Abstract
All-perovskite tandem solar cells (APTSCs) offer the potential to surpass the Shockley-Queisser limit of single-junction solar cells at low cost. However, high-performance APTSCs contain unstable methylammonium (MA) cation in the tin-lead (Sn-Pb) narrow bandgap subcells. Currently, MA-free Sn-Pb perovskite solar cells (PSCs) show lower performance compared with their MA-containing counterparts. This is due to the high trap density associated with Sn2+ oxidation, which is exacerbated by the rapid crystallization of MA-free Sn-containing perovskite. Here, a multifunctional additive rubidium acetate (RbAC) is proposed to passivate Sn-Pb perovskite. We find that RbAC can suppress Sn2+ oxidation, alleviate microstrain, and improve the crystallinity of the MA-free Sn-Pb perovskite. Consequently, the resultant Sn-Pb PSCs achieve a power conversion efficiency (PCE) of 23.02%, with an open circuit voltage (Voc) of 0.897 V, and a filling factor (FF) of 80.64%, and more importantly the stability of the device is significantly improved. When further integrated with a 1.79-electron volt MA-free wide-bandgap PSC, a 29.33% (certified 28.11%) efficient MA-free APTSCs with a high Voc of 2.22 volts is achieved.
Similar content being viewed by others
Introduction
All-perovskite tandem solar cells (APTSCs) are promising to overcome the Shockley-Queisser (S-Q) limit of single-junction solar cells at low cost1,2,3,4. Over the past few years, the power conversion efficiencies (PCEs) of monolithic APTSCs have demonstrated rapid improvement and surpassed that of the single-junction counterparts5,6,7,8,9. However, the operational stability of APTSCs, which is critical for real-world application, is still inferior to that of its single-junction counterparts10,11,12. As the bottom subcells for APTSCs, the efficiency and stability of the tin-lead (Sn-Pb) narrow bandgap (NBG) PSCs directly influence the ultimate performance of the constructed APTSCs13,14,15. Currently, high-efficiency Sn-Pb PSCs generally contain methylammonium (MA) in the composition, which is beneficial for growing high quality perovskite films16,17,18. Nevertheless, MA is thermally unstable, and these MA-containing PSCs are prone to degrade after continuous work under light illumination19,20. Thus, to achieve stable APTSCs, it is critical to develop high-efficiency MA-free Sn-Pb PSCs. The key challenge in fabricating efficient MA-free Sn-Pb PSCs is that the removal of MA from the Sn-Pb perovskite composition leads to more rapid crystallization, which results in non-uniform nucleation with a large number of grain boundaries21,22,23. These non-uniform perovskite surfaces and defective grain boundaries can accelerate Sn2+ oxidation, which deteriorates the performance of the resultant PSCs.
To address this issue, various methods have been developed, for example, tuning the compositions and using two-dimensional additives to improve the quality of the MA-free Sn-Pb NBG perovskite films12,24,25,26,27,28. The rubidium (Rb) related salts such as RbI, RbCl and RbSCN are becoming popularly explored as additives in PSCs research recently29,30,31,32. Despite these efforts, the PCEs of MA-free Sn-Pb NBG PSCs are still much lower than that of their MA-containing counterparts33,34,35,36. Here, we introduce a multifunctional salt rubidium acetate (RbAC) to improve the performance of MA-free Sn-Pb PSCs37,38,39. With the addition of RbAC, the carrier lifetime of Sn-Pb perovskite has been significantly enhanced, increasing from under 1 µs to 5.01 µs, which can be attributed to the improved oriented growth, released strain, suppressed Sn2+ oxidation, and reduced defect density. Consequently, the PCE of the MA-free Sn-Pb PSCs increased from 19.07 to 23.02%. We integrated this MA-free NBG cell with a MA-free 1.79 eV wide-bandgap (WBG) PSC to construct MA-free two-terminal APTSCs and achieved a champion PCE of 29.33% (certified 28.11%), which is the highest PCE for MA-free APTSCs. Moreover, the stability of both MA-free single-junction Sn-Pb PSCs and APTSCs are improved substantially.
Results
Suppress of Sn2+ oxidation and release strain
The oxidation of Sn2+ to Sn4+ during the preparation of Sn-Pb perovskite precursor solutions and films poses a significant challenge in achieving high-quality Sn-Pb perovskite thin films. It leads to short carrier lifetime in the Sn-Pb films and poor charge carrier extraction in the Sn-Pb PSCs.
