Abstract
The application of high-voltage positive electrode materials in sulfide all-solid-state lithium batteries is hindered by the limited oxidation potential of sulfide-based solid-state electrolytes (SSEs). Consequently, surface coating on positive electrode materials is widely applied to alleviate detrimental interfacial reactions. However, most coating layers also react with sulfide-based SSEs, generating electronic conductors and causing gradual interface degradation and capacity fading. To address this, we propose a Li2ZrF6 coating layer on LiCoO2, which exhibits minimal reaction with SSEs, and its decomposition products are electron-conductive-free. Furthermore, this coating layer also efficiently mitigates the layered-to-spinel/rock-salt surface structural transformation in LiCoO2. As a result, the In-Li|Li6PS5Cl | Li2ZrF6-LiCoO2 all-solid-state cell demonstrates an initial areal capacity of 5.2 mAh cm−2 and a capacity retention of 80.5% after 1500 cycles at 70 mA/g with high LiCoO2 areal mass loading (30.19 mg cm−2) and a cut-off voltage of 3.9 V (corresponding to potential of 4.5 V versus Li+/Li), at 25 °C.
Similar content being viewed by others
Introduction
Rechargeable batteries constitute pivotal technologies in the realm of clean energy storage and electric vehicle deployment1. While conventional lithium-ion batteries have long powered a multitude of contemporary tools, they have increasingly raised safety apprehensions due to their utilization of flammable organic liquid electrolytes. In response, there has been a growing shift towards enhancing safety by replacing these organic liquid electrolytes with solid-state electrolytes (SSEs) to produce all-solid-state lithium batteries (ASSLBs). This transition is regarded as a promising strategy, given the substantially improved safety and the potential for achieving high energy densities in ASSLBs2,3.
Among various types of SSEs, sulfide SSEs are the most attractive candidate for the next generation of energy storage technology due to their high ionic conductivity up to 10−2 S cm−1 at room temperature, and favorable mechanical ductile nature4,5,6,7,8. In pursuit of high energy densities in ASSLBs, the integration of high-voltage positive electrode active materials is generally implemented, such as LiCoO2 (LCO), LiNi0.8Mn0.1Co0.1O2, and lithium-rich layered oxides9,10,11,12,13,14,15,16. Unfortunately, multiple challenges have been encountered at the interface between positive electrode materials and sulfide SSEs. These challenges encompass the electrochemical oxidation of sulfide SSEs stemming from their inherent narrow electrochemical windows, undesired chemical reactions between positive electrode materials and sulfide SSEs due to the thermodynamic instability, and structural degradation of positive electrode materials resulting from O loss and the formation of spinel/rock-salt phase17,18,19,20,21,22. All these issues adversely affect the electrochemical performance of ASSLBs and require urgent solutions.
The replacement of sulfide SSEs with halide SSEs may be a promising solution, given the latter possesses high stability against electrochemical oxidation and good chemical compatibility with high-voltage positive electrode materials23,24. However, their metal cations can be easily reduced, leading to the formation of a mixed Li-ion and electronically conductive interlayer at the negative electrode interface, therefore necessitating the incorporation of an additional layer of sulfide SSEs25. This will not only increase the difficulties of the fabrication process, but also lower the cell energy density. In addition, surface modification of sulfide SSEs is a possible method, but at the expense of lower ionic conductivity10. In addition to modifying SSEs, constructing a protective layer on the surface of positive electrode materials is a widely adopted and promising approach in common practice. For example, Liang et al.11 showed gradient coating of LiNi0.8Mn0.1Co0.1O2 with Li3P1+xO4S4x can hinder the structural degradation during cycling and enable the stable electrochemical performance over 250 cycles. Additionally, Wang et al.15 presented a LiNiO2-based ASSLB with high areal capacity and stable cycling performance for over 200 cycles, achieved through the enhancement of structural stability and interfacial dynamics of positive electrode materials by a LixAlyZnzOδ protective layer. Furthermore, it has been demonstrated that prominent electrochemical performance can also be attained through the application of various other protective layers, such as Li0.35La0.5Sr0.05TiO326, TiNb2O713, Li2CoTi3O827, and the most representative LiNbO3 (LNO)28,29,30,31,32,33.
However, despite the use of these protective layers, sulfide-based ASSLBs still face challenges with capacity decay during cell operation. As shown in Supplementary Table 1, the majority of sulfide-based ASSLBs demonstrate restricted cycle life, often not exceeding 250 cycles, even under conditions of low positive electrode materials mass fraction and loading. Since the underlying factors are still obscure, it is essential to carefully evaluate the additional characteristic of coating materials other than ionic/electronic conductivity and electrochemical stability window. Two crucial factors are frequently underestimated when choosing coating materials: the reaction energy between the coating material and the electrolyte, and the electronic conductivity of the resultant reaction products between them. The reaction energy between previously reported coating materials and sulfide SSEs exhibits a notable decrease compared to that between positive electrode materials and sulfide SSEs, yet it cannot be disregarded, especially when the reaction products contain electronically conductive species. Since the reaction products are most probably ion conductive, the presence of electronically conductive species will eliminate kinetic barriers of the reaction. As a result, the differences in chemical potential of various elements between coating materials and sulfide SSEs propels the continuous progression of the reaction until the coating material is entirely consumed to form mixed Li-ion and electronically conductive cathode-electrolyte interlayer (CEI) and fails in its protection role. This is particularly significant, considering the protective layer is only a few nanometers thick. Once the protective layer transforms into a mixed conductive interlayer, the disparity of elemental chemical potential between positive electrode materials and sulfide SSEs (such as Li, Co, O) drives persistent structural degradation of positive electrode materials and decomposition of sulfide SSEs, ultimately leading to failure of ASSLBs. Hence, the meticulous selection of coating materials plays a crucial role in enabling ASSLBs to achieve the electrochemical performance necessary for commercial applications.
