Abstract
Covalent organic frameworks are attractive candidates for the next generation films in technical applications. However, due to their crystallization nature, insolubility in common solvents as well as infusible at high temperatures make it challenging to grow them spontaneously or process them into films. Herein, we report an efficient strategy to fabricate covalent organic framework films based on a modulator-solvent induced polymerization process. The addition of modulator slows down the nucleation rate during the initial stages of covalent organic framework growth, resulting in the formation of fluidic precursors that are easy to process. Subsequently, a suitable drying process is introduced to balance the evaporation rate of solvent and the crystallization rate of modulator induced, resulting in the formation of covalent organic framework films with a mixture of amorphous and crystalline structures. This strategy is universal for the fabrication of several types of covalent organic framework films with large-scale and freestanding state. Moreover, covalent organic framework films with asymmetric structure can function as organic vapor-triggered actuators, offering excellent repeatability and reversibility. By introducing functional molecules such as fluorescence, chirality and catalyst during the nucleation process, versatile functional covalent organic framework films can be easily fabricated, which endow them with broader application prospects.
Similar content being viewed by others
Introduction
The modern concept of polymers as covalently bonded macromolecular structures was proposed in 19201. Since then, advancements in monomer design and enrichment of polymerization principles have greatly improved the diversity and performance of materials, driving the rapid development of science and industry2,3,4. Polymer materials have expanded their applications in separation5, energy storge6, biomaterials7, and display technology8 due to their excellent film-forming properties. Covalent organic frameworks (COFs)9,10,11,12, emerging crystalline porous polymers constructed from organic monomers via reversible covalent bonds, which inherited the structural and chemical versatility, as well as the network-like structure of traditional polymers13,14,15. More importantly, COFs possess well-defined and predictable pore structures, nanometer-sized pores and high surface areas16,17, endowing them with functions such as efficient separation18,19, catalysis20,21 and specific sensing22, enabling them a new force for the state-of-the-art platforms. Unfortunately, guided by kinetic and thermodynamic rules23,24, COFs usually exist in the form of micro/nano-sized crystalline powders with poor processibility due to their insolubility in common solvents as well as infusibility at high temperatures, which severely limit their application prospects in membrane/film-based devices25.
To date, several common strategies have been reported for preparing COF films including layer-by-layer stacking26, in-situ growth of COFs on surfaces27 and interfacial polymerization28,29,30. Although more uniform nucleation sites are provided by these ways, the growth process of COF films still suffers from complicated growth steps, time mismatch between polymerization and crystallization, limiting the quality and expanded applications of film. However, blending method ingeniously utilized the convenient and flexible industrial fabrication process of thin films, avoiding matching issues of the polymerization and crystallization. COFs and polymers are combined by mixing and linking to leverage the continuity and processability of polymers along with the functionalities of frameworks. For example, Rahul and coworkers firstly added chemically stable COFs (TpPa-1 and TpBD) as active phases into polymer matrix to prepare self-supporting hybrid films31. Similarly, Jiang and coworkers assembled the two-dimensional (2D) COF nanosheets and one-dimensional cellulose nanofibers into COF hybrid films, which exhibiting enhanced stability32. Although the blending method overcomes the problem of inferior processability COF film fabrication, the interfacial gaps between two different materials, often leading to discontinuous COF-based membranes cannot realize the potential of the pore structures of COFs for practical applications.
Practically, the growth process of most COFs involves rapid transitions between initial amorphous network and subsequently crystalline states23, which is similar to the composition of COFs hybrid films prepared by the blending method. At first, amorphous structure with low-energy and low surface area is built. Then, this material transforms into a crystalline phase through an error-correction mechanism33. Specifically, the amorphous state with short- range ordered structure and the crystalline structure with long-range ordered framework are similar to the amorphous polymer materials and porous frameworks, respectively. If intervening in the growth process of COFs to keep the two distinct growth states, with the amorphous state providing polymer-like properties and the crystalline state offering porous frameworks characteristics in the same reaction system, could potentially yield new functional COF films.
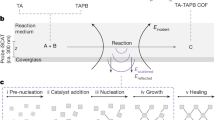
In order to demonstrate our conjecture, we develop a modulator-solvent induced strategy to regulate the growth equilibrium of COFs for the formation and functionalization of COF films (Fig. 1). The selected modulators are introduced to slow down the formation of imine bonds by producing the modulator-stabilized carboxylic acid salt. Subsequently, a suitable drying process is introduced to balance the evaporation rate of solvent and the reorganization rate of metastable amorphous network, resulting in the formation of COF films featuring with large-scale and freestanding state. Notably, the quick evaporation of surface layer solvent will form a compact film with an amorphous structure due to the lack of suitable growth environment of COFs, and yet this compact surface film structure provides an environment conducive to slow solvent evaporation, facilitating the reorganization of inner amorphous network into crystalline COFs induced by suitable amount of modulator. Hence, the obtained COF films exhibit an asymmetric structure combining amorphous network states with crystalline porous states, and display the advantages of reversible organic molecules stimulus-responsive driving behavior. In addition, functional molecules such as fluorescence, chirality and catalyst molecules can be easily encapsulated into COF matrix to obtain functional COF films. Such modulator-solvent induced polymerization approach will open up an avenue for the development of COF films with tailored functionalities.