For the Sn-Pb perovskite, we used a MA-free perovskite composition of FA0.75Cs0.25Pb0.5Sn0.5I3 as the baseline. The presence and distribution of RbAC in the perovskite film was shown in Supplementary Fig. 1. To analyze the interaction between perovskite and RbAC, we performed Fourier-transform infrared spectroscopy (FTIR) (Fig. 1a). When RbAC mixed with SnI2, the peak position of C=O shifts from 1659 cm−1 to 1652 cm−1, which can be attributed to the interaction between C=O bonds and undercoordinated Sn2+ through Lewis acid-base interaction. To further investigate the anti-oxidation effect of AC− on Sn2+, we conducted X-ray photoelectron spectroscopy (XPS) measurement (Fig. 1b). The Sn 3d peaks shifted from 486.40 eV to 486.75 eV, indicating an interaction occurred between AC− and Sn2+. Both FTIR and XPS results demonstrate that AC− interacts with Sn2+, which may suppress Sn2+ oxidation40,41. The Sn 3d fitting curves are shown in Fig. 1c, d, and Supplementary Table 1. The Sn4+/Sn2+ ratio in the with RbAC additive (referred to as target from hereon) perovskite film is significantly lower than that in the without RbAC (referred to as control from hereon) additive film. As shown in Supplementary Fig. 2, the I 3d core levels of the target film exhibited an upshift, suggesting that I vacancies were filled by AC−.
To experimently verify the antioxidant effect of RbAC on Sn2+, we prepared a fresh yellow perovskite precursor containing tin iodine (SnI2), tin fluoride (SnF2), lead iodine (PbI2), formamidinium iodide (FAI), and cesium iodide (CsI) in a mixed solvent of dimethylformamide (DMF) and dimethylsulfoxide (DMSO). It can be seen from Supplementary Fig. 3 that the peak of the Rb element appeared in the target film, which indicates that RbAC has successfully dissolved into the perovskite precursor solution. The precursor without RbAC (control) rapidly turned red upon exposure to air, while the one with RbAC (target) maintained its bright yellow color (Supplementary Fig. 4). Similarly, when exposed to air, Sn-Pb perovskite films exhibited a significantly slower rate of color change to red compared to the control films (Supplementary Fig. 5a–f). Additionally, the water contact angle of the RbAC perovskite film was greater than that of the control film. (Fig. 1e, f), which could effectively enhance the stability of Sn-Pb perovskite in air condition. These results proved that RbAC additive could prevent Sn2+ oxidation of both the precursors and films.
X-ray diffraction (XRD) was employed to examine the impact of RbAC on the crystallinity of MA-free Sn-Pb perovskite (Fig. 2a–f). Both the control and target perovskites exhibited diffraction peaks at 14°, 24°, 28°, 31° and 40°, corresponding to (100), (202), (220), (310) and (224) planes of the perovskite. This indicates that the introduction of RbAC does not result in the formation of new perovskite phase. After adding RbAC, the primary diffraction peaks of Sn-Pb perovskite shifted to larger angles, reflecting that Rb+ ions were embedded in the crystal lattice (Fig. 2b–f). The Rb+ embedded into the perovskite crystal lattice offers several advantages. Firstly, the medium-size Rb+ could occupy interstitial halogen defects in the grain and at grain boundaries, thus reducing the density of deep traps. Secondly, Rb+ as an alkali metal ion with a smaller ionic radius compared to Cs+ and FA+, could form CsFARb triple cation A-site when alloyed with CsFA. This CsFARb triple cation A-site can help release the microstrain in the perovskite. Figures 2g and 2h show that the target films exhibited both smaller full-width at half maximum (FWHM) and reduced microstrain, calculated using the Williamson-Hall plot29,42. The results further prove that RbAC additive improved the MA-free Sn-Pb film quality (Supplementary Table 2).
a XRD patterns of the control and target perovskite films. b–f XRD patterns of the control and target samples at the diffraction positions of the (100), (202), (220), (310), and (224), respectively. g Microstrain statistics of Control and target films. h FWHM statistics of the control and target films.
Modulate perovskite growth
Scanning electron microscopy (SEM) and atomic force microscopy (AFM) were utilized to examine the effect of RbAC on the morphology and microstructure of Sn-Pb perovskite films. The target film showed more compact, uniform grains compared to the control film (Fig. 3a–c and Supplementary Table 3). Cross-section SEM of the target and control devices (ITO/PEDOT:PSS/FA0.75Cs0.25Pb0.5Sn0.5I3/C60/BCP/Ag) showed that the control perovskite had more grain boundaries throughout the film, while the target perovskite displayed fewer grain boundaries and a more orderly vertical alignment (Fig. 3d, e). AFM measurements further revealed that the root-mean-square (RMS) roughness of the perovskite film with RbAC was 27.5 nm, significantly lower than that of the control film (32.5 nm) (Fig. 3f, g). The increased grain size and decreased surface roughness are important for better interfacial contact and better charge transfer at the interface43. Importantly, the XRD intensity ratio of (100) surface to the (202), (310), (224) for the target film are larger than that of control film, indicating that RbAC additive could promote the preferred growth along the (100) plane (Supplementary Table 4 and Supplementary Fig. 6). We attribute this oriented growth to the hydrogen bonding between FA+ and AC−, which likely slowed down the reaction rate between FAI and SnI2, thereby modulating the crystallization and retarding perovskite growth (Supplementary Fig. 7)27,44,45.
a Mechanism of crystallization improvement. Top-view SEM images of b control and c target perovskite films (with inset showing the grain size histogram of the b control and c target perovskite films). Cross-sectional SEM images of d the control and e target PSCs. f, g AFM images of f control and g target perovskite films.