Herein, we introduce Li2ZrF6 (LZF) as a protective layer on the surface of LCO, and investigate its electrochemical performance in comparison with the representative protective layer of LiNbO3 (LNO). The LZF material is characterized by its wide electrochemical stability window and minimal reaction energy when in contact with Li6PS5Cl (LPSCl). Most importantly, the reaction products resulting from the interaction between LZF and LPSCl do not include any electronically conductive species, which provides assurance regarding the kinetic stability of their interface and the sustained function of LZF. Benefiting from these merits, LZF can protect efficient ion transport across the LCO/LPSCl interface from the chemical decomposition of LPSCl and structural degradation of LCO. In contrast, the representative LNO protective layer loses its function prematurely due to the non-negligible reaction energy with LPSCl and the high volume fraction of electronic conductor in their decomposition products. As a result, the ASSLBs with a composite positive electrode containing 80 wt.% LZF-coated LCO exhibit remarkable rate capability (96.2 mAh g−1 at 700 mA g−1) and cycling retention (88.5% after 2492 cycles) with an LCO loading of 11.58 mg cm−2 at a cutoff potential of 4.5 V versus Li+/Li. In particular, available areal capacity exceeding 3.8 mAh cm−2 can be achieved at 70 mA g−1 with LCO loading of 30.19 mg cm−2 and retain 80.5% after 1500 cycles.
Results
The LCO particles were coated with 1 wt.% LZF via a simple wet-chemistry method followed by annealing. For comparison, the conventionally employed 1 wt.% LNO-coated LCO (LNO-LCO) was also prepared following the procedures reported in existing literature34. X-ray diffraction (XRD) was employed to examine the crystal structure of the materials. The XRD patterns (Supplementary Fig. 1) demonstrate that both LNO-LCO and LZF-coated LCO (LZF-LCO) samples correspond well to the hexagonal R\(\bar{3}\)m structure, indicating no structural damage occurred during the coating process. Scanning electron microscopy (SEM) images (Supplementary Fig. 2) depict that the LCO consists of particles ranging from 2–10 μm in size. After being modified with LNO or LZF, the surface of particles appears hazy due to the covering of a protective layer. The homogeneous distribution of Nb on the surface of LNO-LCO was also detected by energy dispersive spectroscopy (EDS) mapping (Supplementary Fig. 3).
To investigate the composition and structure of the protective layer on LZF-LCO unequivocally, high-resolution transmission electron microscopy (HRTEM) was employed (Fig. 1a). A thin layer with a thickness of 6–13 nm is observed to uniformly cover the LCO particle. Enlarged images of the region of interest clearly exhibit nanocrystalline with lattice fringes, which match well with (002), \((\bar{1}\bar{1}1)\), and (201) planes of LZF with trigonal P\(\bar{3}\) 1m structure. The corresponding fast Fourier transform (FFT) further confirms the presence of LZF. Scanning transmission electron microscopy (STEM) also verifies the structure of the protective layer consisting of LZF nanocrystalline embedded in an amorphous matrix (Supplementary Fig. 4). While other regions are mainly composed of amorphous phases. In order to identify the composition of the large amounts of amorphous matter in the coating, we carefully examined the coating layer with STEM and EDS mapping. We found that Zr and F element cover the entire surface of LCO uniformly, rather than being sporadically distributed (Fig. 1b). This indicates that our target product (i.e., LZF) includes not only nanocrystalline structures but also a significant amount of amorphous matter in the coating layer. Density of states (DOS) result indicated the nanocrystalline embedded in amorphous matrix can help reduce electronic conductivity of coating layer (Supplementary Fig. 5 and Supplementary Data 1). Meanwhile, an about 3 nm reconstruction from layered to disordered layered to rock salt structure had occurred on the surface after the coating process (Fig. 1b). In addition, the EDS mapping results from different scales (Supplementary Figs. 6–8) reveal a uniform distribution of Zr and F elements on the surface of LCO particles after coating process as well. The X-ray photoelectron spectroscopy (XPS) spectra of LZF-LCO powders also show signals of Zr4+ and F−, further corroborating the presence of LZF (Supplementary Fig. 9). Furthermore, the morphology of LNO coating layer was also characterized. The STEM and EDS results clearly show that the LNO coating layer with a thickness of 10–18 nm homogeneously covers the LCO particles (Supplementary Fig. 10). We noticed that the thickness of our LNO coating layer falls within the optimal range for LNO (9–18 nm) in terms of electrochemical performance35.
The electrochemical performance of In-Li|LPSCl|LZF-LCO and In-Li|LPSCl|LNO-LCO all-solid-state cells with an LCO loading of 11.07–11.58 mg cm−2 was investigated to validate the efficacy of the LZF protective layer. It is worth noting that the mass fraction of LCO in composite positive electrode is as high as 80 wt.%. As shown in Fig. 2a–d, the In-Li|LPSCl|LZF-LCO exhibits an initial discharge capacity of 154.1 mAh g−1 between 2.6 and 4.5 V at 70 mA g−1. Surprisingly, a capacity of 96.2 mAh g−1 can still be delivered even at a high rate of 700 mA g−1. In stark contrast, the In-Li|LPSCl|LNO-LCO displays a significantly larger overpotential than In-Li|LPSCl|LZF-LCO (Fig. 2c). Consequently, it exhibits a much lower initial discharge capacity of 127.1 mAh g−1 at 70 mA g−1, and only a capacity of 35.2 mAh g−1 can be maintained at 700 mA g−1. It is noted that the electrochemical performance displayed by In-Li|LPSCl|LNO-LCO is comparable to that reported in previous studies28,29,30,31,32,33, proving the successful coating of LNO. The high-voltage cycling stability was also evaluated at 70 mA g−1 (Fig. 2e). The In-Li|LPSCl|LNO-LCO displays substantial capacity decay and only retains 69% of its initial capacity after 100 cycles. While this severe performance deterioration can be considerably alleviated by replacing LNO with the LZF protective layer, achieving 90% capacity retention after 100 cycles.
a Galvanostatic charge-discharge voltage profiles of In-Li|LPSCl|LZF-LCO at different rates. b Rate capability of In-Li|LPSCl|LZF-LCO. c Galvanostatic charge-discharge voltage profiles of In-Li|LPSCl|LNO-LCO at different rates. d Rate capability of In-Li|LPSCl|LNO-LCO. e Cycling stability and Coulombic efficiency of In-Li|LPSCl|LZF-LCO and In-Li|LPSCl|LNO-LCO.
In order to elucidate the basis for the significant performance gap between LZF-LCO and LNO-LCO, we have synthesized pure LNO and LZF powders and compared their ionic and electronic conductivity more comprehensive insights (Supplementary Figs. 11–13, Supplementary Tables 2−3). The ionic conductivity of LNO, as determined from electrochemical impedance spectroscopy (EIS) tests, is 3.4 × 10−8 S cm−1, while LZF exhibits a lower ionic conductivity of 7.6 × 10−9 S cm−1. Both coating materials exhibit similar electronic conductivity, with LNO having a value of 2.8 × 10−10 S cm−1 and LZF having 1.8 × 10−10 S cm−1. According to the ionic and electronic conductivity test results, the LNO-LCO should have delivered similar or even better electrochemical performance compared to the LZF-LCO. However, this was not observed in our experiments.
