Abstract
The risk of cardiovascular outcomes following SARS-CoV-2 infection has been reported in adults, but evidence in children and adolescents is limited. This paper assessed the risk of a multitude of cardiac signs, symptoms, and conditions 28-179 days after infection, with outcomes stratified by the presence of congenital heart defects (CHDs), using electronic health records (EHR) data from 19 children’s hospitals and health institutions from the United States within the RECOVER consortium between March 2020 and September 2023. The cohort included 297,920 SARS-CoV-2-positive individuals and 915,402 SARS-CoV-2-negative controls. Every individual had at least a six-month follow-up after cohort entry. Here we show that children and adolescents with prior SARS-CoV-2 infection are at a statistically significant increased risk of various cardiovascular outcomes, including hypertension, ventricular arrhythmias, myocarditis, heart failure, cardiomyopathy, cardiac arrest, thromboembolism, chest pain, and palpitations, compared to uninfected controls. These findings were consistent among patients with and without CHDs. Awareness of the heightened risk of cardiovascular disorders after SARS-CoV-2 infection can lead to timely referrals, diagnostic evaluations, and management to mitigate long-term cardiovascular complications in children and adolescents.
Similar content being viewed by others
Introduction
Recent research has highlighted post-acute sequelae of SARS-CoV-2 infection (PASC), a term encompassing post-acute or long-term health issues following SARS-CoV-2 infection. The World Health Organization (WHO) defines post-COVID-19 conditions (PCC) as symptoms occurring 3 months from the onset of infection and lasting at least 2 months, not attributable to alternative diagnoses1,2. In contrast, the National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and Royal College of General Practitioners (RCGP) differentiate between ‘ongoing symptomatic COVID-19’ (4 to 12 weeks) and ‘post-COVID-19 syndrome’ (beyond 12 weeks)3. In the United States, the National Institutes of Health (NIH)4 and the Centers for Disease Control and Prevention (CDC)5 define PASC as new, returning, or ongoing health problems present at least four weeks after infection. Additionally, the EpiCore network and other international organizations have developed diverse definitions tailored to their healthcare settings6. Despite slight variations in definitions across organizations, PASC is recognized as a complex condition affecting multiple organ systems, including the cardiovascular system7,8.
While studies have reported increased long-term cardiovascular risks in adults following SARS-CoV-2 infection9,10,11,12,13,14,15,16, the incidence of PASC-related cardiovascular outcomes in children and adolescents is less clear. Pediatric studies have primarily focused on general cardiorespiratory signs and symptoms8,17, myocarditis, pericarditis, and multisystem inflammatory syndrome in children associated with COVID-19 (MIS-C)18,19,20,21, often with short follow-up periods. In adults, those with pre-existing cardiovascular risk factors or established cardiovascular disease face a higher risk of severe COVID-19 and cardiovascular PASC22,23. In children and adolescents, congenital heart disease (CHD) constitutes the most common congenital disorder24, but its effect on the risk of developing cardiovascular PASC has not been reported.
Our study utilizes data from the Researching COVID to Enhance Recovery (RECOVER) electronic health records (EHR) system to conduct a thorough evaluation of cardiovascular signs, symptoms, and conditions in children and adolescents with and without CHDs, to understand the post-acute cardiovascular impacts 28 to 179 days after SARS-CoV-2 infection. In our study, we followed a definition commonly used in pediatric literature8,25,26,27, focusing on features occurring between 28 and 179 days after the index date. This timeframe aligns with the post-acute phase while capturing meaningful cardiovascular outcomes in children and adolescents.
Results
Cohort identification
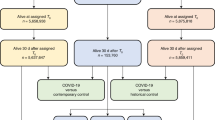
We conducted a retrospective study from March 1, 2020, to September 1, 2023, to evaluate the risk of cardiovascular outcomes in the pediatric population during a standardized follow-up period of 28 to 179 days after SARS-CoV-2 infection. The study included patients under 21 with documented interactions within the healthcare system with certain institutions, excluding those with diagnoses of MIS-C, Kawasaki disease, chronic kidney disease (CKD), or end-stage kidney disease (ESKD). A detailed summary of the participant selection for both SARS-CoV-2 positive and negative groups is shown in Fig. 1.
Flowchart illustrating the selection process for SARS-CoV-2-positive and SARS-CoV-2-negative individuals from March 2020 to March 2023, stratified by CHD status. All individuals identified by this process were included after propensity score stratification and therefore included in the analyses. Abbreviation: PASC post-acute sequelae of SARS-CoV-2 infection, PCR polymerase chain reaction, MIS-C multisystem inflammatory syndrome in children, CKD chronic kidney disease, ESKD end-stage kidney disease, CHD congenital heart defects.
Within the RECOVER network, we identified a total of 297,920 SARS-CoV-2-positive individuals (24.6%; 13,646 with CHDs, 284,274 without) and 915,402 SARS-CoV-2-negative controls (75.4%; 46,962 with CHDs, 868,440 without). Among these patients, 623,806 (51.4%) were male with a mean (SD) age of 7.75 (6.11) years.
The baseline characteristics stratified by SARS-CoV-2 infection and CHD status are detailed in Table 1. Among the SARS-CoV-2-positive cohort, there were 124,301 Non-Hispanic White (NHW) patients (41.7%), 53,446 Non-Hispanic Black (NHB) patients (17.9%), 74,031 Hispanic patients (24.8%), 14,159 Asian American or Pacific Islander (AAPI) patients (4.8%), and others classified as multiple or unknown. In the SARS-CoV-2-negative cohort, 418,445 patients were NHW (45.7%), 154,870 were NHB (16.9%), 196,687 were Hispanic (21.5%), 43,783 were AAPI (4.8%), and the remainder were categorized as multiple or unknown.
The majority of patients entered the cohort between late 2021 and early 2022. Patients with CHD tended to be younger, more often males, had a higher Pediatric Medical Complexity Algorithm (PMCA)28 index indicative of complex chronic condition, were more likely to undergo COVID-19 testing, and were less likely to be vaccinated compared to those without CHD in the database.
