Abstract
The synergistic Cu0-Cu+ sites is regarded as the active species towards NH3 synthesis from the nitrate electrochemical reduction reaction (NO3-RR) process. However, the mechanistic understanding and the roles of Cu0 and Cu+ remain exclusive. The big obstacle is that it is challenging to effectively regulate the interfacial motifs of Cu0-Cu+ sites. In this paper, we describe the tunable construction of Cu0-Cu+ interfacial structure by modulating the size-effect of Cu2O nanocube electrocatalysts to NO3-RR performance. We elucidate the formation mechanism of Cu0-Cu+ motifs by correlating the macroscopic particle size with the microscopic coordinated structure properties, and identify the synergistic effect of Cu0-Cu+ motifs on NO3-RR. Based on the rational design of Cu0-Cu+ interfacial electrocatalyst, we develop an efficient paired-electrolysis system to simultaneously achieve the efficient production of NH3 and 2,5-furandicarboxylic acid at an industrially relevant current densities (2 A cm−2), while maintaining high Faradaic efficiencies, high yield rates, and long-term operational stability in a 100 cm2 electrolyzers, indicating promising practical applications.
Similar content being viewed by others
Introduction
Ammonia (NH3), as a versatile industrial feedstock, is widely used for the production of nitrogen-based fertilizers, chemicals, pharmaceuticals, and polymers. Industrial NH3 production relies on the Haber-Bosch process, under high temperature and high pressure (i.e. 350–450 °C and 150–200 bar)1. The carbon-intensive and highly energy-consuming Haber-Bosch process consumes about 1% of the world’s annual energy output and generates 1.3% of global carbon dioxide emissions2,3. It is urgent and necessary to develop environmentally sustainable and affordable routes for NH3 synthesis. Recently, the nitrate (NO3−) electrochemical reduction reaction (NO3−RR) has attracted increasing attention to produce NH3 under mild conditions4. However, the performance of NO3−RR is hindered by the sluggish kinetics, which involves an eight-electron transfer, and the complex reaction pathways with multiple by-products, such as NO2−, N2, N2H4, and H25. Moreover, the competitive hydrogen evolution reaction (HER) also hampers the Faradaic efficiency6. Thus, it is crucial to explore and develop a highly efficient electrocatalyst to compress the HER process and enable the desired conversion of NO3− into high-valued NH3.
Recently, oxide-derived (OD) Cu catalyst has been widely studied due to excellent NO3−RR activity towards valuable NH37. Mechanistic studies have demonstrated that OD Cu catalyst would be electrochemically reduced to unsaturated Cu species under negative potential, and the synergistic Cu0–Cu+ sites is regarded as the active species towards NH3 synthesis during the NO3−RR process8,9. Therefore, numerous researches has been conducted to synthesize OD Cu catalyst with Cu0–Cu+ sites, and revealed the synergistic mechanism in the NO3−RR process9,10,11,12. The studies also showed that the interfacial motifs would determine the adsorption mode of nitro-intermediates and protons to significantly influence nitrogen selectivity10,11. However, the reaction mechanism and individual roles of interfacial Cu0–Cu+ sites are still unclear. One of the big obstacles is that it is challenging to effectively regulate the interfacial Cu0–Cu+ structure, since the structural motif of OD Cu is unstable under negative potential. Some studies have be devoted to control interfacial structure of Cu0−Cu+ sites by the strong metal-support interaction13, while the electronic interaction between support and active Cu sites would bring to inevitable interference to identify the individual roles of interfacial Cu0–Cu+ sites. Therefore, with the goal of enhancing the NO3-RR performance of Cu-based electrocatalysts, it is of great necessary to understand the interface behavior and reaction mechanism by controllable Cu+–Cu0 sites.
Herein, we developed a feasible and efficient strategy to construct tunable Cu+–Cu0 interfacial motifs by modulating the size effects of Cu2O nanocube catalysts. We revealed the design principle of Cu+–Cu0 pairs by correlating the macroscopic particle size with the microscopic localized coordinated structure properties by in situ electrochemical Raman and X-ray absorption near edge structure (XANES) characterization. Based on the controllable construction of the Cu+–Cu0 interfacial structure, we elucidated the individual and synergic roles of Cu+ and Cu0 sites during the NO3−RR process by combining electrochemical measurement and DFT calculations. Then, we developed an efficient paired-electrolysis system to simultaneously achieve the efficient production of NH3 and 2,5-furandicarboxylic acid (FDCA) by coupling the cathodic NO3−RR process and anodic HMF electrochemical oxidation reaction (HMFOR) process at an industrially relevant current density, while maintaining high Faradaic efficiencies (FENH3 75.6%, FEFDCA 71.2%), yield rates of NH3 (5.20 mmol h−1 cm−2) and FDCA (0.47 mmol h−1 cm−2), and long-term operational stability (20 h) in a 100 cm2 anion exchange membrane (AEM) electrolyzers, indicating promising practical applications. The techno-economic analysis demonstrates the potential of this system.
Results
Structural characterization
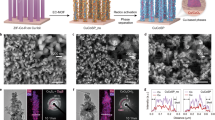
The Cu2O nanocubes with different particle sizes are synthesized through the liquid phase reduction method, by adjusting the localized pH environment of the synthetic solution14, three kinds of Cu2O catalysts with different particle sizes were obtained, denoted as small Cu2O nanocube (S-Cu2O), medium Cu2O nanocube (M-Cu2O) and large Cu2O nanocube (L-Cu2O), respectively (Supplementary Fig. 1). The Cu+–Cu0 sites is controllably constructed by the in situ electrochemical reduction of Cu2O nanocube precursors. The morphology and particle size of the Cu2O nanocube precursors were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), both Cu2O sample exhibit cube-like nanocrystals (Fig. 1a–c), and the average size are concentrated in ~166 nm (S-Cu2O), ~235 nm (M-Cu2O) and ~760 nm (L-Cu2O), respectively (Fig. 1d). Energy dispersive spectroscopic (EDS) elemental mapping further revealed that Cu and O elements are evenly distributed in the Cu2O nanocubes and the ratio of Cu to O elements is close to 2:1. (Supplementary Fig. 2-4). The crystal structure of the Cu2O nanocube was confirmed by high-resolution transmission electron microscopy (HRTEM) and X-ray diffraction (XRD). The lattice distance of 0.242 nm was found in the HR-TEM images (Supplementary Figs. 2–4), indicating that the dominant lattice plane is not influenced by the particle size, and (111) lattice plane is dominated on both Cu2O samples. The XRD patterns confirmed the Cu2O cubic phase (JCPDS: 78-2076) on both samples without the impurity phase (Fig. 1e).
a–c SEM images of S-Cu2O, M-Cu2O, and L-Cu2O. Inset is the corresponding TEM images. d Statistical size distribution of nanoparticles of S-Cu2O, M-Cu2O and L-Cu2O. e XRD patterns of S-Cu2O, M-Cu2O, and L-Cu2O. f EPR patterns of S-Cu2O, M-Cu2O and L-Cu2O. Source data for d–f are provided as a Source Data file.
