Abstract
The WHO has given a permissive recommendation for an off-label one-dose human papillomavirus (HPV) vaccine schedule to prevent cervical cancer, based on evidence of comparable protection to two or three doses of vaccine. While neutralizing antibodies are thought to be the primary mechanism of protection, the persistence of immunity and whether other antibody-mediated mechanisms of protection are involved is unclear. Using systems serology, we investigated HPV antibody responses in serum from Fijian girls who were unvaccinated or received one, two or three doses of quadrivalent HPV vaccine six years earlier. We also evaluated their HPV antibody responses 28 days following a dose of bivalent HPV vaccine. After six years, one dose induced lower antibody concentrations but similar antibody profiles and phagocytic function as two or three doses. Following bivalent vaccine, antibody concentrations, particularly IgG1/IgG3, antibody profiles and phagocytic function were similar between previously vaccinated girls, indicating immune memory after one dose. Cross-reactive antibody responses against non-vaccine genotypes (HPV31/33/45/52/58) were lower following one dose than two or three doses. These findings provide novel insights into serological immunity and recall responses following one-dose HPV vaccination.
Similar content being viewed by others
Introduction
Persistent infection with human papillomaviruses (HPV) is the primary cause of cervical cancer, which is the 4th most prevalent cancer among women worldwide with 660,000 new diagnoses and more than half resulting in death in 20221. Due to this high burden, the World Health Organisation (WHO) has issued a call to action to eliminate cervical cancer as a public health problem, with prophylactic HPV vaccines playing an important role in elimination2. HPV vaccines are highly effective at preventing vaccine-type HPV infection and disease, including cervical cancer and genital warts, and has the potential to reduce up to 93% of cervical cancers with the introduction of the nonavalent vaccine (9vHPV)3.
Currently, the WHO has given a permissive recommendation for an off-label one-dose HPV vaccine schedule for girls aged 9-20 years, based on accumulating evidence of non-inferior protection in adolescent girls who received one dose of HPV vaccine compared with two or three doses4,5,6. A randomised controlled trial in Kenyan women aged 15-20 years found that one dose of 9vHPV or bivalent (2vHPV) HPV vaccine had high vaccine efficacy (>95%, ≥98% respectively) against vaccine-type-specific persistent infections up to 3 years among women who were HPV DNA-negative7. Another study in Tanzania found that 99% of girls who received one dose of 2vHPV or 9vHPV vaccine remained seropositive at 24 months8. Other observational studies have documented similar protection for one dose as two or three doses and sustained vaccine-type antibody responses after 10-11 years, despite one dose inducing lower neutralising antibody concentrations than those induced by two or three doses5,9. Collectively, these studies demonstrate that one dose of HPV vaccine appears to provide strong protection against vaccine-type HPV infection and disease, despite lower antibody concentrations. Considering that two or three doses of protein subunit vaccine are generally needed to generate robust immunological memory responses, it is remarkable that one dose of HPV vaccine can provide robust long-term immunity.
While neutralising antibodies induced by vaccination are thought to be the primary mediator of protection for HPV vaccines, the minimum level required is unknown. Apart from directly neutralising viral pathogens, antibodies can engage Fc receptors (FcR) on immune cells to trigger a range of Fc-mediated effector functions, including antibody-dependent cellular phagocytosis (ADCP) of viral particles, antibody-dependent cellular cytotoxicity (ADCC) and complement activation10. Indeed, non-neutralising Fc effector functions have been shown to be protective in other infectious diseases such as from Plasmodium sp. that cause malaria11,12, human immunodeficiency virus (HIV)13,14 and Ebola virus15, particularly at suboptimal neutralising antibody concentrations13,15.
One study has investigated the induction of Fc-mediated effector functions following HPV vaccination, where vaccination with three doses of quadrivalent (4vHPV) HPV vaccine and 2vHPV vaccine was found to generate different antibody profiles16. Differences in antibody profiles following 2vHPV and 4vHPV vaccine are likely due to the different adjuvants used in each vaccine, with the 2vHPV vaccine known to be more immunogenic due to its inclusion of AS04 adjuvant as a potent inducer of TLR4 compared to aluminium adjuvant in the 4vHPV vaccine. It is currently unknown whether Fc-mediated effector functions contributes to protection against HPV, particularly in the context of a one-dose HPV vaccine schedule10. In this study, we used a systems serology approach to profile HPV-specific antibody responses six years following one, two or three doses of 4vHPV vaccine, and evaluated their responses 28 days after a booster dose of 2vHPV vaccine in a cohort of adolescent Fijian girls.
