Abstract
Chronic allodynia stemming from peripheral stump neuromas can persist for extended periods, significantly compromising patients’ quality of life. Conventional managements for nerve stumps have demonstrated limited effectiveness in ensuring their orderly termination. In this study, we present a spatially confined conduit strategy, designed to enhance the self-organization of regenerating nerves after truncation. This innovative approach elegantly enables the autonomous slowing of axonal outgrowth in response to the gradually constricting space, concurrently suppressing neuroinflammation through YAP-mediated mechanotransduction activation. Meanwhile, the decelerating axons exhibit excellent alignment and remyelination, thereby helping to prevent failure modes in nerve self-organization, such as axonal twisting in congested regions and overgrowth beyond the conduit’s capacity. Additionally, proteins associated with mechanical allodynia, including TRPA1 and CGRP, exhibit a gradual reduction in expression as spatial constraints tighten, a trend inversely validated by the administration of the YAP-targeted inhibitor Verteporfin. This spatially confined conduit strategy significantly alleviates allodynia, thus preventing autotomy behavior and reducing pain-induced gait alterations.
Similar content being viewed by others
Introduction
Truncated peripheral nerves in patients with amputations due to warfare, burns1, traffic accidents2, and chronic diseases such as diabetes inevitably undergo spontaneous regeneration3,4, often leading to the formation of peripheral neuroma. This abnormal regrowth of nerve tissue is characterized by disorganized myofibroblast proliferation and axonal sprouting4,5, which causes stump nerves to be entangled in fibrotic scar tissue with a bulbous-end structure2. This traumatic neuroma can result in mechanical allodynia, a type of chronic neuropathic pain that may persist for months to years6,7. Several pathophysiological factors have been proposed to contribute to this allodynia, including demyelinated axons, scar adhesions, reactive oxygen species (ROS) release due to oxidative stress in Schwann cells (SCs), and activated microglial cells in the dorsal horn of the spinal cord8. Despite numerous surgical and non-surgical treatments, including oral analgesics, local radiofrequency ablation, and surgical excision, proposed for painful neuromas, these interventions have not completely resolved this clinical challenge yet, as evidenced by high failure rate or reoccurrence rate9,10. Therefore, reducing the occurrence of neuroma post-surgery has become a crucial focus of effort in the management of painful neuromas.
Historically, many clinical efforts have focused on suturing nerve stumps into neighboring autologous tissues, such as muscle tissue and veins; however, these endeavors have achieved limited success9,11. Recently, biomedical engineering techniques have significantly advanced the application of nerve conduits to cap nerve stumps, shielding them from surrounding tissues. However, conventional nerve conduits fail to manage the orderly termination of the neural stump interface because they are primarily designed to facilitate nerve growth12. This could potentially explain the delayed onset of pain when nerve growth exceeds the distal port of the conduit or twists and turns in the expansive space. Similar issues arise with conduits containing anti-inflammatory drugs that are designed to improve the local inflammatory microenvironment for pain relief13,9. That is, the effectiveness of drug-loaded conduits is entirely dependent on the controlled release of these drugs. Over time, as the effects of the drugs diminish, pain can recur. Consequently, traditional conduits designed to reduce and ultimately halt the cascade of hypersensitivity pain from the peripheral to the central nervous system are significantly limited in clinical effectiveness. Considering the positive effects of nerve conduits on guiding axonal regeneration in an orderly manner14,15,16, we propose a viable strategy to manage stump nerve self-organization by applying spatially confined conduit that is specifically designed to autonomously retard axonal outgrowth from the neural stump interface while ensuring the orderly axonal regrowth, thereby preventing the occurrence of neuroma.
Spatial constraints primarily influence cell migration and proliferation during the regrowth of the neural stump interface17. Evidence suggests that cell migration can be slowed by regulating intracellular signaling pathways within confined physical environments18 and varying chemical compositions19,20,21. In vitro, spatial confinement decelerates cell migration as cells use the nuclear ruler to modulate motile force, which consumes substantial energy and time for migration17,22,23. This suggests that spatial constraints may positively influence the deceleration of nerve regrowth. Furthermore, spatially-confined functionalized hydrogels have been observed to autonomously and orderly promote the development of cartilage-bone bi-layered structures in vivo24. This indicates that spatial constraints may not disturb the alignment of the nerve regrowth within the conduit.
Yes-associated protein (YAP)25, a nuclear relay of mechanical signals linked to the activity of nuclear transcription factors involved in the Hippo signaling pathway, responds to spatial constraints by regulating downstream signaling factors related to organ size26,27, thereby providing theoretical support for potential axonal atrophy in nerves within spatially constrained environments. Additionally, nerve injury has been reported to increase vascular permeability to local macrophages, creating a macrophage-dependent inflammatory microenvironment that further induces oxidative stress in SCs, leading to progressive demyelination28. This results in not only neuronal axons losing the protective myelin sheath formed by SCs, but also pain hypersensitivity triggered by ROS released by SCs29 under oxidative stress9. YAP activation in SCs has been demonstrated to play a vital role in nerve remyelination26. Furthermore, YAP activation in fibroblasts (FBs) can reduce its differentiation into myofibroblast, thereby exerting anti-fibrotic and anti-inflammatory effects in vivo30. Therefore, it is reasonable to hypothesize that SCs and FBs may respond to YAP-mediated mechanotransduction in a constrained in vivo environment, thereby promoting peripheral nerve remyelination, inhibiting fibrotic scarring and reducing ROS release, which ultimately alleviates neuropathic pain.
In this study, we fabricated frustum cone-shaped spatial-constrained conduits (CC) with varying conical degrees using poly(ε-caprolactone) (PCL)31 and mineralized collagen (MC) to cap the nerve stumps in a rat sciatic nerve amputation model. The incorporation of MC improved the cell adhesiveness on PCL. Spatial constraints were found to activate YAP-mediated mechanotransduction within SCs and FBs, thereby reducing the release of ROS. Furthermore, YAP activation in SCs decreased macrophage proliferation, and limited the polarization to M1 macrophages in peripheral nerves and the spinal cord (L4-L5). Compared to traditional cylindrical conduits (TC), the CC 4:2 (ratio between the proximal and distal diameters) showed the best performance in self-organization, including managing the orderly self-termination of the neural stump interface, reducing fibrotic scars, and alleviating pain via YAP-mediated mechanotransduction. This was demonstrated by immunofluorescence staining, autotomy scores, and catwalk analysis. Moreover, proteins associated with mechanical allodynia, including transient receptor potential ankyrin 1 (TRPA1) and calcitonin gene-related peptide (CGRP), exhibited a gradual reduction as spatial constraints tightened in the CC 4:2, a trend inversely validated by the administration of the YAP-targeted inhibitor Verteporfin (VP). Our results suggest that the engineered spatial constraint strategy we developed here offers a viable approach for achieving in vivo pain resolution at the nerve stump interface and holds great potential for clinical translation.
Results
Fabrication and characterizations of spatially-confined conduits
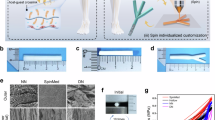
The PCL@MC laminae were initially prepared via a “multiscale cascade regulation” strategy that combined the processes of molecular self-assembly, electrospinning, and mechanical compression from molecular to macroscopic levels, similar with the method previously reported32,33 (Supplementary Fig. 1a, b). These laminae were then rolled into tubes with varying conical degrees. Scanning Electron Microscopy (SEM) results showed that the PCL@MC laminae maintained their oriented alignment of PCL@MC nanofibers and became more compact after mechanical compression (Fig. 1b). Mean orientation angle of the PCL fibers was 8.5° (σ² = 48.5) before compression and 8.2° (σ² = 42.6) after compression (Fig. 1c). The MC microfibrils that formed by a collagen-induced biomimetic mineralization process and uniformly encapsulated within the PCL fibers, exhibited a well-defined morphology and preferential orientation (Fig. 1d).
a The poly(ε-caprolactone) and mineralized collagen (PCL@MC) laminae were firstly prepared via a “multiscale cascade regulation” strategy that combined molecular self-assembly, electrospinning, and pressure-driven fusion processes from molecular to macroscopic levels. b Scanning Electron Microscopy (SEM) revealed that the PCL@MC laminae retained their oriented alignment of PCL@MC nanofibers and became more compact following mechanical compression. c Rose diagrams quantifying the absolute angle values for PCL@MC fibers relative to a vertical line, analyzed using ImageJ software. d Transmission Electron Microscopy (TEM) and Scanning Transmission Electron Microscopy (STEM) images displaying a well-defined morphology and preferential orientation of MC within PCL fibers. e Actual image of the elastic PCL@MC lamina and the rolled nerve conduits. f Fourier Transform Infrared (FTIR) spectroscopy revealed the typical absorption peaks of type I collagen and hydroxyapatite. g Thermogravimetric Analysis (TGA) of the mineral content in the PCL@MC laminae with 12%. h Contact angle experiments to test the hydrophilicity of PCL and PCL@MC laminae. i Tensile test to evaluate the tensile modulus and mechanical properties of PCL and PCL@MC laminae. j Compression test assessing the compressive resistance of nerve conduits with different spatial gradient constraints. The experiments in (b–j) were independently repeated at least three times with similar results.
Figure 1e displayed the gross appearances of a pliable PCL@MC lamina and the rolled nerve conduits (Supplementary Movie 1). The molds used for fabricating nerve conduits were processed into hollow cones with anterior-posterior inner diameter ratios of 4:1, 4:2, 4:3, and 4:4, respectively (Supplementary Fig. 2). Fourier Transform Infrared (FTIR) spectroscopy of PCL@MC lamina revealed the typical absorption peaks of type I collagen and hydroxyapatite (Fig. 1f). The typical absorption peaks of the PCL@MC laminae prepared by compression at 25 °C and 59 °C remained consistent, indicating the stability of MC under 60°C (Supplementary Fig. 3). In addition, the thermogravimetric analysis (TGA) results implied that the PCL@MC laminae had a mineral content of 12% (Fig. 1g), which resulted in a consistently lower water contact angle of 10° to 20° compared to PCL laminae (Fig. 1h). Under tensile testing, the PCL@MC laminae exhibited a tensile strength of 7.3 MPa, which exceeded the strength of 6.3 MPa observed in the PCL membrane (Fig. 1i). The radial compressive resistance of the PCL@MC conduits was represented by the compressive forces at a 30% reduction in conduit anterior diameter, which ranged from 1.5 N to 6.3 N for the conduits with different posterior inner diameter (Fig. 1j; Supplementary Fig. 4), implying their good efficacy in withstanding muscle compression in vivo.
