Abstract
Marvelous natures of alkali and alkaline earth metal hydrides in catalyzing chemical transformations are being discovered. However, the synthesis of (sub)nanostructured metal hydrides, critically important to enhance their catalytic performances, is yet a very challenging task. Herein, we develop a highly reactive heterogeneous catalyst comprising atomically dispersed barium hydrides on MgO support with an ultrahigh barium loading of up to 20 wt% via a convenient preparation method involving liquid-ammonia impregnation followed by hydrogenation. The surface barium hydride species not only exhibits extraordinary reactivity toward H2 activation at room temperature, but also enables the highly efficient hydrogen isotope exchange (HIE) of both sp3 C–H and sp2 C–H bonds in nonactivated alkylarenes using D2 as the deuterium source under mild conditions. The deuteration rate at benzylic site is two orders of magnitude higher than that of bulk BaH2. This study offers an alternative synthetic route for the manufacture of deuterium-labeled compounds using a heterogenous transition metal-free hydride catalyst beyond the widely studied molecular metal complexe catalysts.
Similar content being viewed by others
Introduction
Owing to the unique properties and functionalities of hydridic hydrogen (H−), metal hydrides have shown great promise in many important processes such as energy storage and hydrogen-involved chemical transformations1,2. The physicochemical properties of metal hydrides are closely related to the change in their structures. For example, studies have shown that the H2 desorption enthalpy decreases as particle size decreases3,4. Hence, a promising approach for tuning the properties of a metal hydride is to engineer its (sub)nanostructure. Surface organometallic chemistry has been demonstrated as a powerful and universal technology for the synthesis of well-defined transition metal hydrides on a solid surface5,6. Nevertheless, (sub)nanostructuring of other types of important metal hydrides, e.g., the alkali and alkaline earth metal hydrides, remains highly challenging due to the lack of suitable synthetic methods. The development of methodologies for the fabrication of (sub)nanostructured metal hydrides is of vital importance for enhancing their performances in hydrogen-involved chemical transformations.
Among hydrogen-involved reactions, hydrogen isotope exchange (HIE) is the straightforward approach to synthesize deuterium (or tritium)-labeled organic compounds7,8,9 that are widely utilized for mechanistic studies10, metabolic process11, and new drugs or materials discovery12,13. Both homogenous and heterogenous catalytic approaches for HIE have been investigated using various deuterium sources (Fig. 1). Major efforts have been directed towards exploring the molecular complexes of transition metals as catalysts14,15,16,17, where the modulation of ligands allows for the control of both activity and regioselectivity. In addition, HIE catalyzed by transition metal-based heterogeneous catalysts is highly attractive because of the advantages in product separation and purification. For example, Beller et al. reported Fe18 and Mn-based19 heterogeneous catalysts for deuteration of (hetero)arenes using D2O, showing the potential of cheap metals for HIE reaction. In a recent study, Copéret et al. developed an H/D exchange catalyst based on supported iridium nanoparticles20, with high chemo- and regioselectivity under mild conditions. Supported Ru21 or Pd22,23,24 nanoparticles also achieved selective deuteration of some other valuable substrates, such as alcohols, aldehydes, and olefins. Besides, well-defined surface transition metal hydrides or bimetallic complexes on oxide supports have been proven to be an efficient catalyst system, particularly toward the deuteration of the non-activated sp3 C–H and sp2 C–H bonds25,26,27,28. Alternatively, main group compounds, typically exhibiting limited hydrogenation capability, have been explored intermittently as HIE catalysts/mediators over the years7,29,30,31. Recently, an assortment of molecular amides of alkali and alkaline earth metals (Na, Cs, and Ba)32,33,34,35 have displayed improved deuteration efficiency and selectivity, where certain transient hydride species may form during the reaction. Besides, some metal oxides36,37,38, such as MgO and SiO2/Al2O3, have been used in catalyzing HIE of aliphatic hydrocarbons, albeit the activity is very low under mild conditions. Moreover, bulk metal hydrides39 also show limited reactivity and are considered difficult to achieve catalytic cycle due to their low specific surface area and high lattice energy.
Herein, we present a convenient method for preparing a main group catalyst, i.e., atomically dispersed barium hydride supported on MgO (denoted here as BaH/MgO). This catalyst enables the selective deuteration of benzylic and aromatic C–H bonds in a range of alkylarene substrates at room temperature using deuterium gas (D2) as the deuterium source, which has garnered increasing attention as an alternative model for tritiation studies9. The turnover frequency of benzylic HIE of toluene on the BaH/MgO catalyst surpasses those of the best catalysts reported so far. Mechanistic study indicates the reactive barium hydride species shows multifaceted functionalities in deprotonation of sp3 C–H bonds and nucleophilic attack on the sp2 C atoms leading to regioselective products controlled simply by changing reaction time or reaction temperatures. This study not only reports a simple method for preparing sub-nanostructured metal hydride catalysts, but also offers an efficient and complementary synthetic route to obtain deuterated (or tritiated) alkylarenes under mild conditions. Furthermore, the role of alkali and hydridic hydrogen may provide new insight for future explorations of new functionalities of hydrides in chemical transformations.