Statistical methods
We assessed the incidence of post-acute cardiovascular outcomes in both SARS-CoV-2-positive and negative cohorts. To account for a large number of measured confounding variables collected prior to cohort entry, we employed propensity score stratification29,30,31. To estimate the relative risk (RR) for each outcome comparing the SARS-CoV-2-positive and negative groups, we used a stratified modified Poisson regression model for binary outcomes. All analyses were further stratified based on the presence or absence of CHD to provide subgroup-specific insights.
Incidence of post-acute cardiovascular events
Table 2 presents the incidence of 18 individual and 6 composite post-acute cardiovascular outcomes for SARS-CoV-2-positive compared to SARS-CoV-2-negative patients, stratified by CHD status. The data indicate that SARS-CoV-2-positive patients generally have higher incidences in both CHD and non-CHD groups. For example, the absolute risk of heart failure was 1.61% in SARS-CoV-2-positive patients with CHD, compared to 1.18% in their SARS-CoV-2-negative counterparts. Similarly, chest pain incidence was 1.23% in SARS-CoV-2-positive patients without CHD, versus 0.61% in SARS-CoV-2-negative patients. Overall, the CHD group showed higher absolute risks of any post-acute cardiovascular outcomes than the non-CHD group, with 5.57% for positive and 4.00% for negative patients with CHD, compared to 2.19% and 1.26% respectively in those without CHD.
Adjusted relative risk of post-acute cardiovascular outcomes
The SARS-CoV-2 positive and negative patients achieved empirical equipoise (Figure S2)32,33, implying the majority of individuals in both the treatment (SARS-CoV-2-positive) and control (SARS-CoV-2-negative) groups have similar probabilities of belonging to either group based on their observed characteristics. The two groups have well-balanced characteristics after propensity score stratification (Figure S3). Figure 2 highlights that post-acute cardiovascular outcomes are higher in both CHD and non-CHD groups among patients post-infection compared to negative ones.
Forest plot presenting relative risk (RR) of post-acute cardiovascular outcomes comparing SARS-CoV-2-positive cohort (n = 297,920 patients) with SARS-CoV-2-negative cohort (n = 915,402 patients), stratified by congenital heart defects (CHD) status. Composite outcomes consisted of arrhythmias (atrial fibrillation, ventricular arrhythmias, atrial flutter, and premature atrial or ventricular contractions), inflammatory heart disease (pericarditis and myocarditis), other cardiac disorders (heart failure, cardiomyopathy, cardiac arrest, and cardiogenic shock), thrombotic disorders (pulmonary embolism, deep vein thrombosis, thrombophlebitis, and thromboembolism), cardiovascular-related symptoms (chest pain, palpitations, and syncope), and any cardiovascular outcome (incident occurrence of any cardiovascular outcome studied). The error bars showed the 95% confidence interval (CI) of the estimated RR.
Composite outcomes demonstrated increased risks in both groups. For CHD patients, there were increases across any cardiovascular outcome (RR, 1.63; 95% CI, 1.47–1.80), arrhythmias (RR, 1.52; 95% CI, 1.22–1.89), other cardiac disorders (RR, 1.69; 95% CI, 1.46–1.96), thrombotic disorders (RR, 1.42; 95% CI, 1.13–1.78), and cardiovascular-related symptoms (RR, 1.95; 95% CI, 1.66–2.31). For non-CHD patients, increases were noted in any cardiovascular outcome (RR, 1.63; 95% CI, 1.57–1.69), arrhythmias (RR, 1.47; 95% CI, 1.24–1.75), inflammatory heart disease (RR, 2.92; 95% CI, 2.25–3.78), other cardiac disorders (RR, 1.50; 95% CI, 1.26–1.78), thrombotic disorders (RR, 1.26; 95% CI, 1.09–1.45), and cardiovascular-related symptoms (RR, 1.68; 95% CI, 1.62–1.75).
Individual outcomes also showed increased risks. CHD patients experienced higher risks in hypertension (RR, 1.58; 95% CI, 1.32-1.89), atrial fibrillation (RR, 2.64; 95% CI, 1.15–6.05), ventricular arrhythmias (RR, 1.50; 95% CI, 1.18–1.89), myocarditis (RR, 3.82; 95% CI, 1.35–10.78), heart failure (RR, 1.77; 95% CI, 1.48–2.10), cardiomyopathy (RR, 1.78, 95% CI, 1.33–2.38), cardiac arrest (RR, 1.45; 95% CI, 1.06–1.98), thrombophlebitis (RR, 2.65; 95% CI, 1.18–5.96), thromboembolism (RR, 1.44; 95% CI, 1.14–1.81), chest pain (RR, 2.01; 95% CI, 1.64–2.46), and palpitations (RR, 2.14; 95% CI, 1.57–2.92). Non-CHD patients saw increased risks in hypertension (RR, 1.47; 95% CI, 1.35–1.60), ventricular arrhythmias (RR, 1.49; 95% CI, 1.23–1.81), premature atrial or ventricular contractions (RR, 1.77; 95% CI 1.07–2.95), pericarditis (RR, 1.85; 95% CI, 1.22–2.81), myocarditis (RR, 3.66; 95% CI, 2.67–5.01), heart failure (RR, 1.76; 95% CI, 1.24–2.48), cardiomyopathy (RR, 1.50; 95% CI, 1.19–1.90), cardiac arrest (RR, 1.56; 95% CI, 1.19–2.03), cardiogenic shock (RR, 3.15; 95% CI, 1.54–6.43), pulmonary embolism (RR, 1.61; 95% CI, 1.15–2.24), thromboembolism (RR, 1.30; 95% CI, 1.12–1.52), chest pain (RR, 1.84; 95% CI, 1.75–1.92), palpitations (RR, 1.98; 95% CI, 1.83–2.16), and syncope (RR, 1.30; 95% CI, 1.21–1.40).
Figure 3 demonstrated that the risks of post-acute cardiovascular composite outcomes were evident across age groups, race/ethnicity, sex, obesity status, severity of acute COVID-19, and dominant virus variants.