The feasibility of spatial constraint strategy
SCs and FBs were cultured on PCL and PCL@MC laminae, confirming their excellent biocompatibilities (Fig. 2a, b). The SEM images (Fig. 2c) and rhodamine immunofluorescence (IF) staining of SCs and FBs (Fig. 2d) demonstrated that PCL@MC significantly enhanced cells adhesion, proliferation and orientation arrangement compared to PCL (Fig. 2e–g).
a Live/Dead staining of SCs cultured on PCL and PCL@MC laminae for 1 and 7 days. b Statistical analysis of the viability staining results for SCs cultured on PCL and PCL@MC membranes (n = 3). c SEM images of Schwann cells (SCs) and fibroblasts (FBs) adhered to PCL and PCL@MC laminae after incubation for 1 day. d Cytoskeletal imaging showing the adhesion of SCs and FBs on PCL and PCL@MC laminae after 1 day of incubation. e Quantitative analysis of SCs and FBs adhered to PCL and PCL@MC laminae after 1 day (n = 3). f Cell Counting Kit-8 assay was conducted to assess the proliferation conditions of SCs on PCL and PCL@MC laminae (n = 3). g The absolute angles of fibroblasts on the surfaces of PCL and PCL@MC laminae were quantified through cytoskeletal staining and analyzed using ImageJ, with mean ± SD (n = 3). h Fabrication process of the cell chips, from engraving the template via lithography to the application of a polydimethylsiloxane mask. i The graph of the culminating cell chip used in the experiment. j. Illustration for the loading of chemotactic serum and the seeding of SCs. k Rhodamine-labeled Phalloidin staining of SCs recorded their migration status through gradient-narrowing geometric spaces with ratios of 4:4, 4:3, 4:2, and 4:1, from the cell channel to the medium channel at 2 h. l Visualization of the trajectories of SCs migrating within different gradient geometric spaces of the chip under serum chemotaxis during 2 h. m, n Trajectory lengths and average speed of SCs migrating within gradient geometric spaces of the chip during 2 h, analyzed using ImageJ. o Finite element analysis was used to simulate the impact of spatial constraints on sciatic nerve regrowth in vivo. The image shows the 15th day. Mean values are shown and error bars represent ± s.d., as analyzed by two-sided Student’s t-test in (b–n). The experiments in (k) were independently repeated at least three times with similar results.
To demonstrate the feasibility of spatial constraint on cell behaviors, cell migration was evaluated in vitro using a customized cell chip (Fig. 2h, i; Supplementary Fig. 5). Cells and medium were introduced into the chip channels at predetermined time intervals (Fig. 2j). Rhodamine-labeled Phalloidin staining revealed the migratory patterns of SCs across gradient-narrowing geometric spaces from the cell channel to the medium channel after 2 h (Fig. 2k). In addition, the migration trajectories of the SCs were continuously tracked for 2 h (Fig. 2l). The findings indicated that both the migration velocity and the progression of the cell population decreased as geometric spaces became increasingly constricted (Fig. 2m, n).
Finite element analysis (FEA) was used to simulate the impact of spatial constraints on sciatic nerve regrowth in vivo, assuming a growth speed of 1 mm/day34. After 15 days of simulated growth, the nerve end exerted a force of 4.7 kPa in the CC 4:1, which diminished to 4.1 kPa in the CC 4:2, 2.3 kPa in the CC 4:3, and ~0.5 kPa in the TC 4:4 (Fig. 2o). In the CC 4:1 and CC 4:2, nerve growth ceased after 19 days. In contrast, in the CC 4:3 and TC 4:4, nerves extended to the edge of the conduit by days 12 and 10, respectively (Supplementary Movies 2–5).
In vitro degradation tests on PCL@MC conduits demonstrated that after 4 weeks of immersion in PBS at 37 °C, the conduits remained intact with a well-structured inner wall (Supplementary Fig. 6a). After 4 weeks of exposure to PBS containing 10 μg/mL lipase, morphological changes were observed, including gradual degradation of the inner wall and progressive thinning of the fibers. Supplementary Fig. 6b presents the mass measurements of the conduits under both in vitro and in vivo conditions. The degradation rate of the conduits increased in a concentration-dependent manner when exposed to lipase concentrations ranging from 0 – 100 μg/mL at 37 °C (Supplementary Fig. 6c). No statistically significant difference was found in the in vivo degradation rate (~10%) among conduits with different spatial constraints (Supplementary Fig. 6d).
Regulation of peripheral nerve self-organization
Peripheral nerve self-organization involves axon alignment, myelination, vascularization, and the regulation of fibrosis. A classic example of failed self-regulation is the formation of neuromas at nerve stumps. To determine an appropriate control group that simulates clinical neuroma formation, simple excision was compared with nerve stump suturing to neighboring muscle (muscle sutured group, MS)35 (Supplementary Fig. 7a, b). Supplementary Fig. 7c showed the appearances of the two groups of neuromas at 8 weeks post-surgery. Hematoxylin-eosin (HE) staining revealed disordered fiber morphology inside the neuromas in both groups (Supplementary Fig. 7d), and statistical analysis of fiber orientation showed that the average angle in the simple excision and MS groups were 40° (σ2 = 557.13) and 39.92° (σ2 = 526.47), respectively (Supplementary Fig. 7e). Statistical analysis of neuroma weight showed no significant difference between the two groups (Supplementary Fig. 7f), indicating that suturing the nerve stump to neighboring muscle did not significantly prevent neuroma formation. IF staining for scar tissue (α-smooth muscle actin, α-SMA), macrophage inflammation (F4/80, CD86), nerve axons (neurofilament 200 kDa, NF200), and myelination (Myelin basic protein, MBP) in the simple excision and MS group (Supplementary Fig. 7g, h) were conducted. The results showed that there was no significant difference in the scar-positive area ratio between the two groups (Supplementary Fig. 7i). However, the myelination ratio (MBP/NF200) within the neuromas was higher in the simple excision group compared to the MS group (Supplementary Fig. 7j), suggesting that additional muscle suturing can impede the natural process of myelination. Total macrophage (F4/80) and M1 macrophage counts showed no significant difference between the two groups (Supplementary Fig. 7k). Based on the aforementioned findings, simple excision was applied as a control group in the following studies.
Figure 3a shows a schematic diagram illustrating CC decelerates axonal regrowth and reduces neuroma formation in nerve stumps. At 12 weeks post-operation (Fig. 3b; Supplementary Fig. 8), the neuroma in all groups were shown in Fig. 3c. The newly formed axons were labeled with microtubule-specific class III β-Tubulin (TUJ1) (Fig. 3d). Microtubule densities within the CC groups exhibited a gradient, decreasing from the broader (proximal) to the narrower (distal) ends of the conduits, whereas microtubule in the TC 4:4 displayed a uniform distribution across both the proximal and distal ends. Axons did not reach the outermost edge of the 4:1 and 4:2 conduits, as indicated by the white curve (Supplementary Fig. 9a, b). Mature axons, labeled with NF200, were used for nerve length quantification (Fig. 3e). A gradual reduction in the mass and the axonal lengths of self-organized nerve terminals occurred in response to increasing spatial gradient constraints (Fig. 3f, g).
a A diagram of 4:2 spatially confined conduits, enhancing the directional guidance and reducing scar hypertrophy and vessels proliferation in sciatic nerves. b Images of the animal operation at day 0 and tissue collection at 12 weeks post-surgery. c Specimens of the regenerated nerve. d Immunofluorescence (IF) staining of microtubule (TUJ1, green; nuclei, blue) and e. neurofilaments (NF200, red; nuclei, blue) in vertical sections of the regenerated nerves within the spatial gradient constraint. f Statistical analysis of neuromas weight (n = 6). g Statistical results of the length of regenerated neurofilaments (n = 6). h Hematoxylin and Eosin (H&E) staining of nerve stumps. i Rose diagrams showing the absolute angles of regenerated nerve fibers relative to the horizontal line, quantified through HE staining and analyzed using ImageJ. Mean values are shown and error bars represent ± s.d., as analyzed by one-way ANOVA with Tukey’s post hoc tests in (f, g). The experiments in (d–h) were independently repeated at least three times with similar results.
To evaluate the orientation of regenerated nerves, HE staining was conducted on nerve samples (Fig. 3h), followed by a quantitative analysis of nerve orientation angles using ImageJ12. In comparison to the control group, which exhibited the least aligned orientation (θ = 43.8°, σ2 = 445.4) (Fig. 3i), the organized alignment of nerve tissues in TC 4:4 group demonstrated slightly improved (θ = 23.9°, σ2 = 141.1). The CC 4:2 group exhibited the most organized neural structure (θ = 10.3°, σ2 = 65.9), closely approximating that of normal nerves (θ = 8.5°, σ2 = 64.5) (Supplementary Fig. 10). However, the orientation of nerve fibers regenerated within the 4:1 conduit reached 36.3° (σ2 = 189.2), suggesting that excessive spatial constraint may lead to the bending and winding of nerve fibers. To further verify this finding, we included a gradually sealed confined-conduit group (CC 4:0) corresponding to CC 4:1 (Supplementary Fig. 7l). The orientation angles of the nerve fibers in CC 4:0 and CC 4:1 at 8 weeks post-surgery were 35.5° (σ2 = 204.20), 36.7° (σ2 = 226.92), respectively, deviating from normal nerve orientation (Supplementary Fig. 7m). The statistical analysis of neuronal axon length and neuromas weight showed no significant difference between the two groups (Supplementary Fig. 7n, o). Nevertheless, we found that compared to CC 4:1, the completely sealed CC 4:0 led to an increased accumulation of pain-inducing hydrogen peroxide (H2O2) metabolites within the nerve (Supplementary Fig. 7p). This was speculated to be due to the distal closure that prevented the metabolites from spreading out the conduit space along with body fluid diffusion. Additionally, RECA-1 and α-SMA were used as markers for nerve vasculature and scar tissue, respectively (Supplementary Fig. 11a–f). The results showed that CC 4:1 exhibited the lowest levels of vasculature expression (Supplementary Fig. 11g), while CC 4:2 showed the lowest levels of scar tissue formation (Supplementary Fig. 11h) among all groups. This suggests that moderate spatial constraint could reduce disordered nerve growth by mitigating nerve scarring and inhibiting angiogenesis. Furthermore, the results of remyelination observed through toluidine blue staining (TBS) (Fig. 4a) demonstrated that the CC 4:2 exhibited enhanced remyelination compared to other groups, showing the highest density of myelinated nerve fibers [Fig. 4b (i)]. Transmission electron microscope (TEM) (Fig. 4c) results showed that the myelin sheath thickness was greatest in the CC 4:2 group [Fig. 4b (ii)]. The average G-ratio3 observed in the CC 4:2 group was ~0.6, indicative of the optimal value (Fig. 4d). Therefore, considering the axonal regrowth rate and fiber orientation, a proximal-to-distal inner diameter ratio of 2 mm to 1 mm (4:2) can be considered the optimal spatial limit under the conditions of a nerve diameter of 2 mm and a total conduit length of 10 mm.
a Toluidine blue staining (TBS) images of regenerated nerves at 12 weeks postoperatively. b Myelin sheath quantifications on regenerated nerves displaying (i) average myelin thickness (n = 12) and (ii) number of regenerated axons per square millimeter (n = 3). c TEM images of nerve regeneration at 12 weeks postoperatively. d G-ratio analyses of regenerated axons, categorized by differing diameters. e Immunohistochemical (IHC) staining for C-fos, a protein expressed following noxious stimulation. f IHC staining for substance-P, a neuropeptide that enhances pain signal transmission and triggers inflammatory responses. g Quantification of activated cells per 10,000 μm² in C-fos IHC staining (n = 10). h Quantitative analysis of the positive area ratio per 10,000 μm² in substance-P IHC staining (n = 6). i. Diagram for pain behavior assessment across different time frames. j Result of autotomy scoring across different time frames (n = 6). k Footprints of rat limb gait over a 3 s interval and the intensity of the left hind limb footprints at 12 weeks after surgery. l The ratio of standing time during various time frames within a 5 s interval. m The ratio of maximum contact area during various time frames within a 5 s interval. Mean values are shown and error bars represent ± s.d., as analyzed by one-way ANOVA with Tukey’s post hoc tests in (b–j). Mean values are shown and error bars represent ± SEM, as analyzed by one-way ANOVA with Tukey’s post hoc tests in (l) and (m). The experiments in (c) were independently repeated at least three times with similar results.