Improve optoelectronic properties
To demonstrate the trap passivation effect of RbAC, we performed a space charge limited current (SCLC) analysis by using electron-only (Glass/ITO/C60/ perovskite /C60/Ag) device (Fig. 4a, b). The trap state density (Ntrap) can be calculated by the following equation:
where e signifies the elementary charge, L is the perovskite film thickness, VTFL represents the trap filling limit voltage, ε0 is the vacuum dielectric constant, and ε represents the relative dielectric constant of the perovskite. The calculated defect density for the target NBG perovskite was 2.65 ×1015 cm−3, which is half that of the control (5.41 ×1015 cm−3). This result proves that adding RbAC into the Sn-Pb perovskite could reduce the trap density.
The reduced trap density significantly enhance the optoelectronic properties of the target perovskite film. Time-resolved photoluminescence (TRPL) measurement were undertaken to investigate the charge recombination dynamics in the Sn-Pb perovskites. The carrier lifetime was elongated from 0.9 µs to over 5 µs with the addition of RbAC, indicating the reduction of non-radiative recombination (Fig. 4c and Supplementary Table 5). This was also confirmed by the steady-state photoluminescence (PL) (Supplementary Fig. 8) and photoluminescence quantum yield (PLQY) results (Fig. 4d)46,47,48.
Importantly, the adding of RbAC did not change the bandgap of the Sn-Pb perovskite (Supplementary Fig. 9). Additionally, transient photocurrent (TPC) and transient photovoltage (TPV) measurements were performed to evaluate charge carrier transport and recombination (Fig. 4e, f). The TPC decay time for the target device decreased from 1.27 μs for control to 0.71 μs for target perovskite, indicating improved charge extraction. Moreover, the target device demonstrated a lower photovoltage decay in TPV measurements compared to the control, reflecting reduced surface recombination and enhanced film quality49,50.
Single-junction MA-free Sn-Pb PSCs
The improved film quality of Sn-Pb perovskites enabled us to fabricate efficient and stable PSCs. The device structure is Glass/ITO/PEDOT:PSS/perovskite /C60/BCP/Ag, where PEDOT:PSS is poly (3, 4-ethylenedioxythiophene) -poly (Styrene-sulfonic acid), C60 is fullerene-C60, BCP is bathocuproine and Ag is silver (Fig. 5a). We used PEDOT:PSS as hole transporting layer (HTL) due to its good wetting for perovskite deposition and suitable energy level for hole extraction at the interface (Supplementary Table 6). The current-density (J-V) parameters of the PSCs with different RbAC concentrations as additive are detailed in Supplementary Fig. 10 and Supplementary Table 7. The optimal RbAC concentration was 0.018 mM. As illustrated in Fig. 5b and Table 1, the target device achieved a champion PCE of 23.02%, with a JSC of 31.83 mA cm−2, a VOC of 0.897 V, and a FF of 80.64%. In contrast, the control PSC exhibited a much lower PCE of 19.07%, with a JSC of 31.67 mA cm−2, a VOC of 0.792 V, and a FF of 76.04%. The stable power output (SPO) of the target and the control devices were 22.67% and 17.75%, respectively (Supplementary Fig. 11). The external quantum efficiency (EQE) measurements yielded integrated Jsc values of 31.51 mA cm−2 for the control device and 31.77 mA cm−2 for the target device, respectively, which agreed with the Jsc value in J-V curves (Fig. 5c). These results indicates the RbAC additive could significantly improve the performance of the MA-free Sn-Pb PSCs. We also compared the efficiencies of the devices with other additives, and the RbAC based MA-free Sn-Pb PSC showed the highest efficiency (Supplementary Tables 8 and 9).
a Schematic structure of the NBG PSC. b J-V curves of the NBG PSCs measured under AM 1.5 G solar illumination (100 mW cm−2). c EQE spectra and integrated JSC. d Electrochemical impedance spectroscopy (EIS) curves. e Dark-current measurements. f Mott-Schottky measurements of the control and target devices. g Voc versus light intensity plots. h Stability tracking of uncapsulated devices under continuous 1-sun illumination in N2 glovebox.
To explore the underlying mechanisms for the enhanced performance, we employed electrochemical impedance spectroscopy (EIS) to analyze charge transport and recombination in the Sn-Pb PSCs. Figure 5d presents Nyquist plots for both control and target devices, measured at an applied bias voltage of 0.9 V over a frequency range from 2 MHz to 2 Hz in dark condition. The recombination resistance of the target device (Rrec = 12.77 kΩ) is significantly higher than the control device (Rrec = 6.18 kΩ), suggesting that the RbAC additive could minimize carrier recombination, thereby increasing the Voc of the PSCs51,52. Additionally, the reduction of dark current density by RbAC additive was evidenced by dark J-V measurements (Fig. 5e)53.