Forest plot presenting relative risk (RR) of post-acute cardiovascular outcomes comparing SARS-CoV-2-positive cohort (n = 297,920 patients) with SARS-CoV-2-negative cohort (n = 915,402 patients). The results are stratified according to congenital heart defects (CHD) status, age, race/ethnicity, sex, obesity, severity during the acute phase of COVID-19, and the dominant variant. The error bars showed the 95% confidence interval (CI) of the estimated RR. Source data are provided in Supplementary Table S11. Abbreviation: NHW Non-Hispanic White, NHB Non-Hispanic Black.
Sensitivity analysis
We performed extensive sensitivity analyses to evaluate the robustness of our findings, incorporating death as an outcome, conducting negative control experiments to address systematic bias and control unmeasured confounders, analyzing across different selected populations, assessing potential overlap with other respiratory infections, and carrying out a series of subgroup analyses.
No significant associations were identified in the analysis of death as an outcome (Section S4). Negative control experiments (Section S5) indicated a slight systematic error, as shown by a minor shift in point estimates with wider CIs. Analyses excluding patients solely based on PASC (Section S6), those without prior cardiovascular history within the baseline period (Section S7), those from the first COVID-19 wave (March to May 2020, Section S8), and those at site L (Section S9), all gave similar results as in the primary analyses. Among both SARS-CoV-2 positive and negative groups, the presence or absence of flu or respiratory syncytial virus (RSV) infection did not affect the incidence of cardiovascular outcomes. However, when comparing patients with SARS-CoV-2 infection to those without, regardless of their flu or RSV status, the incidence of most cardiovascular outcomes remained higher in the SARS-CoV-2-positive group (Section S10). Among immunocompromised individuals, a higher risk of cardiovascular outcomes was observed, regardless of the CHD status. (Section S11). Adolescents over 12 and children aged 5 to 11 displayed higher risks of any cardiovascular outcomes compared to children under 5 (Section S12). NHW patients exhibited the highest risks of any cardiovascular outcome, followed by Hispanic and NHB groups (Section S13). Sex differences were noted: females showed higher risks for hypertension, deep vein thrombosis, and cardiac arrest, whereas males were more prone to premature atrial or ventricular contractions, pulmonary embolism, and thrombophlebitis (Section S14). Obesity was associated with increased risks of ventricular arrhythmias, premature atrial or ventricular contractions, cardiogenic shock, pulmonary embolism, and thrombophlebitis, but lower risks of cardiovascular-related symptoms (Section S15). Patients with severe COVID-19 during the acute phase had higher risks across all composite cardiovascular outcomes compared to the non-severe group (Section S16). The pre-Delta period saw the highest risk of any cardiovascular outcome, with consistent risks observed across all dominant virus variants (Section S17). The interaction testing for subgroup analyses is available in Section S18.
Discussion
Our study involved 297,920 children and adolescents with SARS-CoV-2 infection and 915,402 without SARS-CoV-2 infection. We found that those infected with SARS-CoV-2 exhibited increased risks for a range of post-acute cardiovascular outcomes, with RR between 1.26 and 2.92. These outcomes included hypertension, ventricular arrhythmias, myocarditis, heart failure, cardiomyopathy, cardiogenic shock, thromboembolism, chest pain, and palpitations, compared to non-infected controls. These findings applied to patients both with and without CHDs, although children with CHD showed a notably higher risk of atrial fibrillation. Especially noteworthy was the increased risk of myocarditis and pericarditis (inflammatory heart disease) in both groups consistent with prior studies showing an increased occurrence of myocarditis in the months following the diagnosis of COVID-19, across different ages and sexes8,11,34,35,36,37. The composite outcome of inflammatory heart disease was more common in the non-CHD group, suggesting potentially distinct mechanisms of risk in this population that warrant further investigation. Similarly, while cardiogenic shock showed a higher RR in the non-CHD group, it remained a rare event in both CHD and non-CHD groups. A cautious interpretation is needed due to the limited number of cases. Studies have shown that children generally experience fewer acute complications compared to adults. In children, cardiogenic shock, when it occurred, was most often observed in the setting of MIS-C38 or myocarditis8, both of which have been reported more frequently during the post-acute phase (28–179 days) of SARS-CoV-2 infection. Palpitations and chest pain showed high RRs in our study, aligning with the global literature on PPC14,17,39,40,41. Although these symptoms are subjective, they have been consistently reported as part of the PASC in both pediatric and adult populations. Risks were consistently observed regardless of age, sex, race/ethnicity, obesity status, severity of acute COVID-19, or virus variant. Even children and adolescents without a history of any cardiovascular outcomes before SARS-CoV-2 infection showed increased risks, suggesting a broad potential impact on those previously considered at low risk of cardiovascular disease. Our results were robust through extensive sensitivity analyses and negative control experiments.
Our analysis found similar cardiovascular outcomes in children infected with the Delta and Omicron variants, despite some adult studies suggesting higher risks associated with the Delta variant42,43,44. This difference may stem from children’s generally lower baseline risk of severe COVID-19 complications, distinct immune responses, and viral pathogenicity compared to adults. Additionally, increasing vaccination rates, advancements in pediatric COVID-19 care, and evolving public health measures may have reduced potential differences in cardiovascular outcomes between these variants in pediatric populations. These findings highlight the importance of considering age and population-specific factors when studying variant-specific effects.
This study observed an association between obesity and increased risks for certain severe cardiovascular outcomes, coupled with lower risks for cardiovascular symptoms, in children and adolescents. While these findings were not substantial, they highlight the complexity of obesity-related cardiovascular health in pediatric populations. Obesity-related complications, including cardiovascular outcomes, typically evolve over a longer period and may not fully manifest within the follow-up period of 28 to 179 days. Future studies with longer follow-up periods and comprehensive assessments are warranted to better understand the long-term cardiovascular implications of obesity in pediatric populations.
It is important to note that our primary analysis utilized propensity score models to ensure comparability between SARS-CoV-2-positive and SARS-CoV-2-negative patients within the CHD and non-CHD groups separately. However, CHD and non-CHD groups were not balanced against each other; rather, they were analyzed as stratified populations. This approach allowed us to reflect on the increased risk after SARS-CoV-2 infection within these specific subpopulations without direct comparisons between CHD and non-CHD groups.