To determine whether the CC could achieve pain resolution, the behaviors and immunohistochemical staining for classical pain indicators of c-Fos (Fig. 4e) and Substance-P (Fig. 4f) were investigated. The results revealed that the CC 4:2 exhibited the lowest number of activated c-Fos-positive cells (Fig. 4g) and the smallest area of Substance-P (Fig. 4h). Figure 4i illustrates the time points for assessing pain behaviors. The autotomy score results indicated that the CC 4:2 alleviated chronic pain, with the lowest score stabilizing from the 3rd week onward (Fig. 4j). The CC 4:3 group demonstrated performance comparable to that of the TC 4:4 group. In addition, the results of catWalk analysis36 (Fig. 4k) showed that in the CC 4:2 group, the maximum contact area (Fig. 4l) and the standing time (Fig. 4m) of the left nerve injury side were most akin to the preoperative normal condition. Importantly, no significant differences were observed between CC 4:1 and CC 4:0 in partial behavior tests (Supplementary Fig. 7q), including autotomy scores (Supplementary Fig. 7r) and maximum footprint areas (Supplementary Fig. 7s). However, possibly due to the accumulation of hydrogen peroxide, the standing time of the left hind limb in CC 4:0 was shorter than in CC 4:1 (Supplementary Fig. 7t), suggesting that the inability to eliminate harmful metabolic substances may contribute to pain generation.
YAP-mediated mechanotransduction in nerve stumps
To explore the connection between sciatic nerve regrowth in CC and YAP-mediated mechanotransduction, YAP activity was assessed across all groups. S100 and YAP IF co-staining were employed to visualize mechanotransduction in SCs. The results indicated that nuclear translocation of YAP was most prominent in the CC 4:1 and 4:2 groups under spatial constraint (Supplementary Fig. 12a, b). Conversely, in the CC 4:3 and TC 4:4 groups, YAP predominantly remained in the cytoplasm (Supplementary Fig. 12c, d). Statistical analysis revealed no statistically significant differences in nuclear translocation and staining intensity of YAP between the CC 4:1 and CC 4:2 groups, nor between the CC 4:3 and TC 4:4 groups (Supplementary Fig. 12e, f). The expression levels of YAP protein were inversely correlated with phosphorylated YAP (p-YAP) levels across all groups. This suggests that YAP translocates to the nucleus following dephosphorylation. Notably, YAP expression level was highest in the CC 4:2 group (Supplementary Fig. 12g), whereas p-YAP levels were lowest (Supplementary Fig. 12h). This further confirms that the YAP-mediated mechanotransduction process is most prominent in the CC 4:2 group.
YAP-mediated mechanotransduction in vitro
To confirm the activation of YAP-mediated mechanotransduction and its biological functions in SCs in response to different mechanical environments, we cultured SCs on substrates with varying matrix stiffnesses. Figure 5a illustrates the changes in YAP translocation between the nucleus and cytoplasm of SCs in response to different stiffness of PDMS (20 kPa, 0.5 MPa, and 2 MPa) and PCL@MC laminae, treated with VP (50 μM) and its vehicle (PBS solution with 5% DMSO) (Fig. 5b). The expression of YAP and p-YAP in SCs on PCL@MC laminae was reversed by VP (Supplementary Fig. 13). Statistical analysis showed that the average nuclear-to-cytoplasmic ratio of YAP in SCs on a 20 kPa substrate was below 1.0. In contrast, on a 2 MPa PDMS and PCL@MC laminae, the ratio approached 2.0, higher than that on the 0.5 MPa substrate (Fig. 5c). Flow cytometry analysis of ROS in SCs treated with VP and Vehicle revealed that Vehicle treatment did not affect ROS levels, whereas YAP inhibition by VP significantly increased ROS levels (Fig. 5d). Additionally, the activation of YAP-mediated mechanotransduction using gradient stiffness substrates significantly reduced ROS release from SCs (Fig. 5e). These results indicate that YAP-mediated mechanotransduction regulates ROS release by SCs.
a The application of YAP inhibitors, in comparison to the vehicle, influences the nuclear and cytoplasmic expression of YAP in SCs cultured on PDMS substrates with elastic moduli of 20 kPa, 0.5 MPa, and 2.0 MPa, as well as on PCL@MC laminae. b IF staining of YAP nuclear/cytoplasmic expression in SCs. c Statistical analysis of Nuclear/cytoplasmic expression ratio of YAP in SCs (n = 12). d Using flow cytometry, ROS release levels were analyzed in H2O2-pretreated SCs on PCL@MC membranes after 24 h of treatment with either no additional treatment (CON) or 10 μM VP and a vehicle (Veh) (n = 3). e Flow cytometry analysis and statistical analysis of SCs treated with and without H2O2 for 12 h (n = 3). f A diagram of indirect co-culture of H2O2-induced oxidative stress of SCs on substrates of different hardness with HAPI cells in upper well. g A diagram of YAP-mediated mechanotransduction in SCs modulating iNOS+ polarization of microglia. h Flow cytometry and statistical analysis of iNOs+ HAPI cells after 1 h of incubation with DCFH-DA (n = 3). i IF staining displaying YAP-mediated mechanotransduction differences in SCs in the sciatic nerve before and after perineural administration of Verteporfin (VP) in 4:2 and 4:4 groups (SC, green; YAP, red; nuclei, blue). j Statistical analysis of nuclear/cytoplasmic expression ratio of YAP in SCs within sciatic nerve across all groups (n = 12). k Statistical analysis of YAP expression intensity in SC nuclei in the sciatic nerve across all groups (n = 3). l. ELISA results showing YAP protein levels in the sciatic nerve across all groups (n = 3). m ELISA results for phospho-YAP protein levels in the sciatic nerve at postoperative weeks 12 (n = 3). Mean values are shown and error bars represent ± s.d., as analyzed by two-sided Student’s t-test in (c–m). The experiments in (a, b) were independently repeated at least three times with similar results.
To elucidate the effect of ROS released by SCs on microglial polarization37,38,39, the SCs were indirectly co-cultured with highly aggressively proliferative immortalized (HAPI) microglia cells (Fig. 5f). Cell seeding was carefully controlled to ensure uniformity. The increase of YAP nuclear translocation in SCs led to a decrease in the proportion of HAPI polarization to the M1 phenotype, as demonstrated by flow cytometry (Fig. 5g). The lowest counts of iNOS-marked M1-type HAPI cells were also observed in the 2 MPa group (Fig. 5h). These findings suggest that enhancing YAP-mediated mechanotransduction in SCs can decrease ROS release and microglial polarization to the M1 phenotype.
To further clarify the role of YAP-mediated mechanotransduction in ROS release by FBs, which, unlike SCs, are closely associated with neuroma scars, we first examined the effectiveness of YAP-mediated mechanotransduction in FBs using substrates of varying stiffness as physical stimuli (Supplementary Fig. 14a). Statistical analysis of the nucleocytoplasmic ratio of YAP fluorescence intensity revealed the highest ratio in 2.0 MPa and PCL@MC lamella, with no significant difference between them. Additionally, VP treatment downregulated the nucleocytoplasmic ratio of YAP in FBs (Supplementary Fig. 14b). Flow cytometry results indicated that Vehicle treatment did not affect ROS levels (Supplementary Fig. 14c). However, inhibiting YAP nuclear translocation with VP significantly increased ROS levels in FBs (Supplementary Fig. 14d).
Confined-conduit tunes pain responses associated with neuroinflammation in vivo
Chronic inflammation in injured nerves can lead to allodynia39. To investigate the role of CC in alleviating allodynia, we assessed the proliferation status of inflammatory cells in both the stump nerves and the posterior horn of the spinal cord. The CC 4:2 group exhibited the lowest number of total macrophages, marked by F4/80 (Supplementary Fig. 15a). Furthermore, chronically proliferating M1 macrophages were quantified using co-localization staining of CD86, a marker for M1 macrophages, and Ki67, a proliferation marker (Supplementary Fig. 15b). It was found that the counts of total macrophages and proliferating M1 macrophages were lowest in the CC 4:2 group (Supplementary Fig. 15c, d). However, no significant differences in M1 macrophage counts were observed between the TC 4:4 and CC 4:3 groups (Supplementary Fig. 15d). In addition, the CC 4:2 group had the fewest Iba-1-marked microglia in the L4-L5 spinal cord (Supplementary Fig. 15e). Importantly, the Pearson correlation index, for co-localization of Ki67 and CD 86, were >0.3, and the overlap index was above 0.6 in all groups (Supplementary Fig. 15f), indicating that M1-type microglia were still proliferating in the L4-L5 spinal cord at 12 weeks post-peripheral nerve injury. The fewest proliferating M1 microglia were found in the CC 4:2 group. No significant statistical differences were observed between the TC 4:4 and CC 4:3 groups (Supplementary Fig. 15g, h).
YAP inhibitor reverses the anti-inflammation effect of spatial confinement in vivo
To further confirm the link between inflammatory-related function and YAP-mediated mechanotransduction in CC, the YAP inhibitor VP was administered perineurally to both the CC 4:2 and TC 4:4 groups. Compared to the VP-untreated CC 4:2 group, the VP-treated CC 4:2 group showed significant inhibition of YAP nuclear translocation (Fig. 5i, j) and decreased fluorescence intensity (Fig. 5k). Conversely, VP did not affect YAP nuclear translocation or fluorescence intensity in the TC 4:4 group compared to the untreated control. Moreover, in the TC 4:4 group, YAP protein expression was low, while p-YAP expression was high. However, in CC 4:2, YAP protein level was highest and p-YAP level was lowest. VP treatment significantly decreased YAP and increased p-YAP expressions (Fig. 5l, m). In addition, ROS levels, indicated by H2O2 content, were lowest in CC 4:2 and increased with VP treatment (Supplementary Fig. 16). These results suggest that p-YAP undergoes dephosphorylation to become YAP in spatial confinement, which subsequently translocates into the nucleus, and thereby inhibits the release of ROS in nerve.
Staining for total macrophages (F4/80) and M1 macrophages (CD86) showed significantly higher numbers in the VP-treated CC 4:2 group compared to the untreated group (Fig. 6a, b). However, no significant difference was observed between the VP-treated TC 4:4 group and untreated counterparts (Fig. 6c, d). Moreover, staining for total microglia (Iba-1) and M1 microglia (CD86) in the L4-L5 spinal cord revealed significantly higher numbers in the VP-treated CC 4:2 group compared to untreated group (Fig. 6e, f), with no statistical difference between the VP-treated TC 4:4 group and untreated counterparts (Fig. 6g, h). Figure 6i illustrates the role of the CC strategy in resolving allodynia, extending from the peripheral nerves to the spinal cord. This suggests that the CC strategy may reduce neuroinflammation and allodynia through YAP-mediated mechanotransduction.
a IF staining illustrating inflammation with F4/80+ macrophages in sciatic nerves at 12 weeks post-injury. b IF staining of CD86+ M1 macrophages in sciatic nerves at 12 weeks post-injury. c Quantification of F4/80+ inflammatory macrophages per 10,000 μm2 in regenerated nerves at 12 weeks (n = 12). d Quantification of CD86+ M1 macrophages per 10,000 μm2 in regenerated nerves at 12 weeks (n = 12). e IF staining of Iba-1+ microglia in the dorsal horn of the spinal cord at 12 weeks post-operation. f IF staining showing CD86+ microglia in dorsal horn of the spinal cord at 12 weeks post-injury. g Quantification of Iba-1+ microglia per 10,000 μm2 in the spinal cord dorsal horn at 12 weeks (n = 6). h Quantification of CD86+ microglia per 10,000 μm2 in the spinal cord dorsal horn at 12 weeks (n = 6). i Scheme illustrating the role of CC strategy in resolving allodynia, extending from the peripheral nerves to the spinal cord, suggesting that the CC strategy reduces neuroinflammation and allodynia through YAP-mediated mechanotransduction. Mean values are shown and error bars represent ± s.d., as analyzed by one-way ANOVA with Tukey’s post hoc tests in (c–h).