(i) the addition of modulators can slow down the formation of imine bonds by producing the modulator-stabilized carboxylic acid salt. (ii) a gentle drying process is introduced to balance the evaporation rate of solvent and the reorganization rate of metastable amorphous network to form COF films. (iii) COF films with the asymmetric structure have exhibited distinct reversible diffusion behavior of organic molecules, which can be used as sensitive actuators to diverse gases. (iv) versatile functional COF films can be easily synthesized by introducing functional molecules (such as fluorescence, chirality and catalyst) during the nucleation process.
Results
The formation process and characterizations of COF films
We have adopted the modulator-solvent induced polymerization strategy to synthesize 2D imine-based COF-LZU1 (LZU stands for Lanzhou University) which is widely used in many fields due to its high porosity, suitable pore size, and high stability as the representative34. Generally, for synthesizing COF-LZU1 particles, 1,3,5-triformylbenzene (TFB) dissolved in 1,4-dioxane and the other reactant composed of p-phenylenediamine (PPDA) and benzoic acid in 1,4-dioxane are mixed under hydrothermal synthesis. The resulting COF-LZU1 particles exist in the form of powders, scanning electron microscope (SEM) demonstrates spherical morphology with relatively uniform size of ca.1 μm. X-ray diffraction (XRD) spectrum indicates relatively good crystallinity of COF-LZU1 particles as reflected by the characteristic peaks (2θ value of 4.7°)34 (Supplementary Fig. 1). During the synthesis of COF-LZU1 films, the two solutions are rapidly mixed, and then dropped on the glass substrates, which is easy to form COF-LZU1 film under suitable temperature. It is worth noting that two key parameters should be considered. One is the amount of benzoic acid added, which acts as the modulator (the role of competitors and catalysts) to slow down the formation of imine bonds and enhance crystallinity. The other one is the evaporation temperature of the solvent, which plays an important role in balancing the evaporation rate of solvent and modulator, thus achieving compact film. Upon varying the amount of benzoic acid added from 0.1 mmol to 0.7 mmol, an increase in surface roughness and the appearance of cracks are observed in the COF-LZU1 film, regardless of the evaporation temperature being 40 °C, 80 °C or 120 °C (Supplementary Fig. 2), due to the addition excess of benzoic acid enhance the reversibility of imine bond formation and the process of error corrections, and ultimately the surface presented in the form of particles33. At the temperature of 120 °C, COF-LZU1 film exhibits poor crystallinity, which attributed to the rapid evaporation of solvents and benzoic acid, leading to insufficient time for COF-LZU1 film to self-repair and improve crystallinity (Supplementary Fig. 3). In contrast, at the temperature of 40 °C, COF-LZU1 film displays enhanced crystallinity, but remains less dense and prone to form the COF-LZU1 particle. This phenomenon is mainly ascribed to the slower evaporation rate of solvent, which is conducive to the growth of COF crystals in the environment of solvent and benzoic acid. Hence, optimal synthetic conditions for COF-LZU1 films are achieved with 0.3 mmol of benzoic acid and 80 °C heating temperature (Supplementary Fig. 4). The synthesis involves a Schiff base reaction which takes place at 80 °C for 15 min, facilitating instantaneous nucleation of the outermost amorphous polymer layer while allowing relatively sufficient time for the inner solution to convert into crystalline materials through error corrections process.
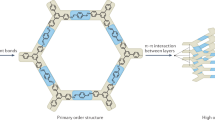
The morphology, crystal structure and porosity of the prepared COF-LZU1 films have been further characterized by SEM, atomic force microscope (AFM), XRD and Brunauer–Emmett–Teller (BET) analysis. From a macroscale view, COF-LZU1 films have smooth and flexible structure with a yellow color similar to the traditional with hydrothermally synthesized COF-LZU1 powders (Fig. 2a, insert image). Analysis of the film’s margin and cross-section (Fig. 2b) reveals a transition from cross-linked particles to dispersed particles with increasing monomer growth time, if we take compact surface as the top side. In terms of the film’s morphology, a compact surface, and relatively compact layers composed of linked spherical particles and dispersed particles are observed. We also investigate the surface roughness of COF-LZU1 films by AFM, in a square area of 25 × 25 µm2, and find that the height amplitude is around ±40 nm, suggesting a flat and compact film state35 (Fig. 2c). XRD analysis indicates that both sides of the asymmetric COF-LZU1 films exhibit an intensive peak at 4.7° corresponding to the (100) plane34, while other minor diffraction peak appears at 2θ values of 8.10, 9.40, 12.25 and 25.55° corresponding to the (110), (200), (210) and (001) facets, respectively (Fig. 2d). Transmission electron microscope (TEM) studies show the layered stacking of the asymmetric COF film (Supplementary Fig. 5). High-resolution TEM (HRTEM) of the asymmetric COF film gives clear lattice with 3.45 Å referring to (001) facets of the eclipsed stacking of COF-LZU1 layers (Supplementary Fig. 6). The porosity of the COF-LZU1 films is evaluated by nitrogen adsorption–desorption measurements (Supplementary Fig. 7). Compared with the reported synthesized COF-LZU1 particles34 (410 m2 g−1), the as-prepared films exhibit lower specific surface area (121 m2 g−1), probably due to the rapid solvent evaporation on the outer surface, resulting in a dense film surface with an amorphous structure, while the specific surface area is mainly attributed to the inner COF-LZU1 film with a crystalline structure.