Our study has several strengths. First, we used the RECOVER EHR database to build a large cohort with and without SARS-CoV-2 infection, providing longitudinal follow-up throughout the post-acute period, which extended past findings in both populations and outcomes definitions. Second, propensity score stratification, accommodating hundreds of covariates while balancing the covariates in the two comparative groups, improved confounder adjustment over traditional linear regression, and reduced non-linear confounder effects31. Third, all analyses were stratified by CHD status, a unique consideration in the pediatric setting. A detailed breakdown of CHD types is also provided, offering valuable insights into how specific congenital heart defects influence post-acute cardiovascular outcomes. We also employed RR as our comparative measure, important for its collapsibility45 and accurate interpretation in clinical research46. Lastly, we conducted extensive sensitivity analyses to examine the robustness of our findings, including negative control experiments47,48 to address systematic bias and control unmeasured confounders, analyses across different selected populations, an evaluation of potential overlap with other respiratory infections, and a series of subgroup analyses.
The COVID-19 pandemic prompted the widespread use of social distancing, masking, subsequently vaccines, and medications. As acute cases declined, attention shifted towards long-term sequelae like cardiovascular PASC, particularly significant in pediatric patients, including competitive athletes with a history of COVID-19 and any subsequent cardiac complications49,50. The substantial risks of post-acute cardiovascular effects of SARS-CoV-2 in pediatrics call for dedicated healthcare resource allocation and long-term monitoring focused on cardiovascular health.
Limitations
This study has several limitations. First, selecting high-quality SARS-CoV-2 controls is challenging due to potential misclassification51; to mitigate this, we incorporated a combination of PCR, antigen, and serology tests, along with diagnosis codes for COVID-19 and PASC, to define our SARS-CoV-2 control group more accurately. Despite these efforts, some individuals in the control group may have experienced asymptomatic or undiagnosed SARS-CoV-2 infections, especially during and after 2021, when exposure became widespread in pediatric populations. This potential misclassification could dilute observed differences between the SARS-CoV-2-positive and control groups, leading to an underestimation of the true impact of SARS-CoV-2 infection on cardiovascular outcomes. In addition, the increased use of at-home rapid antigen tests later in the pandemic may have resulted in underreported testing frequencies in EHRs. On the other hand, although historical or pre-pandemic controls have been suggested, this introduces distinct difficulties for pediatric research due to the dynamic nature of rapid physical growth and physiological, cognitive, emotional, and social development in children and adolescents, which may not align with pre-pandemic benchmarks and could potentially lead to misleading results. Therefore, we used contemporary controls in our primary analyses for a valid comparison. Nonetheless, our sensitivity analyses across different pandemic phases were consistent, showing resilience to the influence of viral variants and mitigating concerns about data capture completeness.
Second, observational studies inherently face the challenge of confounding bias. To mitigate this, we used a propensity-score-based stratification approach and included a large number of potential confounders. We also incorporated the negative control experiments47,48 in our sensitivity analyses to adjust for unmeasured confounders. However, EHR data may still suffer from misclassification biases, such as incorrect documentation of SARS-CoV-2 infection status or incomplete follow-up data. Some efforts to lessen these biases have been made47,48,52,53.
Third, the frequency of hospital visits54 by SARS-CoV-2-positive patients might bias the observed post-acute cardiovascular outcomes upward, as these patients may visit hospitals more frequently. We tried to address this by including healthcare utilization factors such as hospital visits and tests as confounders to balance the comparison groups.
Fourth, this study did not exclude patients who had undergone surgical correction, as these individuals may still have residual or ongoing heart disease, which could influence their risk profile. Further stratification of risks by CHD subtypes and surgical correction status is an important area for future research, particularly in identifying which subgroups are at greatest risk for adverse cardiovascular outcomes following SARS-CoV-2 infection. This information could inform targeted preventive strategies, such as proactive immunization or early interventions, for those most vulnerable.
Fifth, our analysis did not account for reinfection or COVID-19 vaccinations during the study period, which could affect outcomes like myocarditis or pericarditis18,55,56,57. We included the dosage of COVID-19 vaccines and the interval since the last immunization as confounders to partially adjust for these variables to make SARS-CoV-2-positive and SARS-CoV-2-negative groups more comparable, but vaccinations and tests conducted outside hospital systems may not be captured accurately in the EHR.
In addition, cardiovascular diagnoses were based on ICD or SNOMED codes without chart review confirmation, which can lead to misclassification. Also, the absence of family history data in our EHR limits understanding of the predisposition of patients to cardiovascular conditions due to familial trends.
Although this study focuses on pediatric populations in the United States, healthcare practices, testing protocols, and population characteristics differ across countries. While definitions and timeframe for PASC conditions may differ, our findings are consistent with similar reports of post-acute cardiovascular outcomes in pediatric populations globally, including studies from Europe and other regions14,40,41,58,59,60. Validation of these findings in European or UK populations would provide important insights into the generalizability of the observed associations and help address differences in healthcare delivery and surveillance practices.
Lastly, while the RECOVER database includes a diverse pediatric population, it may not fully represent all healthcare providers across the United States. The participating institutions represent a mix of public and private healthcare systems. While some of these institutions may serve insured individuals or fee-paying patients, many also provide care to uninsured or underinsured populations through government programs. Although some institutions may serve specific demographics or geographic areas, the overall dataset reflects a broad and heterogeneous sample of pediatric patients, representing various racial, ethnic, socioeconomic, and geographic backgrounds across the United States. Future studies should incorporate such demographic information to enable direct comparisons with the broader population of individuals under 21 years of age in the United States.
In summary, this study shows a heightened risk of cardiovascular disease in children following SARS-CoV-2 infection, with similar risks observed in those with and without pre-existing congenital heart disease. Awareness of cardiovascular complications in the post-acute phase will improve timely diagnosis and treatment of these conditions.