In addition to ionic and electronic transport properties, Young’s modulus (E) is also considered a critical parameter, as it affects the ability of coating materials to inhibit volume strain and intragranular cracking in positive electrode materials. It can be calculated from Eq. (1).
where the G is shear modulus and the ν is Poisson’s ratio. Using corresponding data from the Materials Project database, we determine the Young’s modulus of LNO to be 126 GPa and that of LZF to be 88 GPa. This result implies that the LNO coating can better resist the volume strain of LCO. The particle integrity of LNO-LCO after 100 cycles has also been verified by STEM images, with no intragranular cracks observed (Supplementary Fig. 14). These results further validate that the mechanical stability of the coatings is not responsible for the distinct electrochemical performance between LNO-LCO and LZF-LCO. Additionally, the LZF-LCO particles also maintain their intact structure after 100 cycles, suggesting the LZF coating is sufficient to inhibit the volume strain of LCO (Supplementary Fig. 15).
Therefore, in order to reveal underlying factors, we conducted potentiostatic electrochemical impedance spectroscopy (PEIS) after each cycle within the initial 50 cycles at 70 mA g−1 (Fig. 3a–b, Supplementary Fig. 16). The In-Li|LPSCl|LNO-LCO cell initially exhibits an acceptable cell impedance of 125.7 Ω cm2 after the first cycle. However, this value continues to increase in each of the subsequent cycles, eventually reaching 346.4 Ω cm2 after 50 cycles. In contrast, the In-Li|LPSCl|LZF-LCO cell exhibits a lower cell impedance of 89.2 Ω cm2 after the first cycle. This value shows a modest increase to 132.8 Ω cm2 after 10 cycles and remains stable for the subsequent cycles. Given that the same negative electrode was utilized in both cells, the substantial difference between them arises entirely from the positive electrode side.
a, b Nyquist plots of In-Li|LPSCl|LNO-LCO (a) and In-Li|LPSCl|LZF-LCO (b) at each discharged state in the first 50 cycles at 70 mA g−1 and 25 °C. c, d Corresponding DRT results of In-Li|LPSCl|LNO-LCO (c) and In-Li|LPSCl|LZF-LCO (d). e Calculated mutual reaction energies of different interface. f The volume fraction of electron conductive species (0 eV band gap) in the decomposition products of LZF/LPSCl, LNO/LPSCl and LCO/LPSCl.
For a more profound understanding, distribution of relaxation time (DRT) analysis was employed to comprehend the evolution of the PEIS spectrum and decouple the contributions of different electrochemical processes (Fig. 3c-d). Five kinetic processes can be distinguished depending on their specific relaxation time. The peaks at 10−5 and 10−4 s are respectively attributed to ionic transport through the grain boundary of the SSE and solid electrolyte interphase (SEI) at the negative electrode side. The multiple peaks at 10−4 to 1 s are assigned to the CEI with complex composition. Charge transfer impedance (Rct) between LCO and SSEs is also identified as a peak located at 1 to 10 s. While the charge transfer process between negative electrode and SSEs is too small to be individually recognized. In addition, the peak at 10 to 100 s is attributed to the diffusional impedance (Rd) of Li+ in LCO36. For In-Li|LPSCl|LNO-LCO cell, it is evident that the impedance related to the electrochemical processes of the positive electrode increases with each successive cycle and does not saturate, including RCEI, Rct, and Rd. The results indicate that the continuous growth of CEI between LCO and SSEs albeit with LNO protective layer, combined with the persistent structural degradation of LCO to impede Li+ diffusion, collectively increase the challenges associated with interfacial charge transfer (Fig. 3c). Conversely, the In-Li|LPSCl|LZF-LCO cell exhibits an increase in RCEI only during the first 10 cycles, with the other impedance contributions remaining stable throughout the entire 50 cycles (Fig. 3d). This highlights the remarkable protective role played by LZF to control CEI growth and preserve layer structure of LCO.
To gain deeper insights into the factors that dictate the divergent electrochemical behaviors, possible interfacial decomposition before and after the incorporation of the LNO/LZF protective layer were investigated (Supplementary Tables 4–8), and corresponding thermodynamic mutual reaction energies were calculated (Fig. 3e). The results disclose that the Li0.33CoO2/LPSCl interface is not thermodynamically stable but undergoes spontaneous decomposition reactions with a maximum decomposition energy of −0.5346 eV·atom−1 (Supplementary Table 4), aligning well with previous literature37. The introduction of an LNO protective layer significantly enhances the interfacial stability of Li0.33CoO2/LNO, as evidenced by a notable decrease in the maximum decomposition energy to −0.0095 eV·atom−1 (Supplementary Table 5). However, it is noteworthy that, despite this improvement, the LNO/LPSCl interface still exhibits a non-negligible maximum decomposition energy of −0.109 eV·atom−1 (Supplementary Table 6). Moreover, from the interfacial decomposition products between LNO and LPSCl, it can be found that NbS3 and Li5(NbS2)7, which possess a band gap of 0 eV (Supplementary Table 7), constitute a significant portion of the resultant products (35.63 vol%). This value is comparable with that of reaction products between Li0.33CoO2 and LPSCl (34.99 vol%). More importantly, we noticed that the S and Nb elements are largely overlapped in the surface particles on LNO-LCO after 100 cycles, which is an evidence to support above calculations (Supplementary Fig. 14). This characteristic expedites the exhaustion of LNO, converting it into a mixed conductive interlayer. This transformation enhances electron mobility and eradicates the kinetic barrier for the chemical interaction between LCO and SSEs, instigated by differences in the chemical potential of various elements. As Co diffuses into LPSCl, it generates electronic conductors such as CoS2, Co3S4, and Co9S8 with a band gap of 0 eV, ensuring a continuous supply of electrons for the persistent reaction (Supplementary Table 8).