The interface properties of the Sn-Pb PSCs were investigated using Mott-Schottky (M-S) measurements (Fig. 5f). The built-in potential (Vbi) increased from 0.65 V to 0.75 V with the addition of RbAC, indicating a reduction in interface defects, which benefits charge transport across the device stack54,55. Figure 5g depicts the light intensity-dependent Voc measurements. The slope of the linear fit is the ideal factor, closely related to non-radiative recombination in the device. The closer of the ideal factor to 1 indicates the less non-radiative recombination. The ideal factor of the target device is 1.17 kT/q, significantly lower than that of the control device (1.45 kT/q)56,57,58. These results indicate that the RbAC additive significantly improve the interface properties of the Sn-Pb PSCs.
The reduced carrier recombination and improved interface properties in the RbAC containing Sn-Pb PSCs could result in substantially improved device performances. The statistics of the photovoltaic parameters of the control and target devices (15 devices each) are shown in Supplementary Fig. 12. Compared with the control devices, the target devices showed improvement almost on all the device parameters. Furthermore, the operational stability of the MA-free Sn-Pb PSCs was measured. As shown in Fig. 5h, a target PSC maintained 80% of its initial PCE after 850 h light illumination, while the PCE of the control device degraded to 55%. We also conducted a thermal stability test at 85 °C, and the results showed that the RbAC additive could improve the thermal stability (Supplementary Fig. 13).
All-perovskite tandem solar cells
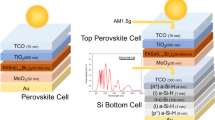
We further fabricated MA-free two-terminal (2-T) APTSCs by integrating a MA-free 1.79 eV FA0.8Cs0.2Pb(Br0.4I0.6)3 wide-bandgap (WBG) PSC with the above MA-free Sn-Pb PSC. The cross-sectional scanning electron microscope (SEM) image for the tandem device is presented in Fig. 6a. The thicknesses are about 400 nm for the WBG and 1 µm for the NBG perovskite layers. As shown in Fig. 6b and Table 2, the control device achieved a PCE of 26.16% under reverse scan and 26.08% under forward scan. The target device reached a PCE of 29.33% under reverse scan, accompanied with an impressive Voc of 2.22 V. The achieved Voc of 2.22 V and PCE of 29.33% represent some of the highest reported performances for MA-free APTSCs (Fig. 6c and Supplementary Table 10). One target device achieved a certified PCE of 28.11% (Supplementary Fig. 14). The integrated Jsc values from the EQE data of the WBG and NBG subcells are 16.31 and 15.78 mA cm−2, respectively, consistent with the Jsc values in the J-V curve (Fig. 6d). The PCEs derived from the J–V curves align well with the stable power output PCE of 29.31% (Fig. 6b and Table 2). The statistics of the APTSCs parameters are presented in Fig. 6e, further confirming the effectiveness of the RbAC additive on improving the device performance of Sn-Pb PSCs. We also compared the operational stability of the control and target tandem cells under ISOS-L-1 N2 conditions with continuous light illumination. After 624 h, the target tandem device maintained 80% of its initial PCE, whereas the control tandem device’s PCE dropped to below half of its initial value within 192 h of illumination. (Fig. 6f).
a Cross-sectional SEM image of the all-perovskite tandem solar cell. b J–V curves of the all-perovskite tandem device measured under simulated AM1.5 solar illumination (100 mW cm−2) and with inset showing the stable power outputs. c Comparison of the Voc and PCE of our PSCs with reported MA-free APTSCs, source data are from Supplementary Table 10. d EQE curves of the all-perovskite tandem cell. e Statistical box plots of the PV parameters. f The long-term stability of unencapsulated all-perovskite tandem cell in an N2-filled glovebox.
Discussion
This work has showcased highly efficient and stable MA-free single-junction Sn-Pb NBG PSCs and all-perovskite tandem solar cells through a multifunctional additive RbAC salt. The AC- anion could coordinate with Sn2+ to suppress Sn2+ oxidation and regulate perovskite crystallization. Moreover, the Rb+ embedded in the perovskite lattice releases the microstrain and passivates traps of the perovskite. As a result, the PCE of the champion single-junction MA-free Sn-Pb PSCs achieved a champion PCE of 23.02%, along with improved operational stability. When integrated with a MA-free 1.79 eV WBG PSC, the MA-free APTSC achieved a PCE of 29.33% (certified 28.11%) with high photovoltage (2.22 V), which is the highest value for MA-free APTSCs. This approach opens up avenues for enhancing the performance of MA-free photovoltaic devices.