Results and discussion
Synthesis and characterization of atomically-dispersed BaH/MgO
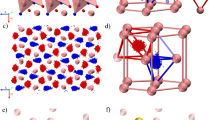
Magnesium oxide (MgO), exhibiting basic properties, is compatible with alkali or alkaline earth metal hydrides, and thus was selected as a support for dispersing metal hydrides. Herein, the MgO support was prepared by calcinating magnesium oxalate in a flow of argon at 550 °C for 5 h40. The obtained MgO has a porous structure with a high specific surface area of ca. 215 m2 g−1 (Supplementary Fig. 1). As shown in Fig. 2a, the BaH/MgO was prepared by deposition of Ba(NH2)2 on MgO followed by hydrogenation of Ba(NH2)2 to BaH species. The pretreated MgO support was impregnated with a barium-ammonia solution in a custom-designed reactor equipped with the observation window, where metallic barium metal can convert to barium amide (Ba(NH2)2). The Ba(NH2)2/MgO sample was obtained after removing the excess ammonia through evacuation. At 300 °C in flowing H2, the Ba(NH2)2/MgO can be hydrogenated to BaH/MgO with the release of NH3 (Supplementary Fig. 2). According to the inductively coupled plasma optical emission spectroscopy (ICP-OES), the loading of Ba in BaH/MgO was 21 wt%, which is close to its nominal content of 20 wt% (Table S1). X-ray diffraction (XRD) pattern (Fig. 2b) of BaH/MgO showed only the diffraction peaks of MgO support, suggesting that barium species is amorphous or highly dispersed on MgO. To examine the structural features of the sample, transmission electron microscopy (TEM) was carried out. MgO nanocrystals with the (111) plane as the main exposed facet can be observed (Fig. 2c), whereas no barium crystal particles are present (Supplementary Fig. 3a), consistent with the XRD result. The aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) was then used to confirm the presence and ___location of barium at sub-nanometer resolution. As shown in the AC-HAADF-STEM images (Fig. 2d and Supplementary Fig. 3b, c), barium appeared as high-density bright dots. This indicates that barium is highly dispersed over the MgO support. Energy-dispersive X-ray (EDX) analysis (elemental mapping) further proves that the Ba species are evenly distributed throughout the whole support ___domain (Fig. 2e). Extended X-ray absorption fine structure (EXAFS) spectroscopy is very sensitive to the local microenvironment and coordinative structure of metal atoms. Accordingly, the Ba–L3 edge spectra for BaH/MgO and a BaO reference are shown in Fig. 2f. The prominent peak at 2.3 Å in BaH/MgO can be attributed to the first Ba–O coordination shell, akin to those observed in BaO, suggesting an interaction between barium and MgO. The absence of a peak at the typical Ba–Ba distance of 3.6 Å suggests that Ba atoms are spatially isolated on MgO41,42. Solid-state 1H nuclear magnetic resonance measurements were employed to identify the existence of hydride in BaH/MgO (Fig. 2g). The BaH/MgO sample shows two peaks centered at 9.6 ppm and 0.7 ppm, respectively. The relatively broad peak at 0.7 ppm should be attributed to the H+ of hydroxyl groups on the sample43. Minor discrepancies in the OH signals between BaH/MgO and bare MgO could stem from the modified surface chemical microenvironment and basicity44,45, resulting from the presence of Ba species on the surface of MgO or the partial formation of Ba–OH. The other peak of BaH/MgO centered at 9.6 ppm was similar to that of Ba–H in bulk BaH2. It should be noted that the interaction between BaH2 and MgO may change the chemical shift of Ba–H bonds, leading to the difference of solid-state 1H NMR spectra of BaH/MgO and BaH2 observed in this study. In addition, we performed FT-IR to probe the signal of Ba–H vibration. As shown in Supplementary Fig. 4, BaH/MgO showed a broad peak centered at 1062 cm−1, which is in accordance with the stretching vibration of Ba–H bonds in bulk BaH2. Upon deuteration, for both BaD/MgO and BaD2 samples, the vibration signal at 1062 cm−1 disappeared, and a new peak at 783 cm−1 was observed. The wavenumber shifted from 1062 cm−1 to 783 cm−1 upon deuteration is consistent with the influence of an isotopic effect, which serves as another experimental evidence to confirm the presence of Ba–H(D) moieties. Compared to bulk materials, the supported samples show different coordination modes, which can be attributed not only to size effect, but also to modifications in the chemical microenvironment of hydridic species.
a Synthetic scheme of the atomically dispersed barium hydride on MgO support. b XRD patterns of bare MgO, BaH2 and BaH/MgO. c A typical HR-TEM image of BaH/MgO. White arrows indicate the lattice spacing (0.247 nm) between the (111) planes of MgO. d AC-HAADF-STEM micrograph of BaH/MgO with a scale bar of 2 nm. e HAADF-STEM image and EDX elemental mappings of BaH/MgO. f EXAFS spectra of BaH/MgO and a BaO reference at the Ba–L3 absorption edge. g Solid-state 1H NMR spectra of BaH2, MgO and BaH/MgO. The signals in the spectra of MgO at −0.7 ppm and −1.4 ppm are ascribed to isolated hydroxyl groups45. Pristine BaH2 shows two peaks at 9.7 ppm and 10.6 ppm, which are attributed to the hydrides (H‾) occupying two nonequivalent sites, i.e., tetrahedral (HT) and octahedral (HO) sites54,55.
Selective deuteration of toluene catalyzed by BaH/MgO
Initially, we investigated dihydrogen activation on BaH/MgO by performing H2–D2 exchange experiment in a flow reactor at 25 °C and 1 bar pressure. A flow of D2 or H2 was fed to the sample alternately and the transient gas products were probed by an on-line mass spectrometer. As shown in Fig. 3a, once D2 was introduced to the reactor, the formation of HD was immediately observed. At this point, the signal of HD grows sharply to a maximum, after which it decreases over a short period due to the complete exchange of hydrogen in BaH/MgO by deuterium. This itinerary production of HD by alternate feeding of D2 and H2 evidences the high dihydrogen activation ability of BaH/MgO at ambient conditions. In contrast, bulk BaH2 and bare MgO support do not show detectable H/D exchange activity under ambient conditions (Supplementary Fig. 5). This indicates that the high dispersion of BaH2 and the interaction between BaH2 and MgO are essential for hydrogen activation.
a H2–D2 isotopic exchange activity on BaH/MgO and BaH2. H2–D2 exchange reactions were performed in a flow reactor at 25 °C. The flow rate of D2 or H2 was 30 mL min−1 and the HD product was probed by its signal at m/z = 3 using a mass spectrometer. b A comparison of the average deuteration rate of benzylic C–H bonds over BaH2 and BaH/MgO, respectively. Reaction conditions: 0.2 M toluene in cyclohexane, 6 bar D2. Ten mol% of BaH2 with respect to toluene was used to conduct the experiment under 120 °C within 6 h, whereas 1 mol% BaH loading of BaH/MgO with respect to toluene was used under 25 °C within 1 h. c Time dependence of deuterium incorporation into toluene catalyzed by BaH/MgO with D2 pressure of 6 bar at 25 °C. One mol% BaH loading with respect to toluene was used. The lines were added to guide the eye. d A comparison of intrinsic deuterium incorporation rates of C–H bonds at different sites of toluene on BaH/MgO. Reactions were performed using 0.2 M toluene in cyclohexane and 6 bar of D2 at 25 °C. BaH loading with respect to toluene was 1 mol%. The initial rates were obtained by the differential of the fitted trend line in Fig. 2c (see Supplementary Section 3.2). e Influence of D2 pressure on the deuterium incorporation rates of benzylic C–H bonds in toluene. f Influence of toluene concentration on the deuterium incorporation rates of benzylic C–H bonds in toluene. Error bars in the figures are the standard deviation.