Consequently, this process results in forming a thick CEI and disintegrating the layered LCO structure. When the LZF protective layer was employed, not only the Li0.33CoO2/LZF interface is thermodynamically stable (maximum decomposition energy is 0 eV·atom−1), but better interfacial stability can also be achieved between LZF and LPSCl, with a negligible maximum decomposition energy of −0.046 eV·atom−1 (Supplementary Table 7. Of greater significance, none of the reaction products are electronic conductors, amongst which the ZrS2 possesses the smallest band gap of 1.04 eV (Supplementary Table 8), thus effectively preventing electron leakage even if the LZF protective layer was decomposed. This property avoids the interfacial reaction between LCO and LPSCl and assures the structural integrity of LCO over an extended period. The interfacial stability was also validated through long-term high-temperature testing (Supplementary Fig. 17 and Supplementary Note 1). In addition, the distinct electrochemical stability windows of LNO and LZF also contribute to the different stability during cycling (Supplementary Fig. 18).
X-ray photoelectron spectroscopy (XPS) was first employed to study the detailed chemical insights into the composite positive electrode of In-Li|LPSCl|LZF-LCO and In-Li|LPSCl|LNO-LCO after cycles (Fig. 4a, b). The samples’ surfaces were cleaned by Ar+ sputtering before the test to remove the possible surface contamination. For comparison, XPS was also conducted on LPSCl powder (Supplementary Fig. 19). The S 2p spectrum and the P 2p spectrum of LPSCl both display doublets, with the S 2p3/2 peak at 161.4 eV and the P 2p3/2 peak at 131.8 eV. These features align well with the PS43− tetrahedra of the argyrodite structure. After 20 cycles, the S 2p spectrum of LNO-LCO composite positive electrode exhibits three new doublets with the 2p3/2 peaks at 162.9 eV, 163.8 eV, and 169 eV, suggesting the formation of bridging sulfur in P2Sx, elemental sulfur (S) and sulfate (SO42−), respectively. Extending the cycling process to 100 cycles leads to the appearance of a new doublet with the 2p3/2 peak at 167.1 eV, which corresponds to sulfite (SO32−), while also intensifying the signal associated with SO42−. These high oxidation state species severely hinder Li+ transport within the composite positive electrode. After substituting LNO with LZF, the chemical/electrochemical formation of these products can be effectively mitigated, with no SO42− detected after 20 cycles. Moreover, even after 100 cycles, the decomposition products still show intensities comparable to those of LNO-LCO composite positive electrode after 20 cycles. It should be noted that the signal of Co9S8 may not be discernible, probably due to its merging with the S signal owing to their close binding energies (~163.9 eV)38. The P 2p core-level XPS data of LNO-LCO composite positive electrode after 20 cycles displays two new doublets at higher binding energy, corresponding to P2Sx and P-O, which show an increase after 100 cycles. Similar to evolution in S 2p spectra, the LZF protective layer efficiently alleviates the generation of these resistive decomposition species. These results demonstrate the capability of LZF to stabilize the interface, thereby achieving better electrochemical performance.
a, b S 2p (a) and P 2p (b) XPS spectra of LNO-LCO and LZF-LCO at discharged state after 20 or 100 cycles. The cells cycled at 70 mA g−1 and 25 °C before disassembly and sampling for the ex situ XPS measurements. c, d S K-edge (c) and P K-edge (d) XANES spectra of pristine composite positive electrode, LNO-LCO composite positive electrode and LZF-LCO composite positive electrode at discharged state after 100 cycles. The cells cycled at 70 mA g−1 and 25 °C before disassembly and sampling for the ex situ XANES measurements. e, f ToF-SIMS secondary ion images of Co+, S−, CoS−, SO3−, PO3− for LNO-LCO (e) and LZF-LCO (f) composite positive electrode at discharged state after 100 cycles. The color bars have been provided in the form of cation mode and anion mode. The scale bar is 10 μm. The cells cycled at 70 mA g−1 and 25 °C before disassembly and sampling for the ex situ ToF-SIMS measurements. g, h DEMS test of SO2 generation in first charge process of In-Li|LPSCl|LNO-LCO (g) and In-Li|LPSCl|LZF-LCO (h) at 7 mA g−1 and 25 °C. i The accumulation of SO2 in the first charge process of In-Li|LPSCl|LNO-LCO and In-Li|LPSCl|LZF-LCO.
X-ray absorption spectra (XAS) were characterized on the composite positive electrode after cycles to further investigate the interfacial stability (Fig. 4c–d). The S K-edge and P K-edge XAS of pristine LZF-LCO composite positive electrode displayed identical characteristics as those of LPSCl, indicating the preserved integrity of the argyrodite structure15,32. However, for LNO-LCO composite positive electrode after 100 cycles, there are notable alterations in the peak shape of S K-edge XAS. These changes include a pronounced increase in the intensity of the peaks at ~2472 eV (typically Li2S) and ~2477 eV (typically SO32−), indicative of severe oxidation reactions and a rearrangement of the local structure of LPSCl. Similarly, the features in P K-edge XAS also display substantial changes, encompassing a decreased intensity of the main peak and the emergence of a new peak at ~2152 eV, ascribed to P4O1039. Additionally, the broad peak around 2170 eV can be attributed to the multiple scattering of P-O40. These results underscore that the LNO protective layer loses its protective function against chemical interactions between LCO and LPSCl prematurely. In the case of the LZF-LCO composite positive electrode after 100 cycles, despite a slight decrease in peak intensity and minor alterations in peak shape, its S K-edge and P K-edge XAS exhibit a significantly improved retention compared to the LNO-LCO composite positive electrode.
Additionally, interphase growth between LCO and LPSCl after 100 cycles was probed by time-of-flight secondary ion mass spectrometry (ToF-SIMS) measurements with LNO or LZF protective layer (Fig. 4e–f). Ar+ sputtering was also applied before the test to avoid misunderstanding. Chemical ion images of Co+ and S− are utilized to locate LCO and LPSCl, respectively. Various other species are also observable, including CoS−, SO3−, and PO3−. CoS− can be recognized as a distinctive component of Co9S8 or Co3S4, SO3− can be attributed to SO32− or SO42−, and PO3− can be associated with P4O10 or PO43−. It is clear that all ions related to decomposition products exhibit higher intensities in the LNO-LCO composite positive electrode when compared to the LZF-LCO composite positive electrode. Normalized intensity of these ionic fragments was also provided (Supplementary Fig. 20). These results provide strong evidence that LNO is not stable enough to inhibit chemical interaction between LCO and LPSCl, leading to thick CEI formation, especially at high voltage. On the other hand, LZF can remarkably reduce chemical degradation and ensure fast Li+ transport within the composite positive electrode.
