Abstract
Synthetic Notch (SynNotch) receptors function like natural Notch proteins and can be used to install customized sense-and-respond capabilities into mammalian cells. Here, we introduce an adaptor-based strategy for regulating SynNotch activity via fluorescein isomers and analogs. Using an optimized fluorescein-binding SynNotch receptor, we describe ways to chemically control SynNotch signaling, including an approach based on a bio-orthogonal chemical ligation and a spatially controllable strategy via the photo-patterned uncaging of an o-nitrobenzyl-caged fluorescein conjugate. We further show that fluorescein-conjugated extracellular matrix (ECM)-binding peptides can be used to regulate SynNotch activity depending on the folding state of collagen-based ECM networks. To demonstrate the utility of these tools, we apply them to activate dose-dependent gene expression responses and to induce myogenic-like phenotypes in multipotent fibroblasts with spatiotemporal and microenvironmental control. Overall, we introduce an optimized fluorescein-binding SynNotch as a versatile tool for regulating transcriptional responses to ligands based on the clinically-approved fluorescein dye.
Similar content being viewed by others
Introduction
Multicellular processes, including development and tissue regeneration, require precise signaling coordination between cells and their surroundings. For example, Notch signaling permits cells to regulate gene expression according to the positional and contextual information they receive via direct cell-cell contacts1,2. Similarly, changes in interactions with the extracellular matrix (ECM) via integrin receptors can trigger diverse cellular processes, ranging from cytoskeletal reorganization and adhesion to stem cell differentiation3. Inspired by these natural mechanisms, researchers have engineered ways to control and reprogram how cells interact with their microenvironments. For example, ‘bio-instructive materials’ can be used to mimic natural ECM properties, and such materials can direct the healing and repair of damaged tissues4,5. Tailored biomaterials can also control engineered cell activities to modulate therapeutic T-cell responses in situ and to facilitate immune cell engineering in vivo6,7,8,9. Yet despite these advances, synthetic biology strategies for programming customized ECM-dependent signaling activities have remained relatively limited, especially in comparison to tools for sensing and programming responses to cell-cell interactions10,11,12,13,14.
Recognizing the engineering utility of cell-ECM signaling components, we aimed to create a versatile approach that could be used to define how cells sense and interpret scaffold-embedded signals within their surroundings. As a basis for this toolset, we exploited the modularity of the synthetic Notch (SynNotch) design, an engineered receptor framework that can link user-specified ligands to customized gene transcription responses10,15. Like natural Notch, SynNotch receptors contain intracellular domains (ICDs) based on transcriptional effector proteins. During signaling, these effectors are proteolytically cleaved from their transmembrane sequences to enable their nuclear translocation for the regulation of target genes. For both native and synthetic Notch, such cleavages require the tension-mediated unfolding of a juxtamembrane segment termed the Negative Regulatory Region (NRR)16,17. In natural contexts, this unfolding is mediated via the tensile forces delivered to Notch receptors during their trans-endocytosis into ligand-expressing cells16,18. Soluble ligands cannot generate the required tensile energies and, therefore, often serve as competitive signaling inhibitors.
Surface and bead-immobilized ligands can also facilitate tension-mediated Notch and SynNotch activation, and by exploiting such ligands, researchers have devised ways to elicit desirable signaling responses from various cell types, in vitro and in vivo19,20,21,22,23. Furthermore, recent work has shown that ligands bound to endogenous and engineered material scaffolds can also facilitate productive signaling. For example, Notch can be activated by immobilized Delta-like 1 (DLL1) ligand copies embedded within photopatterned fibrin-based hydrogels24, and ligands fused with a bone mineral-binding peptide can selectively stimulate Notch in cells surrounding skeletal growth plates in vivo25. From a synthetic biology perspective, SynNotch receptors recognizing native ECM components can be generated using a collagen-II binding antibody fragment26, and strategies involving microcontact printed ligands can direct SynNotch activities with micro-scale spatial precision27,28.
Inspired by the growing repertoire of Notch and SynNotch activation strategies, we set out to devise an adapter-based strategy that could be used to gain versatile and dynamic control over SynNotch signaling via a ‘chemogenetic’ ligand-receptor pair. We reasoned that with such components, one could bypass the need to individually construct and characterize new receptor extracellular domains – the engineering of which can be challenging due to obstacles associated with converting IgGs into well-behaved single-chain sequences. Building upon the previous design of ‘universal’ chimeric antigen receptors (CARs)29,30,31,32,33, we describe a fluorescein-based strategy for regulating the activity of a fluorescein-binding SynNotch with inducible and dose-dependent control. To develop this approach, we first characterize SynNotch constructs fused with distinct fluorescein-binding domains, analyzing their surface levels and quantifying their activities against ligands based on fluorescein isomers and analogs. Using an optimized receptor, we define conditions to maximize ligand-induced gene expression responses while minimizing background (ligand-independent) signaling activities. Roles for the presenilin isoforms (presenilin-1 and presenilin-2) are also investigated in the context of ligand-mediated and ligand-independent receptor activities, and cells expressing our optimized construct are used to define the kinetics of ligand-induced reporter mRNA formation and fluorescent protein expression.
To highlight the versatility of our system, we exploit fluorescein-based tools to regulate SynNotch signaling in biocompatible and spatially controllable ways. Using bio-orthogonal chemistry, we show that tetrazine-functionalized fluorescein can be transformed into a signaling active ligand via its selective ligation with immobilized trans-cyclooctene groups in the presence of live cells. To achieve spatial control over signaling, we employ photo-caged fluorescein as a light-conditional ligand, using photo-masked illumination to generate spatially defined ligand patterns in 2D. Additionally, we exploit fluorescein-conjugated ECM-binding peptides to direct ECM-dependent SynNotch activities in response to native and unfolded collagen-I proteins. Finally, to demonstrate the utility of these tools, we exploit them to induce myogenic-like phenotypes in multipotent fibroblasts with spatial and chemo-biological control.
Results
Fluorescein-binding SynNotch receptors are correctly processed and trafficked to the cell surface
To develop a sensitive and versatile system, we sought an efficiently expressed receptor capable of detecting ligands based on commonly used fluorescein isomers and analogs. Synthetic receptors were generated by fusing fluorescein-binding domains to the extracellular region of the SynNotch scaffold (Fig. 1a). Three previously characterized domains were tested in our designs, including two single-chain variable fragments (scFvs) based on existing anti-fluorescein antibodies—αFITC(E2)34,35 and 4M5.336,37—and a third (non-immunoglobulin) ___domain based on the engineered lipocalin “FluA”38,39,40(Fig. 1b). The resulting fusions are hereafter referred to as αFITC(E2)-SynNotch, 4M5.3-SynNotch, and FluA-SynNotch, respectively. Beyond their distinct binding domains, each construct contained otherwise identical components, including a CD8α signal peptide, an extracellular myc-tag (for surface detection), and a core ___domain based on the Notch1 NRR and its transmembrane helix. We used receptors containing an intracellular ___domain (ICD) based on Gal4-VP64 to quantify ligand-induced signals in cells containing Gal4-dependent reporter genes.
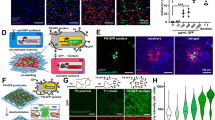
a Schematic depicting the design SynNotch receptors containing the various tested fluorescein binding domains (FBDs). b Dye-bound structures of the various FBDs tested in our designs, including αFITC(E2) (PDB: 2A9N, a bound to the difluorinated fluorescein analog, OregonGreen488), 4M5.3 (PDB: 1X9Q), and FluA (PDB: 1N0S). c Detection of surface-localized SynNotch receptors expressed on transfected HEK293-FT cells. Cells were co-transfected with DNAs encoding the indicated receptors in combination with a mTurq2-encoding plasmid as a co-transfection marker. Cells were immunostained the next day under live-cell conditions using an anti-myc-AlexaFluor647 (anti-myc-AF647) antibody conjugate. Insets represent over-exposed AF647 emissions. Scale bar = 100 µm. Experiment was repeated independently with similar results. d Immunoblot detection of myc-tagged receptors. Whole-cell lysates from transfected HEK293-FT cells were analyzed. Bands corresponding to full-length (FL, higher mass) and furin-cleaved N-terminal (N-term., lower mass) receptor fragments are indicated with arrows. In both (c) and (d), HEK293-FT cells transfected with an mTurq2-encoding plasmid were analyzed as controls to confirm the specificity of the utilized anti-myc antibody probes. The experiment was repeated independently with similar results. e Structures of the fluorescein isomers and analogs used as test ligands against the various receptor designs. f Flow cytometry traces of reporter expression levels in transfected HEK293-FT (UAS:H2B-mCherry) reporter cells expressing the indicated receptors. The transfected cells were grown overnight in microwells containing the indicated ligands and adhesion proteins. Ligand stimulation proceeded overnight prior to flow cytometry analyses; wells were pre-coated the indicated ligands prior to adding cells (see Methods). Traces represent normalized densities of three independent transfections (n = 3 > 5000 cells analyzed per replicate) gated for receptor expression by the mTurq2 co-transfection marker. The dashed black line indicates the fluorescence threshold used to define H2B-mCherry+ cells, as determined via analysis of non-transfected HEK293-FT cells; solid black lines indicate median mCherry intensities for each depicted population. g Median mCherry intensities from mTurq2+ population from (f) of three independent transfections (n = 3>5000 cells analyzed per replicate); Data presented as mean values +/- standard deviation and analyzed with two-way ANOVA (ligand and receptor). All unlabeled comparisons involving fibronectin and gelatin controls versus co-plated ligands have P < 0.0001; labeled NS, P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, otherwise. Data and P values are provided in the Source Data file. FBD fluorescein-binding ___domain, Fluor fluorescein, OG488 OregonGreen488. The cartoon in (a) was created in BioRender87.
Since efficient surface expression is required for sensitive extracellular ligand detection, we first evaluated the presentation of our constructs using transfected HEK293-FT cells. Immunolabeling with a fluorescent anti-myc antibody revealed varying cell surface levels for our designs: αFITC(E2)-SynNotch was the most efficiently presented, followed by FluA-SynNotch, which was moderately reduced by comparison. 4M5.3-SynNotch had the lowest surface levels among the evaluated receptor sequences (Fig. 1c, Supplementary Fig. 1a). Furin is a Golgi-localized enzyme that processes the NRR into a heterodimeric complex before the receptors are presented at the plasma membrane41,42, and blotting of cell lysates showed that full-length receptors were translated to similar levels but were processed by furin to varying degrees (Fig. 1d). The lack of a furin-cleaved band for 4M5.3-SynNotch suggested its retention within the endoplasmic reticulum (ER), which we hypothesized to be due to misfolding of the 4M5.3 scFv. Correspondingly, converting the scFv into the more stable single-chain Fab (scFab) format43 resulted in 4M5.3-scFab-SynNotch, which was efficiently cleaved by furin and trafficked to the surface in multiple transfected cell lines (including HEK293-FT, U2OS, and CHO-K1 cells, Supplemental Fig. 1b, c). Thus, αFITC(E2)-SynNotch and FluA-SynNotch are efficiently trafficked constructs, and our data show that the trafficking inefficiency of 4M5.3-SynNotch can be overcome via substitution of the scFv with a 4M5.3-based scFab. Similar manipulations may help improve the display of other scFv-based antigen-binding receptor systems.