As mentioned above, developing efficient HIE methods and catalysts for inert C–H bonds of aromatics without the aid of directing groups comes at the forefront34,46. Toluene (1) was used as a model substrate to evaluate the selective deuteration of benzylic C–H bonds on BaH2 and BaH/MgO. In a typical reaction, a solution of 1 in cyclohexane was mixed with BaH/MgO powder (1 mol% BaH respect to 1) at 25 °C and 6 bar of D2 for a certain time. As a reference, a mixture of 1 and BaH2 (mole ratio is 10 to 1) in cyclohexane was heated to 120 °C under 6 bar of D2. After the reaction, the reaction mixture was filtered, and analyzed by proton nuclear magnetic resonance (1H NMR), hence the deuterium incorporation degree at a specific site of 1 was quantified by 1H NMR. It is pertinent to note that no by-products were observed in GC analysis throughout the entire deuteration experiment (Supplementary Fig. 6). As depicted in Fig. 3b, under these conditions, the benzylic site of toluene catalyzed by BaH/MgO proceeds with terrific deuteration efficiency, i.e., the average deuteration rate of 1 reached 285 molD molBa−1 h−1 under room temperature, while BaH2 catalyzed benzylic site deuteration with a much lower rate of 0.933 molD molBa−1 h−1 even at 120 °C. The significant differences in deuteration performance closely parallel the hydrogen activation ability. Additionally, a series of control experiments (Table S2) were conducted to determine the essential role of supported BaH species in the superior deuteration activity. Bare MgO exhibited a toluene deuteration activity of 1.77 molD molcat−1 h−1, in line with that reported in an early work36. The BaH/MgO sample either passivated in air or calcinated in O2 at 200 °C didn’t not present any detectable activity.
The time dependence of deuteration of toluene is shown in Fig. 3c. The deuterium enrichment of 27% was achieved in the benzylic methyl group in just 5 min; with the increase of the reaction time to 1 h, deuterium enrichment reached 97%. Notably, deuterium incorporation into the aromatic C–H bonds of 1 was not detectable within 1 h, supported by the result of 2H-NMR spectrum (Supplementary Fig. 7), indicating the high regioselectivity in the HIE reaction of 1 using BaH/MgO. Interestingly, when prolonging the reaction time to 6 h, deuterium incorporation into the aromatic C–H bonds was observed, giving 22% and 12% of deuterium enrichment of meta-position and para-/ortho-position, respectively. Note that the deuterium incorporation rate at the meta-site of toluene is faster than that at para- or ortho-site. It has been known that the hydrocarbon C–H bond activation without directing groups usually proceeds via an electrophilic pathway, and hence the electron-rich site (like ortho- or para-site) would be more accessible than the electron-deficient meta-site47. The results shown here suggest a distinct reaction pathway involving a hydride nucleophilic aromatic substitution mechanism on the BaH/MgO catalyst (see mechanistic study section). A comparison of the intrinsic deuteration rates at different positions of 1 is presented in Fig. 3d. The deuterium enrichment rate of benzylic C–H bonds is 2 orders of magnitude higher than those of aromatic C–H bonds, indicating the kinetic differences result in the high site selectivity at sp3 C–H bonds. Supplementary Fig. 8 displays the time dependences of different site deuteration over the catalyst with the catalyst loading of 10 mol%, which reflects the possibility of affording the perdeuterated toluene. The influence of D2 partial pressure and toluene concentration on the deuterium incorporation activity of benzylic C–H bonds on BaH/MgO was investigated in detail. As shown in Fig. 3e, the deuterium incorporation rate increases from 738 molD molBa−1 h−1 to 1247 molD molBa−1 h−1 with the rise of D2 pressure from 3 bar to 8 bar. On the other hand, the slight promotion of deuteration rate by the increase of toluene concentration can be observed as well (Fig. 3f). Based on these results, reaction orders with respect to D2 and toluene were determined to be 0.39 and 0.18, respectively (Supplementary Fig. 9). Such small values suggest that the adsorption of toluene is favorable on the BaH/MgO surface. After the reaction, neither could Ba particles be observed on AC-HAADF-STEM images nor could the diffraction peak of BaH2 be detected by XRD (Supplementary Fig. 10a–c). ICP-OES (Table S3) showed that barium content in the catalyst remained unchanged. These results indicate the robust stability of BaH/MgO catalyst during deuteration reactions under the conditions applied. On the other hand, to rule out the possibility that molecular barium complexes may be formed and act as catalysts, we tested the deuteration activity of a liquid reaction mixture that was collected after treating toluene with BaH/MgO in 6 bar H2 at 25 °C for 24 h. The liquid reaction mixture was stirred for 24 h at 25 °C under 6 bar of D2. However, no measurable deuterated products can be detected by either 2H-NMR or 1H-NMR spectra (Supplementary Fig. 10d). This demonstrates that the deuteration reaction using BaH/MgO is a heterogeneous catalytic process.
As depicted in Table S4, turnover number (TON) values were calculated for the catalytic deuteration of the sp3 C–H bonds in toluene on BaH/MgO. The value can reach up to 285 at 25 °C for a reaction period of 1 h, which is superior to previously reported molecular complex catalysts and comparable to that of the noble metal Pd catalyst at 110 °C. More interestingly, the turnover frequency (TOF) of the BaH/MgO catalyst (285 h−1) is markedly higher than both homogeneous and heterogeneous ones by at least a factor of 10. Complemented to directed protocols for sp2 C–H bond deuteration, a nondirected method for unbiased C–H bond activation has spurred research toward transition metal-catalyzed methods very recently. Moreover, the BaH/MgO catalyst also exhibits an outstanding HIE rate on the sp2 C–H sites of toluene. The catalytic performances such as TONs and TOFs at room temperature in this work rival those of the catalytic systems where heating was usually required for this H/D exchange reaction (Table S5).
In addition, we explored the deuteration activity of other alkalies (alkaline earth) metal hydride materials (Table 1). Various bulk alkali and alkaline earth metal hydrides were tested for reactions of toluene and D2 at 120 °C (Entries 1–4). KH can achieve a deuterium enrichment of 41% at benzylic position within 6 h, whereas LiH, NaH, and CaH2 showed negligible activity. The sluggish kinetics of bulk hydride materials were attributed to their high lattice energies, which pose significant barriers to achieving efficient catalytic activity39. Given that preparing supported forms of some other alkali (alkaline earth) metal hydrides is more difficult compared to BaH2, we adopted the ball milling method to prepare MgO-supported alkali (alkaline earth) hydrides and compare their deuteration activity. As shown in Table 1, BaH/MgO-bm exhibited the highest H/D exchange activity at room temperature among ball-milled samples (Entries 6–9). Similarly, the ball-milled CaH/MgO catalyst yielded 60% deuterium enrichment within 6 h, while NaH/MgO-bm catalyst gave slightly higher deuterium incorporation of 77% at benzylic position. In contrast, the deuteration rate of ball-milled LiH/MgO was notably slower than that of other alkali and alkaline earth metal hydride-supported catalysts. Therefore, the type of alkali (alkaline earth) metal cations plays an important role in tuning deuteration activity.