The gas generation from the composite positive electrode during charge was also detected by differential electrochemical mass spectrometry (DEMS) experiments (Fig. 4g–i). As depicted in Fig. 4g, SO2 release from the LNO-LCO composite positive electrode was detectable even when LCO was slightly oxidized to Li0.95CoO2 (Fig. 4g). The presence of an LZF protective layer significantly delayed the onset of SO2 generation occurring when LCO underwent oxidation to Li0.7CoO2 (Fig. 4h). Furthermore, the accumulation of SO2 from the LZF-LCO composite positive electrode was notably lower, accounting for only 23.6% of that from the LNO-LCO composite positive electrode (Fig. 4i). It is important to note that the detection of SO2 below 4 V serves as evidence that SO2 originates from a chemical reaction between LCO and LPSCl, rather than being formed by O2 released from LCO interacting with LPSCl. DFT calculations were employed to elucidate the mechanism by which LCO reacts with the electrolytes LPSCl during charging to produce SO2. When a potential of 4 V (versus Li+/Li) is applied, the reaction energy for the formation of SO2 from LCO and LPSCl is -0.592 eV/atom (Supplementary Fig. 21). This suggests that the oxygen from LCO is thermodynamically favored to combine with sulfur to produce SO2 at this potential. Given the spontaneous reaction between LNO and LPSCl, sulfur from the electrolytes can diffuse into LNO coating layer, leading to the formation of electron-conductive niobium sulfide compounds and the fast decomposition of LNO. This process can facilitate the combination of oxygen and sulfur, resulting in the formation of SO2. In stark contrast, the enhanced thermodynamic and kinetical stability between LZF and LPSCl effectively impedes the combination of oxygen and sulfur, preventing the production of SO2. DEMS data for Ar and N2 were also displayed to verify the airtightness of the setup during cell cycling (Supplementary Fig. 22).
Besides the characterization on chemical decomposition and CEI growth, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) was utilized to distinguish the structural transformation of LNO-LCO and LZF-LCO particles after 100 cycles (Fig. 5a–f). These samples were prepared by focused ion beam (FIB). As depicted in Fig. 5a, the layered structure of LCO was reconstructed in the near-surface region despite the presence of the LNO coating. The amplified lattice fringes and the corresponding fast Fourier transform (FFT) further elucidate that this reconstruction phase is dominated by Fd\(\bar{3}\)m spinel Co3O4 (Fig. 5b–c). Additionally, the HRTEM results validate the presence of not only the spinel phase but also a more undesired Fm\(\bar{3}\)m rock-salt phase (Supplementary Figs. 23−24). The formation of these reconstruction phases is associated with O oxidation (O2− to Oα−, 0 <α < 2) at voltages exceeding 4.3 V and subsequent Oα− diffuse and release from the surface due to LNO layer has decomposed and failed in its role41,42. In this case, the chemical reaction between LCO and SSEs also contributes to the formation of the reconstruction layer due to their disparity in O chemical potential. This reconstruction layer not only impedes Li+ diffusion in LCO particles but also leads to the loss of active materials. Moreover, these O species diffuse to SSEs to form resistive products, increasing Li+ transport impedance within the composite positive electrode as discussed above. Excitingly, these layered-to-spinel/rock-salt transformation behaviors were significantly mitigated in LZF-LCO (Fig. 5d–f). The enlarged lattice fringes and corresponding FFT results confirm the well-preserved layered structure of LCO, owing to the LZF protective layer, which effectively prevents the release of Oα− and possesses long-term stability at the interface between LCO and LPSCl.
a–c Cross-sectional STEM images of LNO-LCO showing the reconstructed spinel Co3O4 phase and the corresponding FFT image. d–f Cross-sectional STEM images of LZF-LCO showing the well preserved layered LCO phase and the corresponding FFT image. g, h EELS line scan of O K-edge at the near-surface region of LNO-LCO particle (g) and LZF-LCO particle (h). i, j EELS line scan of Co L2,3-edge at the near-surface region of LNO-LCO particle (i) and LZF-LCO particle (j). All the cells cycled at 70 mA g−1 and 25 °C before disassembly and sampling for the ex situ measurements. And all the samples were characterized at discharged state after 100 cycles.
The electron energy loss spectroscopy (EELS) measurement was further performed to distinguish the states of Co and the loss of O in different depths (Figs. 5g–j, Supplementary Fig. 25). O K-pre-edge features in LCO have been identified as the result of a transition of electrons from the 1 s state to the hybridized O 2p-Co 3 d orbitals43,44. Concerning LNO-LCO particle after 100 cycles, the decreased O pre-edge peak and slight peak shift to higher energy loss within 15 nm from the surface indicate a decrease in the number of unoccupied states in the hybridized O 2p-Co 3 d orbitals, which is assigned to the reduction of transition metal and the sharing of d-orbitals holes with the oxygen ligands, including the associated decrease in covalency15. Moreover, the shift of Co L-edge to the lower energy loss and higher Co L3/L2 area ratio in the near-surface region also manifests the occurrence of undesired spinel/rock-salt phase (Figs. 5i–j, Supplementary Fig. 26). Impressively, only about 7 nm reconstruction layer exists in the surface of LZF-LCO after 100 cycles. Furthermore, the EDS mapping of the interface between LNO-LCO particle and LPSCl reveals the diffusion of Co into LPSCl (Supplementary Fig. 27a). This indicates that the LNO coating layer has already lost its functionality, and Co can readily diffuse into LPSCl, triggering its decomposition due to their differences in Co chemical potential. In contrast, the signals of Co in LZF-LCO remain confined to the LCO region (Supplementary Fig. 27b), once again highlighting the remarkably different protective effects of the two coatings. These results presented here demonstrate that LZF not only more effectively helps mitigate the SSEs decomposition but also plays a more substantial role in alleviating the structural transformation of LCO, jointly facilitating the interfacial charge transfer between LCO and SSEs, in accordance with the finding from the PEIS and DRT results.