αFITC(E2)-SynNotch can sensitively detect ligands based on immobilized fluorescein
Next, we evaluated the signaling activities of our receptors using a model ligand based on fluorescein-5-isothiocyanate (FITC)-conjugated bovine serum albumin (BSA-5-FITC). Reporter cells containing a Gal4-dependent gene (HEK293-FT:UAS-H2B-mCherry) were transiently transfected with receptor-encoding plasmids and grown overnight in BSA-5-FITC-coated microwells. Flow cytometry quantification of H2B-mCherry levels confirmed the activation of our receptors by the immobilized BSA-5-FITC, with controls showing that such signals were fluorescein-dependent (Fig. 1e–g). Dose-dependent analyses with BSA-5-FITC showed that αFITC(E2)-SynNotch produced the most sensitive responses, followed by 4M5.3-scFab-SynNotch, 4M5.3-SynNotch, and FluA-SynNotch, respectively (Supplementary Fig. 2). Signaling levels in 4M5.3-scFab-SynNotch cells were higher than those expressing the scFv-containing 4M5.3-SynNotch, likely due to the increased surface levels of the scFab-containing design.
To investigate the kinetics of signal activation, we directly probed time-dependent reporter mRNA formation using the hybridization chain reaction (HCR)44. Nuclear intensities corresponding to nascent mCherry transcripts were detected within 1–2 h following ligand-induced ICD release (Supplementary Fig. 3). These results show that αFITC(E2)-SynNotch activation and its downstream reporter gene expression proceed along timescales consistent with those reported for Notch nuclear complex assembly and gene transcription45,46,47. To assess the durability of signaling, we analyzed cells after four days of growth in BSA-5-FITC-coated microwells. Numerous transcript copies were detected in the cytoplasmic and nuclear compartments of HCR-labeled cells, indicating that initial ligand levels were sufficient to drive continued reporter expression for at least four days (Supplementary Fig. 3c). Together, these results confirm the fluorescein-dependent activities of our receptor designs and provide insight into the timescales of synthetic signaling-induced transcript formation.
αFITC(E2)-SynNotch facilitates a versatile detection of fluorescein isomers and analogs
Fluorescein bioconjugates (including antibodies, etc.) can contain chemically distinct dye isomers and derivatives, some of which may not interact with the ligand binding domains used in our designs. Thus, to identify a highly-versatile receptor, we next tested our receptors against commonly used fluorescein isomers and analogs. We first analyzed responses to ligands based on 5-fluorescein versus 6-fluorescein—two widely used fluorescein isomers that differ in the positioning of where linker handles are attached to the carboxyphenyl ring (Fig. 1e). Isomerically pure dyes were used to generate BSA-5-fluorescein and BSA-6-fluorescein conjugates (see Methods), and responses to these ligands were quantified as described above. Following overnight stimulation, measurement of H2B-mCherry levels revealed striking differences in the isomer binding preferences of our designs (Fig. 1f–g). Most notably, FluA-SynNotch was activated by BSA-5-fluorescein but not the BSA-6-fluorescein, revealing a strict binding selectivity for the 5-isomer by the lipocalin-derived ___domain. Cells expressing 4M5.3-SynNotch and 4M5.3-scFab-SynNotch were heavily biased toward 5-fluorescein-based ligands, though low activity levels were detected in cells treated with BSA-6-fluorescein (Fig. 1f).
In stark contrast to FluA-SynNotch cells, cells expressing αFITC(E2)-SynNotch produced potent responses to BSA-5-fluorescein and BSA-6-fluorescein, as well as a ligand based on the difluorinated fluorescein analog OregonGreen48848 (OG488, gelatin-OG488; Fig. 1e–g). Together, our analyses revealed several advantageous properties for αFITC(E2)-SynNotch, including its efficient surface display and sensitive and unbiased recognition of fluorescein isomers and OG488. Given these advantages, we proceeded with αFITC(E2)-SynNotch, further evaluating its sensitivity to OG488 using cell-bead and cell-cell-based trans-activation assays. Here, “sender” cells expressing a biotinamide-binding ligand protein (anti-bio-SNAP-TMD-DLL1) were cocultured with αFITC(E2)-SynNotch “receivers,” and treatment with biotin-based bridging compounds was used to template the ligand-receptor interactions in trans49 (Supplementary Fig. 4). Note that a synthetic ligand containing the DLL1 intracellular tail was utilized, given its ability to facilitate the epsin-dependent trans-endocytosis of receptor ectodomains16,18,49. Using co-cultured cells, we evaluated the sensitivity of bridges based on biotin-FITC versus biotin-OG488 conjugates, finding a more potent activity for biotin-OG488, which triggered 192-fold greater H2B-mCherry levels compared to biotin-FITC in response to 20 pM treatment concentrations (Supplementary Fig. 4f). This result suggests OG488 could potentially be a more potent adapter ligand for “universal” CAR designs involving the αFITC(E2) ___domain. Of note, treatment of cocultures with a short-linked fluorescein conjugate (biotin-ethylenediamine-fluorescein) did not lead to detectable reporter activities, suggesting that sufficient spacing between biotin and dye handles is needed for efficient templating of ligand-receptor complex between cells (Supplementary Fig. 4g). In addition to cell-mediated trans-activation, treatment with fluorescein- and OG488-decorated microbeads also resulted in potent signaling activities (Supplementary Fig. 5). Overall, these results highlight the versatility and sensitivity of αFITC(E2)-SynNotch to fluorescein isomers and analogs.
Expression levels influence ligand-dependent and ligand-independent receptor activities
Receptor overexpression can lead to ligand-independent signaling, limiting the dynamic range of bona fide signaling responses50. Thus, to facilitate the reliable adaptation of our tools, we optimized expression conditions needed to achieve low background and high receptor inducibility in using transfected and transduced reporter cells. First, HEK293-FT reporter cells were transfected with varying amounts of receptor-encoding plasmid (pcDNA3-αFITC(E2)-LaG17-SynNotch-Gal4VP64), and we evaluated H2B-mCherry following overnight incubation in BSA-5-FITC-coated versus uncoated microwells (Supplementary Fig. 6). By comparing reporter levels between ligand-treated versus untreated cells, we identified 25 ng of the pcDNA3-based plasmid as an optimal amount for transfecting 150,000 HEK293-FT reporter cells (see Methods). Under these conditions, ligand stimulation led to a 191-fold increase in H2B-mCherry expression compared to untreated (receptor-expressing) reporters. Increased plasmid levels led to higher ligand-independent reporter levels, whereas lower amounts reduced ligand-induced reporter yields. In contrast to transient transfection, receptor expression by lentiviral transduction resulted in potently inducible cells without discernible increases in background activities over a 20-fold viral supernatant dose range (Supplementary Fig. 7).
Ligand-mediated αFITC(E2)-SynNotch signaling mediated primarily by presenilin-1 gamma-secretase (PS1-GS) in HEK293-FT cells
Work by others has implicated gamma-secretase (GS) cleavage as a key mediator of the ‘leaky’ (ligand-independent) activity of cells expressing SynNotch receptors in excess50. GS is a multi-subunit enzyme composed of APH1, PEN-2, and nicastrin subunits in combination with a catalytic subunit based on one of two presenilin (PS) isoforms--PS1 or PS2, encoded by PSEN1 and PSEN2, respectively51. To dissect the contributions of PS1 and PS2 in SynNotch processing, we used Cas9-editing to individually and doubly knockout (KO) PS expression in our HEK293-FT reporter cells (generating PS1-KO, PS2-KO, and PS1/PS2-double KO (DKO) lines) (Supplementary Fig. 8a). Signaling analyses in the cells showed that ligand-induced activities were substantially diminished in PS1-lacking cells, whereas those of PS2-KO and non-KO cells were comparable (Supplementary Fig. 8b–e). This result suggests a more prominent role for PS1-GS proteolysis in the ligand-induced activation of αFITC(E2)-SynNotch in HEK293-FT cells. In contrast, ligand-independent activities proceeded to comparable levels across individual presenilin KO and non-KO cells, being diminished only in PS1/PS2 DKO cells (Supplementary Fig. 8d, f). Thus, ligand-induced SynNotch activities arise primarily due to PS1-mediated cleavage in HEK293-FT cells, whereas ligand-independent signals can derive from either PS1-GS, PS2-GS, or both enzymes to comparable degrees.
Inducible signaling via bioorthogonal bond-formation between tetrazine and trans-cyclooctene handles
Having characterized αFITC(E2)-SynNotch, we next exploited fluorescein-based ligands to devise methods for inducible receptor activation and gene expression control. Given that immobilized ligands can activate signaling, we predicted that soluble fluorescein units could be converted to signaling-competent ligands using cell-compatible bioorthogonal ligation reactions. To test this possibility, we exploited bioorthogonal chemical ligation between tetrazine (Tz) and trans-cyclooctene (TCO) handles to immobilize a Tz-functionalized fluorescein dye to microwells containing an immobilized TCO-BSA conjugate (Fig. 2a, b). Tz and TCO groups can react via an ultrafast inverse electron–demand Diels–Alder ligation reaction compatible with live cells and in vivo conditions52,53. Consistent with our expectations, Tz-5-fluorescein treatment triggered dose-dependent reporter activities upon its addition to 2D cell cultures grown in TCO-BSA-coated microwells. Measurement signaling levels showed that 2 nM treatment doses triggered a 487x-fold change in H2B-mCherry levels compared to Tz-5-fluorescein-untreated cells grown in TCO-BSA wells (Fig. 2c–f). In contrast, cells grown without TCO-BSA were refractory to Tz-5-fluorescein treatment. In live cell time-lapse imaging, ligand treatment led to synchronous and uniform cell activity across TCO-BSA-containing wells (Supplemental Movies 1–2). We also tested a fluorescein conjugate bearing a 6-methyl-tetrazine (MeTz) group, which has improved stability in aqueous media compared to the Tz handle54. Treatment with low nanomolar MeTz-fluorescein also resulted in potent reporter activity in cells grown with TCO-BSA (Supplementary Fig. 9). These results show that bioorthogonal chemistry can be exploited to convert soluble fluorescein dyes into immobilized (and thus signaling-active) ligand handles.
a Chemical structures of Tetrazine-5-Fluorescein (Tz-5-fluorescein) and the trans-cyclooctene (TCO) reactive handle depicted as conjugated to BSA (TCO-BSA). b Schematic depiction of the bio-orthogonal ligation-mediated immobilization of Tz-5-fluorescein upon its reaction to surface-adsorbed TCO-BSA. Generation of the immobilized fluorescein ligand results in productive αFITC(E2)-SynNotch activation and H2B-mCherry reporter expression. c Dose-dependent analysis of Tz-5-fluorescein-mediated H2B-mCherry expression from clonal αFITC(E2)-SynNotch cells grown in TCO-BSA coated microwells. Traces represent normalized densities of three independent treatments at varying doses are shown. The dashed black line indicates the H2B-mCherry+ threshold set based on analysis of control HEK293-FT cells; solid black lines indicate median mCherry intensities for each condition. d Median mCherry emission intensities from untreated and Tz-5-fluorescein-treated cells grown with or without immobilized TCO-BSA from (c). Three independent drug treatments (n = 3 >5000 cells analyzed per replicate); Data presented as mean values +/- standard deviation and analyzed with two-way ANOVA (ligand and drug concentration). Labeled NS, P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data and P values provided in Source Data file. e Fluorescence images of clonal HEK293-FT (UAS:H2B-mCherry) reporter cells expressing αFITC(E2)-SynNotch-mTurq2, as treated under the indicated conditions with 2 nM Tz-5-Fluorescein. Scale bar = 100 µm. f Immunoblot detection of signaling-induced H2B-mCherry levels. Tz-fluorescein concentrations were tested between 2 pM and 200 nM, with variation between adjacent lanes by a factor of 10-fold. For both (e) and (f), treatment with gamma secretase inhibitor (GSI, DAPT at 10 μM) led to diminished Tz-5-fluorescein-induced reporter H2B-mCherry levels. Experiments in (e) and (f) were repeated independently with similar results. The cartoon cells in (b) were created in BioRender87.