The stability of COF-LZU1 films is closely related to its practical application, thus we further investigate the solvent, thermal and structure stability of COF-LZU1 films. By soaking the as-synthesized COF-LZU1 films in commonly used solvents such as ether, tetrahydrofuran, dichloromethane, ethanol, acetone, and deionized water, we find no obvious damage on the morphology and crystal structure of the films (Supplementary Figs. 8 and 9). The thermogravimetric analysis (TGA) shows that the as-synthesized COF-LZU1 film is relatively thermally stable up to around 390 °C while the residual weight is close to 90%, which can meet the demands for potential applications to a certain extent (Supplementary Fig. 10). The mechanical property of the as-prepared COF-LZU1 film was studied by AFM. The Young’s modulus for the free-standing films within a scanning range of 5 μm × 5 μm were calculated to be 731.4 MPa (Supplementary Fig. 11). The thickness of COF films thickness can be controlled by changing the volume of precursor solution added (Supplementary Fig. 12). Additionally, this strategy allows the fabrication of COF films with dimensions up to 60 square centimeters, demonstrating good and dimensional stability in the COF-LZU1 film (Supplementary Figs. 13–15).
The formation mechanism of COF-LZU1 films
In order to elucidate the underlying mechanism of COF-LZU1 film formation, the growth essence of COFs is re-examined, which belong to covalent organic polymers. William R. Dichtel et al. reported the formation of an amorphous network in the initial reaction steps of imine-linked COFs24. However, such COF network intermediates usually exist for a very short time before undergoing imine exchange and crystallization into crystals with a layered 2D network. If the process from the metastable amorphous network to crystallization can be stabilized by modulators in a processable state, the construction of film-state COFs would be feasible. Therefore, we investigate the interaction between the modulator and monomers, evaporation rate of solvent, and the transition from metastable amorphous network to crystalline structure to reveal the mechanism of COF film formation. Firstly, during the fabrication process of COF-LZU1 films, the introduced benzoic acid molecules as modulator are found to be indispensable. The interaction between the benzoic acid and PPDA is much easier than that between the two reacted monomers, as verified by the density functional theory (DFT) calculation, mainly due to its lower binding energy (benzoic acid with PPDA: −0.68 eV; TFB with PPDA: −0.21 eV) (Fig. 3a). We further investigate the effect of the sequence of addition benzoic acid on nucleation time (Supplementary Fig. 16). These results suggest that PPDA firstly binds with benzoic acid in the initial reaction steps of synthesizing COF-LZU1, which slow down the diffusion rate of PPDA and delay crystallization28. Secondly, benzoic acid also functions as a catalyst for COF-LZU1 films during the transition from the metastable amorphous network to crystalline structure. As for modulators, the ones that do not evaporate quickly during the evaporation of solvent is ideal, and thus suitable boiling point of modulators is very important. Benzoic acid with high boiling temperature (249 °C) will still retain its modulator function in the open and high temperature conditions. Hence, traditional acid catalysts with low boiling temperature (acetic acid) and relatively high boiling temperature (p-toluenesulfonic acid and citric acid) are chosen as control samples. The acid with low boiling temperature fails to produce high crystallinity as it evaporates quickly in the heating and open conditions. On the contrary, acids with high boiling temperature can produce compact surface and good crystallinity because they remain their modulator function in the growth process (Fig. 3b, Supplementary Fig. 17). Thirdly, the instant nucleation induced by the evaporation of outer solvent is also important for synthesizing COF-LZU1 films, relying on an open environment. The compact surface of COF-LZU1 film were quickly formed at evaporating for three minutes, and the morphology of surface showed no obvious change in a different time period (Supplementary Fig. 18). We attempt to create a relatively closed condition to hinder evaporation which can confirm the formation mechanism of compact surface. In the closed condition, the surface of the as-synthesized sample is rough and obvious grainy, despite of its good crystallinity (Fig. 3c, Supplementary Fig. 19). The main reason behind this phenomenon is that the solvent under this condition evaporates slowly which provides a favorable growth environment for COF-LZU1 particles, further confirming the above experimental results that the roughness of COF films surface gradually increases with the decrease of reaction temperature. This demonstrates that compact films cannot be formed without rapid solvent evaporation. Lastly, the growth procedures of modulator-solvent induced strategy for COF-LZU1 films focus on the polymerization and crystallization, which is confirmed by the time-dependent changes between of the functional groups and crystallinity. The time-dependent Fourier transform infrared spectroscopy (FTIR) as a direct tool to confirm the changes of functional groups reflects the consumption of the reactants and the formation of products. In our work, the intensity of N-H bonds (~3415 cm−1) and C=O bonds (~1695 cm−1) originating from monomers gradually decreases with prolonging the time, but that of the peak of COFs products at 1618 cm−1 (C=N bond) increases, indicating the formation of imine bonds36 (Fig. 3d). The residual signals at these wavenumbers correspond to the terminal aldehyde and amino groups at the edges of the COF-LZU1 films, respectively. These bands resemble those of COFs synthesized under solvothermal conditions in the literature37. The differences in time interval spectra further confirm that the bonding manner of COF-LZU1 films is similar to that of COF-LZU1 powders. To further reveal this formation mechanism, time-resolved XRD was adopted to explore the crystalline change. The COF-LZU1 precursor is nearly amorphous in the beginning, and several minutes later, main peaks of COF-LZU1 (2θ value of 4.7°) appear corresponding to the (100) plane. Notably, the (100) lattice grows sharply in the subsequent drying process, while the (110) and (200) plane start to appear at the final stages indicating the coexistence of the regrowth process and the self-repair of COF-LZU1 crystallinity (Fig. 3e). In a word, the formation of COF-LZU1 films involves the polymerization and crystallization, predominantly regulated by the modulator (benzoic acid) and solvent. The selected modulator is used to slow down the formation of imine bond by producing the modulator-stabilized carboxylic acid salt. After that, a suitable drying process is introduced to balance the evaporation rate of solvent and the reorganization rate of metastable amorphous network to form COF films. Notably, the quick evaporation of surface layer solvent will form compact film with amorphous structure under unsuitable growth environment of COFs, and this compact surface film structure further decelerates the solvent evaporation rate, favoring to the reorganization of the inner amorphous structure to form crystalline COFs. Thus, COF films with asymmetric structure are obtained (Fig. 3f).