Methods
Ethics and inclusion
This study constitutes human subject research. Institute Review Board (IRB) approval was obtained under Biomedical Research Alliance of New York (BRANY) protocol #21-08-508. As part of the BRANY IRB process, the protocol has been reviewed in accordance with the institutional guidelines. The BRANY waived the need for patient-informed consent and HIPAA authorization.
Data sources
This study is part of the National Institutes of Health (NIH) funded RECOVER Initiative (https://recovercovid.org/), which aims to learn about the long-term effects of COVID-19. The RECOVER pediatric cohort draws from EHRs provided by large national healthcare networks within the United States, covering regional catchment areas across 41 states. For this analysis, data were obtained from nineteen US children’s hospitals and health institutions, collectively covering over 30 million patients under 21, including more than 3 million individuals under 21 affected by COVID-19. The EHR data cover a wide range of healthcare interaction information routinely collected and stored by hospitals.
The institutions include Cincinnati Children’s Hospital Medical Center, Children’s Hospital of Philadelphia, Children’s Hospital of Colorado, University of Iowa Healthcare, Ann & Robert H. Lurie Children’s Hospital of Chicago, University of Michigan, University of Missouri, Montefiore, Medical University of South Carolina, Nationwide Children’s Hospital, Nemours Children’s Health System (in Delaware and Florida), OCHIN, Inc., Ochsner Health System, Ohio State University, Seattle Children’s Hospital, Stanford Children’s Health, Temple University, University of California, San Francisco, and Vanderbilt University Medical Center. The participating institutions represent a mix of public and private healthcare systems. While some of these institutions may serve insured individuals or fee-paying patients, many also provide care to uninsured or underinsured populations through government programs. Although some institutions may serve specific demographics or geographic areas, the overall dataset reflects a broad and heterogeneous sample of pediatric patients, representing various racial, ethnic, socioeconomic, and geographic backgrounds across the United States.
The EHR data was standardized to the PCORnet Common Data Model (CDM) and extracted from the RECOVER Database Version s10. More details are available in the Supplementary Materials Section S1.
Cohort construction and selection criteria
We conducted a retrospective study from March 1, 2020, to September 1, 2023, with a cohort entry period extending from March 1, 2020, to March 6, 2023, ensuring at least a 179-day follow-up for observing post-acute cardiovascular outcomes.
In our study, documented SARS-CoV-2 infections were defined by positive polymerase-chain-reaction (PCR), serology, antigen tests, or diagnoses of COVID-19, or diagnoses of PASC. The index date for SARS-CoV-2-positive patients was set as either the earliest date of positive tests, COVID-19 diagnoses, or 28 days before a PASC diagnosis. For SARS-CoV-2-negative patients, we required all tests to be negative, no evidence or diagnosis of COVID-19 or PASC during the study period, and at least one negative COVID-19 test within the cohort entry period. The index date for the comparator group was a randomly selected date from their negative tests to align the distribution of index dates between the two groups, controlling for time effects.
We included patients under 21, who had at least one healthcare visit within the baseline period, defined as 24 months to 7 days before the index date, and at least one encounter within the follow-up period, defined as 28 to 179 days after the index date, with certain institutions, to ensure active interaction with the healthcare system and adequate follow-up to assess post-acute outcomes.
Patients diagnosed with MIS-C, Kawasaki disease, CKD, or ESKD were excluded61,62,63,64,65,66. Although MIS-C is considered a PASC, it was not included in this study’s post-acute cardiovascular outcomes for two reasons: children with MIS-C can be effectively treated with minimal post-acute cardiac sequelae67, and MIS-C has been and continues to be extensively studied with significantly declining incidence over the past year68.
Defining CHD
In this study, we included a range of CHD types, including: aortic valve stenosis (AVS), atrial septal defect (ASD), atrioventricular septal defect, bicuspid aortic valve, coarctation of aorta (CoA), dextro-transposition of the great arteries, double outlet right ventricle, Ebstein anomaly, hypoplastic left heart syndrome, interrupted aortic arch, mitral insufficiency, mitral stenosis, patent ductus arteriosus (PDA), pulmonary atresia, pulmonary valve stenosis, tetralogy of Fallot, total anomalous pulmonary venous connection (TAPVC), total anomalous pulmonary venous return (TAPVR), tricuspid atresia, truncus arteriosus, ventricular septal defect (VSD). Details of the code sets and the risk table detailing the breakdown of CHD types are available in Tables S1-S2.
Defining cardiovascular outcomes
We identified 18 post-acute cardiovascular outcomes for our study, including hypertension, atrial fibrillation, ventricular arrhythmias, atrial flutter, premature atrial or ventricular contractions, pericarditis, myocarditis, heart failure, cardiomyopathy, cardiac arrest, cardiogenic shock, pulmonary embolism, deep vein thrombosis, thrombophlebitis, thromboembolism, chest pain, palpitations, and syncope. These outcomes were assessed during the follow-up period in patients without a history of the specific condition during the baseline period.
We also grouped related cardiovascular outcomes into categories, including arrhythmias (atrial fibrillation, ventricular arrhythmias, atrial flutter, and premature atrial or ventricular contractions), inflammatory heart disease (pericarditis and myocarditis), other cardiac disorders (heart failure, cardiomyopathy, cardiac arrest, and cardiogenic shock), thrombotic disorders (pulmonary embolism, deep vein thrombosis, thrombophlebitis, and thromboembolism), cardiovascular-related symptoms (chest pain, palpitations, and syncope), and any cardiovascular outcome (any incident cardiovascular condition studied).
The cardiovascular outcomes analyzed in this study were identified using validated diagnostic codes (ICD-10-CM, ICD-10, ICD-9-CM, and SNOMED) applied across 19 institutions using the PCORnet Common Data Model (CDM)69,70,71. The EHR data in this study were primarily entered by physicians and healthcare providers during routine clinical care. No free-text entries were used to identify cardiovascular outcomes in this study; all outcomes were derived directly from these validated diagnostic codes stored in the EHR systems. We used validated diagnostic codes confirmed by two board-certified pediatricians (DT, CF), with details of the code sets available in Supplementary Materials Table S1.