Methods
Materials
All materials were used as recived without further purification. Formamidinium iodide (FAI, 99.5%), Cesium iodide (CsI, 99.99%), Lead bromine (PbBr2, 99.99%), PEDOT:PSS (AI4083), C60 (>99.5%), and BCP were purchased from Xi’an p-OLED Crop. Rubidium acetate (RbAC, 99.5%) was purchased from Shanghai Maclin Biochemical Technology Co.Ltd.[4-(3,6-Dimethyl-9H-carbazol-9-yl)butyl]phosphonic Acid (Me-4PACz, >99.0%) and Lead iodide (PbI2, 99.99%) were purchased from TCI. Other chemicals were purchased from Sigma-Aldrich.
Device fabrication
Single-junction solar cells
Indium tin oxide (ITO) coated glass, procured from Yingkou Youxuan Commercial & Trading Co., Ltd, were sequentially cleaned with detergent, deionized water, and isopropanol (IPA) for 10 min in each step in an ultrasonic cleaner. Subsequently, the cleaned ITO-coated glass was treated with UV-ozone for 15 min and cooled with nitrogen (N2) before use. PEDOT: PSS (AI4083) was deposited on the pre-cleaned substrates by spin coating with a spin speed of 4000 r.p.m. for 30 sec, followed by annealing at 130 °C for 30 min. The perovskite precursor, with a chemical formula of FA0.75Cs0.25Pb0.5Sn0.5I3 (1.8 M), was dissolved in a mixed solvent (DMF and DMSO with a volume ratio of 4:1). In detail, FAI (232.06 mg), CsI (116.91 mg), SnI2 (335.25 mg), PbI2 (414.9 mg) and SnF2 (14.10 mg) were dissolved in 800 μL DMF and 200 μL DMSO. The perovskite precursor solution was then stirred overnight in a nitrogen glove box. The RbAC additive with a concentrations of 0.009 mM, 0.018 mM, 0.036 mM were added to the precursor solution and stirred over night. The precursor solution was spin-coated on the substrate at an acceleration of 2000 r.p.m. and a spin coating speed of 5000 r.p.m. for 80 sec. 250 μL of chlorobenzene was dripped onto the wet film 25 s after the start of spinning and then annealed at 120 °C for 15 min. Finally, C60 (25 nm), BCP (7 nm), and Ag (100 nm) were sequentially deposited by thermal evaporation in a vacuum state (<9 × 10−4 Pa).
All-perovskite tandem solar cells
1.79 eV Device fabrication. ITO was cleaned by UV-ozone for 15 min, followed by spin-coating Me-4PACz (0.5 mg/ml, dissolved in ethanol) at 3,000 rpm for 30 s, and annealed at 100 °C for 10 min. The perovskite precursor (1.79 eV) with a concentration of 1.2 M was prepared in a mixed solvent (DMF/DMSO = 3:1 volume ratio) with a chemical formula of Cs0.2FA0.8Pb(I0.6Br0.4)3. The precursor solution was spin-coated on the substrate at 2,000 r.p.m. for 10 s and 5000 r.p.m. for 30 s, with 100 μL chlorobenzene anti-solvent was dripped 17 s before the end of the spin-coating process, and then annealed at 100 °C for 10 min. After cooled down to room temperature, 30 uL Phenethylammonium bromide (PEABr) solution with a concentration ratio of 2 mg ml−l dissolved in IPA was dynamically spin-coated on the as-prepared perovskite films at 5000 rpm for 30 s, then annealing at 100 °C for 5 min. The spin-coating processes were conducted in a N2-filled glovebox. Then, LiF (1 nm), and C60 (25 nm) layers were consecutively deposited using thermal evaporation under a vacuum pressure of less than 9 × 10−4 Pa. Next, SnO2 (20 nm) was deposited in the samples by atomic layer deposition (ALD). The substrate was transferred into a thermal evaporation system to deposit a 1 nm Au cluster layer on SnO2. Then, the NBG subcell was fabricated by the same process as the single-junction cells.