Mechanism study
Encouraged by the extraordinarily high activity of the BaH/MgO catalyst for the deuteration of toluene, DFT calculations were performed to elucidate the mechanism of deuterium incorporation into toluene over the catalyst. A Ba3H6 model with a stable triangle structure was constructed on a three-layer Mg24O25H6 cluster cutting from MgO(111) surface (Fig. 4a and Supplementary Fig. 11). This model finds support from STEM image (Fig. 2d), showing local barium densities on the surface of the MgO to be ca. 3.3–4.5 Ba nm−2, close to the calculated value of around 5 Ba nm−2 based on the Ba3H6/Mg24O25H6 cluster model. Two pathways have been evaluated: (1) H/D exchange involving sp3 C–H bonds at benzylic site via a deprotonation/protonation mechanism with D2; (2) H/D exchange involving aromatic sp2 C–H bonds with D2. For the deuterium incorporation into the benzylic position (Fig. 4b), the deprotonation of the benzylic group in toluene to form a [Ba3D5∙∙∙CH2Ph] intermediate and HD (1 → TS-a1 → Int-a1) encounters a moderate free energy barrier of 19.3 kcal mol−1, which could occur under room temperature. The reaction of [Ba3D5∙∙∙CH2Ph] intermediate and D2 to produce a deuterated benzyl group and a restored Ba3D6 is slightly exergonic (−3.9 kcal mol−1) with a lower barrier. A similar reaction pathway has been considered for the reaction of H2 and toluene mediated by KH39. On the other hand, the H/D exchange of the sp2 C–H bonds at aromatic sites may proceed via a hydride nucleophilic attack route. As shown in Fig. 4c, the nucleophilic attack of hydridic D in Ba3D6 to the sp2 C of meta-, para-, or ortho- sites, forming Meisenheimer anion intermediates (Int-b1), encounters energy barriers of 16.6 kcal mol−1, 17.4 kcal mol−1, and 17.5 kcal mol−1, respectively, which may explain the order of deuteration rates, i.e., r(meta) > r(ortho/para) (Fig. 3c, d). We note these energy barriers are slightly lower than those for the deuteration of benzylic site. Generally, the nucleophilic attack to electron-rich π-systems such as alkylarenes is disfavored. These low energy barriers are attributed to the cation-π interaction between Ba2+ and the aromatic rings, which reduces the electron density on the arene and stabilizes the intermediates. It has been reported that lithium hydride (LiH)48 and some alkaline earth metal (Ca, Sr, and Ba) complexes32,49,50 could facilitate the hydrogenolysis of aniline, alkylation reaction, and H-D exchange of benzene via unusual nucleophilic aromatic substitution. The subsequent T-shape flip (via TS-b2) of Int-b1 needs to overcome a high free energy barrier of ca. 21.5 kcal mol−1 (relative to the initial state), which likely accounts for the lower deuteration rates at aromatic sites (Fig. 3c, d). After the flip, the toluene with one deuterium incorporation desorbs from the surface of the cluster. Subsequently, D2 dissociation proceeds and completes the full deuteration of substrates sequentially. In addition, the calculated adsorption energy of toluene (−2.8 kcal mol−1) is lower than that of H2 (3.1 kcal mol−1), suggesting that the adsorption of toluene is stronger than H2 on BaH/MgO. This is consistent with the fact that the reaction order for toluene (0.18) is lower than that for H2 (0.39) (Fig. 3e, f).
a Top view of the model of the Ba3H6/MgO cluster. b Calculated free energy diagram of the deprotonation/protonation reaction pathway for the HIE at benzylic position of toluene. c Calculated free energy diagram of the nucleophilic aromatic substitution reaction pathway for the HIE at aromatic positions. Numbers in this profile indicate the free energy of each intermediate or transition state. The schematic structural models of selected intermediates and transition states are shown above.
Substrate scope study
The outstanding catalytic performance of BaH/MgO in the deuteration of toluene encourages us to expand the substrate scope by investigating various toluene derivatives (Fig. 5). Xylenes (2, 3) and mesitylene (4) subjected to deuterium incorporation yield up to ca. 97% enrichment of benzylic C–H bonds. Generally, the secondary or tertiary C–H bonds in the benzylic position exhibit weak acidity due to the strong electron-donating effect of adjacent groups. As a result, deuterium enrichment will be hampered to some extent30,51. However, substrates with secondary (5, 6, 7) and tertiary (8, 9) benzylic C–H bonds on BaH/MgO also exhibited a high degree of deuteration. Substrate 7 yielded a product with high deuterium incorporation (97%) at both the primary and secondary benzylic positions. The deuterium incorporation in 9 is 86%, likely due to the steric hindrance of the bulky cyclohexyl group. And slight deuterium incorporation at meta-sites was observed in 8 and 9. Reactions of diphenylmethane (10) and 4-methylbiphenyl (11) for 12 h resulted in 99% and 97% of deuterium enrichment, respectively. In 2-methylnaphthalene (12), 96% D incorporation of the benzylic methyl group was achieved using 10 mol% BaH (relative to the amount of 12), which is probably due to the unfavorable hydridic D attack on 2-methylnaphthalene with a higher π-electron density.
The optimized reaction conditions were adopted to ensure the deuteration of less active substrates to a greater extent. Reaction conditions, unless otherwise noted: 0.4 mmol of the substrate, 2 mol% BaH with respect to the substrate (5.6 mg BaH/MgO), 2 mL of cyclohexane as solvents, and 6 bar of D2 in a 50 mL autoclave, 25 °C, 6 h. Percent deuterium incorporations shown in parentheses were determined by 1H NMR integration relative to the peaks from an unlabeled C–H site or 1,3,5-trimethoxylbenzene as the internal standard substrate. Steelblue spots: deuteration of benzylic site; Orange spots: deuteration of aromatic site. a4 mol% of BaH (11.2 mg BaH/MgO), 6 h; b10 mol% of BaH (27.8 mg BaH/MgO), 12 h. c10 mol% of BaH (27.8 mg BaH/MgO), 6 h; d20 mol% of BaH (55.6 mg BaH/MgO), 12 h. e45 °C, 10 mol% of BaH (27.8 mg BaH/MgO), 12 h.