Genetic mechanism of pain-relief in spatially-constrained strategy
To explore the genetic mechanisms of CC in pain relief, mRNA sequencing was conducted on nerve stumps. Venn diagrams illustrated the variation in shared genes (Fig. 7a) and differentially expressed genes (Fig. 7b). Gene Ontology (GO) annotation analysis between the CC 4:2 and TC 4:4 groups indicated the distinct presence and development of axonal structures within cellular components (Fig. 7c). Furthermore, these cellular components were mainly engaged in positively regulating T-helper 1 type immune responses, interleukin-10 production, neuroinflammatory regulation, remyelination processes, and negatively regulating axon extension (Fig. 7d). It was found that the CC 4:2 significantly slowed down axon growth compared to the VP-treated CC 4:2 and TC 4:4 groups, whose axonal regrowth extended beyond the outer edge of the conduits (Supplementary Fig. 17a, b). The VP-treated TC 4:4 showed axon regeneration indistinguishable from VP-untreated TC-4:4 (Supplementary Fig. 17c). Supplementary Fig. 17d illustrated the principal component analysis (PCA) of neural samples from four groups: CC 4:2, CC 4:2 + VP, TC-4:4, and control. Axon guidance genes (Supplementary Fig. 18a) and negative axonal extension genes (Supplementary Fig. 18b) showed the highest expression levels in CC 4:2, as shown in the correlative heatmaps. Additionally, HE staining of liver and kidney samples proved that VP did not induce toxicological effects in vivo (Supplementary Fig. 19).
a Visualization of common and differentially expressed genes among groups treated with 4:4 traditional conduits (TC), 4:4 traditional conduits + VP inhibition (TC + VP), 4:2 spatially confined conduits (CC), 4:2 spatially confined conduits + VP (CC + VP), and untreated control group. b Number of differentially expressed genes in comparisons between CC vs. TC, and CC + VP vs. CC. [*P < 0.05, |log2 (fold change) | > 1]. c GO annotations analysis focusing on synaptic parts, development processes, cellular component organization, localization, and immune system processes. d GO enrichment analysis targeting T-helper 1 type immune responses, interleukin-10 production, regulation of neuroinflammatory responses, remyelination processes, and negative regulation of axon extension. e KEGG pathway enrichment analysis for cell adhesion molecules, NF-kappa B signaling pathway, axon guidance, and Hippo signaling pathway. f Volcano plot displaying all differentially expressed genes between CC and TC groups, with specific annotations for genes involved in anti-inflammatory, pro-inflammatory, mechanotransduction, remyelination, and pain activation pathways. g GSEA highlighting various processes of concern. These processes were compared between CC and TC, as well as between CC + VP and CC. All pairwise comparison groups exhibited P-values <0.05. h Heatmap of gene expression related to the Hippo signaling pathway across experimental groups. (n = 3). i Quantitative RT-PCR analysis of genes associated with mechanotransduction, remyelination, immune response, and pain resolution. (n = 3). Mean values are shown and error bars represent ± s.d., as analyzed by two-sided Student’s t-test in (i). Data are presented as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
The differential signaling pathways between the CC 4:2 and TC 4:4 primarily involved cell adhesion molecules, NF-kappa B signaling, axon guidance, and Hippo signaling (Fig. 7e). Significantly differential genes were broadly categorized into four groups related to inflammatory responses, mechanotransduction, remyelination, and pain activation (Fig. 7f). Furthermore, Gene set enrichment analysis (GSEA) was used to enrich concrete gene sets across these four categories. The results indicated that, compared to TC 4:4, genes in the pain-resolving gene sets, such as those involved in the negative regulation of cytosolic calcium ion concentration40, remyelination, and YAP-mediated mechanotransduction of the Hippo signaling pathway, were highly expressed in the CC 4:2. Additionally, genes in the inflammatory gene set were expressed at lower levels in the CC 4:2. These four gene sets in the VP-treated CC 4:2 showed an inverse trend compared to the CC 4:2 (Fig. 7g).
The YAP signaling (Hippo pathway) gene set was significantly enriched in the heatmap (Fig. 7h). Genes involved in mechanotransduction, such as ERK, YAP1, CYR61 (a TEAD family member), ATF3, ATF4, NOTCH1, and WNT9a, were highly expressed in the CC 4:2 group and markedly suppressed after VP treatment (Fig. 7i). In terms of remyelination, numerous genes were depicted in the heatmap (Supplementary Fig. 18c). The qPCR results verified that genes, including MBP, MPZ, MPDZ, NCMAP, and PMP22, contributed to remyelination in the CC 4:2 group (Fig. 7i). More than that, the MBP gene was further validated at the protein level through IF staining (Supplementary Fig. 20a), showing that remyelination was most pronounced in the CC 4:2 group, but significantly decreased after VP administration (Supplementary Fig. 20b). In terms of immune response, the CC 4:2 modulated a positive immune response with the highest expression levels of IL-10 and the lowest levels of IL-12a, IL-2, TNF-α, CSF, and CCL2 (Fig. 7i). Additional genes related to the enrichment of IL-12a, IL-2, and CCL2 were shown in heatmaps (Supplementary Fig. 21a, b, and Fig. 22). The CC 4:2 significantly downregulated the genes directly associated with pain hypersensitivity, such as Apolipoprotein E (APOE) and TRPA1, while upregulating analgesia genes like ATP2b1 and ATP1a2. Importantly, these pain-related genes were closely associated with YAP signaling, as qPCR results demonstrated that VP treatment dramatically reversed their expression (Fig. 7i).
The YAP-mediated biological function genes in H2O2-pretreated SCs and FBs were then validated in vitro using qPCR. The detected genes included those involved in YAP-mediated mechanotransduction (YAP, Cyr61, Wnt6, Wnt9a, Notch-1), specific inflammatory factors (CCL-2, IL-2, IL-12a, TNF-α), myelin regeneration (MBP, MPDZ, MPZ, Ncmap, Pmp22), and pain-related pathways (ApoE, ATP1a2, ATP2b1, TRPA1). The use of the vehicle showed no difference compared to the blank control; however, the application of VP in SCs reversed the expression of related genes (Supplementary Fig. 23a). This was consistent with the qPCR results of in vivo nerve samples, indicating that the YAP-mediated mechanotransduction process in neural tissue within a spatially constrained environment is closely related to SCs. Additionally, VP application in FBs was found to reduce the expression of inflammatory factors α-SMA and TGF-β1, which induce scar hyperplasia as well as other scar-related inflammatory factors (CCL-2, TNF-α, IL-2, IL-12a) (Supplementary Fig. 23b). This partially explains why spatial constraints can reduce scar deposition in regenerating neural tissue.
Spatial distribution of pain-related proteins and newly formed axons in conduits
TRPA1 and CGRP are considered essential proteins involved in mechanical allodynia41. To examine their expression under different spatial constraints, IF staining intensity from proximal to distal ends was analyzed, followed by Pearson correlation analysis to assess the relationship with distance. Co-localization of TRPA1 with CGRP confirmed the presence of TRPA1 in sensory nerve fibers (Fig. 8a). Additionally, microtubules in newly formed axons, marked by TUJ1, exhibited co-localization with TRPA1 (Fig. 8b). Supplementary Fig. 24a showed that neuronal axons progressively increased from the proximal to the distal ends in the control group. In the TC 4:4 group, no significant difference was observed in axon distribution, regardless of VP administration. However, a sequential decrease in axon density was noted from the proximal to the distal ends in both the CC 4:2 and CC 4:2 + VP groups. This suggests that the longitudinal decrease in axon density in the CC 4:2 group is independent of YAP (Supplementary Fig. 24b). In addition, TRPA1 and CGRP exhibited high expression levels in the control group (Fig. 8c), with a gradual increase from the proximal to distal ends (Fig. 8d). However, the expression levels of TRPA1 and CGRP were reduced in CC 4:2 (Fig. 8e), with a progressive decrease from the proximal to the distal ends (Fig. 8f). In the VP-treated CC 4:2 group, CGRP expression showed no significant spatial correlation, whereas TRPA1 expression increased from the proximal to the distal ends (Fig. 8g, h), indicating that VP treatment more effectively reversed TRPA1 expression than CGRP. In the TC 4:4 group, the expressions of TRPA1 and CGRP did not exhibit significant spatial correlation, regardless of VP administration (R < 0.3 or R > -0.3) (Fig. 8i-l). Furthermore, the expression levels of CGRP did not significantly differ in stump nerves at the proximal portions between groups, regardless of VP administration (Fig. 8m, n). However, TRPA1 and CGRP expression levels in both the proximal and distal nerves of the VP-treated 4:2 group were significantly higher than in the VP-untreated 4:2 group (Fig. 8n, o). Taken together, the above results indicate that the expression of pain-related proteins, TRPA1 and CGRP, decreases with spatial narrowing via YAP signaling activation, whereas the decrease in axons regeneration within CC 4:2 is independent of YAP signaling.
a IF staining colocalization of CGRP and TRPA1. b IF colocalization of TUJ1+ neuronal fibers with nociceptor TRPA1. c IF staining of CGRP and TRPA1 in the control group. d Linear regression of the spatial distribution of CGRP and TRPA1 through IF intensity from proximal to distal ends in the control group. e IF staining of CGRP and TRPA1 in CC 4:2. f Linear regression of the spatial distribution of CGRP and TRPA1 IF intensity from proximal to distal ends in CC 4:2. g IF double staining of CGRP and TRPA1 in CC 4:2 treated with VP inhibitor. h Linear regression of the spatial distribution of CGRP and TRPA1 IF intensity from proximal to distal ends in the 4:2 + VP group. i IF staining of CGRP and TRPA1 in TC. j Linear regression of the spatial distribution of CGRP and TRPA1 through IF intensity from proximal to distal ends in TC 4:4. k IF staining of CGRP and TRPA1 in TC 4:4 + VP group. l Linear regression of the spatial distribution of CGRP and TRPA1 through IF intensity from proximal to distal ends in the 4:4 + VP group. m Comparative IF staining of CGRP and TRPA1 in different groups at similar proximal and distal distances. n Quantitative analysis of proximal positive area of CGRP and TRPA1 across different groups (n = 6). o Quantitative analysis of distal positive area of CGRP and TRPA1 across different groups (n = 6). Mean values are shown and error bars represent ± s.d., as analyzed by one-way ANOVA with Tukey’s post hoc tests in (n, o). The experiments in (c–k) were independently repeated at least three times with similar results.