Light-inducible signaling using a photocaged fluorescein ligand
We next asked whether fluorescein-based ligands could be used to define spatial gene expression patterns in our engineered cells. To test this possibility, we implemented a photocaged (PC)-fluorescein as a light-conditional ligand, anticipating that photolytic uncaging of the compound could be used to control receptor binding and, as a result, define gene expression patterns in 2D (Fig. 3a–c). Using BSA conjugated with a bis-5-carboxymethoxy-2-nitrobenzyl (bis-CMNB) modified 5-fluorescein (BSA-PC-5-fluorescein)55, we compared signaling responses to the caged (light-protected) and photo-uncaged versions of the compound. Flow cytometry, imaging, and western blotting analyses confirmed our expectations, showing that the uncaged ligand, but not its caged precursor, could activate αFITC(E2)-SynNotch signaling and induce H2B-mCherry expression in our HEK293-FT reporter line (Supplemental Fig. 10).
a Structure of photolabile bis-5-carboxymethoxy-2-nitrobenzyl (bis-CMNB) modified 5-fluorescein (PC-5-fluorescein) and UV-light induced photo-uncaged product 5-fluorescein. b Models of αFITC(E2) scFv binding (left) PC-5-carboxy-fluorescein; photolabile caging groups (purple) are hypothesized to prevent ligand binding. (right) 5-carboxy-fluorescein. Models were built by superimposing fluorescein derivative on OG488 ligand in PDB: 2A9N. c Schematic of photo-uncaging plate adsorbed PC-5-Fluorescein leading to αFITC(E2)-SynNotch activation in defined areas. d, e Laser-printed transparency slides were used as photomasks to generate patterns of uncaged BSA-PC-5-fluorescein ligands. αFITC(E2)-SynNotch-T2A-BFP U2OS cells with DsRed2 reporter were seeded and imaged 24–36 h later. Photo-uncaging patterning in (d) and (e) were repeated independently with similar results. The cartoon cells in (c) were created in BioRender87.
Equipped with a light-conditional ligand, we next asked whether we could exploit BSA-PC-5-fluorescein to generate spatially defined gene expression patterns. Here, we devised simple photomasks based on laser-printed transparency slides, exploiting them to create uncaged PC-5-fluorescein-BSA patterns via transillumination with a handheld UV lamp (see Methods). U2OS cells expressing αFITC(E2)-SynNotch and containing a UAS:DsRed2 reporter construct were grown on the patterned surfaces, and fluorescence microscopy was used to visualize reporter expression patterns across 2D monolayers 24 - 36 h after cell seeding (Fig. 3d, e, Supplementary Fig. 11). These analyses confirmed the spatial specificity of our approach, showing that reporter activities were confined to culture areas containing photo-uncaged fluorescein ligands.
We also examined ligand photoactivation in the presence of live cells using time-lapse imaging. Here, reporter activity was recorded following pulse photo-uncaging through a DAPI excitation filter (361–389 nm) (Supplementary Movie 3). Subsequent monitoring of reporter activity confirmed the confinement of signaling responses to illuminated well areas. To compare the time scales of SynNotch-mediated versus chemically-induced gene expression, we conducted time-dependent analyses using U2OS cells containing a TRE3G-mCherry reporter gene. Ligand photo-uncaging was used to activate a receptor containing a TetR-VP48 ICD, and time-lapse imaging was used to analyze reporter expression rates with direct comparison to doxycycline-mediated mCherry expression (via a co-expressed TetON-3G trans-activator protein). In both cases, mCherry expression was detected within 4-6 h following light exposure (1 sec) or doxycycline treatment (200 ng/mL) (Supplementary Fig. 12). These data confirm the live cell compatibility of ligand photo-uncaging, showing that light-mediated signal activation proceeds with comparable kinetics to doxycycline-induced gene expression. Additional tests showed that PC-5-fluorescein-BSA could maintain its caged state over prolonged periods in the presence of cells, remaining inactive over a weeklong duration while retaining its photo-activatability (Supplementary Fig. 13). Overall, our results validate BSA-PC-5-fluorescein as a light-conditional ligand, showing that the caged molecule can be uncaged to direct spatially-defined gene expression in 2D cultures of SynNotch-expressing cells.
Signal activation via ECM-binding bifunctional bridges
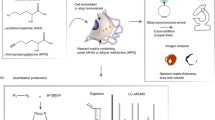
Next, we asked whether fluorescein-based agents could direct synthetic signaling responses to ECM substituent proteins. We reasoned that dye-conjugated ECM-binding proteins could be transformed into signaling-active ligands upon immobilization to ECM protein networks. To test this possibility, we generated an OG488-conjugated version of CNA35, a 35 kDa collagen-binding ___domain derived from the collagen adhesion protein of Staphylococcus aureus56,57 (Fig. 4a). In cells grown on collagen-I substrates, OG488-CNA35 treatment induced dose-dependent αFITC(E2)-SynNotch activation in a manner that was selective for native collagen over gelatin (denatured collagen) and fibronectin (Fig. 4b, c), consistent with CNA35’s well-characterized binding selectivity for folded collagen triple helices58.
a Schematic of OG488-CNA35 (CNA35 in blue, HaloTag in yellow, OG488 in green) bound to natively folded collagen-I (purple). b OG488-CNA35 activates αFITC(E2)-SynNotch specifically on collagen-I coated wells at low nanomolar concentration. HEK293-FT H2B-mCherry reporter cells were transiently transfected with plasmids encoding αFITC(E2)-SynNotch and mTurq2 as a cotransfection marker. Mean percent mCherry positive cells of the mTurq2+ population analyzed by flow cytometry from three independent transfections (n = 3 > 5000 cells per replicate) displayed +/- standard deviation. c Median mCherry fluorescence intensities showing clonal αFITC(E2)-SynNotch-mTurq2 cells specifically activated by 3 nM OG488-CNA35 bound to triple-helical collagen-I. Three independent OG488-CNA35 treatments (n = 3 > 5000 cells analyzed per replicate). Data presented as mean values +/- standard deviation and analyzed by one-way ANOVA, labeled NS: P > 0.05, ****: P < 0.0001. d Schematic of CHP-5-fluorescein bound to denatured collagen (gray). e CHP-5-fluorescein activates on heat-denatured collagen-I. HEK293-FT H2B-mCherry reporter cells were transiently transfected with αFITC(E2)-SynNotch and mTurq2 cotransfection marker. The mean percent mCherry positive cells of the mTurq2+ population analyzed by flow cytometry from three independent transfections (n = 3 > 5000 cells per replicate) displayed +/- standard deviation. f Median mCherry fluorescence intensities showing clonal αFITC(E2)-SynNotch-mTurq2 cells specifically activated by 2 µM CHP-5-fluorescein bound to heat-denatured collagen-I. Three independent CHP-5-fluorescein treatments (n = 3>5000 cells analyzed per replicate). Data presented as mean values +/- standard deviation and analyzed by one-way ANOVA, labeled NS: P > 0.05, ****: P < 0.0001. Data and P values, and non-linear sigmoidal curve fit are provided in the Source Data file. Components of the cartoons in (a) and (d) were created in BioRender87.
To complement the native-collagen sensing method above, we also designed a strategy to enable cells to detect damaged collagens in their microenvironments and activate synthetic gene expression as a response. Here, a fluorescein-conjugated version of the Collagen Hybridizing Peptide (CHP-5-fluorescein) was used as a bridging agent to immobilize receptor-binding fluorescein ligands to denatured collagen proteins. CHP is a short glycine-proline-hydroxyproline repeat that can hybridize with unfolded collagen α-chains59 and previous work has shown that CHP conjugates can be used to trace denatured collagens in vivo60,61,62 and to localize therapeutic molecules to diseased tissues63,64 (Fig. 4d). Growth of αFITC(E2)-SynNotch cells on CHP-5-fluorescein-treated collagen substrates triggered dose-dependent responses to denatured collagen-I, with cells on native collagen producing only marginal responses, limited to wells treated with mid-micromolar-range peptide-conjugate concentrations (Fig. 4e, f). Together, these results show that ligand-conjugated ECM-binding components can be exploited as ECM-receptor adapters to enable cells to detect the composition and folding state of the ECM, directing them to activate synthetic gene transcription activities in response.
Fluorescein-based ligands can facilitate the myogenic conversion of multipotent C3H10T1/2 cells
To demonstrate the utility of our tools, we implemented them to induce myogenic-like phenotypes in embryonic mesenchymal C3H10T1/2 cells containing a SynNotch-inducible p65-MyoD fusion gene (Fig. 5a, b, Supplementary Fig. 14a). MyoD is a master myogenic regulator, and p65-MyoD expression in C3H10T1/2 cells facilitates their efficient conversion into multinucleated syncytial myoblasts28,49,65. As a first test, we grew cells on BSA-5-FITC versus control surfaces, later evaluating them for reporter expression and syncytialization. Consistent with our expectations, growth on BSA-5-FITC resulted in the formation of red fluorescent syncytia, with HCR labeling confirming the continued transcription of the synthetic target gene following four days of ligand stimulation (Supplementary Fig. 14b). We also examined whether Tz-5-fluorescein treatment could trigger similar syncytialization between cells grown on TCO-BSA-coated substrates. Indeed, treatment with Tz-5-fluorescein at nanomolar concentrations triggered DsRed2-expression and syncytialization in the engineered fibroblasts in a TCO-BSA-dependent manner (Fig. 5c, Supplementary Fig. 14c, d). Immunoblotting of cells following 4 days of growth with TCO-BSA and MeTz-(PEG)5-fluorescein confirmed the expression of multiple myogenic markers, including that of MHC, desmin, and troponin-T (Supplemental Fig. 14e). Analysis of MeTz-(PEG)5-fluorescein-treated cells grown without TCO-BSA further confirmed the requirement of ligand immobilization in signal activation and myogenic gene expression.
a Schematic depicting αFITC(E2)-SynNotch containing a TetR-VP48 ICD. The receptor is expressed in C3H10T1/2 fibroblasts containing a TRE-regulated target gene encoding p65-MyoD-T2A-DsRed2. b Depiction of the signaling-mediated conversion of C3H10T1/2 fibroblasts in response to fluorescein-induced αFITC(E2)-SynNotch activation. Expression of p65-MyoD-T2A-DsRed2 results in the formation of red fluorescent syncytia exhibiting myogenic phenotypes. c C3H10T1/2 fibroblasts grown in TCO-BSA coated wells were left untreated (left) or treated with 2 nM Tz-5-Fluorescein (right). Cells were imaged 48 h following treatment. d C3H10T1/2 fibroblasts were grown in microwells containing immobilized and photo-patterned BSA-PC-5-fluorescein. Cells were imaged after 72 h of growth on the patterned surfaces. Expression of the myogenic marker protein myosin heavy chains (MHC) was confirmed via immunofluorescence staining. e Schematic depicting the denaturation of collagen triple helices followed by the binding of CHP-5-fluorescein to immobilized collagen strands. f Microwells containing native (left) or denatured (right) collagen-I proteins were treated with CHP-5-fluorescein (2 µM) prior to the addition of C3H10T1/2 cells. Cells were imaged after 48 h of growth. Experiments in c, d, and f were repeated independently with similar results. The schematic in (a) and cartoon components from (b) were created in BioRender87.