X-ray photoelectron spectroscopy (XPS) was used to investigate the chemical states and electronic structure of Cu and O on the various Cu2O nanocubes (Supplementary Fig. 5). In Cu 2p XPS spectrum, there are two peaks in both Cu2O samples at 952.42 and 932.6 eV, which are attributed to Cu+/Cu0 species (Supplementary Fig. 6)15. Further, the Auger electron spectroscopy (AES) was employed to distinguish Cu+ and Cu0 (Supplementary Fig. 7), it was found that Cu+ (~569.6 eV) characteristic Auger peaks dominated on the Cu2O surface, the signal of Cu0 was not detected on the AES spectrum9. The content of surface oxygen species was identified by the O 1s XPS spectra (Supplementary Fig. 8), it is interesting that the higher content of chemisorbed oxygen groups (~531.0 eV), which belonged to defect-oxide groups, was found on the M-Cu2O and S-Cu2O sample16. The concentration of defect sites was further evaluated by the electron paramagnetic resonance (EPR), a representative EPR signal of g = 2.003 was found in both Cu2O samples (Fig. 1f), revealing the electron trapped by oxygen vacancies9. The sequence of signal intensity is S-Cu2O > M-Cu2O > L-Cu2O, indicating the higher content of oxygen vacancies on the small-size Cu2O nanocubes, which is consistent with the XPS results. According to the principle of surface chemistry, the nanosized material not only shows a higher specific surface area, but also exposes higher content of unsaturated coordination sites, such as grain boundaries and edge sites, on the surface, and the concentration of unsaturated coordination sites is negatively related to the crystal size of materials. Therefore, it is rational speculate that the negative correlation of the concentration of defect sites and the particles size of Cu2O nanocubes.
Then, the Cu0–Cu+ sites was constructed by a constant potentiostatic reduction on Cu2O precursor in 1 M KOH and 0.1 M NO3- solution (pH =13.8) under a negative potential of −0.6 V vs RHE, ensuring to acquire the stable Cu-Cu+ interfacial sites. The morphology structure and composition was observed in the SEM and TEM images, it was found that the Cu2O catalysts retained the morphology of the nanocube, while the surface roughness increased after the electrochemical reduction process (Supplementary Figs. 9–12). Then, the structural evolution is investigated by the XRD patterns and HRTEM images, the results show that the crystal structure of Cu2O and Cu could be simultaneously observed on both Cu2O samples, indicating that the Cu2O precursor is partially transferred to metallic Cu species for the formation of Cu/Cu2O interface structure during the electrochemical reduction process (Supplementary Figs. 10–13), the as-obtained Cu2O catalysts with Cu/Cu2O interface structure is denoted as S-Cu/Cu2O, M-Cu/Cu2O, and L-Cu/Cu2O, respectively.
Further, the in situ Raman spectroscopy was conducted to identify the dynamic phase transformation during the electrochemical reduction process. As shown in Fig. 2a and Supplementary Fig. 14, the characteristic peaks at 147, 218, 415, and 639 cm−1 were observed at the open circuit potential, which is attributed to the T1u, Eu, Au, and T2g structural mode of Cu2O precursors17. When the bias potential is tuned to −0.6 VRHE, the signal of Cu2O is rapidly dropped in the first two minutes and gradually tends to be stable, suggesting the structural evolution of Cu2O to Cu/Cu2O interface structure. The relative intensity of the Cu2O signal could be used for evaluating the relative content of Cu2O and identifying the Cu/Cu2O interfacial structure after the electrochemical reduction process. Interestingly, the relative intensity follows the order of S-Cu/Cu2O < M-Cu/Cu2O < L-Cu/Cu2O (Fig. 2b), indicating that the small particle size Cu2O (S-Cu2O), which owns a high concentration of defect sites, is more tended to transform to metallic Cu under electroreduction process.
a In situ Raman spectra collected during the electrochemical reduction process on various Cu2O samples. b The relationship between the intensity of characterized Raman signals and the time of electrochemical reduction. c AES spectra of Cu LMM over various Cu2O nanocube catalysts after the electrochemical reduction process. d Normalized Cu K-edge XANES spectra. Inset is the magnification of Cu K-edge spectra. e Fourier transforms of Cu K-edge EXAFS spectra with optimal fitting results for various Cu2O nanocube catalysts after electrochemical reduction process. f Structural coherence changes in EXAFS coordination number of Cu–Cu bonds and Cu–O–Cu bonds. g Schematic diagram of structural transformation of Cu2O with different sizes. Source data for a–f are provided as a Source Data file.
Though in situ Raman spectroscopy is an efficient strategy to identify the bending mode for Cu–O bonds, it is difficult to accurately identify metallic Cu species during the electrochemical reduction process. Thus, the electronic structure and valence states of the Cu/Cu2O structure is carefully evaluated by the AES spectrum. By quantifying the content of Cu+ and Cu0 in the Cu LMM spectra (Fig. 2c), the highest proportion of Cu0 was found in S-Cu/Cu2O is 78.9%, followed by M-Cu/Cu2O (53.1%) and L-Cu/Cu2O (38.4%), which is consistent with the in situ Raman spectra. Further, the XANES and extended X-ray absorption fine structure (EXAFS) were used for investigating the coordination environment and local electronic structure of Cu2O catalysts after electroreduction (Fig. 2d, e). The secondary adsorption band (8983 eV), corresponding to a 1s → 4p transition concurrent with ligand to metal charge transfer character, is used to evaluate the coordination structure of Cu0–Cu+ sites in XANES spectra18. After the electrochemical reduction process, the lower intensity of the secondary adsorption band was found on both Cu2O samples, suggesting the appearance of unsaturated coordination structures. It was found that the S-Cu/Cu2O shows the lowest adsorption intensity than M-Cu/Cu2O and L-Cu/Cu2O indicates a high degree of local structural disorder with a low oxidation state. Further, the fine coordination environment and geometric interface structure on the Cu0–Cu+ sites is observed in the EXAFS spectra. As shown in Fig. 2e, the characteristic coordination shell of the Cu–O bond (~1.5 Å) and Cu–O–Cu bond (~2.8 Å) were observed in Cu2O precursors19. Interestingly, after reduction electrolysis, an extra coordination shell was found at ~2.5 Å20, which contributed to the Cu0–Cu bond on the metallic Cu species, indicating the appearance of Cu0–Cu+ interfacial structure during the electroreduction process. To accurately identify the fine coordination structure, the EXAFS spectra were fitted according to the optimal structure parameters, the corresponding fitting curves and structural information are displayed in Supplementary Figs. 15 and 16 and Supplementary Table 1. According to the EPR results, the S-Cu/Cu2O has the higher concentration of unsaturated coordination structure, confirmed by the coordination number in Fig. 2f. The unsaturated defect Cu–O species on the Cu2O surface, which own higher surface energy, tended to be reduced to metallic Cu species during the electrochemical reduction process. Thus, it was found that the coordination number (CN) of the Cu–O–Cu bond is dramatically reduced to metallic Cu species on S-Cu/Cu2O with the appearance of the higher content of the Cu–Cu bond (Fig. 2f), indicating that the formation of metallic-Cu-dominated Cu/Cu2O interface structure after the electroreduction process. On the contrary, owing to low defect concentration on large-sized Cu2O, we found that the Cu2O is slightly reduced to metallic Cu species on L-Cu/Cu2O sample to form Cu+-dominated Cu/Cu2O interface structure. Therefore, by rational tuning the particle size of Cu2O precursors, the Cu/Cu2O interface structure with a flexible ratio of Cu0 and Cu+ could be effectively constructed.