Results
Study cohort characteristics
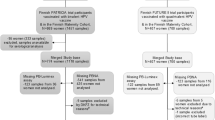
Between February and March 2015, a total of 200 girls aged 15-19 years living in the Greater Suva area of Fiji who were unvaccinated or previously received one, two or three doses of 4vHPV vaccine were recruited into the observational cohort study (Clinicaltrials.gov identifier NCT02276521), with equal distribution of the two main ethnicities in Fiji; indigenous Fijians (iTaukei) and Fijians of Indian Descent17. Serum samples were collected six years following the last dose of 4vHPV vaccine, and 28 days following a booster dose of 2vHPV vaccine to assess immunological memory. Due to logistical limitations and sample availability, a random subset of samples from each dosage group (n = 20/group at each timepoint; total 160 samples) with equal distribution of ethnicity (Indigenous Fijians; iTaukei and Fijians of Indian Descent) from the original study cohort were selected for this analysis. The characteristics of these participant samples are detailed in Table 1. The median age at first dose of 4vHPV vaccine was 10 or 11 years for previously vaccinated girls. Other demographic factors such as age at recruitment and BMI were similar across all dosage groups. Participant characteristics between the dosage groups and between this subset of samples and the overall cohort were similar17.
Immunological memory generated following one dose of 4vHPV vaccine
We assessed vaccine-type HPV16 and HPV18 serum antibody responses to 8 antibody isotypes and subclasses (IgG, IgG1, IgG2, IgG3, IgG4, IgA1, IgA2, IgM) using a multiplex immunoassay that we have previously shown to correlate strongly with neutralising antibodies18. Antibody responses above the median of a negative control antigen, bovine papillomavirus (BPV), were considered to be positive. After six years, one dose of 4vHPV vaccine induced IgG and IgA1 concentrations to both HPV16 and HPV18 that were higher than unvaccinated girls, but lower compared to two or three doses although this was not significant (Fig. 1A, C). HPV18 antibody responses were generally weaker compared to HPV16 as previously documented17. Significantly higher HPV16-specific IgA1 was seen for previously vaccinated girls (≥1 dose) than unvaccinated girls, although this was not significant for one dose. A similar trend was also seen for HPV16 or HPV18-specific IgA2 and IgM, although these levels were low. For IgG subclass responses, IgG1, IgG2, IgG3 and IgG4 MFI to both HPV16 and HPV18 were similar between previously vaccinated girls and remained higher compared with unvaccinated girls after six years (Fig. 1B, D). We found robust IgG1 responses to both HPV16 and HPV18, but HPV16-specific IgG3 MFI in girls previously vaccinated girls were 10-fold higher than for HPV18. IgG2 and IgG4 MFI to HPV16 and HPV18 were lower than IgG1 and IgG3, but higher among previously vaccinated girls compared to unvaccinated girls after six years. A dose-dependent increase was found for HPV16 and HPV18-specific IgG2 in girls previously vaccinated with 4vHPV vaccine, although this was not significant. Overall, we found that HPV vaccine-type antibodies persisted for at least six years, characterised by high concentrations of IgG1 for HPV16/18 and IgG3 for HPV16.
Violin plots show total IgG, IgM, IgA1, IgA2 MFI for HPV16 (A) and HPV18 (C), and IgG subclass MFI i.e. IgG1, IgG2, IgG3, IgG4 for HPV16 (B) and HPV18 (D) median fluorescence intensity (MFI) or International Units/mL (IU/mL) six years following the last dose of 4vHPV vaccine (Pre) or 28 days post-2vHPV booster (Post). The median of each group is denoted by a black horizontal line. Statistical significance was calculated using the Kruskal-Wallis test and corrected for multiple comparisons with Dunn’s test. A positive antibody response is defined as being above median BPV (negative control antigen) MFI or IU/mL (IgG). Definitions: 0D 0-dose (n = 20), 1D 1-dose (n = 20), 2D 2-dose (n = 21), 3D 3-dose (n = 19).