BaH/MgO can also enable the deuteration of toluene derivatives substituted with heteroatom-containing functional groups. For example, the methyl-substituted anisoles (13, 14, 15) underwent benzylic deuteration readily, and the aromatic C–H bonds at the ortho-position of the methoxy group were also labeled. Under the same conditions, the deuteration of the benzylic position in benzyl methyl ether (16) exhibited a selectivity of 76%. The substrates with N, N-dimethyl group (17–19) were also facilely deuterated at their benzylic positions (>90%), and the arene sites were also labeled albeit with relatively low enrichment. The methoxy and amino groups are considered strong electron-donating groups, whereas the halogen groups such as fluorine are typical electron-withdrawing groups. The reaction of p-fluorotoluene (20) afforded 42% deuterium enrichment in its methyl group even with 20 mol% BaH. However, the degree of deuteration incorporation in the ortho C–H bonds of fluorine reached 93%. These results disclose that BaH/MgO has a good functional group tolerance for the deuteration of benzylic or aromatic sites. Moreover, the deuteration of arene sites is independent of the electronic properties of heteroatomic functional groups, possibly due to the coordination between the Ba site and the heteroatoms. For trimethyl-p-tolylsilane (21), only benzylic position showed deuterium labeling with 98% D enrichment, whereas no deuterium incorporation was observed in the Si-Me groups.
Next, we moved our attention to evaluating the deuteration of the sp2 C–H bonds of benzene derivatives with different functional groups. Standard reaction condition was chosen with 10 mol% of supported BaH and 12 h. Benzene is the simplest aromatic compound but with the highest C–H bond dissociation energy (113 kcal mol−1)52. However, in our study, the deuterium enrichment of benzene (22) can be achieved to 96% at room temperature within 12 h. The tert-butylbenzene (23) achieved full deuterium incorporation (95%) at the meta and para positions, but only 11% incorporation at the ortho position caused by the steric effect of the tert-butyl group. P-xylene (24) also achieved a deuterium substitution of 70% in the sp2 C–H bonds as expected. The deuteration efficiency decreased for benzene, toluene, and p-xylene. This can be attributed to the methyl groups, which enrich the electron density and increase the steric hindrance of aromatic rings, thereby hindering the nucleophilic attack process. Biphenyl (25) can be deuterated but with lower isotopic incorporation at various positions. N, N-Dimethylaniline (26) showed moderate D enrichment at the ortho and para positions (42%) and the meta position (20%). Furthermore, the reaction temperature of 23, 25, and 26 was evaluated to 45 °C, resulting in significant improvement of deuterium incorporation at aromatic positions, as indicated by the italic numbers. The substrates with acidic protons such as phenols and anilines are not amenable to deuteration under catalytic conditions. This is probably due to the tendency of acidic protons to react with hydridic hydrogen on the catalyst, forming barium phenoxide or anilide salts.
Compared to the state-of-the-art deuteration methods, we have established a valuable and convenient method for producing various substrates with deuterium incorporation into both sp2 C–H and sp3 C–H bonds under ambient temperature. Furthermore, we evaluated some practical aspects of our catalyst system. As shown in Supplementary Section 3.5, benzene was selected as a benchmark substrate for gram-scale preparation, resulting in the fully-labeled product. It should be noted that 4–5 mL of pure labeled product (deuterium incorporation content > 90%) can be obtained in a single run and easily collected only through catalyst centrifugation.
Hence, this strategy is expected to be a competitive alternative to more traditional procedures relying on noble metal catalysts or molecular complex catalysts, which often require higher temperatures or complicated separation procedures. On the other hand, similar to some molecular complexes, the supported hydrides reported here are sensitive to oxygen, moisture, and substrates with acidic protons. Nevertheless, this feature creates a space for the design of active sites in hydrides that exhibit enhanced tolerance to oxygen, moisture, and substrates.
Bulk alkali and alkaline earth metal hydrides have long been considered to be inactive for the hydrogen isotope exchange (HIE) reaction. This work reports a convenient method for preparing an atomically dispersed barium hydride catalyst and its applications in the synthesis of deuterated alkylarenes typically using D2 as a deuterium source. The barium hydride catalyst affords high reactivity toward D2 dissociation, deprotonation/protonation on the benzylic site, and nucleophilic attack on the aromatic sites, leading to outstanding performance for H/D exchange of inert alkylarenes under ambient temperature. As compared to the widely studied homogeneous metal complex catalysts, the heterogeneous barium hydride catalyst has the advantages of high activity and easy separation, and moreover, this catalyst could be potentially used for tritium incorporation into alkylarenes because of the easily available T2 gas. In light of the rich compositions and structures of solid metal hydrides, this work may open an avenue for the development of hydride catalysts for HIE reactions involving small organic substrates and key pharmaceutical molecules. In addition, the development of new methods for preparing (sub)nanostructured metal hydrides could pave the way for their future applications in many other processes such as nitrogen fixation, selective hydrogenation, functionalization of alkylarenes, and hydrogen storage, etc., which are worthy of future studies.
Methods
Chemicals and materials
The moisture- and oxygen-sensitive manipulations were carried out either in an oven-dried autoclave or in a glovebox purged with argon atmosphere. The reagents used in this work are commercially available. BaH2 was synthesized via hydrogenation of metallic barium at 20 bar H2 pressure under room temperature for 2 days followed by heat treatment at 400 °C for 5 h. MgO was prepared by calcining the precursor magnesium oxalate in an argon flow at 550 °C for 5 h. The magnesium oxalate was synthesized by reacting magnesium acetate tetrahydrate with oxalic acid dihydrate as the acidic precipitant. The substrates were stored in the glovebox and dried using activated 4 Å molecular sieves. Deuterium gas (99.999%) was obtained from Dalian Special Gas Co., Ltd.
Procedure for the preparation of supported barium hydride catalysts
The synthesis procedure of the typical catalyst 20 wt% BaH/MgO was as follows.
Inside an argon-filled glove box, 30 mg of metallic Ba was mixed with 120 mg of MgO, and the mixture was loaded into a custom-designed stainless-steel reactor (20 mL) equipped with a quartz liner and an observation window crafted from sapphire. The reactor was sealed, removed from the glovebox, and evacuated using a vacuum pump. Subsequently, the reactor was placed in a liquid nitrogen bath for cooling down. NH3 was introduced into the reactor and liquefied. The amount of liquefied NH3 used was ca. 5 mL to enable NH3 to impregnate all solid materials. Upon impregnation, a blue solution of NH3-solvated Ba can be observed. After this, the reactor was shaken using an auto-shaker for 5 h, yielding Ba(NH2)2/MgO in a transparent ammonia solution.
After the removal of excess NH3, 150 mg of the as-prepared Ba(NH2)2/MgO sample was placed in a quartz-lined stainless steel fixed-bed reactor in the glovebox. Hydrogenation was performed in H2 with a flow rate of 30 mL min−1, at a pressure of 10 bar and a temperature of 300 °C for 8 h. The H2 stream used was purified by a Na-NaCl solid mixture.
Various supported alkali/alkaline earth metal hydride (LiH, NaH, CaH2, and BaH2) catalysts were synthesized via a ball-milling method. A mixture of MgO and alkali/alkaline earth metal hydride was ball milled in an air-tight vessel filled with argon using a Retsch planetary ball mill (PM 400) at 150 rpm for 3 h.