Electrocatalytic performance for ammonia synthesis
Above, the electrocatalyst with Cu/Cu2O interface structure has been constructed by modulating the size effect of Cu2O catalysts. Then, the NO3−RR performance of as-synthesized Cu/Cu2O catalysts was studied in a standard three-electrode H-type electrolytic cell. The electrochemical behavior was identified by the linear sweep voltammetry (LSV) curve. In the 1 M KOH solution, L-Cu/Cu2O exhibits higher hydrogen evolution reaction (HER) activity than M-Cu/Cu2O and S-Cu/Cu2O (Supplementary Fig. 17), which is attributed to the higher content of Cu+ species on the L-Cu/Cu2O. The Cu+ species has a lower energy barrier for the water-dissociation process than the Cu0 species, resulting in rapid HER dynamics21. The NO3-RR performance is evaluated in the 1 M KOH with 0.1 M KNO3 solution. As shown in Fig. 3a, the M-Cu/Cu2O exhibits a higher current density than S-Cu/Cu2O and L-Cu/Cu2O, which is inconsistent with HER activity, indicating that the NO3-RR activity is not only influenced by the formation of adsorbed *H from water dissociation, but also strongly related to the adsorption of NO3- and NO3−RR intermediates. The intrinsic NO3−RR performance was evaluated by normalized by the electrochemically active surface area (ECSA), which investigated by non-faradaic double layer capacitance (Cdl) in Supplementary Fig. 18. It was found that M-Cu/Cu2O still shows the best intrinsic activity, indicating that the M-Cu/Cu2O has the optimal Cu0–Cu+ interface structure for catalyzing NO3−RR process (Supplementary Fig. 19). To investigate the NO3−RR dynamic process, the Tafel slope and electrochemical impedance spectroscopy (EIS) were conducted. The results of Tafel slopes shown that the M-Cu/Cu2O has a faster electron transfer rate and rapid kinetics for NO3−RR (Supplementary Fig. 20). The electrochemical impedance was performed and fitted by optimal equivalent circuit, and the resistance of each component was shown in Supplementary Table 2. M-Cu/Cu2O showed the lowest charge transfer resistance (Rct), indicating that the appropriate Cu0–Cu+ interface structure would accelerate the charge transfer process and promote the NO3−RR kinetics (Supplementary Fig. 21).
a LSV curves of various Cu/Cu2O catalysts in the 1 M KOH with 0.1 M KNO3 solution with the scan rate of 5 mV s−1. The potential value is non-iR corrected. Faradaic efficiency (b) and yield rate (c) of NH3 over various Cu/Cu2O catalysts at different applied potentials. Data are presented as mean ± s.d. (n = 3) with the error bars representing the s.d. d Comparisons of NH3 yield rates and Faraday efficiency between the M-Cu/Cu2O and typically reported NH3-synthesis electrocatalysts. e Faradaic efficiency of NH3 production and NO3- reduction rates in the 1 M KOH with various concentrations of KNO3 solution over M-Cu/Cu2O catalysts at −0.2 V vs. RHE. f The Faradaic efficiency and yield rate of NH3 over M-Cu/Cu2O catalysts during the stability measurement. Source data for a–f are provided as a Source Data file.
The performance for the transformation of NO3− to NH3 is evaluated by the electrochemical chronoamperometry measurement over S-Cu/Cu2O, M-Cu/Cu2O, and L-Cu/Cu2O. The substrates, intermediates, and products were quantified by ultraviolet-visible (UV–vis) spectrophotometry, and the calibration curves are displayed in Supplementary Figs. 22–24. It was found that M-Cu/Cu2O exhibits a maximum NH3 Faradaic efficiency (FEs) of 95% at −0.2 VRHE in Fig. 3b, which is much higher than that of S-Cu/Cu2O (80%) and L-Cu/Cu2O (86%). Moreover, an increasing yield rate of NH3 and NO3− reduction rate was observed with the increase of applied negative potentials (Fig. 3c and Supplementary Fig. 25), while reached 0.29 mmol h−1 mg-1 and 0.25 mmol h−1 mg-1 towards NO3− reduction and NH3 production, respectively, at the potential of −0.2VRHE on M-Cu/Cu2O, which is better than recently reported NO3RR electrocatalysts (Fig. 3d and Supplementary Table 3). To enhance the reliability of size strategy, the M2-Cu/Cu2O is synthesized with size (~402 nm) between M-Cu/Cu2O and L-Cu/Cu2O (Supplementary Fig. 26). Then, the electrocatalytic NO3−RR performance towards NH3 production is evaluated in the 1 M KOH with 0.1 M KNO3 solution. As shown in Supplementary Fig. 27, the NH3 Faradaic efficiency (FEs) of M2-Cu/Cu2O (87.6%) is slightly lower than M-Cu/Cu2O (95%) and higher then L-Cu/Cu2O (86%), indicating that size-effect strategy is convincing.
To confirm the adaptability of NH3 production capability, the NO3RR performance was studied at different NO3− concentrations over M-Cu/Cu2O catalysts (Fig. 3e). It was found that the FE of NH3 would also keep ~80% in a low concentration of NO3- solution (5 mM), indicating that M-Cu/Cu2O also has a potential application prospect in the electroreduction of wastewater to NH3. Furthermore, it was found that the NH3 FE and yield rate remained stable after 10 electrolytic cycles (Fig. 3f and Supplementary Fig. 28). Notably, we observed that the crystal structure and phase composition of M-Cu/Cu2O catalysts after electrolysis is similar to the fresh M-Cu/Cu2O catalysts, indicating excellent stability of M-Cu/Cu2O (Supplementary Fig. 29).