a Binding energy between reacted molecules. b Selection of modulators. SEM images of synthesizing COF-LZU1 films with (i) citric acid and (ii) p-toluenesulfonic acid. c SEM images of COF-LZU1 film in (i) closed condition and (ii) open condition. Insert is the digital image of COF-LZU1 film in the (i) closed condition and (ii) open condition. d FTIR spectra of COF-LZU1 films at different reaction time. e XRD patterns of COF-LZU1 films at different time. f Schematic representation of the COF films formation mechanism.
The universality of the proposed strategy to other COFs
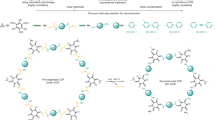
More interestingly, we discover that the modulator-solvent induced strategy can be extended to other COFs with different monomers and linkages. For examples, 1,3,5-triformylphloroglucinol (Tp)-4,4’-azodianiline (Azo) COFs and 1,3,5-tris(4-aminophenyl)benzene (TPB) -2,5-dimethoxyterephthalaldehyde (DMTP) COFs are successfully prepared using this strategy. The synthetic conditions including acid amounts and temperatures are optimized to ensure the formation of freestanding COF films with continuous films structures. By using a large amount of benzoic acid as catalyst and modulator with suitable solvent, we can also synthesize COF films with ideal film properties. For example, Tp-Azo-COF is first synthesized by Rahul and coworkers using 1,3,5-triformylphloroglucinol and 4,4’-azodianiline based on β-ketoenamine linkage38. Under suitable synthetic conditions, Tp-Azo COF films exhibit high crystallinity and crack- free surface (Fig. 4a, d, Supplementary Fig. 20). TPB-DMTP-COF synthesized by Jiang and coworkers is famous for expanding the possibilities of different structures39. The as-synthesized TPB-DMTP-COF films also demonstrate good crystallinity and continuous properties (Fig. 4g, j, Supplementary Fig. 21). In general, benzoic acid acts as a nucleation modulator which interacts with the multifunctional amine-based COF building units through H bond, and these COF films can be theoretically synthesized through this strategy. To further demonstrate the versatility and general applicability of this method, we have further extended two β-ketoenamine linkage COFs films, namely Tp-2,2’-bipyridine-5,5’-diamine (Bpy)-COF (Fig. 4b, e, Supplementary Fig. 22) and Tp-p-phenylenediamine (PPDA)-COF (Fig. 4c, f, Supplementary Fig. 23), and two imine-linked COFs films, namely TPB-TFB-COF (Fig. 4h, k, Supplementary Fig. 24) and TPB-terephthalaldehyde (PDA)-COF (Fig. 4i, l, Supplementary Fig. 25). The XRD and SEM analysis exhibit well-defined crystalline nature and continuous film structure. In addition, owing to the good processability of fluidic COF-LZU1 precursor, COF films with desired structures can be grown on various substrates, such as leather, nickel foam and glass fiber (Supplementary Fig. 26). Taking advantage of this universality, the modulator-solvent induced polymerization strategy holds great prospects for the operation and utilization of COF films in specific applications.
a Chemical structure, and d XRD spectrum of Tp-Azo film (insert is the digital image of Tp-Azo film). b Chemical structure, and e XRD spectrum of Tp-Bpy film (insert is the digital image of Tp-Bpy film). c Chemical structure, and f XRD spectrum of Tp-PPDA film (insert is the digital image of Tp-PPDA film). g Chemical structure, and j XRD spectrum of TPB-DMTP film (insert is the digital image of TPB-DMTP film). h Chemical structure, and (k) XRD spectrum of TPB-TFB film (insert is the digital image of TPB-TFB film). i Chemical structure, and l XRD spectrum of TPB-PDA film (insert is the digital image of TPB-PDA film).
The functionalization of COF films
The vapor stimuli-responsive actuators using smart materials triggered by chemical vapors have shown broad application prospects in various high-tech fields such as aerospace, robotics, biomedical engineering, and sensors40,41,42,43. These smart materials display reversible deformations under the influence of chemical vapors, such as expansion, contraction, bending, twisting and rolling. The combination of the continuity and processability features of the polymers as well as the designability and porosity properties of the framework materials allows the obtained COF films with asymmetric structures to exhibit vapor-triggered actuation performance. Herein, the as-synthesized COF-LZU1 film is selected to further explore the vapor-responsive behavior. It is found that COF-LZU1 films show good response to various chemical vapors (Supplementary Fig. 27). Specifically, when placed over the liquid ethyl acetate (EA) phase, COF-LZU1 films with a width of 1 mm and length of 5 mm bend quickly into closed loops in the first 3 s and gradually stretch back to their original shape upon exposure back to air in 3 s (Fig. 5a). To demonstrate the practical application of COF films, COF-LZU1 films are built into a flower. The open flower prepared from COF-LZU1 films can gradually close in an EA vapor atmosphere and bloom rapidly after being exposed back to an air atmosphere (Fig. 5b). In addition, the bending-recovering process can be repeated for at least 20 cycles, and even up to 400 cycles, with only minimal deformation in its shape. The XRD and SEM analysis show no significant changes after testing the stimulus response testing (Fig. 5c, Supplementary Fig. 28). Moreover, COF films with a total organic backbone have organic chemicals affinity. In the EA vapor environment, the porous structure on the bottom side of COF-LZU1 films allows the solvent molecules to adsorb and diffuse into the structure, while the compact and non-porous top structure prevents the diffusion of EA vapor. This results in the inward bending of the compact and non-porous top surface due to the different structures between the top and bottom. In the degassing step, EA molecules diffuse easily out through the porous structure, leading to the recovery of the original shape (Fig. 5d). These films have great potential to serve as detectors of organic solvents leakage.