H2/D2 exchange tests on BaH2, MgO, and BaH/MgO
The H2/D2 exchange experiments were conducted in a stainless-steel flow reactor with a quartz liner that operated with a supply of a continuous flow of pure H2 or D2 gas. The as-prepared BaH/MgO or other samples were loaded into the reactor. D2 or H2 was fed to the sample alternately at room temperature and the transient gas products were monitored with an online mass spectrometer (MS, Hiden HPR20).
General procedure for the hydrogen isotope exchange reactions
All the catalysts and reagents were stored in a glovebox filled with argon.
Unless otherwise indicated, all H/D exchange reactions of aromatic substrates were performed as follows. In a glovebox, a stainless-steel autoclave (50 mL) equipped with a quartz lining was charged with the corresponding substrates, supported alkali/alkaline earth metal hydrides, cyclohexane (2 mL), and a Teflon-coated oval magnetic stirring bar. The autoclave was sealed, moved out of the glovebox, and connected to a D2 gas manifold. Upon evacuation and refilling of the supply line with D2, the autoclave was flushed with D2 three times and pressurized to the desired value (such as 6 bar). The reaction mixture was stirred at 500 rpm at room temperature (25 °C) for a specific duration time. At the end of the reaction, the gas was carefully released. The mixture was filtered to remove the solid catalysts, and the solvent solution was directly analyzed by 1H NMR, 2H NMR, and 13C NMR spectroscopy without further purification.
Quantitative analysis of deuterium-labeled compounds
Deuterium incorporation was quantified by the decrease of integral intensities in 1H-NMR at specified positions compared to the raw materials. The deuterium incorporation of substrates was determined by 2H NMR. For selectively deuterated products, the integral intensities were calibrated against a peak of the position that does not undergo H/D exchange. For the perdeuterated products, the deuterium incorporation level was determined by 1H NMR analysis of unpurified reaction mixtures, using 1, 3, 5-trimethoxybenzene as an internal standard. Considering the experimental precision of NMR integration analysis, the positions with 10% or more deuterium incorporation are classified as deuterated.
Analytic information on NMR, GC-MS, and HRMS
The 1H and 13C NMR spectra were recorded at room temperature on a JEOL spectrometer operating at 400 MHz. 1H NMR spectra: 400.1 MHz, 13C NMR spectra: 101 MHz. The 1H and 13C NMR spectra were recorded at room temperature in CDCl3. 2H NMR spectra were recorded at room temperature on a JEOL 400 MHz (61 MHz for 2H) instrument in cyclohexane (δ 1.43 ppm). All 1H NMR signals are reported as chemical shifts (δ ppm) relative to the signal of tetramethyl silane (TMS) at 0 ppm. The peak at 1.43 ppm corresponds to cyclohexane. The peaks at 6.03 ppm and 3.69 ppm correspond to 1, 3, 5-trimethoxybenzene. 13C NMR signals are reported as chemical shifts (δ ppm) relative to CDCl3 at 77.16 ppm. The peak at 27.07 ppm corresponds to cyclohexane. The peaks at 162.04 ppm, 93.14 ppm, and 54.98 ppm correspond to 1, 3, 5-trimethoxybenzene. 2H NMR signals are reported as chemical shifts (δ ppm) relative to the residue solvent signal of C6D12 at 1.43 ppm. NMR data is expressed as follows: chemical shift in ppm (δ), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, brs = broad singlet, and m = multiplet), coupling constant (Hz), and integration.
GC-MS measurements were performed on Shimadzu GCMS-QP2020 NX with an Electron Ionization (EI) resource. HRMS data were obtained with Agilent 8890-7250 (GC-MS). GC analysis was conducted by Fu Li 9790-II equipped with a flame ionization detector (FID) and a capillary column (OV-1701).
Reactivity of different positions in toluene
The reaction rates (r) for deuterium incorporation were defined as the number of moles of deuterium atoms incorporated per mole of barium per hour. These rates were calculated based on the percentage of deuteration observed in the C–H bonds at specific positions. The equation is shown as follows:
The turnover numbers (TON) and turnover frequency (TOF) were determined using the methods reported in the literature32,53.
TON was calculated as the moles of deuterium incorporated per mol of catalyst, as shown as follows:
TOF was calculated as follows:
Kinetic measurements
The reaction was performed as outlined in the general procedure for the hydrogen isotope exchange reactions. The reaction orders of D2 and toluene were determined based on the average deuterium incorporation rate within 5 min, where the deuterium incorporation yield was ≤50% and far from thermodynamic equilibrium.
All measurements were conducted at 25 °C. Measurements for the reaction order with respect to D2 were conducted at a constant toluene concentration of 0.2 mol L−1 while changing the pressures of D2 ranging from 3 bar to 8 bar. The reaction order with respect to toluene was measured at a constant D2 pressure of 6 bar while changing the concentration of toluene from 0.25 mol L−1 to 1 mol L−1.
Characterization of catalysts
To obtain the compositional and structural features of the supported barium hydride catalyst (BaH/MgO), investigations were performed using inductively coupled plasma optical emission spectroscopy (ICP-OES), powder XRD, high-resolution transmission electron microscopy (HRTEM), aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM), EXAFS, and solid-state 1H nuclear magnetic resonance (1H MASNMR) spectroscopy.
Nitrogen adsorption/desorption isotherms of MgO were measured using a Micromeritics ASAP 2020 analyzer at −196 °C. The specific surface areas were calculated according to the Brunauer–Emmett–Teller (BET) method.
The inductively coupled plasma optical emission spectrometry (ICP-OES) analyses were performed on an ICPS-8100 (Shimadzu Corporation).
Powder XRD patterns were recorded on a PANalytical X’pert diffractometer using a self-made sample cell covered with KAPTON film to avoid air or moisture contamination.
High-resolution transmission electron microscopy (HRTEM) images were acquired using a JEM-2100X microscope operated at an accelerating voltage of 200 kV.
Diffuse reflectance infrared Fourier transformations (DRIFT) measurements were conducted on a Brucker Tensor II unit with a scan resolution of 4 cm−1 and an accumulation of 32 scans each time.
The transmittance FTIR measurement was conducted on a Brucker Tensor II unit with a scan resolution of 4 cm−1 and an accumulation of 16 scans each time. Samples were loaded to an airtight Specac Omni cell equipped with KBr windows and sealed before bringing out from the Ar glovebox.
Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) coupled with EDX spectroscopy elemental mapping was performed on a JEM-ARM200F using high-angle annular dark field (HAADF) and annular bright field (ABF) detectors for imaging, equipped with a CEOS probe corrector operating at the accelerating voltage of 200 kV.
