H and C−C activations. Angew. Chem. Int. Ed. 59, 23537–23543 (2020).
Guo, J.-H. et al. Site-selective C–C cleavage of benzocyclobutenones enabled by a blocking strategy using nickel catalysis. Angew. Chem. Int. Ed. 60, 19079–19084 (2021).
Xu, T. & Dong, G. Rhodium-catalyzed regioselective carboacylation of olefins: a C−C bond activation approach for accessing fused-ring systems. Angew. Chem. Int. Ed. 51, 7567–7571 (2012).
Chen, P.-H., Xu, T. & Dong, G. Divergent syntheses of fused β-naphthol and indene scaffolds by rhodium-catalyzed direct and decarbonylative alkyne–benzocyclobutenone couplings. Angew. Chem. Int. Ed. 53, 1674–1678 (2014).
Xu, T., Savage, N. A. & Dong, G. Rhodium(I)-catalyzed decarbonylative spirocyclization through C–C bond cleavage of benzocyclobutenones: An efficient approach to functionalized spirocycles. Angew. Chem. Int. Ed. 53, 1891–1895 (2014).
Xu, T. & Dong, G. Coupling of sterically hindered trisubstituted olefins and benzocyclobutenones by C–C activation: total synthesis and structural revision of cycloinumakiol. Angew. Chem. Int. Ed. 53, 10733–10736 (2014).
Sun, T. et al. Rhodium(I)-catalyzed carboacylation/aromatization cascade initiated by regioselective C−C activation of benzocyclobutenones. Angew. Chem. Int. Ed. 57, 2859–2863 (2018).
Zhu, Z. et al. Cobalt-catalyzed intramolecular alkyne/benzocyclobutenone coupling: C–C bond cleavage via a tetrahedral dicobalt intermediate. ACS Catal. 8, 845–849 (2018).
Qin, Y., Zhan, J.-L., Shan, T.-T. & Xu, T. Total synthesis of penta-Me amurensin H and diptoindonesin G featuring a Rh-catalyzed carboacylation/aromatization cascade enabled by C−C activation. Tetrahedron Lett. 60, 925–927 (2019).
Zhang, Y., Shen, S., Fang, H. & Xu, T. Total synthesis of galanthamine and lycoramine featuring an early-stage C–C and a late-stage dehydrogenation via C–H activation. Org. Lett. 22, 1244–1248 (2020).
Zhang, J., Wang, X. & Xu, T. Regioselective activation of benzocyclobutenones and dienamides lead to anti-bredt bridged-ring systems by a [4+4] cycloaddition. Nat. Commun. 12, 3022 (2021).
Wang, Y., Ma, P., Ma, N. & Wang, J. Ligand-controlled nickel-catalyzed reactions of benzocyclobutenones with alkynyltrifluoroborates: diverse construction of polysubstituted naphthols. Org. Lett. 25, 3527–3532 (2023).
Zhang, J. et al. Reversing site-selectivity in formal cross-dimerization of benzocyclobutenones and silacyclobutanes. CCS Chem. 5, 1753–1762 (2023).
Jiang, C. et al. Type I [4σ+4π] versus [4σ+4π−1] cycloaddition to access medium-sized carbocycles and discovery of a liver X receptor β-selective ligand. Angew. Chem. Int. Ed. 63, e202405838 (2024).
Chen, P.-H., Sieber, J., Senanayake, C. H. & Dong, G. Rh-catalyzed reagent-free ring expansion of cyclobutenones and benzocyclobutenones. Chem. Sci. 6, 5440–5445 (2015).
Juliá-Hernández, F., Ziadi, A., Nishimura, A. & Martin, R. Nickel-catalyzed chemo-, regio- and diastereoselective bond formation through proximal C-C cleavage of benzocyclobutenones. Angew. Chem. Int. Ed. 54, 9537–9541 (2015).
Okumura, S., Sun, F., Ishida, N. & Murakami, M. Palladium-catalyzed intermolecular exchange between C–C and C–Si σ-bonds. J. Am. Chem. Soc. 139, 12414–12417 (2017).
Li, R. et al. A ring expansion strategy towards diverse azaheterocycles. Nat. Chem. 13, 1006–1016 (2021).
Ochi, S., Zhang, Z., Xia, Y. & Dong, G. Rhodium-catalyzed (4+1) cycloaddition between benzocyclobutenones and styrene-type alkenes. Angew. Chem. Int. Ed. 61, e202202703 (2022).
Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 108, 1052–1103 (2008).
Xuan, J. & Xiao, W.-J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 51, 6828–6838 (2012).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Schultz, D. M. & Yoon, T. P. Solar synthesis: prospects in visible light photocatalysis. Science 343, 1239176 (2014).
Brimioulle, R., Lenhart, D., Maturi, M. M. & Bach, T. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 54, 3872–3890 (2015).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Zhou, Q.-Q., Zou, Y.-Q., Lu, L.-Q. & Xiao, W.-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 58, 1586–1604 (2019).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Yu, X.-Y., Chen, J.-R. & Xiao, W.-J. Visible light-driven radical-mediated C–C bond cleavage/functionalization in organic synthesis. Chem. Rev. 121, 506–561 (2021).