a Bending-recovering behavior of COF-LZU1 films (5 mm × 1 mm) placed in an ethyl acetate vapor or air atmosphere at room temperature. b Images of a flower with a closed-open-closed transformation upon alternate exposure to ethyl acetate vapor or air. c Plot of the reversible deformation cycles of the COF-LZU1 films. d Schematic illustration of the bending-unbending actuation of a COF-LZU1 films.
Interestingly, such strategy can also be used to encapsulate fluorescence molecules, chiral molecules and organic catalysts for fabricating the functional COF films (Fig. 6a). For example, a typical aggregation-induced emission (AIE) molecule benzophenone (BP) is firstly selected to be encapsulated in the COF-LZU1 structure during film formation. The crystal structure and fluorescence of BP@COF-LZU1 film are further characterized by XRD and fluorescence measurement, respectively. Simultaneously, as revealed by XRD measurements, BP@COF-LZU1 film composites exhibit a well-defined crystal structure identical to the patterns of COF-LZU1 film (Fig. 6b). The influence of the amount of introduced BP is investigated by fluorescence spectra. A redshift of emission wavelength is observed with the increase of BP concentration (Supplementary Fig. 29). Compared with the prepared COF-LZU1 film, BP@COF-LZU1 film shows good fluorescence properties (Fig. 6c). In order to further analysis the encapsulation status of BP molecules, the BET analysis was employed. Compared with the as-synthesized COF-LZU1 films, the as-prepared BP@COF-LZU1 films exhibit lower BET surface area (43 m2 g−1), and the corresponding pore volumes decreases from 0.061 to 0.025 cm3 g−1 (Supplementary Fig. 30). This result can be inferred that the BP molecules with small size might be embedded within the COF channel. In addition, chiral molecule L(+)-Tartaric acid (L(+)-TA), and a second-generation Grubbs catalyst (Grubbs-II) can also be efficiently integrated into the COF-LZU1 structure during film formation. L(+)-TA@COF-LZU1 film and Grubbs-II@COF-LZU1 film exhibit similar crystal structure to COF-LZU1, as characterized by XRD measurements (Fig. 6b). The chirality of L(+)-TA@COF-LZU1 films is studied by circular dichroism (CD) spectroscopy, exhibiting a strong CD signal in the range of 200–600 nm, which is similar to the CD signal of pure L(+)-TA in the liquid state (Fig. 6d). The presence of Ru, N and Cl characteristic elements in Grubbs-II@COF-LZU1 films is confirmed by SEM-mapping (Fig. 6e). In summary, different functional molecules can be controllably encapsulated in COF-LZU1 films, indicating the versatility and potential applications of this strategy in the fields of chemical sensing, drug delivery, and catalysis.
Discussion
In summary, we have designed a modulator-solvent induced strategy to fabricate COF films by balancing the regulation role of benzoic acid and solvent, wherein benzoic acid as competitive modulator slows down the imine bond formation, while the suitable solvent evaporation causes compact surface to supply reorganization environment for the inner amorphous structure to form crystalline COFs. Such strategy is not only applicable to fabricate kinds of COF films but also the prepared COF precursor solution can be deposited on variety of substrates. Based on this unique film structure with different states, COF-LZU1 films exhibit a good stimulus-responsive toward organic chemical molecules. COF-LZU1 films as the actuators can offer fast response and high recyclability due to their unique asymmetric structure. The further functionalization of COF films is achieved by encapsulating functional species in COF structures, which is promising for constructing COF films in applications such as optical devices, chiral catalysis and sensors. We anticipate that the method reported herein for forming COF films and functional COF films will broaden their realm of application.