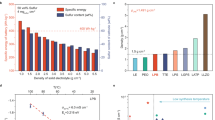
Since the veiled factors that made a significant difference between LNO and LZF protective layers have been clarified, further testing was conducted on the In-Li|LPSCl|LZF-LCO to fully exploit its potential. Its cycling durability at a high rate was demonstrated after evaluation of its rate capability (Fig. 6a, c). The voltage profile displays a high degree of overlap, particularly in the initial 1000 cycles. Impressively, highly stable cycling performance can be maintained for 2492 cycles at 420 mA g−1 with a capacity retention of 88.5%. To further verify the capability of LZF at an areal capacity of commercial interest (typically > 3 mAh cm−2), high-loading In-Li|LPSCl|LZF-LCO with an LCO areal loading of 30.19 mg cm−2 was evaluated (Fig. 6b, d). An areal capacity of 5.2 mAh cm−2 can be delivered at 7 mA g−1 in the first five cycles. When the current density is increased to 70 mA g−1, an areal capacity of up to 3.8 mAh cm−2 can be achieved. Excitingly, a capacity retention of 80.5% can be obtained after 1500 cycles. Another high-loading In-Li|LPSCl|LZF-LCO with an LCO areal loading of 30.82 mg cm−2 can display a high areal capacity of 2.95 mAh cm−2 at a high rate of 140 mA g−1, and achieve good capacity retention of 80% after 320 cycles (Supplementary Fig. 28). Such encouraging results again highlight the interfacial design principle for high-voltage all-solid-state batteries.
a, b Galvanostatic charge-discharge voltage profiles of In-Li|LPSCl|LZF-LCO at 420 mA g−1 and LCO loading of 11.32 mg cm−2 (a) and at 70 mA g−1 and LCO loading of 30.19 mg cm−2 (b). c, d Electrochemical cycling stability of In-Li|LPSCl|LZF-LCO at 420 mA g−1 and LCO loading of 11.32 mg cm−2 (c) and at 70 mA g−1 and LCO loading of 30.19 mg cm−2 (d). The LCO mass fraction in composite positive electrode is 80 wt.%. The experiments were performed between 2.0-3.9 V versus Li+/LiIn, corresponding to ~2.6−4.5 V versus Li+/Li. All cells were operated at 25 °C.
Following the established design criteria for coating materials, we have also investigated 69 types of LixMyFz compounds using data from the Materials Project. According to the basic evaluation criteria, which include electrochemical windows, band gap, and reaction energies of LixMyFz/Li0.33CoO2 and LixMyFz/LPSCl, an initial computational screening identified 18 types of LixMyFz (Supplementary Table 9). Subsequently, we calculated the reaction products between 18 types of LixMyFz and LPSCl and analyzed their band gaps (Supplementary Table 10). Our analysis revealed that Li2TiF6 and Li3CrF6 should be excluded due to the formation of electron-conductive reaction products. Ultimately, we identified LixMyFz compounds with M = Lu, Y, Zr, Be, Al, Th, Cs, Sc, and Ba as suitable coating materials. We observed that the electron transport properties of the metal sulfide in reaction products (e.g., LixMySz, MxSy) is a key factor in choosing LixMyFz coating materials, highlighting the critical role of elements M (e.g., Zr in this work).
Discussion
In summary, an LZF protective layer was proposed to coat on the surface of LCO to tackle the long-standing challenge at the interface of positive electrode materials and SSEs for sulfide ASSLBs. The LZF coating layer is 6–13 nm in thickness, with some nanocrystalline clusters embedded in the amorphous matrix. The minimal thermodynamic mutual reaction energy between LZF and LPSCl and their electron insulative decomposition products make the LZF protective layer exist stably during long-term cycling. In contrast, the conventionally employed LNO materials lose their functionality prematurely due to their transformation into a mixed conductive interlayer, which removes the kinetic barrier for progressing interaction between LCO and SSEs. Consequently, compared to LNO, the LZF protective layer is more crucial in reducing the accumulation of unfavored side products with high electronic conductivity and high oxidation state species with low ionic conductivity. Simultaneously, it provides more effective mitigation of the surface reconstruction of LCO. As a result, the In-Li|LPSCl|LZF-LCO all-solid-state cell with high LCO mass fraction (80 wt.%), substantial LCO areal loading (30.19 mg cm−2), and cutoff potential of 4.5 V versus Li+/Li, shows a notable areal capacity (5.2 mAh cm−2 at 7 mA g−1, 3.8 mAh cm−2 at 70 mA g−1) and a good capacity retention (80.5% after 1500 cycles at 70 mA g−1) at 25 °C at the same time. Meanwhile, all-solid-state cell with an LCO loading of 11.58 mg cm−2 can deliver a specific capacity of 96.2 mAh g−1 at 700 mA g−1 and retain 88.5% after 2492 cycles. This work highlights a critical factor that should be taken into account during the meticulous selection of coating materials and demonstrates a high-quality protective layer with long-term stability for the challenging 4.5V-class ASSLBs, which presents promising opportunities for their widespread utilization.
Methods
Material synthesis
The LZF protective layers were coated on the LCO particles via a facile liquid phase method followed by annealing. Before coating process, LCO powders were annealed at 700 °C for 5 hours in ambient air. The heating rate for all sintering steps was 3 °C per minute, and the samples were naturally cooled after sintering. For preparing LZF solution, 3 mg of Li2CO3 (99.9%, Aladdin) and 21 mg of H2ZrF6 solution (45 wt.%, Macklin) were mixed in 30 mL of deionized water. The pH of the solution is tested to be 3.45 at 25 °C. After that, 1 g of LCO powders (99.5%, Aladdin) was slowly added into the aforementioned solution and subjected to ultrasonication for ten minutes. Then, the mixture was vacuum-heated using a rotary evaporator at 60 °C for 1 hour with all the water had evaporated entirely. Last, the powders were collected and annealed at 700 °C for 2 hours in ambient air. The LNO protective layers were coated on the LCO particles as reported in previous literature34. Typically, 0.5 mg of Li metal (99.9%, China Energy Lithium Co., Ltd) was dissolved in 30 mL of anhydrous ethanol (Titan) and mixed with 21.5 mg of niobium pentaethoxide (99.5%, Aladdin). Next, 1 g of LCO powder was gradually introduced into the solution and subjected to ultrasonication for ten minutes. Subsequently, the mixture was vacuum-heated using a rotary evaporator at 30 °C for 1 hour until all the ethanol had evaporated entirely. Last, the powders were collected and annealed at 400 °C for 2 hours in ambient air. The mass fraction of LZF/LNO in the resulting positive electrode powder was about 1%.