To verify the source of NH3, controlled measurements were conducted by electrolysis in 1 M KOH solution with the absence of NO3−. It was found that the NH3 produced is negligible, indicating that the acquirement of NH3 originated from NO3−RR (Supplementary Figs. 30 and 31). Moreover, isotope labeling experiments were carried out with K14NO3 and K15NO3 as nitrates (Supplementary Fig. 32), and the products were determined by 1H NMR spectroscopy. The results show that the typical two peaks of 14NH4+ and the typical three peaks of 15NH4+ in 1H NMR, which confirms that the synthesized NH3 comes from NO3− in solution rather than environmental contamination.
Mechanistic studies
Firstly, the possible reaction pathway and multiple intermediates were preliminarily evaluated via in situ Attenuated Total Refraction Fourier Transform Infrared Spectrometer (ATR-FTIR). As displayed in Fig. 4a and Supplementary Fig. 33, under the applied potential of −0.2 VRHE, the peak at 1240 cm–1 and 1640 cm–1 were assigned to the N−O antisymmetric stretching of NO2– and the adsorption of *NO intermediates, and the doublets of 1423 cm–1 and 1457 cm–1 is attributed to NH4+ species22,23, indicating that the pathway of *NO3− → *NO2− → *NO → *N → *NH → *NH2 → *NH3 is dominated on Cu/Cu2O interfacial catalysts. Moreover, in situ differential electrochemical mass spectrometry (DEMS) is used to confirm the key intermediates during the NO3RR process. As shown in Supplementary Fig. 34, the six possible NO3RR intermediates could be observed on the DEMS signals at m/z 46 (*NO2), 30 (*NO), 14 (*N), 15 (*NH), 16 (*NH2), and 17 (*NH3), respectively24.
a In situ FTIR spectra of M-Cu/Cu2O catalyst during the nitrate reduction at various electrolytic times. b The evaluation of nitrogen selectivity and conversion rate over various Cu/Cu2O catalysts at −0.2 V vs. RHE. c, d ESR spectra of DMPO adducts over various Cu/Cu2O nanocube catalysts in the absence and presence of nitrate, the signal is collected after 15 min electrolysis at -0.2 V vs. RHE. e The transformation of NO3− over various Cu/Cu2O nanocube catalysts with the presence of 10 mM TBA solution. f LSV curves of M-Cu/Cu2O catalyst in the absence and presence of SCN− solution. The potential value is non-iR corrected. g Gibbs free energy diagrams of the conversion of nitrate to ammonia over various Cu/Cu2O catalysts; h the kinetic energy barriers of potential limiting steps over the various Cu/Cu2O catalysts with CI-NEB method. Source data for a–h are provided as a Source Data file.
To identify the mechanism of NO3−RR on various Cu0–Cu+ interfacial catalysts, the product distribution of NO3−RR is evaluated at the applied potential of −0.2 VRHE, it was found that the M-Cu/Cu2O shows the highest NH3 selectivity (93.6%) and NO3− conversion (86.2%) than S-Cu/Cu2O and L-Cu/Cu2O in Fig. 4b. Besides, the production of NO2−, derived from two-electron NO3− reduction process, is founded in the solution, which is attributed to the weakly reduction ability of NO2− to NH3. Further, by revealing potential-dependent production selectivity, it was found that S-Cu/Cu2O tended to generate NO2− at low potential, resulting in higher NO2− FEs than M-Cu/Cu2O and L-Cu/Cu2O at the same potential (Supplementary Fig. 35), suggesting that the NO2− electrochemical reduction step is sluggish on the metallic-Cu-dominated Cu/Cu2O interface structure, which leads to the abundant NO2− accumulation during the NO3−RR process. On the contrary, the rapid NO2− electrochemical hydrogenation is observed on the Cu+-dominated Cu/Cu2O interfacial catalysts, indicating that the Cu2O is the active species for the NO2− → NH3 process.
It is known that two possible mechanisms of NO3− reduction reaction: direct electron transfer process and atomic H*-mediated indirect reduction process. For the atomic *H-mediated indirect reduction process, the water molecule would be firstly dissociated to *H and *OH on the surface of electrode, then a sequential electrochemical NO3_ hydrogenation process is conducted by utilizing the adsorbed *H species. Different from the *H-mediated pathway, the NO3- adsorbed on catalysts would be electrochemically reduced by directly accepting electrons from catalysts without the involvement of water dissociation via the indirect reduction process. The electron spin resonance (ESR) is used to confirm the pathway during the NO3->RR process. Without adding NO3− in catholyte, the ESR spectra display typical 9 signals with intensity ratios of 1:1:2:1:2:1:2:1:1 (Fig. 4c), which confirms the formation of DMPO-H in the solution. However, when NO3− was added to the solution, the typical 9 signals were decreased (Fig. 4d), indicating that the adsorbed H* is consumed by the NO3−RR intermediates via atomic *H-mediated indirect reduction pathways over various Cu/Cu2O catalysts. The sequence of DMPO-H signal intensity is S-Cu/Cu2O < M-Cu/Cu2O < L-Cu/Cu2O, indicating Cu2O species is tended to produce sufficient atomic *H adsorbed to participate in the hydrogenation of NO3RR intermediates. The quenching experiments are conducted to identify the possible pathway on the various Cu-Cu2O interfacial catalysts, and tertiary butyl alcohol (TBA) serves as a scavenger to capture atomic *H25. As shown in Fig. 4e, a decrease of NO3− kinetics on various Cu/Cu2O catalysts was observed when 10 mM TBA was added into the solution, indicating that the atomic *H-mediated indirect reduction pathway is dominated. Compared to S-Cu/Cu2O, M-Cu/Cu2O and L-Cu/Cu2O catalysts showed a rapid NO3− RR kinetics suggesting that Cu2O species would provide sufficient atomic hydrogen for NO3RR process, which is consistent with ESR results.
Besides, it is expected to identify the roles of Cu sites on the Cu0–Cu+ interfacial catalysts, a poisoning experiment was implemented using KSCN species19. Since the SCN− is a strongly Lewis alkalinity, which would be selectively adsorbed on the metallic Cu sites and competed with NO3−, when SCN- ions were added to the solution, the current density of NO3RR decreased sharply (Fig. 4f), suggesting that the metallic Cu sites, in Cu0–Cu+ interface, play a vital role during the NO3−RR process.