Covariates
We examined a detailed set of patient characteristics as measured confounders collected before cohort entry, to be adjusted through propensity score stratification29 to balance the comparison groups. These included demographic factors, including age at index date, sex (female, male), and race/ethnicity (NHW, NHB, Hispanic, AAPI, Multiple, Other/Unknown); clinical factors, including obesity status, a chronic condition indicator defined by the Pediatric Medical Complexity Algorithm28 (PMCA, no chronic condition, non-complex chronic condition, complex chronic condition), and a list of pre-existing chronic conditions8,72; health care utilization factors collected 24 months to 7 days before index date, including the number of inpatient visits, outpatient visits, emergency department (ED) visits, unique medications, and negative COVID-19 tests (0, 1, 2, ≥3); vaccine information, including dosage of COVID-19 vaccine before index date (0, 1, ≥2) and interval since the last COVID-19 immunization (no vaccine, <4 months, ≥4 months); year-month of cohort entry (from March 2020 to March 2023); indicators from the 19 data-contributing sites.
Statistical analysis
We calculated the incidence of post-acute cardiovascular outcomes in SARS-CoV-2-positive and negative cohorts, stratified by CHD status. For each outcome, incidence rates were calculated by dividing new cases during the follow-up period by the total number of patients, excluding those with the specific outcome at baseline.
We presented distributions of preference scores—a transformation of propensity scores that accounts for prevalence differences between populations—to assess empirical equipoise32,33. Preference scores, unlike traditional propensity scores, measure the relative likelihood of exposure versus non-exposure and adjust for differences in exposure prevalence. Empirical equipoise is achieved for SARS-CoV-2 positive and negative patients when the majority of individuals in both groups have preference scores ranging from 0.3 to 0.7, indicating substantial overlap in baseline characteristics29,32. This overlap supports valid causal inference by ensuring comparisons are made within regions of the data with adequate representation.
To mitigate the effects of confounding, we used propensity score stratification29,30,31 to adjust for a large number of measured confounders collected before the index date. We fitted a logistic regression model by regressing the response variable, SARS-CoV-2 infection status, on covariates, including demographic, clinical, and healthcare utilization factors as listed in the study variables. The predicted probabilities from the logistic regression model give the PS for each patient, representing their likelihood of belonging to the SARS-CoV-2 positive group given the observed covariates. The population was then stratified into five equally sized strata (quintiles) based on the distribution of propensity scores. Each stratum represented a distinct level of risk for SARS-CoV-2 positivity, making more comparable groups across strata. After stratification, we assessed the standardized mean difference (SMD) of each covariate between SARS-CoV-2 positive and negative patients, with an SMD of 0.1 or less indicating acceptable balance29,73.
Within each stratum, the RR was estimated using a modified Poisson regression model for binary outcomes74. To combine estimates across strata, a weighted average was applied, where the weights were proportional to the number of individuals in each stratum. This approach ensures that the combined RR reflects the population distribution accurately. RR is a collapsible measure, meaning the measure of association conditional on some factors remains consistent with the marginal measure collapsed over strata, which is crucial for accurate interpretation in clinical research45,46.
We conducted an analysis stratified by immune status (immunocompromised versus not) within both the CHD and non-CHD groups was conducted. Additionally, subgroup analyses were performed across various demographic and clinical factors, including age (0–4, 5–11, 12–20 years), race/ethnicity (NHW, NHB, Hispanic), sex (male and female), obesity status (obese and non-obese), severity of acute COVID-1975 (“non-severe” including asymptomatic and mild, “severe” including moderate and severe), and estimated time frames corresponding to dominant virus variants (pre-Delta, Delta, Omicron). Specifically, the pre-Delta variant spanned March 1, 2021, to June 30, 2021; Delta from July 1, 2021, to December 31, 2021; and Omicron from January 1, 2022, to March 6, 2023, with a minimum 179-day follow-up to observe PASC outcomes76.
Sensitivity analysis
We conducted extensive sensitivity analyses to examine the robustness of our findings. We assessed death as an outcome in Section S4. While death is a major cardiac outcome, it is fortunately rare in the pediatric population. Negative control outcome experiments29,47,48 were performed to calibrate the residual study bias from unmeasured confounders and systematic sources, in which the null hypothesis of no effect was believed to be true utilizing a list of 36 negative control outcomes determined by two board-certified pediatricians (DT, CF). The empirical null distribution and calibrated risks were reported in Supplementary Materials Section S5. Patients included solely based on a PASC diagnosis (Section S6) or those without any cardiovascular outcomes within the baseline period (Section S7) were excluded from additional analyses to examine the influence of potential selection bias and baseline differences. Given limited SARS-CoV-2 testing availability during the first wave of COVID-19 (March to May 2020), we conducted analyses excluding patients whose index dates fell within this period (Section S8). Furthermore, we excluded data from site L where the population is truncated at the ambulatory level (Section S9), to assess whether differences in data capture influenced the findings. To address the possibility that observed symptoms or manifestations attributed to PASC might overlap with those from other respiratory infections, we also compared the incidence of cardiovascular outcomes following SARS-CoV-2 infections with those following influenza or RSV infections (Section S10). All analyses were performed using R version 4.0.2. Statistical significance was set at a p-value < 0.05 (two-tailed).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The results reported in this study are based on detailed individual-level patient data compiled as part of the RECOVER program. Due to the high risk of reidentification based on the number of unique patterns in the data, patient privacy regulations prohibit us from releasing the data publicly. The data are maintained in a secure enclave, with access managed by the program coordinating center to remain compliant with regulatory and program requirements. Please direct requests to access the data, either for the reproduction of the work reported here or for other purposes, to the RECOVER EHR Pediatric Coordinating Center ([email protected]).
Code availability
The code used for the analysis in this study is available and can be accessed in a public repository at https://doi.org/10.24433/CO.5543902.v1.
References
World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus (WHO, 2021).
World Health Organization. A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus (WHO, 2023).
Shah, W., Hillman, T., Playford, E. D. & Hishmeh, L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372, n136 (2021).