Ba–L3 edge XAFS analyses were performed with Si(111) crystal monochromators at the BL14W Beamline at the Shanghai Synchrotron Radiation Facility (SSRF) (Shanghai, China). Before the analysis at the beamline, samples were placed into aluminum sample holders and sealed using Kapton tape film. The XAFS spectra were recorded at room temperature using a 4-channel Silicon Drift Detector (SDD) Bruker 5040. Ba–L3 edge extended X-ray absorption fine structure (EXAFS) spectra were recorded in transmission mode. The spectra were processed and analyzed by the software code Athena.
The 1H MAS NMR experiments were recorded with a Bruker AVANCE III 500 spectrometer in an air atmosphere, equipped with a 1.3 mm probe and with a spinning rate of 45 kHz. A Hahn-echo pulse (90° pulse-τ-180° pulse-τ) was used.
Mechanistic study
Based on a Mg24O25 cluster cutting from MgO(111), we have attempted to construct a series of models involving different configurations of surface barium hydride species (BaH2, Ba2H4, and Ba3H6). However, the model of BaH2/MgO and Ba2H4/MgO failed to be built in DFT calculations because all the hydridic H in barium hydrides preferred to migrate to the neighboring MgO surface and thus forming Mg-OH moieties (as shown in Supplementary Fig. 12). Our experimental results indicated that the use of oxidized BaH/MgO with abundant surface hydroxy groups was unable to facilitate HIE. Notably, when a triangular arrangement of barium atoms formed on the MgO surface, the resulting Ba3H6 cluster created a stable geometry through optimization. In this structure (Ba3H6), three hydridic hydrogen atoms spilled over to adjacent oxygen atoms, while the three residual hydrogen atoms remained attached to barium, forming surface [–O–Ba–H] species.
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Mohtadi, R. & Orimo, S.-I. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2, 16091 (2016).
Wang, Q., Guo, J. & Chen, P. The power of hydrides. Joule 4, 705–709 (2020).
Zaluski, L., Zaluska, A. & Ström-Olsen, J. O. Nanocrystalline metal hydrides. J. Alloy. Compd. 253–254, 70–79 (1997).
Berube, V., Chen, G. & Dresselhaus, M. S. Impact of nanostructuring on the enthalpy of formation of metal hydrides. Int. J. Hydrog. Energy 33, 4122–4131 (2008).
Vidal, V., Theolier, A., ThivolleCazat, J., Basset, J. M. & Corker, J. Synthesis, characterization, and reactivity, in the C–H bond activation of cycloalkanes, of a silica-supported tantalum(III) monohydride complex: (≡SiO)2TaIII–H. J. Am. Chem. Soc. 118, 4595–4602 (1996).
Pelletier, J. D. A. & Basset, J.-M. Catalysis by design: well-defined single-site heterogeneous catalysts. Acc. Chem. Res. 49, 664–677 (2016).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. C–H functionalisation for hydrogen isotope exchange. Angew. Chem. Int. Ed. 57, 3022–3047 (2018).
Kang, Q. K. & Shi, H. Catalytic hydrogen isotope exchange reactions in late-stage functionalization. Synlett 33, 329–338 (2022).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Gómez-Gallego, M. & Sierra, M. A. Kinetic isotope effects in the study of organometallic reaction mechanisms. Chem. Rev. 111, 4857–4963 (2011).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 57, 1758–1784 (2018).
DeWitt, S. H. & Maryanoff, B. E. Deuterated drug molecules: focus on FDA-approved deutetrabenazine. Biochemistry 57, 472–473 (2018).
Di Martino, R. M. C., Maxwell, B. D. & Pirali, T. Deuterium in drug discovery: progress, opportunities and challenges. Nat. Rev. Drug Discov. 22, 562–584 (2023).
Kerr, W. J., Knox, G. J. & Paterson, L. C. Recent advances in iridium(I) catalysis towards directed hydrogen isotope exchange. J. Label. Compd. Radiopharm. 63, 281–295 (2020).
Pony Yu, R., Hesk, D., Rivera, N., Pelczer, I. & Chirik, P. J. Iron-catalysed tritiation of pharmaceuticals. Nature 529, 195–199 (2016).
Palmer, W. N. & Chirik, P. J. Cobalt-catalyzed stereoretentive hydrogen isotope exchange of C(sp3)-H bonds. ACS Catal. 7, 5674–5678 (2017).
Zarate, C., Yang, H., Bezdek, M. J., Hesk, D. & Chirik, P. J. Ni(I)-X complexes bearing a bulky α-diimine ligand: synthesis, structure, and superior catalytic performance in the hydrogen isotope exchange in pharmaceuticals. J. Am. Chem. Soc. 141, 5034–5044 (2019).
Li, W. et al. Scalable and selective deuteration of (hetero)arenes. Nat. Chem. 14, 334–341 (2022).
Bourriquen, F., Rockstroh, N., Bartling, S., Junge, K. & Beller, M. Manganese-catalysed deuterium labelling of anilines and electron-rich (hetero)arenes. Angew. Chem. Int. Ed. 61, e202202423 (2022).
Yao, C. & Copéret, C. Site-selective and late-stage deuteration of (hetero)arenes with supported iridium nanoparticles. ACS Catal. 15, 2822–2826 (2025).
Palazzolo, A. et al. Tuning the reactivity of a heterogeneous catalyst using N-heterocyclic carbene ligands for C–H activation reactions. Angew. Chem. Int. Ed. 59, 20879–20884 (2020).
Esaki, H. et al. Efficient H/D exchange reactions of alkyl-substituted benzene derivatives by means of the Pd/C–H2–D2O system. Chem. Eur. J. 13, 4052–4063 (2007).
Kurita, T. et al. Efficient and convenient heterogeneous palladium-catalyzed regioselective deuteration at the benzylic position. Chem. Eur. J. 14, 664–673 (2008).
Pfeifer, V. et al. Palladium nanoparticles for the deuteration and tritiation of benzylic positions on complex molecules. Angew. Chem. Int. Ed. 60, 26671–26676 (2021).
Lefort, L., Copéret, C., Taoufik, M., Thivolle-Cazat, J. & Basset, J. M. H/D exchange between CH4 and CD4 catalysed by a silica supported tantalum hydride, (≡SiO)2Ta-H. Chem. Commun. https://doi.org/10.1039/A910239F (2000).
Casty, G. L., Matturro, M. G., Myers, G. R., Reynolds, R. P. & Hall, R. B. Hydrogen/deuterium exchange kinetics by a silica-supported zirconium hydride catalyst: evidence for a σ-bond metathesis mechanism. Organometallics 20, 2246–2249 (2001).
Lassalle, S. et al. Metal-metal synergy in well-defined surface tantalum-iridium heterobimetallic catalysts for H/D exchange reactions. J. Am. Chem. Soc. 141, 19321–19335 (2019).