Next, we tested the utility of photopatterned BSA-PC-5-fluorescein surfaces in directing syncytialization with spatial control. Following three days of growth in photopatterned microwells, cells in photo-activated well areas exhibited phenotypic markers of receptor activation and myogenesis, including DsRed2 fluorescence, multinucleation, and expression of the myogenic marker protein myosin heavy chain (MHC; Fig. 5d, Supplementary Fig. 15a, b). In contrast, cells residing in light-protected well regions remained in fibroblast-like states, as evident by their mononuclear morphologies and lack of anti-MHC immunoreactivity. Similar treatment of C3H10T1/2 cells containing a TRE:DsRed2 reporter gene (in place of TRE:p65-MyoD-T2A-DsRed2) resulted in confined DsRed2 expression patterns without discernible syncytia formation, verifying that the observed conversion of TRE:p65-MyoD-T2A-DsRed2 cells was due to signaling-mediated p65-MyoD expression (Supplementary Fig. 15c).
Finally, we asked whether treatment with CHP-5-fluorescein could be used to selectively direct the syncytialization of cells grown on denatured but not native collagen-I-based substrates. Consistent with our fluorescent reporter data, the growth of cells on denatured collagen in the presence of CHP-5-fluorescein led to p65-MyoD-T2A-DsRed2 expression and syncytium formation. In contrast, cells in wells with native collagen remained mononuclear and largely lacked detectable DsRed2 (Fig. 5e, f, Supplementary Fig. 16a). Note that western blotting showed that MHC expression was induced by CHP-5-fluorescein in cells on denatured and non-denatured collagen, with levels of the myogenic marker being substantially elevated in cells grown with the denatured ECM protein (Supplementary Fig. 16b). The low MHC expression in cells treated on non-denatured collagen likely stems from residual CHP-5-fluorescein levels, which could be overcome by further optimizing the CHP-5-fluorescein reagent. The result also suggests an elevated sensitivity for targets encoding transcription factor-encoding genes. We note that the TCO/MeTz ligation method exhibited superior specificity compared to CHP-5-fluorescein, evident by its ability to tightly control MHC, troponin T, and desmin expression following four days of MeTz-(PEG)5-fluorescein treatment in the presence of TCO-BSA. Overall, our results show that fluorescein-based ligands can be used to activate synthetic gene expression responses via distinct mechanisms and in potentially useful ways.
Discussion
In this study, we leveraged exogenously administered ‘adapter’ compounds to devise ways to regulate SynNotch activity via bio-orthogonal and photo-chemical reactions and in response to the folding state of natively-derived collagen-I proteins. To do so, we combined an optimized fluorescein-binding SynNotch with biocompatible ‘adapters’ based on fluorescein- and OG448-conjugates. Using this approach, we demonstrated spatial, temporal, and dose-dependent control over αFITC(E2)-SynNotch signaling and its downstream outcomes. As a utility of these methods, we exploited them to direct the myogenic conversion of multipotent fibroblasts into myoblasts using diverse triggers, including bio-orthogonal bond formation, photo-patterned ligands, and unfolded collagen—a disease biomarker associated with multiple pathological conditions59.
The FDA-approved and bio-compatible nature of fluorescein may facilitate facile extensions of this approach to in vivo settings, where fluorescein- and OG488-based adapters could activate and confine therapeutic cell activities to targeted sites within the body. For example, TCO-functionalized materials could be combined with exogenous Tz-5-fluorescein administration to generate signaling-competent ligands within disease-relevant sites containing implanted biomaterial scaffolds. Additionally, TCO-containing nanoparticles could be directed to tumor sites via enhanced permeability and retention (EPR)66 and subsequently modified via Tz-5-fluorescein dosing to confine αFITC(E2)-SynNotch agonists to malignant sites in vivo.
Using a bacterially derived CNA35 ___domain, we directed cells to sense folded collagen-I and enact dose-dependent responses based on gene transcription. Additionally, by anchoring CHP-5-fluorescein to unfolded collagen strands, we devised a synthetic signaling strategy mimicking that of natural ECM regulation, in which mechanical unfolding is used to reveal cryptically buried signals in ECM proteins67,68. While heat-denatured collagen-I was applied as a model in our studies, CHPs are reported to bind diverse forms of denatured and damaged collagens, including protease-digested strands and mechanically ruptured fibrils, which are hallmarks of conditions such as aging and cancer69,70. In future studies, our approach could be extended to enable the detection of diverse disease-specific ECM-based signatures (beyond denatured collagens), including post-translationally modified ECM components, cleavage-generated neo-epitopes, or alternatively-spliced disease-associated ECM protein isoforms71,72. The detection of such species could be facilitated through fluorescein-labeled binding peptides, bacterial proteins, or fluorescein-modified antibody conjugates.
While the fluorescein adapter-based approach makes this system highly versatile, users should be aware of potential limitations when applying or adapting the techniques that are described herein. Firstly, heterobifunctional compounds are susceptible to the dose-mediated hook effect, in which treatment with adapter concentrations in excess can result in the non-productive occupation of binding sites with reduced or without the desired ligand-receptor bridging. This effect was observed when using Tz-Fluorescein at high doses (200 nM, Fig. 2d). Given this effect, bifunctional bridging compounds should be screened across various doses to identify suitable concentrations, which may vary depending on each compound, their applications, or the media compositions in which they are tested. New agents should be evaluated to confirm their independent binding to target components (including sender and receiver cells, immobilized scaffolds, ECM proteins, etc.) before cell-signaling measurements. Second, while the vast majority of commercial fluorescein conjugates will be based on 5- and 6-linked isomer derivatives, dyes linked via alternative positions may not be recognized by the αFITC(E2) scFv. Thus, chemical structures for conjugated dyes should be inspected or requested from vendors before use. Third, while o-nitrobenzyl caged compounds have been used in various applications, they are limited by their requirement of UV light and by their generation of reactive photoproducts (which may react with cells or media components)73,74. Thus, minimized light doses should be applied when uncaging PC-5-fluorescein in the presence of live cells. In future work, these limitations could be overcome by designing caged ligands bearing alternative photo-protecting groups, including those that can be photolyzed using visible or near-infrared wavelengths74.
While we used CMNB-caged fluorescein to generate αFITC(E2)-SynNotch activity patterns in 2D, the approach could be readily extended to 3D applications, where techniques like multiphoton laser-scanning lithography75 could be leveraged to define gene expression patterns in hydrogel-based systems, organ-on-a-chip models76, and in the manufacturing of lab-grown meat77. Lastly, while we exploited immobilized agents to induce signaling activation, approaches involving the regulated disassembly of tethered ligands (via chemical, optical, or enzyme-based triggers) could be exploited as strategies to mediate ligand inactivation.
Overall, we developed chemogenetic components based on fluorescein and a fluorescein-binding SynNotch, demonstrating their utility in controlling gene expression activities in mammalian cells in response to a diverse set of chemical triggers and biological cues. Diverse fluorescein-based indicator dyes have been previously developed as sensing agents to report on an array of biological signals and activities, and many of these agents could be combined with our system to facilitate synthetic sense-and-respond activities in the future. For example, fluorescein-based compounds with sensitivity to H2O278, or those designed to detect the activity of proteases, esterases79, and glycosidases could be employed as micro-environmental sensors capable of directing gene expression activities in αFITC(E2)-SynNotch cells80,81,82. In future work, ligands with dual-input requirements (i.e., ‘AND’ gate ligands) could also be developed by modifying fluorescein’s phenolic oxygens with independent caging moieties, similar to the design of existing dual-input probes based on Virginia Orange83. Through such approaches, existing chemical biology dyes originally developed as fluorescent sensors could be leveraged in synthetic biology applications as inducers and actuators.
Methods
General
Full product details (including product numbers and supplier information for the utilized materials), step-by-step protocols for the synthesis, immobilization, and uncaging of BSA-PC-5-fluorescein, and amino acid sequences for the generated receptors are provided in the Supplementary Information.
Mammalian cell culture
Mammalian cell lines were cultured in a humidified incubator maintained at 37 °C with 5% CO2. Cell media based on Dulbecco’s Modified Eagle Medium (DMEM) was supplemented with 10% fetal bovine serum (FBS), 1x GlutaMAX, 1x non-essential amino acids, and 1x Penicillin-Streptomycin. For experiments involving cells containing doxycycline-inducible reporter genes, media containing 10% Tet-Approved (or otherwise-doxycycline-free) FBS was used in place of regular FBS (see Supplementary Information for details).
Flow cytometry
Cells were analyzed using an Attune NxT flow cytometer v2.6 and v3.1 flow cytometry software (ThermoFisher) as in (Sloas et al. 2023)49. Live cells were identified, setting FSC-A and SSC-A thresholds, and singlets were identified by a polygon gate set by FSC-A versus FSC-H, respectively (gating scheme example shown in Supplementary Fig. 17). Positively transfected or transduced cells were identified based on the expression of a co-transfection marker or that of a co-translated T2A-fluorescent protein; positive cells were identified based on gates set using signals from control (non-expressing) cells ( > 99th percentile). To improve the detection of cells expressing both a receptor of interest and co-transfection marker in transiently transfected cells, positively transfected cells were further gated for those bearing marker fluorescence emission at levels above the median of all transfected cells. Flow cytometry data were analyzed and quantified using the open-source ggCyto software (version 1.27.1).
Data collection and analysis software
Fluorescence images were collected using the Zen 2.3 Pro (Blue Edition) imaging software and analyzed in ImageJ v2.0.0. Western blots were collected and analyzed using the QuantityOne(4.5.2) immunoblotting software or the iBright Imaging System software v1.4.0. The data shown in the figures are representative examples of results that were repeated in at least two independent experiments. Statistical analyses were performed using the GraphPad Prism v9.0.0 software.
Live time-lapse Imaging
For live time-lapse imaging, cells were cultured in FluoroBrite DMEM supplemented with 5% FBS, 1X GlutaMAX, 1X non-essential amino acids, and containing 20 mM HEPES (pH 7.4). Imaging was performed in media containing Hoechst-Janelia Fluor 646 (Hoechst-JF646; gift from Luke Lavis of Janelia Farm) at 500 nM and verapamil at 10 μM; both the stain and verapamil were added to pre-warmed imaging media and mixed vigorously to ensure full dissolution before cell application. A layer of mineral oil was applied atop the imaging media to prevent evaporation during live cell microscopy.
DNA constructs and transfections
DNA constructs were generated using standard cloning procedures, typically by Gibson assembly reactions and T4 ligations. Inserts were generated by PCR amplification or acquired as custom-synthesized DNA fragments (Integrated DNA Technologies). Plasmid backbones were linearized by digestion with restriction enzymes. Cloning of lentiviral backbones and sequences containing repeat regions was performed using NEB Stable Competent E. coli cells (New England Biolabs) as the transformation host.
DNA transfections were carried out using Lipofectamine 3000 Reagent (L3000001, ThermoFisher) according to the manufacturer’s instructions. For analyses involving transient transfection of HEK293-FT derived reporter cells (UAS:H2B-mCherry), 10 ng of receptor-encoding plasmid DNA was used in combination with 10 ng of a separate plasmid encoding a mTurquoise2 fluorescent protein as a co-transfection marker, combined with 30 ng of salmon sperm filler DNA. Mixtures containing 50 ng of total DNA were used to transfect ~60,000 reporter cells to be plated in a 96-well. In cases where receptor-T2A-BFP fusions were used, ~60,000 reporter cells were transfected with 10 ng of receptor-T2A-BFP plasmid with 40 ng of salmon sperm filler DNA.