Material characterization
X-ray powder diffraction (XRD) patterns were obtained by means of a Bruker D8 advance diffractometer equipped with Cu Kα radiation (λ = 1.54178). Kapton films were used to isolate air during XRD test. Scanning electron microscopy (SEM, Hitachi S4800) in conjunction with energy-dispersive X-ray spectroscopy (EDS) was used to study the morphology and element distribution. X-ray photoelectron spectroscopy (XPS) measurements were carried out by means of a Thermo Scientific K-Alpha+ with monochromatic Al Kα radiation (1486.6 eV). The collection of all test electrodes was carried out by slowly applying pressure on the negative electrode side using a mold in Ar-filled glovebox, thereby extracting the entire solid-state battery. Time-of-flight secondary-ion mass spectrometry (ToF-SIMS) was performed by means of a PHI nanoTOF II. For the XPS and ToF-SIMS tests, the chamber was directly connected to the argon glovebox to prevent the sample surface from contacting air and moisture. Ar+ ion sputtering was also applied before the test to remove possible contamination. The XAS at the P K-edge and the S K-edge were measured using fluorescence yield mode at 16 A beamline (1800 − 7500 eV) at the Taiwan Synchrotron Radiation Research Center. An inert atmosphere sample holder was used to transfer the samples. For atomic structural analysis, the particles were first sliced by a focused ion beam (FIB/SEM, FEI Scios 2 HiVac), and then high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images and electron energy loss spectroscopy (EELS) spectra were obtained using a JEM-ARM300F microscope. For FIB and TEM/STEM characterization, the samples were briefly exposed to air for less than one minute. For DEMS test, the all-solid-state cell (10 mm diameter, 25 mg cm−2 LCO loading) was assembled as it was for the electrochemical test and then carefully demolded. The demolded cell was subsequently placed inside the DEMS setup. Electrochemical cycling was performed at a rate of 7 mA g−1 and 25 °C in the voltage range of 2.0–3.9 V (versus Li+/LiIn) and 2.6–4.5 V (versus Li+/Li) using a Land battery test system. A 10 h open-circuit voltage period was included in the beginning. The flow of carrier gas was controlled to be 1.0 mL min−1. Due to the distance between the cell and the mass spectrum, the lag of the mass spectrum data was about 280 s compared with the battery data.
Electrochemical measurements
All battery assembly procedures were carried out in an argon glovebox with H2O < 0.1 ppm and O2 < 0.1 ppm. The In-Li|LPSCl|LCO all-solid-state cells were tested in a polyether ether ketone cylinder with an inner-wall diameter of 10 mm at 25 °C. To prepare a composite positive electrode, coated LCO, LPSCl, and PTFE (Aladdin, 5–10 μm) with a weight ratio of 80:19:1 were mixed to form a single flake. Subsequently, the flake was hot rolled repeatedly by hand using a stainless-steel cylindrical rod at 80 °C. An 80 mg of LPSCl powder (Hefei Kejing Material Technology Co., Ltd) was first added into the cylinder, followed by uniaxial pressing with 300 MPa for 2 minutes. Then, the composite positive electrode flake with 10 mm diameter was punched out and pressed on one side of the LPSCl pellet at 360 MPa for 2 minutes. The mass loading of LCO was 11.07–11.8 mg cm−2 (~30 µm thickness) and 30.19–30.82 mg cm−2 (~100 µm thickness) for the high-loading tests. After the second pressing step, a 0.1-mm-thick indium foil disc of 10 mm diameter and a 0.1-mm-thick lithium foil disc of 5 mm diameter (Kairui New Material Technology Co., Ltd.) were carefully removed with a punching pin, and then successively added to the other side of the LPSCl pellet. The metal foil was used as received without surface cleaning. The assembly was then pressed at a pressure of 150 MPa for 2 minutes. A constant pressure of ~50 MPa was applied to the cell using the screw of the stainless-steel framework during electrochemical test. Galvanostatic charge/discharge tests of cells were conducted in an Ar atmosphere using a Land battery test system (Wuhan, China) at 25 °C inside a static-heated environmental chamber, between 2.0–3.9 V versus Li+/LiIn, corresponding to ~2.6–4.5 V versus Li+/Li. PEIS was carried out after one complete cycle (discharged state) and 0.5 h of rest to achieve equilibrium in the first 50 cycles with Autolab electrochemical workstation. The applied cycling current density was 70 mA g−1 and the testing temperature was 25 °C. An amplitude of 10 mV was chosen for measurements in the frequency range of 1 MHz to 20 mHz with 10 points per decade. For the DRT analysis, a MATLAB code contributed by Prof. Francesco Giucci was employed45.
Theoretical studies
The DFT calculations were performed using Vienna Ab initio Simulation Package. The initial crystal structure was obtained from the Materials Project46 (Li2ZrF6 (mp-4002)). The chemical reactions (LixMyFz and Li0.33CoO2, LixMyFz and Li6PS5Cl) were analyzed by Pymatgen47. Precomputed data used in the phase diagram construction can be obtained from the Material Project database. The band gap of different LixMyFz are obtained from Material Project.
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 6, 123–134 (2021).
Wang, M. J., Kazyak, E., Dasgupta, N. P. & Sakamoto, J. Transitioning solid-state batteries from lab to market: linking electro-chemo-mechanics with practical considerations. Joule 5, 1371–1390 (2021).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682 (2011).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Kraft, M. A. et al. Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1-xGexS5I for all-solid-state batteries. J. Am. Chem. Soc. 140, 16330–16339 (2018).
Adeli, P. et al. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution. Angew. Chem. Int Ed. 58, 8681–8686 (2019).
Li, Y. et al. A lithium superionic conductor for millimeter-thick battery electrode. Science 381, 50–53 (2023).
Wang, L. et al. Bidirectionally compatible buffering layer enables highly stable and conductive interface for 4.5 V sulfide‐based all‐solid‐state lithium batteries. Adv. Energy Mater. 11, 2100881 (2021).
Zhang, X. et al. Spontaneous gas-solid reaction on sulfide electrolytes for high-performance all-solid-state batteries. Energy Environ. Sci. 16, 1091–1099 (2023).
Liang, J. et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun. 14, 146 (2023).
Wu, Y. Q. et al. Highly reversible Li2RuO3 cathodes in sulfide-based all solid-state lithium batteries. Energy Environ. Sci. 15, 3470–3482 (2022).
Sun, N. et al. Surface-to-bulk synergistic modification of single crystal cathode enables stable cycling of sulfide-based all-solid-state batteries at 4.4 V. Adv. Energy Mater. 12, 2200682 (2022).
Jang, J. et al. Enabling a Co-Free, High-voltage LiNi0.5Mn1.5O4 cathode in all-solid-state batteries with a halide electrolyte. ACS Energy Lett. 7, 2531–2539 (2022).
Wang, L. et al. High-energy all-solid-state lithium batteries enabled by Co-free LiNiO2 cathodes with robust outside-in structures. Nat. Nanotechnol. 19, 208–218 (2024).