Pain-associated behaviors alleviated under spatial constraint
Autotomy scoring and catwalk analysis2,9 were used to evaluate pain-associated behaviors. Supplementary Fig. 25a (i) shows a 2-cm surgical incision mark. The distal end of the sciatic nerve was transected at 1 cm [Supplementary Fig. 25a (ii)]. One day after surgery, the left hind paws of the rats showed an inability to extend [Supplementary Fig. 25a (iii)]. Supplementary Fig. 25a (iv) depicts the method of VP injection administration. Supplementary Fig. 25a (v) provides time points for behavioral data collection. Autotomy was assessed at 3, 6, and 12 weeks postoperatively [Supplementary Fig. 25b (i)]. Supplementary Fig. 25b (ii) outlines the criteria for autotomy scoring. Autotomy behavior in the VP-treated CC 4:2 group exhibited significantly more severe than the VP-untreated group. In contrast, the autotomy behavior in the VP-treated TC 4:4 group did not significantly differ from the untreated TC 4:4 group (Supplementary Fig. 25c), indicating that VP impedes pain resolution within spatially confined conduits, and neither the administration method nor the vehicle of VP exacerbates the pain. Additionally, gait analysis revealed that the maximum contact area and standing time of the left hind foot in the VP-treated 4:2 group were significantly smaller and shorter than those in the VP-untreated group at 3 weeks postoperatively (Supplementary Fig. 25d, e). These observations were consistent with the autotomy scores discussed previously. Conversely, the maximum contact area and the standing time of the left hind foot in the VP-treated TC 4:4 group were indistinguishable from the VP-untreated TC 4:4 group at 7 weeks postoperatively (Supplementary Fig. 25d, e). These findings suggest that spatially confined conduits effectively alleviate limping behavior resulting from neuropathic pain through YAP signaling activation.
Discussion
Neuromas at amputated nerve stumps, exhibiting spontaneous activity, frequently cause neuropathic pain triggered by light touch39,42,43. Nerve capping has shown promise as a treatment method due to its beneficial effects on nerve stumps protection9,44,45. However, the excessive growth of spontaneously regenerated nerves within the capping conduit contradicts the goal of limiting nerve regrowth. Here, we propose a viable spatial constraint strategy designed to decelerate nerve regrowth and manage the orderly termination of the neural stump interface.
This strategy effectively averted failure modes in nerve self-organization including axonal twisting in overcrowded areas and overgrowth exceeding the conduits capacity. Recently, many innovative nerve conduits featuring aligned topographic structures, such as channels or nanofibers, have demonstrated excellent performance in promoting rapid nerve regeneration and orientation14,15,16. In our study, the aligned PCL/MC nanofibers of the CC conduits are expected to facilitate orderly axon extension. Under this premise, the improved performance to regulate spontaneous growth deceleration can be attributed to the mechanical and biological confinements imposed by their structural design with gradually spatial constraints. Additionally, compared to commercial closed capping, which can induce H2O2 accumulation within conduits, PCL@MC conduits with appropriate constraints (CC 4:2) and distal openings facilitate H2O2 release out of the conduit, enhance neural self-regulation, and decelerate nerve growth more effectively than other configurations with ratios of 4:4, 4:1, or 4:3. Furthermore, this purely physical intervention provides greater sustainability and stability than pharmaceutical pain management, without medication-associated side effects. Thus, this strategy may represent a promising therapeutic potential for managing neuroma formation and neuropathic pain at amputation sites in clinical translation.
In this study, spatial constraints were found to activate YAP-mediated mechanotransduction within SCs and FBs, which consequently influenced the processes of remyelination, scar formation and neuroinflammation. At the injury site, the demyelination of SCs impairs sensory nerve functions and leads to neuropathic pain28,46. Previous studies indicate that remyelination is highly dependent on mechanotransduction, potentially regulated by the mechanical effector YAP of the Hippo pathway26,47. This study demonstrated that the activation of YAP-mediated mechanotransduction in SCs is essential for promoting myelination in vitro and in vivo, thereby enabling sensory neurons to resist pain stimuli in the inflammatory microenvironment following nerve injury. Additionally, YAP-mediated mechanotransduction reduced the expression of scar-related genes in FBs in vitro, suggesting that spatial constraints in vivo may reduce scar hyperplasia through YAP signaling. Moreover, it is well-established that neuroinflammation impedes SC proliferation and remyelination41,47 and promotes scar formation2,28. However, the relationship between YAP-mediated mechanotransduction in SCs or FBs has not been well addressed previously. Our study demonstrates that the activation of YAP-mediated mechanotransduction in oxidative-pretreated SCs through modulating substrate stiffness in vitro significantly reduces ROS release. Although YAP-mediated mechanotransduction in FBs also reduces ROS release, the effect is less pronounced than in SCs. Furthermore, peripheral neuroinflammation-induced pain hypersensitivity elevates the number of microglia in the spinal dorsal horn, thereby intensifying pain perception8. In this study, we observed a significant reduction in macrophage differentiation into the M1 phenotype in peripheral nerves and the L4-L5 dorsal horn of the spinal cord in CC 4:2 group. Therefore, it can be concluded that YAP-mediated mechanotransduction in nerves significantly decreases neuroinflammation and alleviates allodynia. Although we were unable to systematically exclude the impact of mechanotransduction in unstudied cells on neuropathic pain relief, our findings confirm the crucial role of SCs and FBs in alleviating pain after peripheral nerve transection via YAP-mediated mechanotransduction.
Notably, the activation of YAP-mediated mechanotransduction under spatial constraints reduced the expression of pain-related genes and proteins. TRPA1 is a nociceptor channel expressed by peptidergic primary sensory neurons, serving as a primary peripheral target for mechanical allodynia39,48,49. CGRP, by activating endosomal TRPA1, maintains the heightened sensitivity of primary sensory neurons linked to neurogenic pain41. Peripherally acting anti-CGRP drugs have been shown to reduce migraine pain partly by targeting allodynia, which is driven by CGRP-mediated endosomal signaling50,48. Our study shows that YAP-mediated mechanotransduction in CC 4:2 alleviates pain by significantly reducing TRPA1 and CGRP expression levels. Additionally, C-C motif chemokine ligand 2 (CCL2) can rapidly increase neuronal hypersensitivity by directly stimulating primary sensory neurons, thereby enhancing mechanical allodynia51,52. This study demonstrates that neural tissue in CC 4:2 can decrease CCL2 expression, thereby reducing neuropathic pain. Furthermore, APOE is highly co-expressed in both humans and mice with chronic neuropathic pain8. ATP2b1 and ATP1a2 are calcium ion feedback regulators that are crucial for pain relief52. Our strategy significantly reduced the protein expression levels of APOE and enhanced the levels of ATP2b1, and ATP1a2 in nerve stumps. These findings effectively connect pain-related genes in the Hippo pathway with YAP-mediated mechanotransduction, facilitating the resolution of pain at the nerve stump interface.
Building on the aforementioned findings, further in-depth research into the mechanisms of YAP and its association with pain is still warranted. Spatial transcriptomic sequencing is planned to elucidate multicellular functions within their spatial context. Given that YAP and TAZ function as co-effectors of the Hippo pathway, which regulates multicellular development by integrating chemical and mechanical signals, we plan to conduct more comprehensive and systematic comparative studies in future research to elucidate the potential differences between TAZ and YAP, as well as their downstream signaling pathways, particularly within confined spatial conduits. Furthermore, given the variability in nerve fiber diameters and growth rates across different species, especially in large animals and various types of peripheral nerves, the extent of spatial restriction in nerve guidance conducts requires further validation and optimization to achieve improved therapeutic outcomes prior to clinical application.
In summary, our spatial constraint strategy offers a viable solution for patients suffering from chronic allodynia at the clinical neural stumps. We propose the use of spatially confined conduits to regulate peripheral nerve self-organization. This process involves guiding nerves to converge orderly until growth cessation, reducing disordered nerve fibers, suppressing neuroinflammation, promoting remyelination, and eliminating allodynia via YAP-mediated mechanotransduction. Additionally, this spatial constraint strategy may also be a promising treatment for spontaneous neurofibromas and schwannomas. Furthermore, the concept of spatial constraints can also be applied in other fields, such as organ-on-a-chip technology, which simulates and examines the pathophysiological mechanisms by which human organs adapt to spatial limitations in vivo. As a result, our strategy offers an engineered and practical approach to controlling uncontrolled nerve growth and alleviating traumatic neuropathic pain.
Methods
Finite element analyses
The schematic drawings of frustum cone-shaped spatial-constrained conduits (4:1-4:4) for sciatic nerves are shown in Fig. 2o. The truncated cone-shaped conduit models and columnar nerve models were established as deformable axisymmetric models using the commercial software Abaqus (SIMULIA, Providence, RI). The mechanical responses of the nerves and conduits were calculated using finite element methods (FEM) throughout the nerve growth process until growth termination. The elastic modulus was set to 10 MPa for the nerve models and 1 GPa for the conduit models. Gradient-confined conduits and nerves were modeled using four-node bilinear axisymmetric quadrilateral elements with reduced integration (Abaqus element type CAX4R). Refined meshes were designed to enhance the convergence and of calculation accuracy. A penalty contact approach was used to simulate the interaction between the conduits and the nerve with a friction coefficient of 0.2. It was noted that the simulation results were insensitive to the friction coefficient due to the steep slope. The nerve growth rate was set to 1 mm/day. The finite element simulation of peripheral nerve growth within the gradient-confined conduit was shown in Supplementary Movies 2–5.
Preparation of MC microfibrils
The MC microfibrils were synthesized using the method previously reported32. Briefly, bovine type I collagen (≥99%, Collagen Biotechnology Co. Ltd, China) was dissolved in a 12 mM H3PO4 solution (Aladdin, Shanghai, China) at a concentration of 0.03% (w/v). The collagen solution was stirred for 24 h at 37 °C to ensure complete dissolution. Ca (OH)2 was dissolved in pre-cooled (4 °C) deionized water (20 mM) and stirred for 1 h. Tris-HCl was dissolved in deionized water at 37 °C to create a buffer solution (10 mM) with a pH of 8.5. Then, the collagen/H3PO4 and Ca(OH)2 solutions were slowly dropped at a volume ratio of 1:1 (Ca/P = 1.67) into the pre-prepared Tris-HCl buffer solution. The mixture was gently stirred at 200 rpm and 37 °C for 24 h. The precipitate was centrifuged and washed three times with deionized water at 720 x g for 10 min each. Subsequently, the MC microfibrils were collected by centrifugation at 3840 x g for 10 min. Reagent catalog numbers and corresponding details are provided in Supplementary Data 1.
Electron microscopic observation of MC and PCL@MC laminae
Throughout the mineralization process of MC, samples from the mixture were periodically collected and placed on thin copper sheets. The samples were then left to air-dry at room temperature for 12 h prior to the observation of the MC morphology under scanning transmission electron microscope (STEM, JEM-2100F, Japan) (Supplementary Fig. 26a). The morphologies of MC inside the PCL were observed by TEM (JEOL, Japan) (Supplementary Fig. 26b). The orientation morphologies of PCL@MC fibrils were observed using SEM (Merlin, Zeiss, Germany) (Supplementary Fig. 26c).
Fabrication of spatially-confined conduits
First, 0.5 g of freeze-dried MC powders (Supplementary Fig. 26d) were added to 20 mL of 10% (w/v) polycaprolactone solution (PCL, Mw=250,000, Jinan Daigang Biomaterial Co., Ltd) in hexafluoroisopropanol (HFIP) (Aladdin, Shanghai, China) and stirred vigorously for over 48 h to form a homogeneous electrospinning sol. Then, the PCL@MC sols were loaded into two 10 mL syringes and extruded through stainless-steel nozzles with an inner diameter of 500 μm at a constant flow rate of 0.05 mL/min using a micro-infusion pump for electrospinning (Sharp Banner, Beijing, China) under a high DC voltage of +15 kV/-4 kV (Supplementary Fig. 26e). The process was conducted at room temperature with a relative humidity of ~10%. The working distance between the emitter needle and the rolling collector was set to 20 cm. The rolling collector was operated at 2000 rpm to produce aligned fibrils. To remove residual organic solvent, the electrospun PCL@MC fibril layers were vacuum-dried for 30 min at 60 °C. Subsequently, the PCL@MC fiber layers were stacked with fibril alignment in parallel and then compressed under 40 kPa pressure using a hydraulic press to obtain PCL@MC laminae. The resulting PCL@MC laminae were fitted and crimped using a pre-processed tungsten steel mold to produce nerve conduits with different spatial constraints. The spatially confined conduits had consistent length of 10 mm and anterior (proximal) inner diameter of 2 mm, while varying posterior (distal) inner diameter of 0.5 mm, 1 mm, 1.5 mm, and 2 mm, which were designated as CC 4:1, CC 4:2, CC 4:3, and TC 4:4, respectively.