Methods
Chemicals and materials
All the chemicals were received from suppliers without further purification unless otherwise noted, including 1,3,5-triformylbenzene (97%, Shanghai Tensus Biotech Co., Ltd.), p-phenylenediamine (97%, Sigma Aldrich), 4,4’-azodianiline (98%, Adamas), 1,3,5-triformylphloroglucinol (97%, Shanghai Tensus Biotech Co., Ltd.), 1,3,5-tris(4-aminophenyl)benzene (97%, Macklin), 2,5-dimethoxyterephthalaldehyde (97%, Sigma Aldrich), 2,2’-bipyridine-5,5’-diamine (98%, Adamas), 1,3,5-tris(4-aminophenyl)benzene (98%, Adamas), terephthalaldehyde (98%, Adamas), benzoic acid (99.5%, Shanghai Lingfeng Chemical Reagent Co., Ltd), 1,4-dioxane (99.5%, Sinopharm Chemical Reagent Co., Ltd), acetic acid (99.8%, Sinopharm Chemical Reagent Co., Ltd), p-toluenesulfonic acid monohydrate (98%, Energy Chemical), benzophenone (95%, Shanghai Apeptide Co., Ltd), L(+)-Tartaric acid (99%, Adamas), Hoveyda-Grubbs II (98%, Adamas), ether (analytical pure, Shanghai Lingfeng Chemical Reagent Co., Ltd), ethyl acetate (95%, Sigma Aldrich), tetrahydrofuran (99%, Shanghai NO.4 Reagent & H.v.chemical Co., Ltd), dichloromethane (99.9%, J&K Scientific), acetone (analytical pure, Sinopharm Chemical Reagent Co., Ltd), ethanol (99.7%, Sinopharm Chemical Reagent Co., Ltd), methanol (analytical pure, Sinopharm Chemical Reagent Co., Ltd), citric acid (98.5%, Sinopharm Chemical Reagent Co., Ltd), N,N-dimethylformamide (analytical pure, Sinopharm Chemical Reagent Co., Ltd), glass fiber (No. 1820-047, Whatman), potassium bromide (99.5%, Innochem), and nickel foam (analytical pure, Thermo Fisher Scientific). Deionized water was made in our laboratory.
Fabrication of COF-LZU1 particles
0.10 mmol of 1,3,5-triformylbenzene and 0.15 mmol of p-phenylenediamine were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Then, benzoic acid (0.3 mmol) was added to a 1,4-dioxane solution of p-phenylenediamine under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and p-phenylenediamine was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 1,3,5-triformylbenzene was poured into the mixed solution. The obtained solution was sonicated until evenly mixed, and placed in an 80 °C oven for 15 min and cooled to room temperature. Finally, the yellow product was washed three times with DMF and THF respectively, and soaked in THF for 12 h. The obtained product was dried overnight in a 120 °C vacuum oven.
Fabrication of COF-LZU1 films
Similarly, 0.10 mmol of 1,3,5-triformylbenzene and 0.15 mmol of p-phenylenediamine were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Then, benzoic acid (0.3 mmol) was added to a 1,4-dioxane solution of p-phenylenediamine under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and p-phenylenediamine was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 1,3,5-triformylbenzene was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of the fresh mixed solution was dropped on 1 cm2 clean glass plate, heated at 80 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of Tp-Azo COF films
0.05 mmol of 1,3,5-triformylphloroglucinol (Tp) and 0.075 mmol of 4,4’-azodianiline (Azo) were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Additional benzoic acid (0.6 mmol) was added to a 1,4-dioxane solution of 4,4’-azodianiline under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and 4,4’-azodianiline was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 1,3,5-triformylphloroglucinol was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of the fresh mixed solution was dropped onto 1 cm2 clean glass plate, heated at 150 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of TPB-DMTP COF films
0.04 mmol of 1,3,5-tris(4-aminophenyl)benzene (TPB) and 0.06 mmol of 2,5-dimethoxyterephthalaldehyde (DMTP) were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Additional benzoic acid (0.6 mmol) was added to a 1,4-dioxane solution of 1,3,5-tris(4-aminophenyl)benzene under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and 1,3,5-tris(4-aminophenyl)benzene was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 2,5-dimethoxyterephthalaldehyde was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of fresh mixed solution was dropped onto 1 cm2 clean glass plate, heated at 160 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of Tp-Bpy COF films
0.04 mmol of 1,3,5-triformylphloroglucinol (Tp) and 0.06 mmol of 2,2’-bipyridine-5,5’-diamine (Bpy) were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Additional benzoic acid (0.6 mmol) was added to a 1,4-dioxane solution of 2,2’-bipyridine-5,5’-diamine under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and 2,2’-bipyridine-5,5’-diamine was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 1,3,5-triformylphloroglucinol was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of the fresh mixed solution was dropped onto 1 cm2 clean glass plate, heated at 150 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of Tp-PPDA COF films
0.04 mmol of 1,3,5-triformylphloroglucinol (Tp) and 0.06 mmol of p-phenylenediamine (PPDA) were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Additional benzoic acid (0.6 mmol) was added to a 1,4-dioxane solution of p-phenylenediamine under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and p-phenylenediamine was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 1,3,5-triformylphloroglucinol was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of the fresh mixed solution was dropped onto 1 cm2 clean glass plate, heated at 150 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of TPB-TFB COF films
0.10 mmol of 1,3,5-tris(4-aminophenyl)benzene (TPB) and 0.10 mmol of 1,3,5-triformylbenzene (TFB) were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Additional benzoic acid (0.6 mmol) was added to a 1,4-dioxane solution of 1,3,5-tris(4-aminophenyl)benzene under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and 1,3,5-tris(4-aminophenyl)benzene was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of 1,3,5-triformylbenzene was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of fresh mixed solution was dropped onto 1 cm2 clean glass plate, heated at 100 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of TPB-PDA COF films
0.10 mmol of 1,3,5-tris(4-aminophenyl)benzene (TPB) and 0.15 mmol of terephthalaldehyde (PDA) were dispersed into 0.5 mL 1,4-dioxane solution respectively. The above solution was ultrasonically dispersed for 5 min until fully dissolved. Additional benzoic acid (0.6 mmol) was added to a 1,4-dioxane solution of 1,3,5-tris(4-aminophenyl)benzene under sonication for several minutes. 100 μL of a mixed solution of benzoic acid and 1,3,5-tris(4-aminophenyl)benzene was added to a 2 mL centrifuge tube, and another 100 μL of 1,4-dioxane solution of terephthalaldehyde was poured into the mixed solution. The obtained solution was shaken for 10 s. Lastly, 50 μL of fresh mixed solution was dropped onto 1 cm2 clean glass plate, heated at 100 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain COF films.