Further, the DFT calculations were performed to elucidate the mechanism over the Cu0–Cu+ interface structure. Owing to strong electron correlation, the Hubbard-U parameter is used to prevent the delocalization of Cu d-orbitals. To accurately simulate the Cu0–Cu+ interface structure, the model of S-Cu/Cu2O, M-Cu/Cu2O, and L-Cu/Cu2O catalysts is constructed and optimized based on the atomic ratio of Cu0/Cu+ species according to the AES and EXAFS results (detailed in the Supplementary information and Supplementary Data 1). Based on the ATR-FTIR results, the pathway of *NO3− → *NO2− → *NO → *N → *NH → *NH2 → *NH3 is proposed to investigate the reaction mechanism, and the optimal adsorption configuration of the reaction intermediates on various Cu/Cu2O catalysts is displayed in Supplementary Figs. 36–40.
The adsorption behavior of NO3− reactant is investigated on the active Cu0–Cu+ sites, it was found that NO3− tends to adsorb on the metallic Cu0 sites rather than Cu+ sites or Cu0–Cu+ interface sites over various Cu/Cu2O catalysts (Supplementary Fig. 36). Owing to the oxyphility of metallic Cu species, its tends to produce strong binding interactions with oxygenated species, resulting to the strong adsorption of NO3− reactant (Supplementary Fig. 37)11,26. It was found that the NO3‒ adsorption energy of L-Cu/Cu2O (−0.35 eV) is lower than M-Cu/Cu2O (−0.70 eV) and S-Cu/Cu2O (−0.79 eV), suggesting that introduction of Cu2O benefits to weaken the NO3− adsorption on the surface of Cu sites. The open circuit potential (OCP) measurement is also used to identify the adsorption of NO3− on the various Cu/Cu2O electrocatalysts27,28. After the injection of KNO3, the S-Cu/Cu2O (0.14 V) showed a significant decrease in OCP than the M-Cu/Cu2O (0.11 V) and L-Cu/Cu2O (0.06 V), indicating that Cu+ helps to weaken NO3− adsorption (Supplementary Fig. 41), which is consistent with the results of DFT calculation.
Then, according to the proposed reaction pathway, the free energy of NO3RR was investigated to identify the mechanism of synergistic Cu0–Cu+ motifs on various Cu/Cu2O electrocatalysts. As shown in Fig. 4g, it was found that the hydrogenation step of *NH2 → *NH3 is regarded as the potential limiting step (PLS) on the M-Cu/Cu2O and S-Cu/Cu2O, while the hydrogenation step of *NH → *NH2 is considered as PLS on the L-Cu/Cu2O. It is interesting that the free energy change of PLS for M-Cu/Cu2O (0.11 eV) is slightly lower than S-Cu/Cu2O (0.22 eV) and L-Cu/Cu2O (0.20 eV), indicating that the NO3RR process is thermodynamically favorable on the M-Cu/Cu2O electrocatalysts. Moreover, owing to the high energy barrier (0.96 eV), we have excluded possible pathway of *NO coupling for the formation of nitrogen gas (N2) on the M-Cu/Cu2O materials (Supplementary Fig. 42).
It was noticed that the water-dissociation reaction (H2O → *H + *OH) process is crucial to provide atomic hydrogen species for the hydrogenation of NO3RR intermediates29,30, while the H2O molecules may be competitive adsorbed on the surface of electrode. Therefore, we considered the adsorption and activation behavior of H2O molecules, it was found that the dissociation of the H2O molecule needs to overcome a high kinetic energy barrier of 1.21 eV on the M-Cu/Cu2O (Supplementary Fig. 43). Such a high energy barrier clearly reflects the sluggish water-dissociation kinetics and poor HER activity (Supplementary Fig. 17). Besides, the OH- species may also be competitive adsorbed on the Cu sites, we found that the direct reduction of adsorbed *OH species also requires endothermic 0.24 eV, which is higher than NO3RR (0.11 eV), demonstrating that M-Cu/Cu2O exhibits the high selectivity for NO3RR.
Further, we also explore the kinetic energy barrier for the PLS of the different Cu/Cu2O materials. As shown in Fig. 4h, the kinetic energy barrier of the PLS is 0.51 eV on the M-Cu/Cu2O, which is lower than S-Cu/Cu2O (0.64 eV), and L-Cu/Cu2O (1.29 eV), indicating that the M-Cu/Cu2O, with the optimal interface structure, is favorable in kinetics for NO3 electrochemical reduction process.
Paired-electrolysis NO3 −RR//HMFOR system
Traditional electrocatalytic nitrate reduction systems involve a cathodic nitrate reduction reaction and anodic oxygen production reaction (OER). Though the efficient transformation of NO3− to NH3 has been achieved by modulating the size effect of the Cu2O electrocatalyst, the sluggish anodic OER process would dramatically enhance the energy consumption for NH3 production. Thus, it is expected to explore promising anodic reactions for the replacement of the OER process to reduce energy consumption. The electrooxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) has been attracted to replace the traditional OER process to reduce cell voltage and achieve the production of high-value chemicals. Since the Ni-based catalyst has excellent performance of HMF electrochemical oxidation reaction (HMFOR), the Ni3Se4, as a representative Ni-based material31,32, was selected as anode electrocatalyst to assemble a paired electrolysis NO3-RR//HMFOR system (Fig. 5a).
a, b Scheme and digital photo of NO3−RR//HMFOR paired-electrolysis system. c The LSV curves of overall water splitting systems and NO3-RR//HMFOR Paired-electrolysis system. Potential-dependent Faradaic efficiency and yield rate of NH3 (d) and FDCA (e) in AEM electrolyzer. f Stability measurement of NO3−RR//HMFOR Paired-electrolysis system at the current density of 2 A cm−2. g Techno-economic analysis of NO3−RR//HMFOR paired-electrolysis system. Source data for c–g are provided as a Source Data file.
To further verify the NO3−RR coupled HMFOR strategy, a simple scale-up test was carried out in an AEM electrolytic cell with a working area of 100 cm2, and the M-Cu/Cu2O and Ni3Se4 served as cathode and anode catalyst, respectively. The crystal structure and morphology is characterized by XRD pattern, SEM, and TEM images in Supplementary Figs. 44 and 45, and the HMFOR performance is evaluated in Supplementary Fig. 46. Digital photos and structural diagrams of AEM are shown in Fig. 5b. When coupling anodic HMFOR process and cathodic NO3RR process, the cell voltage is significantly reduced (Fig.5c), indicating that the high energy conversion efficiency of NO3RR//HMFOR system. The product measurement was performed at the different applied potentials as shown in Fig. 5d, e. The highly NH4+ yield rates (5.20 mmol h−1 cm−2) and FE (75.6 %) with excellent FDCA yield rates (0.47 mmol h−1 cm−2) and FE (71.2 %) were achieved at the potential of 2.1 V. Moreover, the NH4+ and FDCA could be continuously and stably produced at the current density of 2 A cm-2, indicating the excellent stability of this electrolysis system (Fig. 5f). Further, we assembled a product extraction system to extract the cathode product (NH4+) and the anode product FDCA from the electrolyte. The purified product was confirmed by the XRD pattern in Supplementary Fig. 47. Based on the above results, it can be determined that the NO3RR//HMFOR system would significantly improve energy efficiency, ensuring the economy and practicability, TEA results show that the economic benefits generated by the system are greater than the cost input (Fig. 5g).