Assessment of the degradation
The conduits were immersed in PBS solutions containing lipase with concentrations of 0, 10, 25, 50, and 100 µg/mL (≥100 units/mg, QASEG, China) at 37 °C. The conduits were then freeze-dried at various time intervals over a period of 4 weeks to assess in vitro degradation by weighing. For the in vivo studies, SEM was employed to observe morphological changes after 12 weeks. After 12 weeks, the neural tissue surrounding the implanted nerve conduits (CC 4:1, CC 4:2, CC 4:3, TC 4:4) was excised and subsequently freeze-dried. SEM was then used to observe surface micro-degradation changes, and the mass was measured and compared to the preoperative mass.
Contact angle measurements
Contact angle measurements on the surfaces of PCL and PCL@MC laminae were performed at room temperature with a humidity of 40%. Prior to the experiment, the specimens were dried for 12 h to ensure complete dryness. First, a 5 μL droplet of deionized water was deposited onto the surface using a syringe. Then, the contact angle on the surface was measured using an automatic microscopic contact angle meter (KRUSS DSA25S, Germany) (Supplementary Fig. 26f). The process of measuring the contact angle of water droplets on the surfaces of the PCL and PCL@MC laminae is illustrated in Supplementary Movies 6 and 7, respectively.
Fabrication of microchannel chip
A 10-μm-thick layer of SU-8 photoresist (MicroChem, USA) was applied on a mechanical-grade silicon wafer using a WS-650-23 Spin Coater (Laurell, USA). The wafer was subsequently spun for 20 s at a speed of 650 rpm (Analysis, HC150SE, China) to achieve a thickness of 15 μm. This wafer was then heated to 65 °C for 15 min followed by 95 °C for 30 min, after which the patterning was etched using lithography (Suss MicroTec MA6, Germany). To create the pattern, UV light filtered through a photomask induced cross-linking of the SU-8 photoresist. Non-cross-linked SU-8 was then removed using SU-8 developer solution, and the wafer was rinsed with isopropyl alcohol. Polydimethylsiloxane (PDMS) was mixed at a 10:1 ratio with a curing agent. The mixture was poured onto the silicon master and incubated in a sealed vacuum container for 3 h. The PDMS was baked for 1 h at 65 °C to facilitate curing, then peeled off the negative master, cut to dimensions of 12 × 26 mm (W × L), and pierced to form inlet and outlet ports (4 mm diameter). The pre-cut PDMS blocks and clean glass (500 × 24 mm) were activated by oxygen plasma treatment (SAT-5D, Beijing, China) for 1 min (30 W; 300 mL/min) and then irreversibly sealed. The devices were sterilized with an autoclave (STIK, IMJ-78A, Shanghai, China) for subsequent use.
Cells migration on a chip
The following operations were conducted in a laminar flow workstation to ensure an aseptic environment. 50 μL of extracellular matrix (20 μg/mL type I collagen, Sigma) in phosphate-buffered saline (PBS) was introduced into the migration chamber via capillary action in the microchannel. The entire device was then incubated at 37 °C in a cell incubator (Supplementary Fig. 26g) for 1 h to facilitate collagen adsorption. Rat Schwann cells (RSCs) were obtained from the National Infrastructure of Cell Line Resource (NICLR, RSC-96; 3111C0001CCC000664; Beijing, China). The RSCs were detached and suspended in a solution at a concentration of 2 × 106 cells/mL. A volume of 50 μL (1 × 105 cells) was added to the cell channel through the cell inlet port using a micro-infusion pump (KD Scientific, USA) for 5 min, followed by an additional 5 min of incubation. The remaining cell suspension was then removed from the cell outlet port, and the cell channel was filled with serum-free medium (SFM, ThermoFisher) added from the medial medium inlet. The topmost medium inlet was filled with 10% fetal bovine serum (FBS, Thermo Scientific) medium to create a chemoattractant gradient. These operations were conducted inside a laminar flow hood to ensure a sterile environment for the RSCs in the chip. The chip device was placed in an incubator (37°C, 5% CO2; Zeiss, LSM980, Germany) to ensure the survival of RSCs during migration along the chemoattractant gradient (100 μm). The process was recorded using a Confocal Transmitted Photomultiplier Tube Channel (T-PMT, Zeiss, LSM980, Germany) for 2 h. Data on RSCs migration speed, distance, and directionality were extracted and analyzed from the image series using Fiji (NIH, USA). To document cellular migration trajectories, the cell center was identified as the midpoint between the poles of the cell body and tracked for changes in x and y positions at 5 min intervals. Using this method, RSCs morphology after 2 h was recorded via IF staining of the cytoskeleton (Rhodamine, Solarbio, China). This process was repeated for at least 50 cells per condition per experimental trial. Each experiment was conducted in triplicate.
Cells adhesion
Prior to cell culture, the circular slices of PCL and PCL@MC laminae were sterilized by soaking in a 75% alcohol solution for 24 h, followed by drying at 60 °C for 1 h and washing several times with PBS to remove any residual alcohol. FBs were obtained from the National Biomedical Experimental Cell Resource (NBECR, 1101RAT-PUMC000688; Beijing, China). FBs and RSCs were seeded at a density of 4000 cells per well in a 48-well plate containing either PCL or PCL@MC laminae and cultured for 1 day. After washing with PBS, the cells were fixed with 2.5% glutaraldehyde for 3 h, followed by three times of additional washing with PBS (15 min each). The samples were sequentially dehydrated using ethanol solutions with varying concentrations (50%, 70%, 80%, 90%, 95%, and 100%), then immersed in tert-butanol for 2 h and finally subjected to overnight lyophilization (Alpha 1-2 LDplus, Martin Christ Germany) for observation of cell adhesion via SEM.
Fourier transform infrared spectroscopy (FTIR)
The PCL@MC laminae, obtained through hydraulic pressing at 25 °C and 59 °C were measured using a FTIR spectrometer (Bruker, Germany). The measured wavelength range was 4000 to 600 cm-1.
Thermogravimetric (TGA)
The PCL@MC laminae samples were assessed using a thermogravimetric analyzer (Q5000IR, TA INSTRUMENTS, USA). The samples, each weighing 50 mg, were cut into small pieces and subjected to weighing during heating at a rate of 10 °C/min from 25°C to 800 °C in an air atmosphere.
Mechanical property test
Tensile strength was tested using a small-capacity tensile testing machine (EZ-LXHS, Shimadzu, Japan) with a maximum load of 100 N to obtain a stress-strain curve. Three samples from each group were tested to ensure data accuracy.
The Young’s modulus (Ey) of PCL and PCL@MC laminae were measured using a tensile testing machine (EZ-LXHS, Shimadzu, Japan). The radial compressive resistance of spatially-confined PCL@MC nerve conduits were also evaluated by a high-sensitivity compression testing machine (Instron, Shimadzu, Japan). The compressive force corresponding to the diameter of conduits reduced by 30% were recorded for comparison. Samples of each group were repeated three times.
Biocompatibility evaluation
To evaluate the potential cytotoxicity of PCL and PCL@MC on nerves in vivo, live/dead staining was performed on RSCs over different time periods. Cells were seeded at a density of 4000 cells/well on a 48-well plate with either PCL or PCL@MC laminate at the bottom. To label live cells, Calcein AM (2 μM, Invitrogen, USA) was added to the wells, followed by incubation at 37 °C in a 5% CO2 atmosphere for 40 min. Subsequently, Propidium iodide (PI, 5 μM, Invitrogen, USA) was added for dead cell staining and incubated at room temperature for 10 min, followed by washing with PBS to remove excess dye. Images of the live (green) and dead (red) cells were captured during a fluorescence microscope (Zeiss, LSM980, Germany). The results from day 1 and day 7 were analyzed by comparing the ratio of green to red fluorescent cells to assess cell viability.
The CCK-8 assay (Dojindo, Japan) quantifies cell viability based on metabolic activity. RSCs were seeded at a density of 2000 cells per well in a 96-well plate containing either PCL or PCL@MC laminate that were fixed at the bottom of the well using silicon rings. After the addition of 10 μL of CCK-8 solution to each well, the cells were incubated at 37 °C for 1 h. The CCK-8 solution reacts with cellular dehydrogenases to produce a yellow formazan dye. Absorbance at 450 nm was measured using a microplate reader to assess cell proliferation and viability on day 1, day 3, and day 5.
Ethics statement
Animal experiments in this study were conducted at the Laboratory Animal Resources Center, Tsinghua University, and were carried out following a protocol approved by Tsinghua University Animal Ethics Committee (no. 22-WXM7). Sample size in each experiment was illustrated in corresponding figure legends.
Animal experiment
One hundred and fourteen 8-week-old male Sprague-Dawley rats, weighing 260 ± 5 g (mean ± SD), were obtained from the Laboratory Animal Resources Center, Tsinghua University. All animals were individually housed at 25 °C ± 1 °C with ad libitum access to food and water, under a 12 h light-dark cycle, with lights on daily at 7:30 AM. Masked gas anesthesia was used during the surgical procedures. Isoflurane was mixed with oxygen at concentrations ranging from 1.5% to 3%, with a flow rate maintained at 2 liters per minute. Adjustments were made according to the animals’ responses to ensure that the anesthesia was both safe and effective. The left thighs and buttocks of the rats were shaved and disinfected, where 2 cm linear incisions were made. The sciatic nerve was exposed and quickly transected proximal to the nerve sheath valgus, with distal nerve segments cut and discarded at 1 cm intervals. In the initial phase of the study, we investigated the impact of engineered gradient spatial confinement within nerve conduits on neuronal self-organization and central pain responses in rats. We established three gradient confined conduits with ratios of 4:1, 4:2, and 4:3, along with one traditional cylindrical conduit with a ratio of 4:4 (12 rats per group). In the simple excision control group, the sciatic nerve was severed to simulate a standard neuroma resection procedure. In the stump suturing control group, the proximal nerve was sutured to the adjacent muscle, with samples collected at 8 weeks (n = 6). Additionally, fully sealed 10 mm conical conduits, designated as CC 4:0 (n = 6) and CC 4:1 (n = 6), underwent an 8 week evaluation period. The proximal inner diameter of the implant was set to 2 mm to accommodate the diameter of the sciatic nerve in 8 week-old SD rats. Four internal diameters of the conduits were selected, with the distal inner diameter being 0.5 mm, 1 mm, 1.5 mm, and 2 mm, each corresponding to CC ratios of 4:1, 4:2, 4:3, and 4:4, respectively. The distal autologous nerve was not anastomosed to the conduit.
To investigate the mechanisms by which optimal spatially-confined conduits affect nerve self-organization and central allodynia, we examined the role of Yes-associated protein (YAP), a functional protein responsive to mechanical forces involved in Hippo signaling, and administered its specific inhibitor, VP (Sigma). VP was perineurally administered at a dose of 10 mg/kg body weight per week, beginning on the day of surgery. All surgeries were performed by a same surgeon (X.L.). Pain relief in the rats was achieved through subcutaneous injection of Meloxicam at 0.05–0.2 mL/100 g initially upon awakening post-surgery, followed by daily injections for 5 days. On the day of surgery and for the subsequent 2 days, potassium penicillin was administered intramuscularly to prevent infection. Throughout the 12-week postoperative period, cotton pads were placed at the bottom of the housing cages to prevent frictional injuries between the bedding and the paws of the animals.