Film and device characterizations
The X-ray photoelectron spectroscopy (XPS) spectra was tested by an X-ray photoelectron spectrometer (Thermo Fischer, ESCALAB Xiþ). The fourier-transform infrared spectroscopy (FTIR) was measured by Nicolet6700. The SEM images were acquired using Hitachi SU8010 field-emission scanning electron microscopy. The perovskite films were also characterized by X-ray diffractometer (XRD, D8 Advance), UV-Vis spectrometer (lambda 750 S, PerkinElmer), photoluminescence (PL), and time-resolved PL spectra (TRPL, Micro Time 200 PicoQuant GmbH). The current density–voltage (J–V) curves were measured by a Keithley 2450 source meter. The J–V curves were measured in a N2 glove box using an air mass 1.5 global (AM 1.5 G) solar simulator (Oriel Sol3A Class AAA). The intensity of the solar simulator was calibrated to 100 mW cm–2 using a reference silicon solar cell (Oriel, VLSI (Very Large Scale Integration) standards. The J–V curves of both single-junction and tandem solar cells were taken with reverse and forward voltage scans with the scan speed of 0.1 V s (voltage steps of 10 mV and a delay time of 100 ms). Prior to J–V characterization, all devices were first preconditioned by continuous illumination under 1 sun (AM 1.5 G, 100 mW cm–2) for 10 sec. Single-junction devices were tested with a black metal aperture with a mask area of 0.058 cm2, and all-perovskite tandem devices were tested with a black metal aperture with a mask area of 0.09 cm2. The EQE measurement was obtained on a computer-controlled quantum efficiency measuring instrument (QE-R, Enlitech). Photoluminescence quantum yield (PLQY) was tested by Enlitech with a 405 nm laser light source. The TRPL and PL of the perovskite films were tested using the device structure of: Glass/Perovskite. The time-of-flight secondary ion mass spectrometry (TOF-SIMS) measurements were conducted with a TOF-SIMS IONTOF M6 instrument (IONTOF GmbH). A Bi3+ beam (30 keV, 0.43 pA) with a raster size of 100 μm × 100 μm was used as the primary beam to detect the samples, sputtering with an Ar+ beam (10 kV, 700 μm × 700 μm). Negative ion mode was used during measurement.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided as a Source Data file. Source data are provided with this paper.
References
Rühle, S. Tabulated values of the Shockley–Queisser limit for single junction solar cells. Sol. Energy 130, 139–147 (2016).
Brinkmann, K. O. et al. Perovskite–organic tandem solar cells with indium oxide interconnect. Nature 604, 280–286 (2022).
Lin, R. et al. All-perovskite tandem solar cells with 3D/3D bilayer perovskite heterojunction. Nature 620, 994–1000 (2023).
Lin, R. et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature 603, 73–78 (2022).
Wang, J. et al. Halide homogenization for low energy loss in 2-eV-bandgap perovskites and increased efficiency in all-perovskite triple-junction solar cells. Nat. Energy 9, 70–80 (2024).
Zhou, J. et al. Mixed tin-lead perovskites with balanced crystallization and oxidation barrier for all-perovskite tandem solar cells. Nat. Commun. 15, 2324 (2024).
Sun, Q. et al. Surface charge transfer doping of narrow-bandgap Sn–Pb perovskites for high-performance tandem solar cells. Energy Environ. Sci. 17, 2512–2520 (2024).
Tong, J. et al. Carrier lifetimes of >1 μs in Sn-Pb perovskites enable efficient all-perovskite tandem solar cells. Science 364, 475–479 (2019).
Zhu, J. et al. A donor–acceptor-type hole-selective contact reducing non-radiative recombination losses in both subcells towards efficient all-perovskite tandems. Nat. Energy 8, 714–724 (2023).
Jiang, Q. et al. Surface reaction for efficient and stable inverted perovskite solar cells. Nature 611, 278–283 (2022).
Zhu, J. et al. Custom-tailored hole transport layer using oxalic acid for high-quality tin-lead perovskites and efficient all-perovskite tandems. Sci. Adv. 10, eadl2063 (2024).
Tong, J. et al. Carrier control in Sn–Pb perovskites via 2D cation engineering for all-perovskite tandem solar cells with improved efficiency and stability. Nat. Energy 7, 642–651 (2022).
Liu, H. et al. Indium Iodide Additive Realizing Efficient Mixed Sn─Pb Perovskite Solar Cells. Adv. Energy Mater. 14, 2304234 (2024).
He, R. et al. Improving interface quality for 1-cm2 all-perovskite tandem solar cells. Nature 618, 80–86 (2023).
Zhou, S. et al. Aspartate all-in-one doping strategy enables efficient all-perovskite tandems. Nature 624, 69–73 (2023).
Cao, J. et al. High-Performance Tin–Lead Mixed-Perovskite Solar Cells with Vertical Compositional Gradient. Adv. Mater. 34, 2107729 (2022).
Zhou, W. et al. A Soldering Flux Tackles Complex Defects Chemistry in Sn-Pb Perovskite Solar Cells. Adv. Mater. 36, 2405807 (2024).
Zhao, J. et al. Suppressed Defects by Functional Thermally Cross-Linked Fullerene for High-Efficiency Tin-Lead Perovskite Solar Cells. Adv. Mater. 36, 2406246 (2024).
Leijtens, T. et al. Tin-lead halide perovskites with improved thermal and air stability for efficient all-perovskite tandem solar cells. Sustain. Energy Fuels 2, 2450–2459 (2018).
Turren-Cruz, S.-H., Hagfeldt, A. & Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 362, 449–453 (2018).
Li, C. et al. Low-bandgap mixed tin–lead iodide perovskites with reduced methylammonium for simultaneous enhancement of solar cell efficiency and stability. Nat. Energy 5, 768–776 (2020).