Discussion
In summary, we developed a promising strategy to achieve the controllable construction of Cu0/Cu+ interfacial structure by modulating the size effect of Cu2O nanocube electrocatalysts. Combining the in situ electrochemical Raman and X-ray absorption near edge structure (XANES) characterization, the design principle of Cu0–Cu+ pairs has been revealed by correlating the macroscopic particle size with the microscopic localized coordinated structure properties. Based on the controllable construction of the Cu0–Cu+ interfacial structure, we elucidated the synergic roles of Cu+ and Cu0 sites during the NO3−RR process by combining electrochemical measurement and DFT calculations. The results reveal that Cu0 is the main active site in the Cu0–Cu+ motifs, Cu+ helps to weaken NO3− adsorption, provides atomic *H species to accelerate potential limiting step of the hydrogenation of NH2 to NH3. Based on the design of Cu+–Cu0 interfacial electrocatalyst, we developed an efficient NO3−RR//HMFOR paired electrolysis system to simultaneously achieve the efficient production of NH3 and FDCA at an industrially relevant current density, while maintaining high Faradaic efficiencies (75.6% for NH3, 71.2% for FDCA), yield rates (5.20 mmol h−1 cm−2 for NH3, 0.47 mmol h−1 cm−2 for FDCA), and long-term operational stability (20 h) in AEM electrolyzers with an area of 100 cm2. This work provides a strategy to design energy-effective and eco-friendly electrocatalysts for large-scale industrial electrolytic synthesis of high-value-added products.
Method
Chemical reagents
KNO3 (Kermel, GR, 99%), KNO2 (Kermel, GR, 99%) NH4Cl (Aladdin, AR, 99%), 5-hydroxymethylfurfural (98%) were purchased from Energy Chemical. 5-hydroxymethyl-2-furancarboxylic acid (98%), 5-formyl-furancarboxylic acid (99%), 2,5-diformyfuran (98%), and 2,5-furandicarboxylic acid (98%) were obtained from Aladdin. Cu(CH3COO)2·H2O (Aladdin, AR, 99%), L-Ascorbic acid (Macklin, AR, 99%), and NaOH (Macklin, AR, 99%) were used without further purification. Deionized water (UPR series super pure water purification system, resistivity >18.2 MΩ cm) was used to prepare all solutions.
Materials preparation
Synthesis of Cu2O nanocubes
In a typical synthesis, a mixture of 10.0 mL of 0.009 M ascorbic acid solution and 16.0 mL of 0.113 M NaOH solution was added into 16.0 mL of 0.005 M copper acetate solution. The mixed solution was stirred vigorously for 30 min, enabling the solution became yellow and turbid, indicating the formation of Cu2O nanocubes. Finally, the precipitates were collected and washed through centrifugation. All the experiments were carried out at room temperature (298 K).
Synthesis of Cu/Cu2O nanocubes
The Cu/Cu2O nanocubes were synthesized from Cu2O nanocubes by a constant potentiostatic reduction process at the potential of −0.6 V vs RHE for 300 s in 1 M KOH and 0.1 M NO3− solution. This process was implemented in a three-electrode system, where carbon paper, loaded on the Cu2O nanocubes, graphite rod, and Hg/HgO electrode was used as the working electrode, counter electrode, and reference electrode. After the electrochemical reduction process, the obtained Cu/Cu2O nanocubes were washed with absolute ethanol and blown dry by nitrogen.
Materials characterization
XRD patterns were collected via an X-ray diffractometer (Shimadzu XRD-7000) using monochromatic Cu Kα radiation (λ = 1.5406 Å) to investigate the phase structure transformation. Scanning electron microscopy (SEM; JSM-7610F) and transmission electron microscopy (TEM; JEOL F200 equipped with energy dispersive spectrometer (EDS)) were employed to observe the microstructure and elemental distribution of the catalysts. The chemical composition and surface valence of the catalysts were analyzed through X-ray photoelectron spectroscopy with an Al-Kα X-ray source (E = 1486.6 eV, XPS, Thermo Scientific Escalab), and the binding energy was corrected with a C 1s spectral of 284.8 eV. Raman spectroscopy was collected on a laser Raman spectrometer (Horiba LabSpec6, 532 nm laser). The ultraviolet-visible (UV-Vis) absorbance spectra were measured on a spectrophotometer (Beijing Purkinje General T6 new century).
Electrochemical measurement
Typically, 1 mg catalyst was dispersed in a mixed solvent containing 500 μL of ethanol, deionized water (450 μL), and 50 μL of 5% Nafion solution. After ultrasonic treatment for 20 min, the catalyst slurry was dropped uniformly onto a piece of carbon paper (1 cm2, 1 mg cm−2). After drying at room temperature (298 K), the catalyst-covered carbon paper was utilized as the working electrode in NO3−RR measurements. The NO3−RR measurements were performed with a three-electrode system in an H-type electrochemical cell separated by Nafion 117 membrane using a CHI electrochemical workstation (CHI 760E) at room temperature (298 K). The Nafion 117 membrane was pre-treated by immersing it in water, 5% H2O2 solution, and 0.5 M H2SO4 at 80 °C for 1 h. The Cu/Cu2O was used as the working electrode, a carbon rod as the counter electrode, and Hg/HgO as the reference electrode. The NO3−RR measurement was conducted in 1.0 M KOH with 0.1 M KNO3 solution (pH was 13.8 ± 0.2, which determined by pH meter). All potentials were recorded against the reversible hydrogen electrode (RHE, ERHE = 0.0591 × pH + 0.098 V + EHg/HgO) without special explanation. The linear sweep voltammetry (LSV) test was performed at a rate of 5 mV s−1, and the potential was not iR corrected. Constant potential electrolysis was conducted at each potential for 3 h. Electrochemical surface area (ECSA) was calculated from scan rate dependence of CV with scan rates of 10–90 mV s−1. The electrochemical impedance spectroscopy (EIS) measurements were carried out with frequencies from 0.01 Hz to 100 kHz at an amplitude of 10 mV. For flow electrochemical measurement, the customized two-electrode flow cell (as shown in Fig. 5b) is used for the NO3-RR//HMFOR paired-electrolysis system, the cathode and anode were separated by a anion exchange membrane (Fumasep FAA-3-20), which is directly used for electrochemical measurement without pre-treatment, and the voltages was not iR corrected. The Ni3Se4, dropped on the carbon felt (10 × 10 cm2, 2 mg cm-2), and M-Cu2O, dropped on the carbon felt (10 × 10 cm2, 2 mg cm-2), were used as anode and cathode, respectively. Catalyst inks (Ni3Se4 or M-Cu2O, 200 mg) were prepared by dispersing catalyst powder in a mixture of isopropanol (10 mL), pure water (9 mL), and Nafion (1 mL). Subsequently, the catalyst inks were dropped on the carbon felt and dried at room temperature (298 K). The anodic electrolyte of 0.1 M HMF in 2.0 M KOH was prepared before pumping into the anode chamber with a flow rate of 20.0 mL min−1, and the cathodic electrolyte of 0.1 M KNO3 in 2.0 M KOH was prepared before pumping into the cathode chamber with a flow rate of 20.0 mL min−1. The electrolyte pumping out from the anode and cathode were collected, and the product was analyzed using HPLC and UV–Vis, respectively.