Histology and immunofluorescence staining
After 12 weeks, fresh nerve, liver, and kidney tissue samples were fixed in 4% paraformaldehyde for 48 h. They were then dehydrated with 15% and 30% sucrose solutions for over 48 h. The samples were embedded in an optimal cutting temperature (OCT) compound and stored at −80 °C. The embedded samples were then sectioned into 8 μm thick cryosections. Subsequently, the nerve, liver, and kidney tissue samples underwent HE staining, following the methods outlined in our previous publications53.
Nerve samples were subjected to IF staining as follows. First, the samples were soaked in a blocking buffer (10% goat serum, 0.3% Triton in PBS) for 1 h. Next, the samples were incubated with a mixture of PBS and primary antibodies overnight at 4 °C. The sections were washed three times with PBS and then incubated with secondary antibodies and DAPI in a blocking buffer for 1 h at room temperature. The sections were then rinsed three times with PBS. The following primary antibodies were used: anti-TUJ1 (rabbit, 1:400, Abcam ab18207); anti-NF-200 (mouse, 1:200, Sigma, SAB4200747); anti-MBP (rabbit, 1:200, Abcam ab218011); anti-Yap (mouse, 1:200, Santa sc-376830); anti-S100 (rabbit, 1:200, Sigma SAB4502708); α-SMA (rabbit, 1:200, Proteintech 14395-1-AP); Reca-1 (mouse, 1:200, Santa sc-52665); anti-Ki67 (rabbit, 1:150, Cell Signaling 9129S); anti-CD86 (mouse, 1:200, Santa sc-28347); anti-F4/80 (mouse, 1:200, Santa sc-377009); Iba-1 (rabbit, 1:150, Abcam ab178846); anti-CGRP (rabbit, 1:400, Abcam ab283568); anti-TRPA1 (mouse, 1:200, Santa sc-376495).
Secondary antibodies, including anti-mouse and anti-rabbit, were diluted at a ratio of 1:800. Nuclei were stained with DAPI-Aqueous (Abcam, ab104139). Immunostaining of nerve samples was captured using a high-sensitivity SCOMS monochrome camera integrated into an automated digital slide scanning system (Axio Scan.Z1 Zeiss) at a scanning resolution of 0.22 μm/pixel. Partial high-definition images were acquired with a 20× numerical aperture (NA) objective (LSM980, Carl Zeiss). All images were captured at room temperature and optimized using the Arivis Vision4D software (Carl Zeiss).
For RSC cytoskeleton staining, samples were washed three times with PBS, fixed with 4% paraformaldehyde for 2 h, washed again with PBS, and then treated with rhodamine (1:200, Invitrogen R415). The samples were subsequently washed with PBS and observed under a fluorescence microscope with an excitation light wavelength range of 540–565 nm (Zeiss, LSM980, Germany).
Immunofluorescence intensity measurements
To quantify the intensity of YAP immunostaining in nerves regenerated within 12 weeks, the integrated density was measured using ImageJ, a well-established method for measuring fluorescence intensity54,55. Spatial variation in the intensity of pain-associated proteins (CGRP, TRPA1) in regenerating nerves were quantified using the profile function of ZEN software (Carl Zeiss), with selected areas of 3 × 106 μm2 (600 μm × 5000 μm) for wide field and 1.6 × 105 μm2 (400 μm × 400 μm) for narrow field. Each group was measured three times at random positions, maintaining a consistent grid distance of 1.
Immunohistochemistry
For Immunohistochemistry staining, cryosections (8 μm) were fixed in precooled 4% paraformaldehyde for 10 min, followed by antigen unmasking for 25 min at 98 °C in citrate buffer (pH = 6) using the PT module. Endogenous peroxidase was blocked using a 3% hydroperoxide solution (Merck) in methanol for 12 min at room temperature. Endogenous avidin and biotin were blocked using the Endogenous Blocking kit (Invitrogen) for 30 min at room temperature. A blocking buffer was then used to block other nonspecific antigens. Next, the samples were incubated overnight at 4 °C with anti-c-Fos (mouse, 1:150, Abcam ab208942) and anti-Substance-p (rabbit, 1:200, Sigma, S1542). Subsequently, they were treated with biotinylated anti-rabbit and anti-mouse antibodies in blocking buffer, followed by the Standard ABC kit, in this specific order. Horseradish peroxidase (HRP) activity was detected using ImmPACT DAB (Vector Laboratories). The sections were dehydrated and mounted using SafeMount (Labonord).
Enzyme-linked immunosorbent assay (ELISA)
To quantify the proteins (YAP, phospho-YAP) in nerves regenerated within 12 weeks, the following ELISA procedure was employed. Neural tissue was chopped and weighed to 50 mg, then combined with 2 mL of PBS (pH 7.2-7.4). The tissues were manually ground after being frozen with liquid nitrogen. The samples were then centrifuged at 4 °C for ~20 min (480-720 x g), after which the supernatant was removed. The precipitate was washed three times with PBS. The cell suspension was diluted with a small volume of PBS and disrupted using an ultrasonic cell disruptor to release intracellular contents. The resulting material was centrifuged again at 4 °C for 20 min (480-720 x g), and the supernatant was collected for analysis using the ELISA kits for YAP (Meimian MM-72340R1) and phospho-YAP (Meimian MM-72346R1). Finally, the optical density (OD) values were recorded using an enzyme-linked immunosorbent assay reader (Labsystems Multiskan MS, Finland). Each group was tested in triplicate.
Flow cytometry
SCs were subjected to oxidative stress induced by hydrogen peroxide (H2O2) for 12 h. The SCs were then seeded in a 6-well culture plate at a density of 1 × 105 cells/well, with the well bottoms modified to different stiffness levels using PDMS (20 kPa, 0.5 MPa, 2.0 MPa).
Intracellular ROS levels in SCs were quantified using a ROS Assay Kit (S0033, Beyotime, China). In brief, upon reaching 70% confluence, the SCs were incubated with a DCFH-DA solution at a dilution of 1:1000 for 1 h to allow DCF fluorescence labeling for ROS detection. Flow cytometry analysis was then performed using a BD Fortessa flow cytometer (BD Biosciences) to detect the stained cells (Supplementary Fig. 26h). The data obtained were then analyzed using FlowJo 10 (TreeStar).
The effectiveness of YAP was assessed through an inhibitor experiment. SCs or FBs in six-well plates were exposed to a low concentration of hydrogen peroxide for 12 h, followed by the treatment with VP (50 μM), Vehicle (PBS solution with 5% DMSO), and a blank control for 24 h. The cells were washed three times with serum-free medium for 5 min each, then incubated with the DCFH-DA fluorescent probe according to the ROS detection kit instruction. The cells were subsequently separated using a 0.025% trypsin-EDTA solution. Finally, ROS levels in the cells were detected using a BD flow cytometer with a 488 nm laser.
Simultaneously, an indirect co-culture of HAPI cells (upper chamber) and SCs (lower chamber) was performed in a transwell system. This setup was used to observe the differentiation of microglia into the M1 phenotype under the influence of SCs. The M1 phenotype antibodies, iNOS (1:200; sc-7271, Santa Cruz), were added to the upper chamber for fluorescence analysis. This was followed by flow cytometry using the same methods as described above.
Observation of axon and myelination
Axons and myelin in neural tissues were observed using TEM and optical microscopy. For TEM sample preparation, neural tissues were fixed in a 2.5% glutaraldehyde solution for 48 h. After fixation, the samples were dehydrated through a graded series of acetone to remove water. The tissues were then infiltrated with epoxy and sectioned into ultra-thin slices using a Leica EM UC7 ultramicrotome. The 60-nm thick slices were collected on copper grids. The sections were stained with uranyl acetate to enhance contrast and were observed under TEM. For optical microscopic observation with toluidine blue staining, the methods for sample preparation and staining can be found in our prior publications53. The sections were examined using a 3DHISTECH Pannoramic Scan automatic slide scanner.
Evaluation of pain-associated behaviors
The autotomy phenomenon caused by symptomatic stump nerve was assessed using autotomy scoring6,9,44. Briefly, one point was assigned for the loss of one or more toenails or the removal of half a digit, and two points for the loss of both distal and proximal phalanges. Autotomy scores were recorded at postoperative weeks 0, 1, 3, 5, 7, 9, and 12, using the mean values of each group for each testing phase.
It is well known that neuropathic pain is closely linked to limb movement36. Thus, monitoring the dynamic changes in rat gait patterns can indirectly reflect pain levels56,57,58. Catwalk analysis (Catwalk XT, Noldus Netherlands) (Supplementary Fig. 26i) was used to indirectly assess neural pain manifestations in rats at postoperative weeks 0, 1, 3, 5, 7, 9, and 12. Behavioral testing was conducted 3 days post-VP injections to avoid interference.
RNA extraction and quantitative PCR
Freshly frozen pieces of rat sciatic nerves were ground into powder using liquid nitrogen. RNA from SCs, FBs, and nerve tissue homogenate was extracted using the RNeasy Microkit (Qiagen) according to the manufacturer’s protocol. The purified RNA was used to synthesize first-strand complementary DNA with SuperScript II (Invitrogen) and random hexamers (Roche). Quantitative analyses were performed using the Light Cycler 96 (Roche). The primers used in this study are listed in Supplementary Table. 1.
RNA sequencing
Total RNA was extracted from the tissue using TRIzol® Reagent (Qiagen) following the manufacturer’s instructions. RNA quality was determined using the 5300 Bioanalyser (Agilent) and quantified with the ND-2000 (NanoDrop Technologies). Only RNA samples that met specific criteria (OD260/280 = 1.8 ~ 2.2, OD260/230 ≥ 2.0, RQN ≥ 7.0, 28S: 18S ≥ 1.0, >1 μg) were used to construct the sequencing library. RNA purification, reverse transcription, library creation, and sequencing were conducted by Shanghai Majorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China). The mRNA-seq transcriptome library was generated according to the Illumina® Stranded mRNA Prep, Ligation protocol (San Diego, USA), using 1 μg of total RNA. Initially, messenger RNA was isolated using the polyA selection method with oligo (dT) beads and subsequently fragmented with a fragmentation buffer. Subsequently, double-stranded cDNA was produced using a SuperScript double-stranded cDNA synthesis kit (Invitrogen, USA) with random hexamer primers. The resulting cDNA underwent end-repair, phosphorylation, and adapter ligation according to the library preparation protocol. cDNA target fragments of 300 bp were size-selected on 2% Low Range Ultra Agarose and amplified by PCR using Phusion DNA polymerase (NEB) for 15 cycles. After post-quantification using Qubit 4.0, the sequencing libraries were sequenced on either a NovaSeq X Plus platform (PE150) with the NovaSeq Reagent Kit or a DNBSEQ-T7 platform (PE150) with the DNBSEQ-T7RS Reagent Kit (FCL PE150) version 3.0.