Melchiorre, P. Introduction: photochemical catalytic processes. Chem. Rev. 122, 1483–1484 (2022).
Hou, L. Z., Liu, X. H., Cao, W. D. & Feng, X. M. Recent advances in visible light-induced asymmetric transformations of carbonyl compounds into chiral alcohols. ChemCatChem 15, e202300893 (2023).
Dutta, S., Erchinger, J. E., Strieth-Kalthoff, F., Kleinmans, R. & Glorius, F. Energy transfer photocatalysis: exciting modes of reactivity. Chem. Soc. Rev. 53, 1068–1089 (2024).
Norrish, R. G. W. & Kirkbride, F. W. 204. Primary photochemical processes. Part I. The decomposition of formaldehyde. J. Chem. Soc., 1518–1530 https://doi.org/10.1039/JR9320001518 (1932).
Norrish, R. G. W. & Bamford, C. H. Photo-decomposition of aldehydes and ketones. Nature 140, 195–196 (1937).
Dantas, J. A., Correia, J. T. M., Paixão, M. W. & Corrêa, A. G. Photochemistry of carbonyl compounds: application in metal-free reactions. ChemPhotoChem 3, 506–520 (2019).
Cava, M. P. & Spangler, R. J. 2-(Carbomethoxy)benzocyclobutenone. Synthesis of a photochemically sensitive small-ring system by a pyrolytic wolff rearrangement. J. Am. Chem. Soc. 89, 4550–4551 (1967).
Ng, D., Yang, Z. & Garcia-Garibay, M. A. Total synthesis of (±)-herbertenolide by stereospecific formation of vicinal quaternary centers in a crystalline ketone. Org. Lett. 6, 645–647 (2004).
Nicolaou, K. C., Gray, D. L. F. & Tae, J. Total synthesis of hamigerans and analogues thereof. Photochemical generation and Diels−Alder trapping of hydroxy-o-quinodimethanes. J. Am. Chem. Soc. 126, 613–627 (2004).
Okada, M. et al. Photocatalytic one-pot synthesis of homoallyl ketones via a Norrish type I reaction of cyclopentanones. J. Org. Chem. 80, 9365–9369 (2015).
Schiess, P., Eberle, M., Huys-Francotte, M. & Wirz, J. Thermal addition reactions to benzocyclobutenones studied by flash photolysis. Tetrahedron Lett. 25, 2201–2204 (1984).
Wang, Z. Y., Suzzarini, L. & Gao, J. P. Thermal reactions of benzocyclobutenone with alcohols. Tetrahedron Lett. 38, 5745–5746 (1997).
Wurm, T., Turnbull, B. W. H., Ambler, B. R. & Krische, M. J. Thermal hetero-Diels–Alder reaction of benzocyclobutenones with isatins to form 2-oxindole spirolactones. J. Org. Chem. 82, 13751–13755 (2017).
Arnold, D. R., Hedaya, E., Merritt, V. Y., Karnischky, L. A. & Kent, M. E. Benzocyclobutenone: pyrolysis and photochemistry. Tetrahedron Lett. 13, 3917–3920 (1972).
Krantz, A. Laser ultraviolet irradiation of α-pyrone. Extremely rapid isomerization of a transient ketene. J. Am. Chem. Soc. 96, 4992–4993 (1974).
Hacker, N. P. & Turro, N. J. Low temperature photolysis of benzocyclobutanone and 2,2-dihydrocyclobuta[1]phenanthrenone: Evidence for photochromic behavior. J. Photochem. 22, 131–135 (1983).
Bally, T. & Michalak, J. Photochemistry and radiation chemistry of benzocyclobutenone: formation of an o-quinoid ketene and its radical cation. J. Photochem. Photobiol. A 69, 185–190 (1992).
Chou, C.-H., Wu, C.-C. & Chen, W.-K. Synthesis of pyrido[b]cyclobuten-5-one and 1-azafulvenallene by flash vacuum pyrolysis of 3-chloroformyl-2-methylpyridine. Tetrahedron Lett. 36, 5065–5068 (1995).
Chiang, Y., Kresge, A. J. & Zhan, H.-Q. Generation of 6-methylene-2,4-cyclohexadienylidene ketene by flash photolysis of benzocyclobutenone in aqueous solution and study of the reactions of this ketene in that medium. Can. J. Chem. 81, 607–611 (2003).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Heravi, M. M. & Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 10, 44247–44311 (2020).
Lang, K. D., Kaur, R., Arora, R., Saini, B. & Arora, S. Nitrogen-containing heterocycles as anticancer agents: an overview. Anticancer Agents Med. Chem. 20, 2150–2168 (2020).
Kumar, A. et al. Nitrogen containing heterocycles as anticancer agents: a medicinal chemistry perspective. Pharmaceuticals 16, 299 (2023).