National Institutes of Health (NIH). What is Long COVID? Building Our Understanding About Recovery. https://recovercovid.org/long-covid (NIH, 2024).
Centers for Disease Control and Prevention (CDC). Long COVID or Post-COVID Conditions. https://www.cdc.gov/covid/long-term-effects/?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (Centers for Disease Control and Prevention (CDC), 2023).
EpiCore. Obtaining Long COVID Definition Through EpiCore. https://endingpandemics.org/wp-content/uploads/2023/03/EPICORE-Long-Covid-Definitions-NASEM-2023-4.pdf (EpiCore, 2023).
Al-Aly, Z, Xie, Y & Bowe, B High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Rao, S. et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 176, 1000–1009 (2022).
Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590 (2022).
Ayoubkhani, D. et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 372, n693 (2021).
Daugherty, S. E. et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 373, n1098 (2021).
Carfì, A., Bernabei, R. & Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605 (2020).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232 (2021).
Raman, B., Bluemke, D. A., Lüscher, T. F. & Neubauer, S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 43, 1157–1172 (2022).
Wang, W., Wang, C.-Y., Wang, S.-I. & Wei, J. C.-C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. eClinicalMedicine 53, 101619 (2022).
Lim, J. T. et al. Long-term cardiovascular, cerebrovascular, and other thrombotic complications in COVID-19 survivors: a retrospective cohort study. Clin. Infect. Dis. 78, 70–79 (2024).
Rao, S. et al. Postacute Sequelae of SARS-CoV-2 in Children. Pediatrics 153, e2023062570 (2024).
Block, J. P. et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, january 2021–January 2022. Morb. Mortal. Wkly. Rep. 71, 517 (2022).
Abi Nassif, T. et al. Cardiac manifestations in COVID-19 patients: a focus on the pediatric population. Can. J. Infect. Dis. Med. Microbiol. 2021, 5518979 (2021).
Chakraborty, A. et al. Long-term cardiovascular outcomes of multisystem inflammatory syndrome in children associated with COVID-19 using an institution based algorithm. Pediatr. Cardiol. 44, 367–380 (2023).
Wu, Q. et al. Real-world effectiveness of BNT162b2 against infection and severe diseases in children and adolescents. Ann. Intern. Med. 177, 165–176 (2024).
Liu, P. P., Blet, A., Smyth, D. & Li, H. The science underlying COVID-19: implications for the cardiovascular system. Circulation 142, 68–78 (2020).
Bozkurt, B. et al. 2022 AHA/ACC key data elements and definitions for cardiovascular and noncardiovascular complications of COVID-19: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. Circ. Cardiovasc. Qual. Outcomes 15, e000111 (2022).
Van Der Bom, T. et al. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 8, 50–60 (2011).
Razzaghi, H. et al. Vaccine effectiveness against long COVID in children. Pediatrics 153, e2023064446 (2024).
Zhou, T. et al. Body Mass Index and Postacute Sequelae of SARS-CoV-2 Infection in Children and Young Adults. JAMA Netw. Open 7, e2441970–e2441970 (2024).
Wu, Q. et al. Real-world effectiveness and causal mediation study of BNT162b2 on long COVID risks in children and adolescents. EClinicalMedicine 79, 102962 (2025).
Simon, T. D., Haaland, W., Hawley, K., Lambka, K. & Mangione-Smith, R. Development and validation of the Pediatric Medical Complexity Algorithm (PMCA) version 3.0. Acad. Pediatr. 18, 577–580 (2018).
Suchard, M. A. et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet 394, 1816–1826 (2019).
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424 (2011).
Amoah, J. et al. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin. Infect. Dis. 71, e497–e505 (2020).
Walker, A. M. et al. A tool for assessing the feasibility of comparative effectiveness research. Comp. Eff. Res. 3, 11–20 (2013).
Schuemie, M., Madigan, D., Suchard M. & Ryan P. Population-level estimation. in The Book of OHDSI, CH. 12. https://ohdsi.github.io/TheBookOfOhdsi/PopulationLevelEstimation.html (GitHub, 2021).
Murk, W. et al. Diagnosis-wide analysis of COVID-19 complications: an exposure-crossover study. CMAJ 193, E10–E18 (2021).
Boehmer, T. K. et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morb. Mortal. Wkly. Rep. 70, 1228 (2021).
Barda, N. et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 385, 1078–1090 (2021).
Rathore, S. S. et al. Myocarditis associated with Covid-19 disease: a systematic review of published case reports and case series. Int J. Clin. Pr. 75, e14470 (2021).
Alsaied, T. et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 143, 78–88 (2021).
Tanni, S. E., Tonon, C. R., Gatto, M., Mota, G. A. F. & Okoshi, M. P. Post-COVID-19 syndrome: Cardiovascular manifestations. Int J. Cardiol. 369, 80 (2022).
Lee, C. C. E. et al. COVID-19-associated cardiovascular complications. Diseases 9, 47 (2021).
Bisaccia, G. et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J. Cardiovasc Dev. Dis. 8, 156 (2021).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-delta, delta, and omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
Ballouz, T. et al. Post COVID-19 condition after Wildtype, Delta, and Omicron SARS-CoV-2 infection and prior vaccination: pooled analysis of two population-based cohorts. PLoS ONE 18, e0281429 (2023).
Hyams, C. et al. Severity of Omicron (B. 1.1. 529) and Delta (B. 1.617. 2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study in Bristol, United Kingdom. Lancet Reg. Health–Eur. 25, 100556 (2023).
Greenland, S., Pearl, J. & Robins, J. M. Confounding and collapsibility in causal inference. Stat. Sci. 14, 29–46 (1999).
Whitcomb, B. W. & Naimi, A. I. Defining, quantifying, and interpreting “noncollapsibility” in epidemiologic studies of measures of “effect”. Am. J. Epidemiol. 190, 697–700 (2021).
Schuemie, M. J., Hripcsak, G., Ryan, P. B., Madigan, D. & Suchard, M. A. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc. Natl. Acad. Sci. 115, 2571–2577 (2018).
Schuemie, M. J., Ryan, P. B., DuMouchel, W., Suchard, M. A. & Madigan, D. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat. Med 33, 209–218 (2014).