Raw paired-end DNA reads were initially processed for trimming and quality control using the FAST tool with default settings. Subsequently, the purified reads were individually mapped to the reference genome in orientation mode using HISAT2 software. The aligned reads for each sample were then reconstructed using a reference-based method implemented by StringTie. To identify differentially expressed genes (DEGs) between two samples, transcript levels were evaluated using the transcripts per million (TPM) technique, and RSEM was employed to estimate gene abundances. Differential expression analysis was conducted using DESeq2, where DEGs demonstrating a | log2 fold change (FC) | ≥ 1 and a false discovery rate (FDR) < 0.05 (DESeq2) or FDR < 0.001 (DEGseq) were identified as significantly differentially expressed. Additionally, functional enrichment analyses such as GO and KEGG pathway assessments were conducted to determine significant enrichment of DEGs in GO terms and metabolic pathways, with a Bonferroni-corrected P-value < 0.05 against the entire transcriptome background. These analyses were performed using Goatools for GO and a Python script for KEGG pathway analysis.
Statistical analysis
Data presented as means ± SD were analyzed using a two-tailed Student’s t-test or one-way analysis of variance (ANOVA). The Tukey’s post-hoc tests were used to assess statistical significance when analyzing multiple groups, unless otherwise stated. The Benjamini-Hochberg correction was applied to RNA-sequencing data analysis. Statistical analyses were performed using Prism GraphPad 8.0. A p-value <0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary files. The raw RNA sequencing data has been uploaded to the GEO public database (no. GSE280040). Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper.
Code availability
This study simulates physical working conditions by utilizing the built-in simulation functions of the proprietary ABAQUS software to analyze resistance changes during neural growth. As a result, it does not fully replicate real-world conditions. Since no new code was developed, this work does not involve any programming. Nonetheless, the simulation computation program data generated in this study have been deposited in the Figshare under accession code [DOI: 10.6084/m9.figshare.27712467].
References
Kuehlmann, B., Bonham, C. A., Zucal, I., Prantl, L. & Gurtner, G. C. Mechanotransduction in wound healing and fibrosis. J. Clin. Med. 9, 1423 (2020).
Luo, X. et al. A new insight on peripheral nerve repair: the technique of internal nerve splinting. J. Neurosurg. 137, 1406–1417 (2022).
Yang, Q. et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact. Mater. 26, 194–215 (2023).
Diaz-delCastillo, M. et al. Metastatic infiltration of nervous tissue and periosteal nerve sprouting in multiple myeloma-induced bone pain in mice and human. J. Neurosci. 43, 5414–5430 (2023).
Boato, F. et al. Activation of MAP2K signaling by genetic engineering or HF-rTMS promotes corticospinal axon sprouting and functional regeneration. Sci. Transl. Med. 15, eabq6885 (2023).
Zhou, X. et al. An aligned nanofiber nerve conduit that inhibits painful traumatic neuroma formation through regulation of the RhoA/ROCK signaling pathway. J. Neurosurg. 132, 837–846 (2019).
Decrouy-Duruz, V., Christen, T. & Raffoul, W. Evaluation of surgical treatment for neuropathic pain from neuroma in patients with injured peripheral nerves. J. Neurosurg. 128, 1235–1240 (2018).
Tansley, S. et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 13, 843 (2022).
Yi, J. et al. Painful terminal neuroma prevention by capping PRGD/PDLLA conduit in rat sciatic nerves. Adv. Sci. (Weinh.) 5, 1700876 (2018).
Aslami, Z. V. et al. Symptomatic neuroma development following en bloc resection of skeletal and soft tissue tumors: a retrospective analysis of 331 cases. Plastic Reconst. Surg. 153, 873–883 (2023).
Zhou, L. et al. New techniques and methods for prevention and treatment of symptomatic traumatic neuroma: a systematic review. Front. Neurol. https://doi.org/10.3389/fneur.2023.1086806 (2023).
Cao, S. et al. Inversely engineered biomimetic flexible network scaffolds for soft tissue regeneration. Sci. Adv. 9, eadi8606 (2023).
Yang, X. et al. Multifunctional chitin-based hollow nerve conduit for peripheral nerve regeneration and neuroma inhibition. Carbohydr. Polym. 289, 119443 (2022).
Kong, L. et al. Multilevel neurium-mimetic individualized graft via additive manufacturing for efficient tissue repair. Nat. Commun. 15, 6428 (2024).
Wang, X. et al. Biomimetic multi-channel nerve conduits with micro/nanostructures for rapid nerve repair. Bioact. Mater. 41, 577–596 (2024).
Sun, M. et al. 4D oriented dynamic scaffold for promoting peripheral nerve regeneration and functional recovery. Adv. Funct. Mater. 34, 2305827 (2023).
Sala, F., Ficorella, C., Osellame, R., Käs, J. & Martínez Vázquez, R. Microfluidic lab-on-a-chip for studies of cell migration under spatial confinement. Biosensors 12, 604 (2022).
Paul, C. D., Mistriotis, P. & Konstantopoulos, K. Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2016).
Agrawal, R., Windsor, A. & Lammerding, J. The nuclear pore complex. Annu. Rev. Biochem. 64, 329–349 (2022).
Crestani, M., Dini, T., Gauthier, N. C. & Monzo, P. Protocol to assess human glioma propagating cell migration on linear micropatterns mimicking brain invasion tracks. STAR Protoc. 3, 101331 (2022).
Choi, Y. Dendritic cell migration is tuned by mechanical siffness of the confining space. Cells 10, 3362 (2021).
Lomakin, A. J. et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894 (2020).
Zanotelli, M. R. et al. Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat. Commun. 10, 4185 (2019).
Stüdle, C. et al. Spatially confined induction of endochondral ossification by functionalized hydrogels for ectopic engineering of osteochondral tissues. Biomaterials 171, 219–229 (2018).
Aragona, M. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013).
Poitelon, Y. et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 19, 879–887 (2016).
Guo, Y. et al. CK2-induced cooperation of HHEX with the YAP-TEAD4 complex promotes colorectal tumorigenesis. Nat. Commun. 13, 4995 (2022).
Qian, Y., Lin, H., Yan, Z., Shi, J. & Fan, C. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling. Mater. Today 51, 165–187 (2021).
Dong X. et al. An injectable and adaptable hydrogen sulfide delivery system for modulating neuroregenerative microenvironment. Sci. Adv. 9, eadi1078 (2023).
Liu, Y. et al. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J. Hepatol. 71, 719–730 (2019).
Zhan, L. et al. Grooved fibers: preparation principles through electrospinning and potential applications. Adv. Fiber Mater. 4, 203–213 (2021).
Zhao, Y. et al. Hierarchically engineered artificial lamellar bone with high strength and toughness. Small Struct. 4, 2200256 (2022).
Zhao, Y. et al. A multifaceted biomimetic periosteum with a lamellar architecture and osteogenic/angiogenic dual bioactivities. Biomater. Sci. 11, 3878–3892 (2023).
Hadlock, T. A., Cheney, H. J. & Mackinnon, M. SE. Functional recovery after facial and sciatic nerve crush injury in the rat. Arch. Facial Plast. Surg. 7, 17–20 (2005).
Adidharma, W. et al. Sensory nerve regeneration and reinnervation in muscle following peripheral nerve injury. Muscle Nerve 66, 384–396 (2022).
Zhang, Y. et al. Therapeutic effects of peripherally administrated neural crest stem cells on pain and spinal cord changes after sciatic nerve transection. Stem Cell Res. Ther. 12, 180 (2021).
Tansley, S. et al. Arkady Khoutorsky Microglia-mediated degradation of perineuronal nets promotes pain. Science 377, 80–86 (2022).
Tansley, S. et al. Microglia-mediated degradation of perineuronal nets promotes pain. Science 377, 80–86 (2022).
De Logu, F. et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 8, 1887 (2017).
Sun, L. Targeted ubiquitination of sensory neuron calcium channels reduces the development of neuropathic pain. Proc. Natl Acad. Sci. USA 119, e2118129119 (2022).
De Logu, F. et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 13, 646 (2022).
Echeverry, S., Shi, X. Q. & Zhang, J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain 135, 37–47 (2008).
Zhang, H., Zhang, K., Li, M., Shao, Y. & Feng, X.-Q. Force-regulated state transitions of growing axons. Phys. Rev. Lett. 129, 128101 (2022).
Onode, E. et al. Nerve capping with a nerve conduit for the treatment of painful neuroma in the rat sciatic nerve. J. Neurosurg. 132, 856–864 (2019).
Hama, S. et al. Nerve capping treatment using a bioabsorbable nerve conduit with open or closed end for rat sciatic neuroma. Clin. Neurol. Neurosurg. 209, 106920 (2021).
Jessen, K. R. & Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 594, 3521–3531 (2016).
Yang, S. et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF—mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 10, 8227–8249 (2020).
Trevisan, G. et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139, 1361–1377 (2016).
Iannone, L. F., Nassini, R., Patacchini, R., Geppetti, P. & De Logu, F. Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief. Temperature 10, 50–66 (2022).
De Logu, F. et al. Non-neuronal TRPA1 encodes mechanical allodynia associated with neurogenic inflammation and partial nerve injury in rats. Br. J. Pharm. 180, 1232–1246 (2023).
Liu, Z., Murphy, S. F., Wong, L., Schaeffer, A. J. & Thumbikat, P. Neuronal/astrocytic expression of chemokine (C-C motif) ligand 2 is associated with monocyte/macrophage recruitment in male chronic pelvic pain. Pain 161, 2581–2591 (2020).
Lau, M.-T. et al. Molecular dissection of box jellyfish venom cytotoxicity highlights an effective venom antidote. Nat. Commun. 10, 1655 (2019).
Yang, J. et al. 3D bio-printed living nerve-like fibers refine the ecological niche for long-distance spinal cord injury regeneration. Bioact. Mater. 25, 160–175 (2023).
Ellis, S. J. et al. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature 569, 497–502 (2019).
Aragona, M. et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584, 268–273 (2020).
Deuis, J. R., Dvorakova, L. S. & Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. https://doi.org/10.3389/fnmol.2017.00284 (2017).
Bolleboom, A. et al. Novel experimental surgical strategy to prevent traumatic neuroma formation by combining a 3D-printed Y-tube with an autograft. J. Neurosurg. 130, 184–196 (2019).
Masayuki, M. Assessment of pain behavior in a Rat model of intervertebral disc injury using the catwalk gait analysis system. Spine 38, 1459–1465 (2013).
Acknowledgements
We acknowledge the funding support from the National Natural Science Foundation of China (No. 32271414) the Tsinghua Precision Medicine Foundation (No. 2022TS001). We thank Qian Li and Yue Sun from the Cell Biology Facility in Tsinghua University for technical assistance with the 3DHISTECH automatic slide scanner and automatic immunofluorescent slide scanner. We also thank the protein research technology center cellular imaging platform in Tsinghua University.
Author information
Authors and Affiliations
Contributions
Author contributions: X.L., X.W., and Y.P. conceived the idea and designed the research project. X.L., Y.Z., J.Y., T.N. Z.L. and G.D. fabricated the materials and performed the characterization. X.L. and Z.H. performed the cell studies. X.L., J.C., T.N., P.H. performed the animal studies. X.L. and Z.Z. performed the bioinformatics analysis. X.L. and J.W. performed the finite element analysis. X.L. and J.W. analyzed the data. X.L. prepared the figures, and wrote the manuscript. X.L., J.Y., Y.Z., T.N., J.C., P.H., Z.H., L.Z., X.S., Z.Z., Y.P., and X.W. reviewed and revised the manuscript. All authors approved the submitted vision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Cunyi Fan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, X., Yang, J., Zhao, Y. et al. Engineering spatially-confined conduits to tune nerve self-organization and allodynic responses via YAP-mediated mechanotransduction. Nat Commun 16, 66 (2025). https://doi.org/10.1038/s41467-024-55118-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55118-9