Tan, Z. D., Zhu, S. B., Liu, Y. B. & Feng, X. M. Photoinduced chemo-, site- and stereoselective α-C(sp3)−H functionalization of sulfides. Angew. Chem. Int. Ed. 61, e202203374 (2022).
Hou, L. Z. et al. Enantioselective radical addition to ketones through Lewis acid-enabled photoredox catalysis. J. Am. Chem. Soc. 144, 22140–22149 (2022).
Yang, L. K. et al. NickelII-catalyzed asymmetric photoenolization/Mannich reaction of (2-alkylphenyl) ketones. Chem. Sci. 13, 8576–8582 (2022).
Zhan, T. Y. et al. Chiral Lewis acid-catalyzed Norrish type II cyclization to synthesize α-oxazolidinones via enantioselective protonation. CCS Chem. 5, 2101–2110 (2023).
Yang, L. K. et al. Catalytic asymmetric photocycloaddition of triplet aldehydes with benzocyclobutenones. CCS Chem. 135, 11473-6 (2024).
Hou, L. Z. et al. Catalytic asymmetric dearomative [2 + 2] photocycloaddition/ring-expansion sequence of indoles with diversified alkenes. J. Am. Chem. Soc. 146, 23457–23466 (2024).
Leitao Da-Cunha, E. V., Fechine, L. M., Guedes, D. N., Barbosa-Filho, J. M. & Sobral Da Silva, M. Protoberberine alkaloids. alkaloids: Chem. Biol. 62, 1–75 (2005).
Yu, L.-L. et al. Protoberberine isoquinoline alkaloids from arcangelisia gusanlung. Molecules 19, 13332–13341 (2014).
Liu, X. H., Lin, L. L. & Feng, X. M. Chiral N,N’-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 44, 574–587 (2011).
Liu, X. H., Zheng, H. F., Xia, Y., Lin, L. L. & Feng, X. M. Asymmetric cycloaddition and cyclization reactions catalyzed by chiral N,N’-dioxide-metal complexes. Acc. Chem. Res. 50, 2621–2631 (2017).
Chen, D.-F. & Gong, L.-Z. Feng chiral N,N’-dioxide ligands: uniqueness and impacts. Org. Chem. Front. 10, 3676–3683 (2023).
Dong, S. X., Cao, W. D., Pu, M. P., Liu, X. H. & Feng, X. M. Ligand acceleration in chiral Lewis acid catalysis. CCS Chem. 5, 2717–2735 (2023).
Xu, N. et al. Iron-catalyzed asymmetric α-alkylation of 2-acylimidazoles via dehydrogenative radical cross-coupling with alkanes. Angew. Chem. Int. Ed. 62, e202314256 (2023).
Wang, K. X. et al. Asymmetric catalytic ring-expansion of 3-methyleneazetidines with α-diazo pyrazoamides towards proline-derivatives. Angew. Chem. Int. Ed. 62, e202307249 (2023).
Chen, M. et al. Regioselective and asymmetric allylic alkylation of vinyl epoxides for the construction of allylic alcohols via synergistic catalysis. Sci. China Chem. 67, 542–550 (2024).
Padwa, A. Photochemistry of the carbon-nitrogen double bond. Chem. Rev. 77, 37–68 (1977).
Pratt, A. C. The photochemistry of imines. Chem. Soc. Rev. 6, 63–81 (1977).
Kandappa, S. K., Valloli, L. K., Ahuja, S., Parthiban, J. & Sivaguru, J. Taming the excited state reactivity of imines – from non-radiative decay to aza Paternò–Büchi reaction. Chem. Soc. Rev. 50, 1617–1641 (2021).
CCDC: 2293174 (E1), contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Kessar, S. V., Singh, P., Vohra, R., Kaur, N. P. & Venugopal, D. Facile generation and trapping of α-oxo-o-quinodimethanes: Synthesis of 3-aryl-3,4-dihydroisocoumarins and protoberberines. J. Org. Chem. 57, 6716–6720 (1992).
Acknowledgements
We appreciate the National Key Research and Development Program of China (2022YFA1504301, X.M.F.) and the National Natural Science Foundation of China (92256302, W.D.C. and 22071160, W.D.C.) for financial support. We thank Dr. Yuqiao Zhou from Sichuan University for their assistance in X-ray analysis.
Author information
Authors and Affiliations
Contributions
L.K.Y., S.Y.L., H.S.Z., and L.Z. performed the experiments. L.C.N. performed the DFT calculations. L.K.Y., W.D.C., and X.M.F. prepared this manuscript and the supplementary information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shuichi Nakamura, Peng-Fei Xu and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, L., Li, S., Ning, L. et al. Aza-[4 + 2]-cycloaddition of benzocyclobutenones into isoquinolinone derivatives enabled by photoinduced regio-specific C–C bond cleavage. Nat Commun 15, 10866 (2024). https://doi.org/10.1038/s41467-024-55110-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55110-3
This article is cited by
-
Synergistic catalytic synthesis of chiral γ-butenolides by trapping carboxylic oxonium ylides with enones and isatins
Science China Chemistry (2025)