Jiang, T. et al. Efficient MA-free Pb-Sn alloyed low-bandgap perovskite solar cells via surface passivation. Nano Energy 101, 107596 (2022).
Yadegarifard, A. et al. FA/Cs-based mixed Pb–Sn perovskite solar cells: A review of recent advances in stability and efficiency. Nano Energy 112, 108481 (2023).
Zhang, Z. et al. Resolving Mixed Intermediate Phases in Methylammonium-Free Sn–Pb Alloyed Perovskites for High-Performance Solar Cells. Nano Micro Lett. 14, 165 (2022).
Klug, M. T. et al. Metal composition influences optoelectronic quality in mixed-metal lead–tin triiodide perovskite solar absorbers. Energy Environ. Sci. 13, 1776–1787 (2020).
Shi, H. et al. Additive Engineering for High-Performance Two-Dimensional Dion–Jacobson Pb–Sn Alloyed Perovskite Solar Cells. Energy Technol. 10, 2200983 (2022).
Zhang, W. et al. Component Distribution Regulation in Sn-Pb Perovskite Solar Cells through Selective Molecular Interaction. Adv. Mater. 35, 2303674 (2023).
Lv, S. et al. Stability of Sn-Pb mixed organic–inorganic halide perovskite solar cells: Progress, challenges, and perspectives. J. Energy Chem. 65, 371–404 (2022).
Sahamir, S. R. et al. Enhancing the Electronic Properties and Stability of High-Efficiency Tin–Lead Mixed Halide Perovskite Solar Cells via Doping Engineering. J. Phys. Chem. Lett. 13, 3130–3137 (2022).
Khadka, D. B., Shirai, Y., Yanagida, M. & Miyano, K. Attenuating the defect activities with a rubidium additive for efficient and stable Sn-based halide perovskite solar cells. J. Mater. Chem. C. 8, 2307–2313 (2020).
Wang, Z. et al. Regulation of Wide Bandgap Perovskite by Rubidium Thiocyanate for Efficient Silicon/Perovskite Tandem Solar Cells. Adv. Mater. 36, 2407681 (2024).
Zhao, Y. et al. Inactive (PbI2)2RbCl stabilizes perovskite films for efficient solar cells. Science 377, 531–534 (2022).
Zhang, W., Li, X., Fu, S., Zhao, X., Feng, X. & Fang, J. Lead-lean and MA-free perovskite solar cells with an efficiency over 20%. Joule 5, 2904–2914 (2021).
Chen, L. et al. Incorporating Potassium Citrate to Improve the Performance of Tin-Lead Perovskite Solar Cells. Adv. Energy Mater. 13, 2301218 (2023).
Mubarok, M. A. et al. Regulating the Quantum Dots Integration to Improve the Performance of Tin–Lead Perovskite Solar Cells. Adv. Energy Mater. 14, 2304276 (2024).
Liu, B. et al. Imidazole Derivative Assisted Crystallization for High-Efficiency Mixed Sn–Pb Perovskite Solar Cells. Adv. Funct. Mater. 34, 2310828 (2024).
Saliba, M. et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354, 206–209 (2016).
Kubicki, D. J., Prochowicz, D., Hofstetter, A., Zakeeruddin, S. M., Grätzel, M. & Emsley, L. Phase Segregation in Cs-, Rb- and K-Doped Mixed-Cation (MA)x(FA)1–xPbI3 Hybrid Perovskites from Solid-State NMR. J. Am. Chem. Soc. 139, 14173–14180 (2017).
Xu, Z. et al. Synergetic Optimization of Upper and Lower Surfaces of the SnO2 Electron Transport Layer for High-Performance n–i–p Perovskite Solar Cells. ACS Appl. Mater. Interfaces 16, 34377–34385 (2024).
Li, G. et al. Ionic Liquid Stabilizing High-Efficiency Tin Halide Perovskite Solar Cells. Adv. Energy Mater. 11, 2101539 (2021).
Lou, Q. et al. Room Temperature Ionic Liquid Capping Layer for High Efficiency FAPbI3 Perovskite Solar Cells with Long-Term Stability. Adv. Sci. 11, 2400117 (2024).
Zheng, G. et al. Manipulation of facet orientation in hybrid perovskite polycrystalline films by cation cascade. Nat. Commun. 9, 2793 (2018).
Tao, H. et al. A Ferrocene Capping Layer Enabling Highly Efficient and Stable Sn–Pb Mixed Perovskite Solar Cells. Sol. RRL 8, 2400035 (2024).
Meng, X. et al. Highly Stable and Efficient FASnI3-Based Perovskite Solar Cells by Introducing Hydrogen Bonding. Adv. Mater. 31, 1903721 (2019).
Tao, L. et al. Stabilizing wide-bandgap halide perovskites through hydrogen bonding. SCI CHINA CHEM 65, 1650–1660 (2022).
Hu, S. et al. Synergistic Surface Modification of Tin–Lead Perovskite Solar Cells. Adv. Mater. 35, 2208320 (2023).