Product quantification
The UV–Vis spectrophotometer was used to quantified the concentration of nitrate, nitrite, and ammonia by same colorimetric methods. In nitrate quantification, the electrolyte is neutralized and diluted to suitable concentrations. Then, 2 mL of 0.8 w% sulfamic acid solution, 2 mL of Nessler’s reagent followed by 1 M HCl were added to the above solution. After standing at room temperature (298 K) for 20 min, record the absorbance at a wavelength of 420 nm with a UV–Vis spectrophotometer. In nitrite quantification, a mixture of p-aminobenzenesulfonamide (4 g), N-(1-Naphthyl) ethylenediamine dihydrochloride (0.2 g), ultrapure water (50 mL), and phosphoric acid (10 mL, 1.70 g mL−1) was used as a color reagent. A certain amount of electrolyte was extracted from the cathodic chamber and diluted to suitable concentrations. Then, 0.1 mL of color reagent was added to the above solution to further react for 20 min. The absorbance was recorded at a wavelength of 540 nm. In ammonia quantification, Nessler’s reagent was used as the color reagent to determine the quantification of ammonia. Firstly, a certain amount of electrolyte was extracted from the cathodic chamber and diluted to suitable concentrations. Then, 0.1 mL of potassium sodium tartrate solution (ρ = 500 g L−1) and 0.1 mL Nessler’s reagent were subsequently added into the above solution to further react for 20 min. The absorbance was recorded at a wavelength of 420 nm.
Calculation of the yield, selectivity, and Faradaic efficiency
The conversion of NO3− was calculated using:
The selectivity of NH3 and NO2− calculated using:
The yield of NH3 was calculated using:
The Faradaic efficiency was calculated using:
Where \({{{{\rm{C}}}}}_{{{{\rm{NH}}}}_{3}}\) and \({{{\rm{C}}}}_{{{{{\rm{NO}}}}}_{{2}}^{-}}\) are the concentration of \({{{{\rm{NH}}}}}_{3}\) and \({{{{\rm{NO}}}}}_{{2}^{-}}\), \(\Delta {{{\rm{C}}}}_{{{{{\rm{NO}}}}}_{{3}}^{-}}\) is the concentration difference of \({{{{\rm{NO}}}}}_{{3}^{-}}\) before and after electrolysis, C0 is the initial concentration of \({{{{\rm{NO}}}}}_{{3}^{-}}\), V is the electrolyte volume, t is the electrolysis time, m is the mass of catalyst, F is the Faradaic constant (96,485 C mol−1), Q is the total charge passing the electrode.
Theoretical simulation
All density functional theory (DFT) calculations were performed using the Vienna ab initio simulation package (VASP)33. The ion-electron interaction was described with the projector-augmented plane-wave (PAW) method34. The strongly correlated electronic states of Cu-3d orbitals were corrected via a DFT + U method. The effective Hubbard-U parameter (Ueff) for the Cu atom was set to be 5.0 eV35. The kinetic energy cutoff for the plane-wave expansion was set to 400 eV, and the Brillouin zone was sampled with 3 × 3 × 1 for geometry optimization. All the structural models were fully relaxed to the ground state with the convergence of energy and forces setting to 5 × 10−5 eV and 0.05 eV Å−1, respectively. DFT-D3 (D stands for dispersion) with the empirical correction in Grimme’s scheme was employed to describe van der Waals (vdW) interactions36. Solvation effect was considered with the Poisson-Boltzmann implicit solvation model, and the dielectric constant of water was set to 8037.
The Cu2O and Cu crystallographic information files were downloaded from the Materials Project crystal material library. For the Cu2O catalyst, the typical (111) slab with a (3 × 3 × 3) supercell was constructed; the top two layers and NO3RR intermediates were fully relaxed, while the bottom layer was fixed. The bulk Cu was established as nanoclusters to represent the metal Cu species formed during the NO3RR progress. Especially, considering the different structure the Cu0–Cu+ sites, we combine the Cu2O (111) planes and Cu clusters to build the three types of Cu/Cu2O catalysts. Using the Cu cluster of 15, 27, and 43 atoms, to represent L-Cu/Cu2O, M-Cu/Cu2O, and S-Cu/Cu2O according to according to the Auger electron spectroscopy results. The length of the vacuum layer in the Z direction was set to 15 Å to avoid the interactions between periodic images. The Gibbs free energy of each species was calculated by the computational hydrogen electrode (CHE) model38. Here, the chemical reaction considered can be summarized with the reaction equations below.
Where * represents the active site. Then, the reaction free energy can be obtained with the equation below:
Where ΔE is the total energy difference before and after intermediate adsorbed, ΔEZPE and ΔS are the differences of ZPE zero-point energy and entropy, respectively, which can be obtained from the vibrational frequency calculations. T is room temperature (298 K). Besides, the climbing-image nudged elastic band (CI-NEB) method was used to simulate the energy barrier of the NO3RR process39.
Data availability
The data that support the findings of this study are available in the paper and its Supplementary Information. Source data for Figs. 1–5 are provided as a Source Data file. Source data are provided in this paper. Source data are provided with this paper.
References
Chen, J. G. et al. Beyond fossil fuel–driven nitrogen transformations. Science 360, 6611 (2018).
Humphreys, J., Lan, R. & Tao, S. Development and recent progress on ammonia synthesis catalysts for Haber–Bosch process. Adv. Energ. Sustain. Res. 2, 2000043 (2021).
Li, K. et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 374, 1593–1597 (2021).
Fu, X. et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 379, 707–712 (2023).
Wang, D., Lu, X. F., Luan, D. & Lou, X. W. Selective electrocatalytic conversion of nitric oxide to high value‐added chemicals. Adv. Mater. 36, 2312645 (2024).