HCR-FISH
An oligonucleotide probe set against the mCherry transgene mRNA was obtained through Molecular Instruments as part of an “HCR v3.0” kit. Detection was performed using “B1” amplifiers as AlexaFluor647 conjugates. Cells were fixed at room temperature with a 4% paraformaldehyde solution in Dulbecco’s Phosphate-Buffered Saline (DPBS) for 10 minutes, were washed twice with DPBS for 5 minutes each, and permeabilized in ice-cold 70% ethanol (v/v in water) with incubation overnight at -20 °C. The manufacturer’s protocol was followed for probe hybridization and hybridization chain reaction (HCR) steps. The same anti-sense probe set was also utilized in detecting DsRed2-encoding transcripts.
KO cell line generation
Cas9-mediated KO of PSEN1 and PSEN2 were done using the sgRNA provided below. Annealed oligonucleotides corresponding to the sequences were cloned into the pGuide-it-tdTomato vector backbone (Takara, 632604) according to the manufacturer’s protocol. hPSEN1-sgRNA: 5’-CCCTGTGACTCTCTGCATGG-3’. hPSEN2-sgRNA: 5’-GAAGAGCTGACCCTCAAATA-3’. HEK293-FT reporter cells (UAS:H2B-mCherry) were transfected in a 6-well plate with Lipofectamine 3000 using the manufacturer’s protocol. At 24 h post-transfection, tdTomato+ cells were sorted into a 96-well plate at one cell per well using a FACSMelody Cell Sorter (BD Biosciences). PSEN2 KO cells were used to generate the DKO line.
Lentiviral production and transduction
For viral transduction, lentiviral particles were generated using a second-generation lentiviral vector system. HEK293-FT cells were grown to approximately 90% confluence on a fibronectin-coated 6-well dish (coated using a 1 μg/mL fibronectin coating solution in PBS). The wells were transfected with 566 ng of construct-encoding transfer plasmid, alongside 833 ng of packaging (pPax2) and envelope encoding (pVSVG) plasmids using Lipofectamine 3000 reagent. Transfection media was replaced the following morning with 2 mL of fresh media. The viral supernatant was harvested 24 h later and passed through a low protein-binding 0.45 μm filter before immediate use or storage at -80 °C. For viral transduction, cells were transduced in growth media containing diluted and filtered viral supernatant; viral transduction media was replaced with fresh media 24 h later.
Reporter cell line generation
U2OS:TRE3G-mCherry and C3H/10 T½:TRE-DsRed reporter cells were generated in a similar manner as was described previously (Sloas et al. 2023)49. Briefly, lentiviral particles were generated as described above and were used to transduce ~75,000 of U2OS or C3H/10 T½ cells in a 24-well plate. Viral media was replaced with fresh media 24 h after transduction. At 48 – 72 h post-transduction, cells were trypsinized and transferred to a 6-well for selection in antibiotic-containing media (0.5 μg/mL puromycin for the utilized reporter constructs). Care was taken to prevent C3H/10 T½ from reaching confluence. Selection in puromycin typically proceeded for 10-14 days, after which U2OS:TRE3G-mCherry cells were used a stable pool. A single clone of C3H/10 T½:TRE-DsRed2 cells via limited dilution into 96 well plates before use. The activities of the U2OS pool and of the isolated C3H/10 T½ clone were verified by overnight doxycycline treatment (~ 200-1000 ng/μL) followed by fluorescence microscopy detection of fluorescent protein expression. Cell lines bearing doxycycline-inducible reporter genes were maintained in media containing doxycycline/tetracycline-free FBS.
Receptor-expressing cell line generation
Stable receptor-expressing cell lines were generated by lentiviral integration of EF1A-driven constructs based on pLV-EF1a-IRES-Hygro (AddGene #85134). Viral particles generated as described above were used to transduce HEK293-FT:UAS-H2B-mCherry (clone E5) and U2OS:UAS-T2A-DsRed2 (clone 1G4) reporter cells developed in (Sloas et al. 2023)49. Typically, ~300,000 HEK293-FT and ~150,000 U2OS cells were transduced with 500 μL of viral supernatant in a 6-well. Viral media was replaced with fresh media 24 h later. Once the cells reached ~75% confluence, media was replaced with antibiotic selection media (75 μg/mL and 200 μg/mL Hygromycin-B-Gold for HEK293-FT and U2OS, respectively). Cells were sub-passaged once 90% confluent, using 0.25% trypsin solution without EDTA. Media was replaced with fresh antibiotic-containing media daily, or every other day to generate receptor-expressing stable pools; selection typically proceeded for 10–14 days, with trypsinization and dilution as needed. Clonal cell lines were generated by limiting dilution in 96-well plates. Important note: use of 0.25% trypsin solution without EDTA is required to maintain receptor quiescence during trypsinization and sub-passaging (EDTA induces NRR unfolding and receptor cleavage84).
For generating αFITC(E2)-SynNotch-TetR-VP48 cells, a lentiviral construct encoding an αFITC-SynNotch receptor with a TetR-VP48 ICD (a.k.a tTA) (pHR-SFFV-αFITC(E2)-SynNotch-TetR-VP48) was produced in HEK293-FT cells grown in tetracycline (Tet)-free media. Transductions were performed in fibronectin-coated 24-well tissue culture plates using ~100,000 C3H/10 T½ or U2OS TRE reporter cells per well in combination with 200 μL of viral supernatant from frozen stocks stored at -80 °C. After 24 h, the cells were trypsinized using a pre-warmed 0.25% trypsin solution (without EDTA) and transferred to 6 well tissue culture plates before further expansion or analysis.
For generating αFITC(E2)-SynNotch-Gal4-VP64 and αFITC(E2)-SynNotch-Gal4-VP64-mTurq2 cells, HEK293-FT (UAS:H2B-mCherry) cells were transfected with linearized versions of corresponding receptor plasmids based on restriction enzyme cleaved pcDNA3 plasmids. The plasmids contained a hygromycin resistance expressed from a PGK promoter. The PGK promoter was used in place of an original SV40 promoter/origin within the pcDNA3 backbone due to the expression of the Large T-antigen in HEK293-FT cells. Plasmids were linearized by enzyme digestion at positions outside of the receptor and resistance marker cassettes, and the resulting linearized DNA was transfected into HEK293-FT (UAS:H2B-mCherry) cells. At 72 h post-transfection, stable integrants were selected using 75 μg/mL Hygromycin-B-Gold (InvivoGen). Single clones were isolated by limited dilution into 96-well plates. The clonal lines were maintained in media containing 100 μg/mL zeocin (for maintenance of the UAS:H2B-mCherry reporter) and 75 μg/mL Hygromycin-B-Gold (for maintenance of the integrated receptor encoding gene cassettes).
Measurement of receptor surface expression and analysis of furin/S1-processing
For cell surface measurements by flow cytometry, ~60,000 HEK293-FT cells were transfected using Lipofectamine 3000 as the transfection reagent; 100 ng of a receptor-encoding plasmid and 10 ng of pcDNA3-mTurquoise2 as a co-transfection marker. Cells were immunostained at ~24 h after transfection using mouse monoclonal anti-c-myc-AlexaFluor647 (Santa Cruz Biotechnology) diluted into growth medium (1:200 dilution). Staining proceeded for 30 min at 37 °C before removing antibody-containing mediate and washing gently with fresh media (3 x 200 μL washes).
Cells were seeded in fibronectin-coated 8-well glass-bottom imaging dishes to detect cell surface-expressed receptors by fluorescence imaging. HEK293-FT and CHO-K1 cells were seeded at ~120,000 cells per well and U2OS cells at ~75,000 cell per well. Cells were co-transfected 5–6 h later using a mixture containing receptor-encoding plasmid and pcDNA3-mTurquoise2 co-transfection marker (50 ng of each plasmid per well). A no-receptor control was carried out by transfecting cells with 50 ng of pcDNA3-mTurquoise2 in combination with 50 ng of salmon sperm DNA as filler. Cells were stained 24 h after transfection using anti-myc-AlexaFluor647 as described above.
HEK293-FT and U2OS cells were transfected for western blotting using conditions described for live cell imaging. Lysates were prepared by rinsing cells once with PBS, followed by direct lysis in 1xSDS-PAGE sample buffer. Samples were subjected to sonication to shear genomic DNA and reduce sample viscosity. Samples were heated and reduced before separation on SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes and subsequently blocked in a “blocking buffer” solution containing 5% nonfat dry milk (w/v) in PBS containing 0.1% (v/v) Tween-20 (PBS-T). Myc-tagged receptors were detected using rabbit monoclonal anti-myc (Cell Signaling Technology), probing overnight at 4 °C at a dilution of 1:1000 in blocking buffer. A goat anti-rabbit-HRP conjugate (Bio-Rad) was used as a secondary antibody, probing at room temperature for 1 h with agitation at a dilution of 1:3000 in PBS-T. Membranes were developed using SuperSignal West Pico PLUS Chemiluminescent Substrate (ThermoFisher) with recording on a ThermoFisher iBright instrument. Developed membranes were stripped using Restore Western Blot Stripping Buffer (ThermoFisher) per the supplier’s protocol, and stripped membranes were re-blocked with blocking buffer for 1 h before re-probing. The detection of loading control proteins (using anti-GAPDH, etc.) was done after detecting the primary antigens of interest. Loading control proteins were detected using stripped and re-blocked membranes with probing via DirectBlot anti-GAPDH-HRP (BioLegend). Gels were run with PageRuler Prestained Protein Ladder (10 to 180 kDa, ThermoFisher) as the molecular weight marker.
Plating fluorescein ligands
Ligand-coated surfaces for BSA conjugated fluorescein derivatives (BSA-5-FITC, BSA-5-fluorescein, BSA-6-fluorescein) were prepared by diluting the BSA ligand into PBS containing 10 μg/mL fibronectin. Non-TC treated wells (50 μL for 96-well, 100 μL for 8-well) were incubated for 1 h and washed 3 times with PBS before seeding cells; wells were coated using BSA conjugate solutions at 5 μg/mL, unless otherwise indicated. Gelatin ligand-coated surfaces (gelatin-5-FITC, gelatin-5-OG488) were plated similarly using 5 μg/mL solutions in combination with 50 μg/mL unconjugated gelatin in PBS.
Generation of BSA-5-fluorescein and BSA-6-fluorescein ligands
BSA was functionalized with dibenzocyclooctyne (DBCO) handles by reaction with a DBCO-N-hydroxysuccinimidyl ester (DBCO-NHS ester, Click Chemistry Tools). A reaction containing a 10:1 molar ratio of DBCO-NHS ester to BSA was carried out for 2 h at room temperature in 100 mM Borate Buffer (pH 8.4), after which the mixture was dialyzed against PBS using a 2 kDa MWCO Slide-A-Lyzer Dialysis Cassette (ThermoFisher). The dialyzed BSA-DBCO was then reacted overnight with excess amounts of 6-FAM-azide (AAT Bioquest, Catalog No. 133) or 5-FAM-azide (Lumiprobe, B4130). The next day, the mixtures were dialyzed again against PBS as before. Levels of the conjugates were matched based on fluorescein extinction and aliquots were stored at -20 °C until use.