Fabrication of functional COF-LZU1 films
Functional molecules (BP, L(+)-TA and Grubbs-II) were first dissolved in organic solvent with specific concentrations. Then, the as-prepared solution was added into COF-LZU1 metastable amorphous network solution. Then, the obtained solution was sonicated uniformly for several seconds. The mixed solution was dropped on 1 cm2 clean glass plate, heated at 80 °C for 15 min and cooled to room temperature, followed by peeling the film off the glass slide to obtain functional COF films.
Solvent stability of COF-LZU1 films
The optimal preparation conditions are 0.3 mmol of benzoic acid and 80 °C of film preparation temperature. The film was soaked in solvent (ether, tetrahydrofuran, dichloromethane, ethanol, acetone, and deionized water) for 12 h, placed it in a vacuum oven at 120 °C for vacuum drying, and finally attached the sample to the conductive adhesive for SEM testing to observe whether it maintains original morphology and density.
Preparation of organic vapor-triggered actuators
The dried COF films are cut to obtain uniform size (5 mm × 1 mm). Fix one end of the COFs film with tweezers and move it into the range of organic molecules under test. The specific operation is to fix the COFs film above the organic solvent. Observe its changes, record the deformation using a camera, and convert it through video software.
DFT Calculations of binding energy between 1,3,5-triformylbenzene, p-phenylenediamine, and benzoic acid
All the energy calculations were performed based on DFT using Gaussian code by Gaussian 16, Revision C.01, M. The reaction segments were put together, respectively. And then the geometry optimization calculations were performed. The Perdew–Burke–Ernzerhof (PBE) functional was utilized to explore the optimal structure with a minimum energy. The lanl2dz basis functions were applied to the system. The energy differences were obtained after the geometry optimization.
Brunauer–Emmett–Teller (BET) surface area analysis
The specific surface areas (SBET) and the pore size (DP) distributions of the samples were performed on a Micromeritics ASAP 2460 apparatus at 77 K. COF films samples were prepared by breaking large films into pieces gently and pouring them into the testing tube. All the samples were degassed at 393 K for 12 h.
X-ray diffraction (XRD) measurements
The powder XRD patterns were recorded with a Bruker AXS D8 Advance diffractometer with nickel-filtered Cu Kα radiation (λ = 1.5406 Å). For time-resolved XRD, the film sample was tested at intervals of 3 min till it was fully dried at desired temperature.
Scanning electron microscopy (SEM) observation
A field-emission SEM (JEOL JSM-7600F) was used to scan the three-dimensional morphology and size of films with an accelerating voltage of 15 kV. Film samples were formed by drop coating on a silicon wafer or directly attaching to conductive adhesive to observe the three-dimensional morphology and size of COF film materials.
Transmission electron microscopy (TEM) observation
TEM images were taken by JEOL JEM-2100 Plus at an accelerating voltage of 200 kV to observe the morphology of the samples, as well as the microstructure at a certain size.
Atomic force microscopy (AFM) measurements
AFM images with surface roughness were obtained by Park NX20 instrument in a tapping mode. The COF-LZU1 film sample was attaching to a silicon slide for further characterization.
Time resolved Fourier transform infrared spectroscopy (FTIR) measurements
A Nicolet iS10 instrument was employed for tracking the vibration changes of functional groups during the drying process of COF-LZU1 precursor solution. KBr was used as background and the film sample was evenly mixed with KBr. The film sample was tested at intervals of 3 min till it was fully dried at desired temperature.
Performance tests of fluorescent functional COF-LZU1 Film
Steady-state fluorescence spectra and excitation spectra were measured using a FL F-7100 spectrophotometer.
Circular dichroism (CD) spectroscopy measurements
The CD spectrum was tested using a JASCO J-1500 CD spectrometer with a bandwidth of 0.1 nm and a scanning rate of 500 nm/min.
Data availability
The data that support the findings of this study are available in the article and Supplementary Information file. Source Data are provided with this paper. Additional data are available from the corresponding author upon request. Source data are provided with this paper.
References
Staudinger, H. Über Polymerisation. Ber. Der Dtsch. Chemischen Ges. A B Ser. 53, 1073–1085 (1920).
Peplow, M. The plastics revolution: how chemists are pushing polymers to new limits. Nature 536, 266–268 (2016).
Patrick, J. F., Robb, M. J., Sottos, N. R., Moore, J. S. & White, S. R. Polymers with autonomous life-cycle control. Nature 540, 363–370 (2016).
Ding, L. et al. Polymer semiconductors: synthesis, processing, and applications. Chem. Rev. 123, 7421–7497 (2023).
Lai, H. W. H. et al. Hydrocarbon ladder polymers with ultrahigh permselectivity for membrane gas separations. Science 375, 1390–1392 (2022).
Zuo, P. et al. Sulfonated microporous polymer membranes with fast and selective ion transport for electrochemical energy conversion and storage. Angew. Chem. Int. Ed. 59, 9564–9573 (2020).
Yi, J. et al. Water-responsive supercontractile polymer films for bioelectronic interfaces. Nature 624, 295–302 (2023).
Kim, S. D. et al. Poly(amide-imide) materials for transparent and flexible displays. Sci. Adv. 4, eaau1956 (2018).