Pichugov, A. V. et al. Highly selective and efficient perdeuteration of n-pentane via H/D exchange catalyzed by a silica-supported hafnium-iridium bimetallic complex. Angew. Chem. Int. Ed. 63, e202400992 (2024).
Tortajada, A. & Hevia, E. Alkali-metal bases in catalytic hydrogen isotope exchange processes. Catal. Sci. Technol. 13, 4919–4925 (2023).
Hu, Y. et al. A convenient synthesis of deuterium labeled amines and nitrogen heterocycles with KOt-Bu/DMSO-d6. Tetrahedron 71, 1425–1430 (2015).
Zhan, M. et al. A simple and cost-effective method for the regioselective deuteration of phenols. Eur. J. Org. Chem. 2015, 3370–3373 (2015).
Martin, J., Eyselein, J., Grams, S. & Harder, S. Hydrogen isotope exchange with superbulky alkaline earth metal amide catalysts. ACS Catal. 10, 7792–7799 (2020).
Tortajada, A. & Hevia, E. Perdeuteration of arenes via hydrogen isotope exchange catalyzed by the superbasic sodium amide donor species NaTMP·PMDETA. J. Am. Chem. Soc. 144, 20237–20242 (2022).
Du, H.-Z. et al. Cesium amide-catalyzed selective deuteration of benzylic C-H bonds with D2 and application for tritiation of pharmaceuticals. Angew. Chem. Int. Ed. 62, e202214461 (2023).
Du, H.-Z. et al. Directed aromatic deuteration and tritiation of pharmaceuticals by heavy alkali metal amide catalysts. ACS Catal. 14, 9640–9647 (2024).
Hoq, M. F. & Klabunde, K. J. Thermally-activated magnesium-oxide as a selective deuteration catalyst under mild conditions. J. Am. Chem. Soc. 108, 2114–2116 (1986).
McCosh, R. & Kemball, C. Exchange reactions of benzene toluene and m-xylene with deuterium on silica-alumina and alumina catalysts. J. Chem. Soc. A 1555–1560 https://doi.org/10.1039/J19680001555 (1968).
Hargreaves, J. S. J., Hutchings, G. J., Joyner, R. W. & Taylor, S. H. A study of the methane–deuterium exchange reaction over a range of metal oxides. Appl. Catal. A 227, 191–200 (2002).
Xu, M., Jupp, A. R., Qu, Z.-W. & Stephan, D. W. Alkali metal species in the reversible activation of H2. Angew. Chem. Int. Ed. 57, 11050–11054 (2018).
Guan, H. B. et al. Preparation of nanometer magnesia with high surface area and study on the influencing factors of the preparation process. Acta Phys. Chim. Sin. 22, 804–808 (2006).
Gržeta, B. et al. Environment of the Eu3+ ion within nanocrystalline Eu-doped BaAl2O4: correlation of X-ray diffraction, Mössbauer spectroscopy, X-ray absorption spectroscopy, and photoluminescence investigations. Inorg. Chem. 57, 1744–1756 (2018).
Zhang, Y. et al. Enhanced hydrogenation properties of Pd single atom catalysts with atomically dispersed Ba sites as electronic promoters. ACS Catal. 12, 15091–15096 (2022).
Hayashi, K., Sushko, P. V., Hashimoto, Y., Shluger, A. L. & Hosono, H. Hydride ions in oxide hosts hidden by hydroxide ions. Nat. Commun. 5, 3515 (2014).
Aramendía, M. A. et al. Study of MgO and Pt/MgO systems by XRD, TPR, and 1H MAS NMR. Langmuir 15, 1192–1197 (1999).
Chizallet, C. et al. Study of the structure of OH groups on MgO by 1D and 2D 1H MAS NMR combined with DFT cluster calculations. J. Phys. Chem. C. 111, 18279–18287 (2007).
Kang, Q.-K. et al. Rhodium-catalyzed stereoselective deuteration of benzylic C–H bonds via reversible η6-coordination. Angew. Chem. Int. Ed. 61, e202117381 (2022).
Munz, D. et al. Proton or metal? The H/D exchange of arenes in acidic solvents. ACS Catal. 5, 769–775 (2015).
Cai, Y. et al. Transition metal-free hydrogenolysis of anilines to arenes mediated by lithium hydride. J. Am. Chem. Soc. 144, 17441–17448 (2022).
Wilson, A. S. S., Hill, M. S., Mahon, M. F., Dinoi, C. & Maron, L. Organocalcium-mediated nucleophilic alkylation of benzene. Science 358, 1168–1171 (2017).
Rösch, B. et al. Nucleophilic aromatic substitution at benzene with powerful strontium hydride and alkyl complexes. Angew. Chem. Int. Ed. 58, 5396–5401 (2019).
Tie, L., Shan, X.-H., Qu, J.-P. & Kang, Y.-B. α-Trideuteration of methylarenes. Org. Chem. Front. 8, 2981–2984 (2021).
Díaz-Requejo, M. M. & Pérez, P. J. Coinage metal catalyzed C–H bond functionalization of hydrocarbons. Chem. Rev. 108, 3379–3394 (2008).
Emmert, M. H., Gary, J. B., Villalobos, J. M. & Sanford, M. S. Platinum and palladium complexes containing cationic ligands as catalysts for arene H/D exchange and oxidation. Angew. Chem. Int. Ed. 49, 5884–5886 (2010).
Novak, E. et al. Temperature dependent local atomic structure and vibrational dynamics of barium hydride and calcium hydride. J. Phys. Chem. C. 125, 24328–24339 (2021).
Tosello Gardini, A., Raucci, U. & Parrinello, M. A bulk phase transformation drives ammonia synthesis on barium hydride. Preprint at https://doi.org/10.26434/chemrxiv-2024-3n866 (2024).
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (grant no. 21988101), the Youth Innovation Promotion Association CAS (nos. Y2022060), and the Zhejiang Provincial Natural Science Foundation of China (grant no. LR24B030002).
Author information
Authors and Affiliations
Contributions
P.C. and J.G. conceived the project. J.G and F.C. co-supervised the research. J.G., F.C., and Y.C. designed the experiments, analyzed the data, and wrote the manuscript. Y.C. and Y.W. conducted the experimental work. L.R. conducted the DFT calculations. Y.Z. performed the AC-HADDF-STEM measurements. Y.C. analyzed the EXAFS data with the assistance of J.Y. T.H., H.W., J.H., A.W., and B.G. reviewed and edited the paper. All authors participated in the discussion and data analyses.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, Y., Rao, L., Wang, Y. et al. Fabrication of atomically dispersed barium hydride catalysts for the synthesis of deuterated alkylarenes. Nat Commun 16, 1868 (2025). https://doi.org/10.1038/s41467-025-57207-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57207-9