Transactivation coculture assay
For coculture experiments, ~20,000 HEK293-FT-based “sender” cells expressing anti-bio-SNAP-TMD-DLL1 ligand were combined with ~20,000 HEK293-FT (UAS:H2B-mCherry)-based “receiver” cells expressing a receptor based on myc-αFITC(E2)-LaG16-SynNotch-Gal4-VP64-T2A-BFP. Suspended cell mixtures were then added to fibronectin-coated wells in a 96-well plate. At ~6 h post-cell seeding (once cells had adhered), the cocultures were treated with bifunctional bridging compounds at the concentrations indicated within the captions. Cocultures containing non-transduced HEK293-FT cells in place of ligand-expressing senders were used as controls; control cocultures were seeded into microwells and treated with bridging compounds in a similar manner as described for sender-receiver mixtures. Note that biotin-FITC (Cayman Chemical, 25574) and biotin-OG488 were active inducers in transactivation measurements, whereas the short-linked biotin-ethylenediamine-fluorescein (ThermoFisher, B10570) was inactive (see Supplementary Information and Supplementary Fig. 4g).
Preparation and cell stimulation with ligand-decorated beads
Fluorescein-bound microparticles were prepared using streptavidin-coated magnetic beads (Dynabeads M-280 Streptavidin, 2.8-μm, ThermoFisher, 60210). Beads were first rinsed by diluting 10 μL of the commercial suspension into 1 mL PBS. The beads were then collected by magnetic separation and resuspended in 1 mL PBS solution containing 200 nM biotin-FITC or biotin-OG488. The mixture was allowed to incubate for 30 min at room temperature, after which they were magnetically collected and rinsed three times using PBS to remove unbound ligand molecules. A fourth rinse was carried out using 2 mL of cell culture media. The beads were resuspended in a 130 μL volume of culture media before cell application. Based on this protocol, the final mixture corresponded to ~500,000 beads per 10 μl of fully suspended solution; beads were applied to cells using 10 μl volumes per each well of an 8-well imaging dish (~ 500,000 beads per well).
Tetrazine-TCO ligation-mediated gene expression control
TCO-BSA protein conjugation was prepared as described by (McMahan and Ngo 2022)85 using TCO-PEG4-NHS (Click Chemistry Tools). Reactions were carried out using solutions containing 75 μM BSA dissolved in 75 mM sodium bicarbonate buffer (pH 8.2). Protein conjugation was initiated by adding NHS ester from stock solutions dissolved in dry dimethyl sulfoxide (DMSO). Final reaction solutions contained 7.5 mM of the NHS ester and 20% (v/v) DMSO. Coupling reactions were carried out for 1 h at room temperature, followed by removal of unconjugated and hydrolyzed esters via three rounds of dialysis against PBS using 10 kDa MWCO dialysis cassettes (ThermoFisher). Following dialysis, the solution was sterilized by filtration through a low-protein binding 0.2 μm porous membrane filter.
TCO-BSA coated surfaces were prepared in a similar manner as described above for BSA-fluorescein ligands using 5 μg/mL coating solutions. Receptor-expressing cells were seeded on TCO-BSA- coated substrates and allowed to adhere at 37 °C for ~6 h. Cells were exchanged into fresh media before treatment with Tz-5-fluorescein at the concentrations indicated within the captions. Reporter expression analyses were conducted at 24 h post-treatment.
For live timelapse microscopy of Tz-TCO-mediated gene expression, cells were grown in coverslip bottom imaging dishes for recordings via an inverted epifluorescence microscope. Neutravidin was used to immobilize a TCO-PEG4-biotin reagent as follows: wells were pre-coated with fibronectin and biotinylated-BSA using a solution containing 20 μg/mL fibronectin and 25 μg/ml biotinylated-BSA (Sigma-Aldrich, A8549) diluted in PBS. Coating with the solution proceeded for 1 h at 37 °C before removing the solution and rinsing three times with PBS. NeutrAvidin (NA, ThermoFisher) was then immobilized on the coated surfaces via treatment with a 100 μg/mL NA solution in PBS. NA binding proceeded for 30 minutes at room temperature before removal and rinsing three times with PBS. TCO-PEG4-biotin was then immobilized on the NA-containing surfaces by treatment with a solution containing 200 nM TCO-PEG4-biotin in PBS for 20 minutes at room temperature before removal and rinsing three times with PBS. Stable clones of receptor-expressing HEK293-FT(UAS:H2B-mCherry) and U2OS(UAS:dsRed2) were added to the wells and allowed to adhere for ~4 h. Following cell attachment, Tz-5-fluorescein was diluted into the cell and media-containing wells using 2x concentrations solutions to achieve the final concentrations indicated in the captions. Negative (TCO-lacking) control wells were prepared as described above but omitting TCO-PEG4-biotin. Positive control wells were prepared by treatment of NA-bound wells with 200 nM biotin-FITC (in place of TCO-PEG4-biotin and tetrazine-5-FAM).
Uncaging of BSA-PC-5-fluorescein in the presence of live cells
A step-by-step protocol describing the preparation of BSA-PC-5-fluorescein is provided in the Supplementary Information. Microscope-based pulse illuminations were carried out to facilitate ligand photo-uncaging in the presence of live cells. Coverslip glass surfaces were coated with BSA-PC-5-fluorescein and fibronectin before adding cells. Cells were incubated for at least 6 h (or overnight) to permit attachment to the caged ligand- and fibronectin-coated surfaces. For Supplementary Fig. 7b (top row of images), wells were illuminated for 1 second through a 20X/0.8-NA air objective lens (Plan-Apochromat 20x/0.8 WD = 0.55 M27). Samples were illuminated through Zeiss Filter Set 49 (DAPI, EX G365 S free, G365/FT395/ BP445/50) using an illumination source based on a 12 V/100 W/GY6.35 halogen bulb housed in an Illuminator HXP 120 V (Zeiss #423013-9010-000) under 100% lamp power settings. For Supplementary Movie 3, the sample was exposed to a 100 ms pulse illumination through a 63x/1.4-NA oil immersion objective lens (Plan-Apochromat 63x/1.40 Oil DIC M27), followed by a live recording of the illuminated area through a 40x oil immersion objective lens.
Tests of BSA-PC-5-fluorescein stability in the presence of live cells
A HEK293-FT (UAS:H2B-mCherry) reporter cell clone stably expressing αFITC(E2)-SynNotch-Gal4-VP64-mTurq2 was utilized in these analyses. Cells were 8-well imaging chambers for 6-8 days before analysis. Individual wells were coated with (1) fibronectin and BSA, (2) fibronectin and BSA-5-FITC (Sigma-Aldrich, A9771), or (3) fibronectin and BSA-PC-5-fluorescein. BSA and BSA-5-FITC were added to coating solutions at 5 μg/ml; BSA-PC-5-fluorescein was added at 25 μg/ml. Coating proceeded for 1.5 h at 37 °C before the solutions were aspirated and wells rinsed thrice with PBS. Cells in growth media were added to each well at ~7000 cells per well. BSA-PC-5-fluorescein was left caged (without exposure to uncaging light), and cells were incubated for 6 days before analysis. After 6 days, reporter expression levels were recorded using fluorescence microscopy. A similar procedure was used to determine the photo-activatability of BSA-PC-5-fluorescein after growth with cells. Briefly, cells were treated similarly as described, with uncaging on day 7 via illumination through a DAPI excitation filter using a 20X objective lens (see above).
Preparation, cell seeding, and analysis of cells grown on patterned ligand surfaces
A printed photomask was attached to the bottom of the imaging dish. A small volume of mineral oil was applied to the outer bottom of the imaging dishes before the printed photomask was applied. BSA-PC-5-Fluorescein was then photo-uncaged for 2 minutes by direct placement atop a handheld UV lamp (365 nm, 6 W, Research Products International, item # 950006-02). The wells were rinsed two times with PBS following the uncaging step. Cells were applied to the wells in media containing 10 μM N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT). For 8-well imaging dishes, ~100,000 U2OS cells were applied per well. DAPT was removed the next day by gentle aspiration of DAPT-containing media followed by gentle rinsing thrice with DAPT-free media. Cells were returned to the incubator in fresh media and imagined the next day. Imaging to detect co-expressed T2A-BFP was done under live cell conditions. For nuclear counterstaining, imaging was performed in media containing Hoechst-JF646 at 500 nM and containing verapamil at 10 μM; both the stain and verapamil were added to pre-warmed imaging media and mixed vigorously to ensure full dissolution before cell application. After 15-30 minutes of staining, cells were fixed with 4% paraformaldehyde (PFA, w/v, diluted into PBS from a 16% stock). Fixation proceeded for 15 minutes at 37 °C, after which the PFA was removed. Cells were rinsed once with culture media (to quench trace PFA) and again with PBS. When visualizing patterned cells stained with Hoechst 33342, cells were imaged under fixed conditions using exposure times that prevented detection and crosstalk from BFP (the fluorescence of which is diminished upon fixation with PFA).
Expression, purification, and dye-conjugation of HaloTag-CNA35
Starter cultures of BL21-DE3 E. coli cells (ThermoFisher) harboring pET28a-Halotag-CNA35 (AddGene #131128) were grown in 2xYT media containing 35 μg/ml kanamycin. Cultures were grown overnight with incubation at 37 °C and shaking (2 x 5 mL cultures). The next day, ~10 mL of the overnight cultures were used to inoculate fresh 500 mL of 2xYT containing 35 μg/ml kanamycin. Cells were grown at 37 °C with shaking (250 rpm) until a culture density of OD600 = ~ 0.5 was reached, at which point the incubation temperature was adjusted to 27 °C. Protein expression was induced after 15 minutes of growth at 27 °C by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to the culture medium to a final concentration of 0.5 mM. Expression proceeded at 27 °C, shaking (250 rpm) for ~8 h. Cells were harvested by centrifugation in 50 mL tubes. Cell pellets were stored at -80 °C until use. For purification, 2 x 50 mL aliquots were processed using one column from the Ni-NTA Fast Start Kit (Qiagen, 30600). The purity of the protein fractions was assessed by Coomassie staining (GelCode Blue Stain Reagent, Pierce), and the purest fractions were utilized in conjugation reactions with a HaloTag-reactive OG488.
A chloroalkane (CA)-OG488-biotin was prepared by reacting Halo-DBCO (Iris Biotech, RL-3670.0100) and OG488-biotin-azide as follows: a 20 mM stock solution of Halo-DBCO was prepared by dissolving 2.6 mg of dry material in 254 μL of DMSO. An aliquot of this solution was diluted to 2 mM in DMSO, and the remainder of the 20 mM stock was stored at -80 °C with labeling of the date of preparation. The 2 mM Halo-DBCO solution was then reacted with a 2 mM solution of OG488-biotin-azide, also in DMSO. The reaction was initiated by combining 40 μL of Halo-DBCO with 50 μL of OG488-biotin-azide; the excess OG488-biotin-azide was used to ensure complete derivatization of Halo-DBCO. The solution was incubated at room temperature for 3 h before use. For protein conjugation, the reaction mixture was diluted 1:250 by volume into aliquots of purified HaloTag-CNA35 in the Ni-NTA Fast Start Kit elution buffer. The solution was mixed by pipetting, and the reaction proceeded at 4 °C for 3 h before the unligated components were removed by dialysis against PBS using a 10 kDa MWCO dialysis cassette (66380, ThermoFisher). The dialyzed conjugate was then concentrated and exchanged into 50 mM Tris buffer (pH = 8.0) by repeated centrifugation and dilution of the retentate using Amicon Ultra Centrifugal Filters containing a 10 kDa MWCO membrane (Sigma-Aldrich). The conjugate concentration was determined via extinction coefficient (OG488, ϵ = 76,000 cm-1M-1 at 495 nm). Solutions of the conjugated protein (containing 12 μM of product) were then flash frozen by immersion in liquid nitrogen and stored at -20 °C until use. The purity of the isolated protein and confirmation of its successful dye-conjugation were confirmed via SDS-PAGE. Lanes containing dye-conjugated and unconjugated (control) protein samples were compared by in-gel fluorescence detection. The gel was fixed in a fixing solution (50% methanol, 40% water, 10% glacial acetic acid) and rinsed with water before total protein gel fluorescence was detected using an iBright instrument (ThermoFisher). The gel was then stained for total protein using the Coomassie-based GelCode Blue Stain Reagent (Pierce).