Long, J. et al. Direct electrochemical ammonia synthesis from nitric oxide. Angew. Chem. Int. Ed. 59, 9711–9718 (2020).
Ren, Z., Shi, K. & Feng, X. Elucidating the intrinsic activity and selectivity of Cu for nitrate electroreduction. ACS Energ. Lett. 8, 3658–3665 (2023).
Shen, Z. et al. Cu/Cu+ synergetic effect in Cu2O/Cu/CF electrocatalysts for efficient nitrate reduction to ammonia. ACS Sustain. Chem. Eng. 11, 9433–9441 (2023).
Shi, Y. et al. In-situ reconstructed Cu/Cu2O heterogeneous nanorods with oxygen vacancies for enhanced electrocatalytic nitrate reduction to ammonia. Chem. Eng. J. 479, 147574 (2024).
Costa, G. F. et al. Identifying the active site of Cu/Cu2O for electrocatalytic nitrate reduction reaction to ammonia. Chem Catal 4, 100850 (2024).
Zhou, J. et al. Achieving efficient and stable electrochemical nitrate removal by in-situ reconstruction of Cu2O/Cu electroactive nanocatalysts on Cu foam. Appl. Catal. B Environ. 317, 121811 (2022).
Bai, L. et al. Electrocatalytic nitrate and nitrite reduction toward ammonia using Cu2O nanocubes: active species and reaction mechanisms. J. Am. Chem. Soc. 146, 9665–9678 (2024).
Yuan, X. et al. Controllable Cu0-Cu+ sites for electrocatalytic reduction of carbon dioxide. Angew. Chem. Int. Ed. 60, 15344–15347 (2021).
Cao, Y., Xu, Y., Hao, H. & Zhang, G. Room temperature additive-free synthesis of uniform Cu2O nanocubes with tunable size from 20 nm to 500 nm and photocatalytic property. Mater. Lett. 114, 88–91 (2014).
Zhong, W. et al. Modulating surface oxygen species via facet engineering for efficient conversion of nitrate to ammonia. J. Energy Chem. 78, 211–221 (2023).
Lu, Y. et al. Tailoring competitive adsorption sites by oxygen-vacancy on cobalt oxides to enhance the electrooxidation of biomass. Adv. Mater. 34, 2107185 (2021).
Sander, T. et al. Correlation of intrinsic point defects and the Raman modes of cuprous oxide. Phy. Rev. B 90, 045203 (2014).
Liu, X. et al. Self-supported copper oxide electrocatalyst for water oxidation at low overpotential and confirmation of its robustness by Cu K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 120, 831–840 (2016).
Pan, Y. et al. Unveiling the synergistic effect of multi-valence Cu species to promote formaldehyde oxidation for anodic hydrogen production. Chem 9, 963–977 (2023).
Fan, Y. et al. Electrochemical synthesis of formamide by C–N coupling with amine and CO2 with a high faradaic efficiency of 37.5%. Chem 10, 2437–2449 (2024).
Qu, Y. et al. Heterostructured Co‐doped‐Cu2O/Cu synergistically promotes water dissociation for improved electrochemical nitrate reduction to ammonia. Small 20, 2308246 (2024).
Li, M. et al. Electrosynthesis of amino acids from NO and α-keto acids using two decoupled flow reactors. Nat. Catal. 6, 906–915 (2023).
Butcher, Jr. D. P. & Gewirth, A. A. Nitrate reduction pathways on Cu single crystal surfaces: Effect of oxide and Cl−. Nano Energy 29, 457–465 (2016).
Han, S. et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 6, 402–414 (2023).
Duan, W. et al. In situ reconstruction of metal oxide cathodes for ammonium generation from high-strength nitrate wastewater: Elucidating the role of the substrate in the performance of Co3O4-x. Environ. Sci. Technol. 57, 3893–3904 (2023).
Wang, J. et al. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy 86, 106088 (2021).
Pan, Y. et al. Electrocatalytic coupling of nitrate and formaldehyde for hexamethylenetetramine synthesis via C–N bond construction and ring formation. J. Am. Chem. Soc. 146, 19572–19579 (2024).
Lu, Y. et al. Tuning the selective adsorption site of biomass on Co3O4 by Ir single atoms for electrosynthesis. Adv. Mater. 33, e2007056 (2021).
Deng, X., Yang, Y., Wang, L., Fu, X. Z. & Luo, J. L. Metallic Co nanoarray catalyzes selective NH3 production from electrochemical nitrate reduction at current densities exceeding 2 A cm−2. Adv. Sci. 8, 2004523 (2021).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Fangying, D., Menglei, Y. & Jian, Z. Paired electrolysis for inorganic small molecules reduction coupled with alternative oxidation reactions. Chinese J. Org. Chem. 44, 809 (2024).
Wang, R. et al. Three-dimensional Ni3Se4 flowers integrated with ultrathin carbon layer with strong electronic interactions for boosting oxygen reduction/evolution reactions. Chem. Eng. J. 430, 132720 (2022).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys Rev B Condens Matter 47, 558–561 (1993).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953–17979 (1994).
Chang, C.-C., Ku, M.-S., Lien, W.-H. & Hung, S.-F. Unveiling the bonding nature of C3 intermediates in the CO2 reduction reaction through the oxygen-deficient Cu2O (110) surface─ A DFT study. J. Phys. Chem. C 126, 5502–5512 (2022).
Grimme, S. Semiempirical GGA‐type density functional constructed with a long‐range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 22162025; 21925203; 22332002; 22402110; 22222304; 22403048), the Youth Innovation Team of Shaanxi Universities, the Program for Young Scholar Talents of Wenying in Shanxi University, the Program of Technology Innovation of Shanxi Province (2024L007), the Open and Innovation Fund of Hubei Three Gorges Laboratory (SK232001), the Regional Innovation Capability Leading Program of Shaanxi (2022QFY07-03, 2022QFY07-06) and the Shaanxi Province Training Program of Innovation and Entrepreneurship for Undergraduates (S202210719108), the Foundation of State Key Laboratory of Coal Conversion (grant number J24-25-909), the Natural Science Research Foundation of Shanxi Province (202303021211016).
Author information
Authors and Affiliations
Contributions
F.F., H.Y., and C.Y. conceived and supervised the project. Y.L. and F.Y. executed the experiments and collected the data. T.L. and Y.J. conducted the theoretical calculations. Y.-C.H. carried out the EXAFS characterization measurements. All authors edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, Y., Yue, F., Liu, T. et al. Size-effect induced controllable Cu0-Cu+ sites for ampere-level nitrate electroreduction coupled with biomass upgrading. Nat Commun 16, 2392 (2025). https://doi.org/10.1038/s41467-025-57097-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57097-x