Chowdhury, D. et al. Return to activity after SARS-CoV-2 infection: cardiac clearance for children and adolescents. Sports Health 14, 460–465 (2022).
Daniels, C. J. et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 6, 1078–1087 (2021).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol 21, 133–146 (2023).
Chen, Y., Wang, J., Chubak, J. & Hubbard, R. A. Inflation of type I error rates due to differential misclassification in EHR-derived outcomes: empirical illustration using breast cancer recurrence. Pharmacoepidemiol Drug Saf. 28, 264–268 (2019).
Duan, R. et al. An empirical study for impacts of measurement errors on EHR based association studies. in AMIA Annual Symposium Proceedings, Vol. 2016, 1764–1773 (American Medical Informatics Association, 2016).
Asch, D. A. et al. Variation in US Hospital Mortality Rates for Patients Admitted With COVID-19 During the First 6 Months of the Pandemic. JAMA Intern. Med. 181, 471–478 (2021).
Truong, D. T. et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation 145, 345–356 (2022).
Kohli, U. et al. mRNA coronavirus disease 2019 vaccine-associated myopericarditis in adolescents: a survey study. J. Pediatr. 243, 208–213 (2022).
Stowe, J., Miller, E., Andrews, N. & Whitaker, H. J. Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: a self-controlled case series analysis in England. PLoS Med 20, e1004245 (2023).
Sabatino, J. et al. Mid-and long-term atrio-ventricular functional changes in children after recovery from COVID-19. J. Clin. Med 12, 186 (2022).
Ortega-Paz, L. et al. One-year cardiovascular outcomes after coronavirus disease 2019: The cardiovascular COVID-19 registry. PLoS ONE 17, e0279333 (2022).
Radtke, T., Ulyte, A., Puhan, M. A. & Kriemler, S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA 326, 869–871 (2021).
Chavers, B. M., Li, S., Collins, A. J. & Herzog, C. A. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 62, 648–653 (2002).
Sharma, C. et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat. Rev. Rheumatol. 17, 731–748 (2021).
Harahsheh, A. S. et al. Kawasaki Disease in the time of COVID-19 and MIS-C: the International Kawasaki Disease Registry. Can. J. Cardiol. 40, 58–72 (2024).
Peco-Antić, A. & Paripović, D. Renal hypertension and cardiovascular disorder in children with chronic kidney disease. Srp. Arh. Celok. Lek. 142, 113–117 (2014).
Weaver, D. J. & Mitsnefes, M. Cardiovascular disease in children and adolescents with chronic kidney disease. in Seminars in Nephrology, Vol. 38, 559–569 (Elsevier, 2018).
Pawar, S. M. Multi system inflammatory syndrome in children and adolescents temporally related to COVID-19. GFNPSS-Int J. Multidiscip. Res 1, 97–102 (2020).
Matsubara, D. et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J. Am. Coll. Cardiol. 76, 1947–1961 (2020).
Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC. https://covid.cdc.gov/covid-data-tracker (2023).
Forrest, C. B. et al. PEDSnet: a national pediatric learning health system. J. Am. Med. Inform. Assoc. 21, 602–606 (2014).
PCORnet Common Data Model. https://pcornet.org/data/common-data-model/.
PEDSnet Common Data Model. https://pedsnet.org/data/pedsnet-common-data-model/.
Woodruff, R. C. et al. Risk factors for severe COVID-19 in children. Pediatrics 149, e2021053418 (2022).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 28, 3083–3107 (2009).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Forrest, C. B. et al. Severity of acute COVID-19 in children<18 years old March 2020 to December 2021. Pediatrics 149, e2021055765 (2022).
GISAID, via CoVariants.org—processed by Our World in Data. https://ourworldindata.org/grapher/covid-variants-area?time=earliest..2023-03-06&country=~USA (2024).
Acknowledgements
This study is part of the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC). For more information on RECOVER, visit https://recovercovid.org/. We would like to thank the National Community Engagement Group (NCEG), all patients, caregivers, and community Representatives, and all the participants enrolled in the RECOVER Initiative. We would like to thank the patient representatives Megan Carmilani, Nick Guthe, and Leah Baucom for their helpful suggestions and comments. This work was supported in part by the National Institutes of Health (OT2HL161847-01, Y.C.) Y.C.’s effort has also been supported in part by the National Institutes of Health (1R01LM014344, 1R01AG077820, R01LM012607, R01AI130460, R01AG073435, R56AG074604, R01LM013519, R56AG069880, U01TR003709, RF1AG077820, R21AI167418, R21EY034179) and the Patient-Centered Outcomes Research Institute (PCORI) Project Program Awards (ME-2019C3-18315 and ME-2018C3-14899). This content is solely the responsibility of the authors and does not necessarily represent the official views of the RECOVER Initiative, the NIH, PCORI, its Board of Governors, or the Methodology Committee.
Author information
Authors and Affiliations
Contributions
Authorship was determined using ICMJE recommendations. B.Z., D.T., C.B.F., and Y.C. designed methods and experiments; E.A.C., D.A.C., S.F., V.G., S.K., A.S.M.M., M.R.S., B.W.T., D.A.W., and C.B.F. provided datasets for data analysis; B.Z., T.Z., D.Z., and Y.L. conducted data analysis; B.Z. visualized the analysis results; B.Z., D.T., C.B.F., and Y.C. interpreted the results; B.Z., D.T., T.Z., D.Z., Y.L., and Y.C. drafted the main manuscript. B.Z., D.T., T.Z., D.Z., Y.L., J.C., E.A.C., D.A.C., S.F., V.G., S.K., A.S.M.M., M.R.S., B.W.T., D.A.W., Q.W., C.B.F., and Y.C. provided critical edits to the early draft and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Betty Raman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, B., Thacker, D., Zhou, T. et al. Cardiovascular post-acute sequelae of SARS-CoV-2 in children and adolescents: cohort study using electronic health records. Nat Commun 16, 3445 (2025). https://doi.org/10.1038/s41467-025-56284-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56284-0