Collagen adsorption, OG488-CNA-binding, and cell stimulation
A non-tissue culture (non-TC)-treated 96-well plate (Celltreat, 229596) was coated with native collagen-I using a 50 μg/mL solution diluted from a 4.04 mg/mL solution provided by the supplier. Coating proceeded for 1 h at 20 C, after which the collagen solution was gently removed, and wells were rinsed 3 times using PBS. The coated wells were then passivated using a 1% solution of BSA (w/v) in PBS; passivation proceeded for 1 h at room temperature before gently aspirating the BSA solution and rinsing three times PBS. The collagen-coated and BSA-passivated wells were then treated with varying concentrations of CNA-OG488 (as indicated in the figures and captions); CNA binding proceeded for 1 h at room temperature before gentle aspiration of the CNA-containing solution and rinsing 3 times with PBS. Cells suspended in pre-warmed (37 °C) growth media were then added to the treated wells at ~60,000 cells per well. Flow cytometry measurement of reporter activities was carried out 24 h after cell plating. Control wells coated with either fibronectin or gelatin were prepared using coating solutions of 10 μg/mL in PBS (for fibronectin) and 50 μg/mL gelatin in PBS. Fibronectin and gelatin-coated wells were also treated with OG488-CNA as described above, and cells grown in these wells were analyzed as CNA non-binding controls.
Collagen denaturation, CHP-5-fluorescein binding, and cell stimulation
A non-tissue culture (non-TC)-treated 96-well plate (Celltreat, 229596) was coated with a 50 μg/mL solution of native collagen-I (Corning) followed by washing with PBS; a protocol similar to that which is described above was used. To produce wells containing denatured collagen, microwells containing adhered native collagen were subjected to heat denaturation by rinsing wells with sterile double-distilled water (ddH2O) pre-headed to ~90 °C. Three rinses with boiling hot ddH2O were carried out to denature plated collagen immediately before treatment with CHP-5-fluorescein. To produce control wells containing native collagen, wells were treated in a similar manner but rinsed using room-temperature sterile ddH2O (in place of hot ddH2O). The denatured and control wells were then treated with CHP-5-fluorescein. A stock solution containing 50 μM CHP-5-fluorescein in PBS was prepared according to the manufacturer’s protocol; aliquots from this stock were further diluted to PBS to generate binding solutions at the working concentrations indicated in the figures and captions. For CHP-5-fluorescein binding, peptide working solutions were applied to native and denatured collagen-containing wells at a volume of 50 μL per well. Wells were incubated with the solutions for 3 h at room temperature before gently removing the peptide-containing solutions and washing wells 3 times using room temperature PBS. Cells suspended in pre-warmed growth media were then added to the treated wells at ~60,000 cells per well. Flow cytometry measurement of reporter levels was carried out 24 h after cell plating.
Myogenic differentiation of C3H/10 T½ cells
A clonal cell line containing an integrated TRE:p65-MyoD-T2A-DsRed construct was utilized. Generation and isolation of the clone have been previously described (Sloas et al. 2023)49. The DNA construct used in generating the line has also been described (Kabadi et al., 2015; AddGene #60627)65. A control line containing an inducible TRE:DsRed2 reporter (without p65-MyoD) was also generated (Kabadi et al., 2015; AddGene #60623)65. For experiments using C3H/10 T½ reporter lines, cells were transduced with lentiviral constructs based on pHR-SFFV- αFITC(E2)-SynNotch-TetR-VP48. Fusion indices were measured using ViaFuse86.
Experiments using transduced C3H/10 T½ cells were typically initiated at 48–72 h post-transduction as follows: for surface detection of αFITC(E2)-SynNotch expression in transduced C3H/10 T½ cells, cells were plated in fibronectin-coated 8 well coverslip bottom imaging dishes. Antibody staining under live cell conditions was carried out the next day using mouse anti-myc-AlexaFluor647 antibody diluted into tetracycline-free culture media (1:200). Staining was carried for 30 minutes at 37 °C. Cells were washed with pre-warmed culture media (3 x 200 μL washes) and counterstained with Hoechst 33342 in media for 30 minutes at 37 °C. Imaging was done under live cell conditions in PBS. Non-transduced C3H/10 T½ cells were stained and imaged as a labeling control.
For bio-orthogonal Tz/TCO-ligation-induced differentiation, 18 well glass bottom imaging dishes were treated with a PBS solution containing 20 μg/ml fibronectin and 50 μg/mL TCO-BSA for 3 h at 37 °C. Wells were rinsed three times with PBS before adding cells to a density of ~30,000 C3H/10 T½ cells per well. After 2 h, wells were treated with Tz-5-fluorescein by adding dye-containing media solution at 2X concentrations to achieve the final Tz-5-fluorescein indicated in the figures and captions. For anti-troponin-T western blot, cells were transduced with viral particles based on the construct with an mTurq2 coexpression marker (pHR-αFITC(E2)-SynNotch-TetR-VP48-P2A-T2A-mTurq2) and treated with 100 nM of Fluorescein-PEG5-methyltetrazine (BroadPharm).
For patterned differentiation, 8 well glass-bottom imaging dishes were coated with BSA-PC-5-Fluorescein and fibronectin, as described above. Photo-uncaging was carried out for 2 minutes, and wells were rinsed with PBS before adding cells. Transduced C3H/10 T½ cells were trypsinized using pre-warmed 0.25% trypsin solution (without EDTA) and quenched with media before collection by centrifugation (5 minutes at 300 x g). Cells were resuspended in fresh media before seeding into the photo-patterned 8-well dishes. For C3H/10 T½, ~65,000 cells were seeded per well of patterned 8-well dishes. Cells were analyzed at 2–3 days after seeding. See below regarding the immunofluorescence detection of myosin heavy chain expression in differentiated cells.
To verify myosin heavy chain (MHC) expression, cells were fixed using 4% paraformaldehyde (PFA, w/v, diluted into PBS from a 16% stock). Fixation proceeded for 15 minutes at 37 °C, after which the PFA was removed. Cells were rinsed once with culture media (to quench trace PFA) and again with PBS. Cells were permeabilized with a PBS solution containing 0.1% Triton X-100 (v/v) (10 minutes at room temperature), rinsed with PBS, and subsequently blocked using Immunofluorescence Blocking Buffer (Cell Signaling Technologies) for 60 minutes at room temperature. Antibody staining was done with mouse anti-myosin heavy chain antibody at a 1:100 dilution in antibody dilution buffer (PBS containing 0.1% Tween-20, v/v, and 1% BSA, w/v; sterile filtered before use). Staining with the primary antibody proceeded overnight at 4 °C. The primary antibody solution was removed the next day and cells were washed using PBS-T thrice for 10 minutes each. Cells were then labeled with a rabbit anti-mouse AlexaFluor647-conjugate using a 1:1000 dilution in antibody dilution buffer for 1 h at room temperature. The unbound secondary antibody was removed by washing with PBS-T three times for ten minutes each. Cells were counterstained with Hoechst 33342 before imaging in PBS.
For CHP-5-fluorescein-induced myogenic conversion of C3H/10 T½ fibroblast cells, 8 well glass-bottom imaging dishes were coated with 50 μg/ml of collagen-I as described above and rinsed thrice with PBS. Heat-denaturion of the bound collagen was carried out as described above. A solution containing 2 μM CHP-5-fluorescein was applied to the collagen-containing wells, and unbound units were removed by rinsing (both as described above). C3H/10 T½ TRE:p65-MyoD-2A-DsRed2 cells were seeded into the treated wells at ~65,000 cells per well.
Statistics & reproducibility
Individual data points in graphs represent distinct samples. Statistics were calculated in Prism, using one- or two-way ANOVA between groups and Tukey’s multiple comparisons tests. *p < 0.05, **p = <0.01, ***p = <0.001, ****p = <0.0001 with 95% confidence interval. Data and P values can be found in Source Data file. The fluorescence micrographs and immunoblots results shown are representative examples of results that were repeated during at least two independent experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Protein structure coordinates were accessed from the Protein Data Bank: aFITC(E2) (PDB: 2A9N) 4M5.3 (PDB: 1X9Q), and FluA (PDB: 1N0S). The data supporting the findings of this work are available within the paper and the Supplementary Information files. Source data are provided with this paper.
References
Bray, S. J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 17, 722–735 (2016).
Sprinzak, D. & Blacklow, S. C. Biophysics of Notch Signaling. Annu. Rev. Biophys. 50, 157–189 (2021).
Kanchanawong, P. & Calderwood, D. A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat. Rev. Mol. Cell Biol. 24, 142–161 (2022).
Gonzalez-Fernandez, T., Sikorski, P. & Leach, J. K. Bio-instructive materials for musculoskeletal regeneration. Acta Biomater. 96, 20–34 (2019).
Wang, H. & Mooney, D. J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 17, 761–772 (2018).
Agarwalla, P. et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat. Biotechnol. 40, 1250–1258 (2022).
Bressler, E. M. et al. Boolean logic in synthetic biology and biomaterials: towards living materials in mammalian cell therapeutics. Clin. Transl. Med. 13, e1244 (2023).
Grosskopf, A. K. et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 8, eabn8264 (2022).
Adu-Berchie, K. et al. Adoptive T cell transfer and host antigen-presenting cell recruitment with cryogel scaffolds promotes long-term protection against solid tumors. Nat. Commun. 14, 3546 (2023).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 164, 780–791 (2016).
Zhu, I. et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell 185, 1431–1443.e16 (2022).
Zhang, S. et al. Monitoring of cell-cell communication and contact history in mammals. Science 378, eabo5503 (2022).
Nakandakari-Higa, S. et al. Universal recording of cell-cell contacts in vivo for interaction-based transcriptomics. bioRxiv https://doi.org/10.1101/2023.03.16.533003 (2023).
Malaguti, M., Lebek, T., Blin, G. & Lowell, S. Enabling neighbour labelling: using synthetic biology to explore how cells influence their neighbours. Development 151, dev201955 (2024).
Roybal, K. T. et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167, 419–432.e16 (2016).
Gordon, W. R. et al. Mechanical allostery: evidence for a force requirement in the proteolytic activation of notch. Dev. Cell 33, 729–736 (2015).
Wang, X. & Ha, T. Defining single molecular forces required to activate integrin and notch signaling. Science 340, 991–994 (2013).
Langridge, P. D. & Struhl, G. Epsin-dependent ligand endocytosis activates notch by force. Cell 171, 1383–1396.e12 (2017).
Varnum-Finney, B. et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 113 Pt 23, 4313–4318 (2000).
Parks, A. L., Klueg, K. M., Stout, J. R. & Muskavitch, M. A. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127, 1373–1385 (2000).
Conboy, I. M., Conboy, M. J., Smythe, G. M. & Rando, T. A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Gonzalez-Perez, D. et al. Affinity-matured DLL4 ligands as broad-spectrum modulators of Notch signaling. Nat. Chem. Biol. 19, 9–17 (2023).
Shukla, S. et al. Progenitor T-cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1. Nat. Methods 14, 531–538 (2017).
Batalov, I., Stevens, K. R. & DeForest, C. A. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc. Natl Acad. Sci. Usa. 118, e2014194118 (2021).
Xu, C. et al. Induction of osteogenesis by bone-targeted Notch activation. Elife 11, e60183 (2022).