Sun, Q. et al. Surface charge transfer doping of narrow-bandgap Sn-Pb perovskites for high-performance tandem solar cells. Energy Environ. Sci. 17, 2512–2520 (2024).
Zhang, K. et al. Understanding the Limitations of Charge Transporting Layers in Mixed Lead–Tin Halide Perovskite Solar Cells. Adv. Energy Sustain. Res. 3, 2100156 (2022).
Luo, Y. et al. Enhanced Efficiency and Stability of Wide-Bandgap Perovskite Solar Cells Via Molecular Modification with Piperazinium Salt. Adv. Energy Mater. 14, 2304429 (2024).
Hidayat, R. et al. Revealing the charge carrier kinetics in perovskite solar cells affected by mesoscopic structures and defect states from simple transient photovoltage measurements. Sci. Rep. 10, 19197 (2020).
Ye, F. et al. Tailoring Low-Dimensional Perovskites Passivation for Efficient Two-Step-Processed FAPbI3 Solar Cells and Modules. Adv. Energy Mater. 14, 2302775 (2024).
Wu, Z. et al. The tricyclic alkaloid catalyzed crystallization of α-FAPbI3 for high performance antisolvent-free perovskite solar cells. Energy Environ. Sci. 17, 4670–4680 (2024).
Cao, J. et al. Ultrathin Self-Assembly Two-Dimensional Metal–Organic Framework Films as Hole Transport Layers in Ideal-Bandgap Perovskite Solar Cells. ACS Energy Lett. 7, 3362–3369 (2022).
Liu, H. et al. Modulated Crystallization and Reduced VOC Deficit of Mixed Lead–Tin Perovskite Solar Cells with Antioxidant Caffeic Acid. ACS Energy Lett. 6, 2907–2916 (2021).
Zhou, P. et al. Bifunctional Dimethyldichlorosilane Assisted Air-Processed Perovskite Solar Cell with Enhanced Stability and Low Voltage Loss. Sol. RRL 7, 2201067 (2023).
Naik, N. R., Gabritchidze, B., Chen, J. H., Cleary, K. A., Kooi, J. & Minnich, A. J. Investigation of cryogenic current–voltage anomalies in SiGe HBTs: Role of base–emitter junction inhomogeneities. J. Appl. Phys. 135, 164501 (2024).
Barthel, A. et al. Open-circuit voltage degradation and trap-assisted tunneling in electron and proton-irradiated ultra-thin GaAs solar cells. J. Appl. Phys. 135, 224505 (2024).
Li, H., Han, Z., Zhou, X., Xu, G. & Long, S. Negative bias stress stable PtOx/InGaZnOx Schottky barrier diodes optimized by oxygen annealing. J. Appl. Phys. 135, 114501 (2024).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (52202293 and 52330004, J.T.), and the Fundamental Research Funds for the Central Universities (WUT: 2023IVA075 and 2023IVB009, J.T.). C.X. acknowledges the financial support from National Natural Science Foundation of China (52302327, C.X.). Z. R. thanks the financial support from RISE project Grant (Q-CDBK, Z.R.), Start-up Fund for RAPs under the Strategic Hiring Scheme (PoluU) (1-BD1H, Z.R.),PRl Strategic Grant (1-CD7X, Z.R.), and RI-iWEAR Strategic Supporting Scheme (1-CD94, Z.R.). Y.B. acknowledges the financial support from the National Natural Science Foundation of China (No. 52302333, Y.B.), Guangdong Basic and Applied Basic Research Foundation (2023A1515012788, Y.B.), and Shenzhen Science and Technology Program (KQTD20221101093647058, Y.B.).
Author information
Authors and Affiliations
Contributions
J.T. conceived the idea and supervised the project; J.T. and X.L.(Xufeng Liao) designed the experiments and wrote the manuscript. X.L. (Xufeng Liao) fabricated NBG PSCs and all-perovskite tandem cells, and performed film and device characterizations. X.J. helped with the fabrication of WBG subcells in all-perovskite tandem cells and performed all-perovskite tandem cells device characterizations. W.L., J.Z. and X.Z. offered help in film characterization. Y.J. contributed to the NBG PSCs EQE characterization under the guidance of W.H.; C.K. measured the GIWAXS characterization.; X.L. (Xiting Lang) did the AFM measurement under the guidance of C.X.; Q.L. helped with the PL measurement; Q.D., Z.R., Y.B., K.Z., and Y-B.C. provided valuable suggestions for the manuscript; All authors discussed the results and contributed to the revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hang Zhou, Wei Chen, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liao, X., Jia, X., Li, W. et al. Methylammonium-free, high-efficiency, and stable all-perovskite tandem solar cells enabled by multifunctional rubidium acetate. Nat Commun 16, 1164 (2025). https://doi.org/10.1038/s41467-025-56549-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56549-8