Wang, L. et al. In-situ visualization of the space-charge-layer effect on interfacial lithium-ion transport in all-solid-state batteries. Nat. Commun. 11, 5889 (2020).
Zuo, T. T. et al. A mechanistic investigation of the Li10GeP2S12|LiNi1-x-yCoxMnyO2 interface stability in all-solid-state lithium batteries. Nat. Commun. 12, 6669 (2021).
Wang, C. H. et al. Deciphering interfacial chemical and electrochemical reactions of sulfide-based all-solid-state batteries. Adv. Energy Mater. 11, 2100210 (2021).
Zhang, Y. Q. et al. Direct visualization of the interfacial degradation of cathode coatings in solid state batteries: a combined experimental and computational study. Adv. Energy Mater. 10, 1903778 (2020).
Koerver, R. et al. Chemo-mechanical expansion of lithium electrode materials – on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 11, 2142–2158 (2018).
Banerjee, A., Wang, X. F., Fang, C. C., Wu, E. A. & Meng, Y. S. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem. Rev. 120, 6878–6933 (2020).
Li, X. et al. Unravelling the chemistry and microstructure evolution of a cathodic interface in sulfide-based all-solid-state Li-ion batteries. ACS Energy Lett. 4, 2480–2488 (2019).
Zhou, L. D. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Zhang, S. et al. A family of oxychloride amorphous solid electrolytes for long-cycling all-solid-state lithium batteries. Nat. Commun. 14, 3780 (2023).
Riegger, L. M., Schlem, R., Sann, J., Zeier, W. G. & Janek, J. Lithium-metal anode instability of the superionic halide solid electrolytes and the implications for solid-state batteries. Angew. Chem. Int Ed. 60, 6718–6723 (2021).
Cao, D. et al. Stable thiophosphate-based all-solid-state lithium batteries through conformally interfacial nanocoating. Nano Lett. 20, 1483–1490 (2020).
Wang, C. W. et al. Engineering the interface between LiCoO2 and Li10GeP2S12 solid electrolytes with an ultrathin Li2CoTi3O8 interlayer to boost the performance of all-solid-state batteries. Energy Environ. Sci. 14, 437–450 (2021).
Wu, E. A. et al. A Facile, Dry-processed lithium borate-based cathode coating for improved all-solid-state battery performance. J. Electrochem. Soc. 167, 130516 (2020).
Sun, X. et al. Discharge voltage profile changes via physicochemical phenomena in cycled all-solid-state cells based on Li10GeP2S12 and LiNbO3-coated LiCoO2. J. Mater. Chem. A 9, 17905–17912 (2021).
Wang, C. et al. Unveiling the critical role of interfacial ionic conductivity in all-solid-state lithium batteries. Nano Energy 72, 104686 (2020).
Peng, L. et al. LiNbO3-coated LiNi0.7Co0.1Mn0.2O2 and chlorine-rich argyrodite enabling high-performance solid-state batteries under different temperatures. Energy Storage Mater. 43, 53–61 (2021).
Wang, C. H. et al. Manipulating interfacial nanostructure to achieve high-performance all-solid-state lithium-ion batteries. Small Methods 3, 1900261 (2019).
Lu, G. et al. Enhanced electrochemical performances of LiCoO2 cathode for all-solid-state lithium batteries by regulating crystallinity and composition of coating layer. J. Power Sources 468, 228372 (2020).
Ohta, N. et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 9, 1486–1490 (2007).
Bong, W. S. K. et al. Effect of thickness and uniformity of LiNbO3-coated layer on LiNi0.5Co0.2Mn0.3O2 cathode material on enhancement of cycle performance of full-cell sulfide-based all-solid-state batteries. J. Power Sources 577, 233259 (2023).
Lu, Y., Zhao, C.-Z., Huang, J.-Q. & Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 6, 1172–1198 (2022).
Zhu, Y., He, X. & Mo, Y. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 4, 3253–3266 (2016).
He, J., Bhargav, A. & Manthiram, A. High-performance anode-free Li–S batteries with an integrated Li2S–electrocatalyst cathode. ACS Energy Lett. 7, 583–590 (2022).
Redeker, A. et al. Investigation of P4O6, P4O10 and P4O6S by X-Ray absorption spectroscopy at the phosphorus K-edge. Phosphorus, Sulfur, Silicon Relat. Elem. 76, 239–242 (1993).
Franke, R. & Hormes, J. The P K-near edge absorption spectra of phosphates. Phys. B: Condens. Matter 216, 85–95 (1995).
Tan, X. H. et al. High-entropy surface complex stabilized LiCoO2 cathode. Adv. Energy Mater. 13, 2300147 (2023).
Yang, X. R. et al. Pushing lithium cobalt oxides to 4.7 V by lattice-matched interfacial engineering. Adv. Energy Mater. 12, 2200197 (2022).
Wang, Z. et al. In situ STEM-EELS observation of nanoscale interfacial phenomena in all-solid-state batteries. Nano Lett. 16, 3760–3767 (2016).
Roychoudhury, S. et al. Deciphering the oxygen absorption pre‐edge: a caveat on its application for probing oxygen redox reactions in batteries. Energy Environ. Mater. 4, 246–254 (2020).
Wan, T. H., Saccoccio, M., Chen, C. & Ciucci, F. Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim. Acta 184, 483–499 (2015).
Jain, A. et al. Commentary: the materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Ong, S. P. et al. Python materials genomics (pymatgen): a robust, open-source python library for materials analysis. Computational Mater. Sci. 68, 314–319 (2013).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22225201 Y. W.) and the Shanghai Pilot Program for Basic Research-Fudan University 21TQ1400100 (21TQ009 Y. W.). We thank the Taiwan Synchrotron Radiation Research Center for the provision of synchrotron radiation beam time at the 16 A beamline.
Author information
Authors and Affiliations
Contributions
X.Z. and Y.W. conceived and designed the research project. X.Z., K.Z., and Z.L. carried out the material characterization. C.Y.C. and S.K.J. carried out the XANES experiments. X.Z. performed the battery measurements. D.Y. and Y.Z. carried out the calculations. X.Z. wrote the manuscript. Y.Z., Y.X., B.J.H., and Y.W. directed the entire study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, X., Chang, CY., Yu, D. et al. Li2ZrF6 protective layer enabled high-voltage LiCoO2 positive electrode in sulfide all-solid-state batteries. Nat Commun 16, 112 (2025). https://doi.org/10.1038/s41467-024-55695-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55695-9