One month following 2vHPV vaccine, HPV16- and HPV18-specific IgG, IgM, IgA1 and IgA2 increased from pre-2vHPV vaccine MFI for all dosage groups (Fig. 1A, C). No significant differences in HPV16- and HPV18-specific IgG, IgM, IgA1 and IgA2 MFI were observed between girls who previously received one, two or three doses of 4vHPV vaccine. Compared with previously unvaccinated girls, higher IgG but lower IgM and similar IgA1 and IgA2 MFI were observed among girls previously vaccinated with 4vHPV vaccine. Similarly, girls who received at least one dose of 4vHPV vaccine previously had higher IgG subclass (IgG1-IgG4) responses than unvaccinated girls following 2vHPV vaccine (Fig. 1B, D). Importantly, there was no difference in IgG subclass MFI between girls who previously received one, two or three doses of 4vHPV vaccine. We found increased HPV16- and HPV18-specific IgG1 and IgG3 MFI following 2vHPV vaccine in girls who received one, two or three doses of 4vHPV vaccine previously. While HPV16-specific IgG2 and IgG4 responses did not change following 2vHPV vaccine for all dosage groups, significant increases were found for HPV18-specific IgG2 in girls who received one or two doses of 4vHPV vaccine previously.
The proportion of each IgG subclass expressed as a percentage of total HPV-specific IgG within each dose group is presented in Supplementary Table 1. We found HPV18 IgG3 formed a lower percentage of total IgG than HPV16 in previously vaccinated girls both after six years (HPV16: mean 15.61-23.55% IgG3; HPV18: mean 5.17-13.85% IgG3) and 28 days post-2vHPV vaccine (HPV16: mean 36.35-44.57%; HPV18: mean 12.26-21.09%) (Supplementary Table 1).
One dose of 4vHPV vaccine induces robust FcR-mediated antibody responses
We next evaluated HPV-specific Fc-mediated effector function by measuring the engagement of three FcγR (FcγR2A, FcγR2B, FcγR3A) typically associated with ADCP, inhibitory Fc function and ADCC, respectively19. Six years after the last dose of 4vHPV vaccine, we found that girls who received one dose of 4vHPV vaccine induced robust binding to all three FcγRs, which were higher than unvaccinated girls but similar to two or three doses (Fig. 2A). HPV18-specific FcγR responses were weaker in magnitude compared to HPV16, reflecting lower HPV18-specific antibody concentrations. Overall, higher HPV16- and HPV18-specific FcγR2A, FcγR2B and FcγR3A MFI were found among girls who were previously vaccinated with 4vHPV vaccine compared with unvaccinated girls. Following a dose of 2vHPV vaccine, significantly higher HPV16- and HPV18-specific FcγR2A, FcγR2B and FcγR3A antibodies were found among girls who previously received 4vHPV vaccine compared with previously unvaccinated girls, except for FcγR2B in the three-dose group.
Fc receptor responses (FcγR2A, FcγR2B, FcγR3A) and ADCP function against HPV16 and HPV18 (A) were measured at six years following the last dose of 4vHPV vaccine (Pre) or 28 days post-2vHPV booster (Post). The median of each group is denoted by a black horizontal line. A positive response is defined as being above the median of control antigen BPV for Fc receptors, or the median of a seronegative control serum for ADCP. Statistical significance was calculated using the Kruskal-Wallis test and corrected for multiple comparisons with Dunn’s test. Correlation between FcγR2A and ADCP for HPV16 and HPV18 (B) was assessed using Spearman’s rank test. Definitions: 0D 0-dose (n = 20), 1D 1-dose (n = 20), 2D 2-dose (n = 21), 3D 3-dose (n = 19).
While we observed responses to all three FcγR, we evaluated ADCP (FcγR2A-mediated) as the most likely mechanism of immune protection for prophylactic HPV vaccine20,21. We found that ADCP function against HPV16 was higher among girls who were previously vaccinated with 4vHPV vaccine compared with unvaccinated girls after six years, and was higher for girls who received two or three doses of 4vHPV vaccine compared to girls who received one dose although this was not significant. After six years, ADCP against HPV18 was relatively low.
Following a dose of 2vHPV vaccine, ADCP function against HPV16 and HPV18 increased significantly for all dosage groups. Girls who were previously vaccinated with one dose of 4vHPV vaccine had HPV16- and HPV18 specific ADCP function similar to girls who were previously vaccinated with two or three doses and was significantly higher than previously unvaccinated girls, although the small sample size may have precluded statistical significance for HPV18-specific ADCP.
We observed a positive correlation between FcγR2A MFI and ADCP function (Fig. 2B). Correlation analyses between FcγR and ADCP and other antibody features are shown in Supplementary Fig. 2, with HPV16 and HPV18 total IgG, IgG1 and IgG3 strongly correlating with both FcγR binding and ADCP. Furthermore, neutralising antibodies, which were measured previously17, also showed a strong correlation with IgG1 and ADCP (Supplementary Fig. 2).