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Tan, K. T. et al. Covalent organic frameworks. Nat. Rev. Methods Primers 3, 2 (2023).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Ji, C., Kang, C., Patra Bidhan, C. & Zhao, D. Flexible covalent organic frameworks: design, synthesis, and applications. CCS Chem. 0, 1–26 (2023).
Segura, J. L., Mancheño, M. J. & Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chem. Soc. Rev. 45, 5635–5671 (2016).
Guan, X., Chen, F., Qiu, S. & Fang, Q. Three-dimensional covalent organic frameworks: from synthesis to applications. Angew. Chem. Int. Ed. 62, e202213203 (2023).
Haase, F. & Lotsch, B. V. Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 49, 8469–8500 (2020).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
El-Kaderi, H. M. et al. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007).
Halder, A. et al. Ultrastable imine-based covalent organic frameworks for sulfuric acid recovery: an effect of interlayer hydrogen bonding. Angew. Chem. Int. Ed. 57, 5797–5802 (2018).
Zhong, S. et al. Radiation-assisted synthesis of crown ether-modified covalent organic frameworks for lithium isotope separation. CCS Chem. 0, 1–13 (2024).
Sick, T. et al. Oriented films of conjugated 2D covalent organic frameworks as photocathodes for water splitting. J. Am. Chem. Soc. 140, 2085–2092 (2018).
Hao, M. et al. Converging cooperative functions into the nanospace of covalent organic frameworks for efficient uranium extraction from seawater. CCS Chem. 4, 2294–2307 (2022).
Das, G. et al. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 6, 3931–3939 (2015).
Smith, B. J. & Dichtel, W. R. Mechanistic studies of two-dimensional covalent organic frameworks rapidly polymerized from initially homogenous conditions. J. Am. Chem. Soc. 136, 8783–8789 (2014).
Smith, B. J., Overholts, A. C., Hwang, N. & Dichtel, W. R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 52, 3690–3693 (2016).
Kandambeth, S., Dey, K. & Banerjee, R. Covalent organic frameworks: chemistry beyond the structure. J. Am. Chem. Soc. 141, 1807–1822 (2019).
Burke, D. W. et al. Acid exfoliation of imine-linked covalent organic frameworks enables solution processing into crystalline thin films. Angew. Chem. Int. Ed. 59, 5165–5171 (2020).
Fan, H. et al. Covalent organic framework–covalent organic framework bilayer membranes for highly selective gas separation. J. Am. Chem. Soc. 140, 10094–10098 (2018).
Dey, K. et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 139, 13083–13091 (2017).
Sahabudeen, H. et al. Highly crystalline and semiconducting imine-based two-dimensional polymers enabled by interfacial synthesis. Angew. Chem. Int. Ed. 59, 6028–6036 (2020).
Yang, Y. et al. A self-standing three-dimensional covalent organic framework film. Nat. Commun. 14, 220 (2023).
Biswal, B. P., Chaudhari, H. D., Banerjee, R. & Kharul, U. K. Chemically stable covalent organic framework (COF)-polybenzimidazole hybrid membranes: enhanced gas separation through pore modulation. Chem. Eur. J. 22, 4695–4699 (2016).
Yang, H. et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 10, 2101 (2019).
Ma, T. et al. Single-crystal x-ray diffraction structures of covalent organic frameworks. Science 361, 48–52 (2018).
Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19822 (2011).
Li, H. et al. Construction of metal–organic framework films via crosslinking-induced assembly. Adv. Mater. 35, 2209777 (2023).
Fan, H., Gu, J., Meng, H., Knebel, A. & Caro, J. High-flux membranes based on the covalent organic framework COF-LZU1 for selective dye separation by nanofiltration. Angew. Chem. Int. Ed. 57, 4083–4087 (2018).
Zhang, F. et al. Room-temperature synthesis of covalent organic framework (COF-LZU1) nanobars in CO2/water solvent. ChemSusChem 11, 3576–3580 (2018).
Kandambeth, S. et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes. Adv. Mater. 29, 1603945 (2017).
Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Mu, J. et al. Origami-inspired active graphene-based paper for programmable instant self-folding walking devices. Sci. Adv. 1, e1500533 (2015).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Lee, H., Xia, C. & Fang, N. X. First jump of microgel; actuation speed enhancement by elastic instability. Soft Matter 6, 4342–4345 (2010).
Zhao, Q. et al. An instant multi-responsive porous polymer actuator driven by solvent molecule sorption. Nat. Commun. 5, 4293 (2014).
Acknowledgements
We thank Prof. Wenxiong Shi (Tianjin University of Technology) for taking the DFT calculations. This work was supported by the National Natural Science Foundation (62288102, F.W.H.; 21727808, F.W.H.; 22375091, W.N.Z.; 22305077, H.F.L.), and the National Science Foundation for Distinguished Young Scholars (21625401, F.W.H.).
Author information
Authors and Affiliations
Contributions
W.N.Z. and F.W.H. designed the research and supervised experiments. X.R.L. performed the experiments, analyzed experimental data, and drafted the manuscript. X.Y.J. helped characterize the structure of as-prepared COF-LZU1 films and conduct the applications of the films. X.L.Z. designed the scheme diagram and helped draft the manuscript. X.Y.C., H.F.L. helped synthetize the film materials. S.Y.Z. helped design and revise the paper. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Renhao Dong, Manmatha Mahato and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Ji, X., Zhang, X. et al. Construction of functional covalent organic framework films by modulator and solvent induced polymerization. Nat Commun 16, 1223 (2025). https://doi.org/10.1038/s41467-024-55114-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55114-z