Walton, B. L. et al. A programmable arthritis-specific receptor for guided articular cartilage regenerative medicine. bioRxiv https://doi.org/10.1101/2024.01.31.578281 (2024).
Lee, J. C. et al. Instructional materials that control cellular activity through synthetic Notch receptors. Biomaterials 297, 122099 (2023).
Garibyan, M. et al. Engineering programmable material-to-cell pathways via synthetic notch receptors to spatially control differentiation in multicellular constructs. Nat. Commun. 15, 5891 (2024).
Ruffo, E. et al. Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting. Nat. Commun. 14, 2463 (2023).
Tamada, K. et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin. Cancer Res. 18, 6436–6445 (2012).
Kim, M. S. et al. Redirection of genetically engineered CAR-T cells using bifunctional small molecules. J. Am. Chem. Soc. 137, 2832–2835 (2015).
Lee, Y. G. et al. Regulation of CAR T cell-mediated cytokine release syndrome-like toxicity using low molecular weight adapters. Nat. Commun. 10, 2681 (2019).
Ma, J. S. Y. et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc. Natl Acad. Sci. Usa. 113, E450–E458 (2016).
Honegger, A., Spinelli, S., Cambillau, C. & Plückthun, A. A mutation designed to alter crystal packing permits structural analysis of a tight-binding fluorescein-scFv complex. Protein Sci. 14, 2537–2549 (2005).
Vaughan, T. J. et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat. Biotechnol. 14, 309–314 (1996).
Boder, E. T., Midelfort, K. S. & Wittrup, K. D. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proc. Natl Acad. Sci. Usa. 97, 10701–10705 (2000).
Midelfort, K. S. et al. Substantial energetic improvement with minimal structural perturbation in a high affinity mutant antibody. J. Mol. Biol. 343, 685–701 (2004).
Beste, G., Schmidt, F. S., Stibora, T. & Skerra, A. Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. Proc. Natl Acad. Sci. Usa. 96, 1898–1903 (1999).
Korndörfer, I. P., Beste, G. & Skerra, A. Crystallographic analysis of an “anticalin’‘ with tailored specificity for fluorescein reveals high structural plasticity of the lipocalin loop region. Proteins 53, 121–129 (2003).
Vopel, S., Mühlbach, H. & Skerra, A. Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. Biol. Chem. 386, 1097–1104 (2005).
Gordon, W. R. et al. Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One 4, e6613 (2009).
Logeat, F. et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl Acad. Sci. Usa. 95, 8108–8112 (1998).
Koerber, J. T., Hornsby, M. J. & Wells, J. A. An improved single-chain Fab platform for efficient display and recombinant expression. J. Mol. Biol. 427, 576–586 (2015).
Choi, H. M. T. et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Ilagan, M. X. G., Lim, S., Fulbright, M., Piwnica-Worms, D. & Kopan, R. Real-time imaging of notch activation with a luciferase complementation-based reporter. Sci. Signal. 4, rs7 (2011).
Martin, A. P. et al. A spatiotemporal Notch interaction map from plasma membrane to nucleus. Sci. Signal. 16, eadg6474 (2023).
Tveriakhina, L. et al. Temporal dynamics and stoichiometry in human Notch signaling from Notch synaptic complex formation to nuclear entry of the Notch intracellular ___domain. Dev. Cell 59, 1425–1438.e8 (2024).
Sun, W.-C., Gee, K. R., Klaubert, D. H. & Haugland, R. P. Synthesis of Fluorinated Fluoresceins. J. Org. Chem. 62, 6469–6475 (1997).
Sloas, D. C., Tran, J. C., Marzilli, A. M. & Ngo, J. T. Tension-tuned receptors for synthetic mechanotransduction and intercellular force detection. Nat. Biotechnol. 41, 1287–1295 (2023).
Yang, Z.-J., Yu, Z.-Y., Cai, Y.-M., Du, R.-R. & Cai, L. Engineering of an enhanced synthetic Notch receptor by reducing ligand-independent activation. Commun. Biol. 3, 116 (2020).
Wolfe, M. S. Structure and function of the γ-Secretase Complex. Biochemistry 58, 2953–2966 (2019).
Devaraj, N. K., Upadhyay, R., Haun, J. B., Hilderbrand, S. A. & Weissleder, R. Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angew. Chem. Int. Ed. Engl. 48, 7013–7016 (2009).
Devaraj, N. K. & Weissleder, R. Biomedical applications of tetrazine cycloadditions. Acc. Chem. Res. 44, 816–827 (2011).
Karver, M. R., Weissleder, R. & Hilderbrand, S. A. Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation. Bioconjug. Chem. 22, 2263–2270 (2011).
Krafft, G. A., Sutton, W. R. & Cummings, R. T. Photoactivable fluorophores. 3. Synthesis and photoactivation of fluorogenic difunctionalized fluoresceins. J. Am. Chem. Soc. 110, 301–303 (1988).
Foster, T. J., Geoghegan, J. A., Ganesh, V. K. & Höök, M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12, 49–62 (2014).
Aper, S. J. A. et al. Colorful protein-based fluorescent probes for collagen imaging. PLoS One 9, e114983 (2014).
Zong, Y. et al. A ‘Collagen Hug’ model for Staphylococcus aureus CNA binding to collagen. EMBO J. 24, 4224–4236 (2005).
Li, X., Zhang, Q., Yu, S. M. & Li, Y. The chemistry and biology of collagen hybridization. J. Am. Chem. Soc. 145, 10901–10916 (2023).
Li, Y. et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl Acad. Sci. Usa. 109, 14767–14772 (2012).
Liu, L. et al. Molecular imaging of collagen destruction of the spine. ACS Nano 15, 19138–19149 (2021).
Bennink, L. L. et al. Visualizing collagen proteolysis by peptide hybridization: From 3D cell culture to in vivo imaging. Biomaterials 183, 67–76 (2018).
Arlotta, K. J., San, B. H., Mu, H.-H., Yu, S. M. & Owen, S. C. Localization of therapeutic Fab-CHP conjugates to sites of denatured collagen for the treatment of rheumatoid arthritis. Bioconjug. Chem. 31, 1960–1970 (2020).
Chattopadhyay, S., Teixeira, L. B. C., Kiessling, L. L., McAnulty, J. F. & Raines, R. T. Bifunctional peptide that anneals to damaged collagen and clusters tgf-β receptors enhances wound healing. ACS Chem. Biol. 17, 314–321 (2022).
Kabadi, A. M. et al. Enhanced MyoD-induced transdifferentiation to a myogenic lineage by fusion to a potent transactivation ___domain. ACS Synth. Biol. 4, 689–699 (2015).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Saraswathibhatla, A., Indana, D. & Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 24, 495–516 (2023).
Atcha, H., Choi, Y. S., Chaudhuri, O. & Engler, A. J. Getting physical: material mechanics is an intrinsic cell cue. Cell Stem Cell 30, 750–765 (2023).
Hwang, J. et al. In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano 11, 9825–9835 (2017).
Zitnay, J. L. et al. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat. Commun. 8, 14913 (2017).
Barascuk, N. et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin. Biochem. 43, 899–904 (2010).
Jailkhani, N. et al. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl Acad. Sci. Usa. 116, 14181–14190 (2019).
Adams, S. R. & Tsien, R. Y. Controlling cell chemistry with caged compounds. Annu. Rev. Physiol. 55, 755–784 (1993).
Weinstain, R., Slanina, T., Kand, D. & Klán, P. Visible-to-NIR-light activated release: From small molecules to nanomaterials. Chem. Rev. 120, 13135–13272 (2020).
Zorlutuna, P. et al. Microfabricated biomaterials for engineering 3D tissues. Adv. Mater. 24, 1782–1804 (2012).
Park, J. H., Jang, J., Lee, J.-S. & Cho, D.-W. Three-dimensional printing of tissue/organ analogues containing living cells. Ann. Biomed. Eng. 45, 180–194 (2017).
Ma, T. et al. Transdifferentiation of fibroblasts into muscle cells to constitute cultured meat with tunable intramuscular fat deposition. Elife 13, RP93220 (2024).
Dickinson, B. C., Huynh, C. & Chang, C. J. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 132, 5906–5915 (2010).
Breeuwer, P. et al. Characterization of uptake and hydrolysis of fluorescein diacetate and carboxyfluorescein diacetate by intracellular esterases in Saccharomyces cerevisiae, which result in accumulation of fluorescent product. Appl. Environ. Microbiol. 61, 1614–1619 (1995).
Hill, H. D., Summer, G. K. & Waters, M. D. An automated fluorometric assay for alkaline phosphatase using 3-O-methylfluorescein phosphate. Anal. Biochem. 24, 9–17 (1968).
Kramer, D. N. & Guilbault, G. G. A substrate for the fluorometric determination of lipase activity. Anal. Chem. 35, 588–589 (1963).
Plovins, A., Alvarez, A. M., Ibañez, M., Molina, M. & Nombela, C. Use of fluorescein-di-beta-D-galactopyranoside (FDG) and C12-FDG as substrates for beta-galactosidase detection by flow cytometry in animal, bacterial, and yeast cells. Appl. Environ. Microbiol. 60, 4638–4641 (1994).
Grimm, J. B., Gruber, T. D., Ortiz, G., Brown, T. A. & Lavis, L. D. Virginia orange: a versatile, red-shifted fluorescein scaffold for single- and dual-input fluorogenic probes. Bioconjug. Chem. 27, 474–480 (2016).
Rand, M. D. et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell. Biol. 20, 1825–1835 (2000).
McMahan, J. B. & J. T. Ngo. A genetically encodable and chemically disruptable system for synthetic post-translational modification dependent signaling. bioRxiv. https://doi.org/10.1101/2022.05.29.493928 (2022).
Hinkle, E. R. et al. ViaFuse: Fiji macros to calculate skeletal muscle cell viability and fusion index. Skelet. Muscle 11, 28 (2021).
Ngo, J. Figures. Created in BioRender. https://BioRender.com/q75j103 (2025).
Acknowledgements
This work was funded through NIH research grants R35GM128859 (to J.T.N.) and R01HL147585 (to C.S.C. and J.T.N.). J.C.T and R.E.M. were supported through a Cross-Disciplinary Fellowship from BUnano (Boston University Nanotechnology Innovation Center). J.C.T., Z.E.S., and D.C.S. received support through the Boston University training program in Quantitative Biology and Physiology (QBP, NIH grant T32GM008764). D.C.S. and A.M.M. were recipients of National Science Foundation Graduate Research Fellowships. Z.E.S. received support from the National Science Foundation Research Traineeship (NRT) in Biological Feedback Control (NSF-DGE 2244366). Additional support for the work was provided through the Center for Multiscale & Translational Mechanobiology (CMTM, Boston University) and the Multicellular Design Program (MDP, Boston University). Some graphic elements shown were created with Biorender.com.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of experiments and discussed results. J.C.T., C.J.K, A.M.M, R.E.M., Z.E.S., J.B.M., D.C.S., and J.T.N. performed experiments and analyzed data. J.C.T and J.T.N wrote the manuscript with feedback from all the authors. J.T.N. and C.S.C. provided funding support. J.C.T., C.J.K, A.M.M., C.S.C., and J.T.N. edited the manuscript. J.T.N oversaw the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tran, J.C., Kuffner, C.J., Marzilli, A.M. et al. Fluorescein-based SynNotch adaptors for regulating gene expression responses to diverse extracellular and matrix-based cues. Nat Commun 16, 852 (2025). https://doi.org/10.1038/s41467-025-56148-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56148-7