HPV16 and HPV18 antibody signatures are similar between one, two or three doses of 4vHPV vaccine
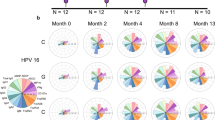
We next evaluated HPV antibody signatures that incorporated all examined features in each dosage group to better visualise the overall HPV antibody profiles in this cohort. The raw data was first normalised within the dynamic range of each individual HPV genotype, antibody feature and timepoint. The median MFI of each antibody feature was then calculated for each dose group and visualised as a polar plot. After six years, one dose of 4vHPV vaccine induced lower magnitude of antibody features, but similar HPV16 and HPV18-specific antibody profiles as two or three doses, characterised by strong IgG, IgG1 and FcγR responses against HPV16 (Fig. 3). HPV18-specific antibody profiles in girls that were previously vaccinated were similar to HPV16 but were weaker.
The polar plots depict the relative magnitude of HPV16 and HPV18 antibody features six years following the last dose of 4vHPV vaccine (Pre) or 28 days post-2vHPV booster (Post). Wedge sizes represent the median of each antibody feature, normalised within HPV genotypes and antibody features, and across all dose groups; 0-dose (n = 20), 1-dose (n = 20), 2-dose (n = 21), 3-dose (n = 19).
Following 2vHPV vaccine, antibody signatures were similar between girls previously vaccinated with one, two or three doses of 4vHPV vaccine, driven by IgG, IgG1, FcγR2A, FcγR2B, FcγR3A and ADCP (Fig. 3). Interestingly, antibody signatures for HPV16 and HPV18 differed, with HPV16 antibody responses characterised by IgG1, IgG3 and IgA1, whereas HPV18 induced a predominately IgG1 response.
Next, we investigated if there were differences in the overall antibody profile between one, and two or three doses of 4vHPV vaccine using principal component analysis (PCA) incorporating all measured antibody features (isotype/subclass, FcγR and ADCP). To determine the fewest antibody features needed to differentiate 4vHPV vaccine dosage groups and avoid overfitting, least absolute shrinkage and selection operator (LASSO) feature selection was first applied to the dataset. PCA on LASSO feature-selected data did not reveal any major difference in HPV16/18 vaccine type antibody profile between girls who received one, two or three doses of 4vHPV vaccine after six years (Fig. 4). Separation between the unvaccinated and vaccinated groups was driven by 5 of the total 24 features, including HPV16-specific IgG1, IgA2, FcγR3A and HPV18-specific IgG and IgG2. Following 2vHPV vaccine, antibody profiles in girls who previously received one, two or three doses of 4vHPV vaccine were indistinguishable, and separated from the unvaccinated group largely by HPV18-specific IgG1. Overall, the antibody profile induced by one, two or three doses of 4vHPV vaccine were similar after six years and following 2vHPV vaccine, with IgG1 being the main contributor to the vaccine-type HPV-specific antibody response.
PCA analyses and loading plots of log-transformed, LASSO-selected HPV16/18 antibody features in unvaccinated (0D, n = 20) or 4vHPV-vaccinated individuals (1D, 1-dose, n = 20; 2D, 2-dose n = 21; 3D, 3-dose, n = 19) six years following their last dose of vaccine, and one month after a 2vHPV booster dose. The mean of each group is represented by the large solid circles in the PCA plot, with coloured ellipses representing the 95% confidence level of each dosage group. Bars on the loading plot show the contribution of each LASSO-selected antibody feature to PC1 in ascending order.
One dose of 4vHPV vaccine induces lower cross-reactive antibody responses than two or three doses
We explored the breadth of cross-reactivity induced by one, two or three doses of 4vHPV by compiling antibody responses to the five HPV genotypes not included in either 2vHPV or 4vHPV vaccines (i.e. HPV31, 33, 45, 52, 58). These additional five HPV types are included in the 9vHPV vaccine and contribute approximately 20% of cervical cancer cases22. Any individual with detectable antibodies to one or more non-vaccine genotypes, defined as MFI ≥ mean ± 2 standard deviations of BPV MFI, were considered to have cross-reactive antibodies. The concentrations of each antibody feature for individual non-vaccine HPV types (HPV31, 33, 45, 52, 58) are shown in Supplementary Figs. 3–7.
After six years, one dose of 4vHPV vaccine induced a weaker IgG cross-reactive response compared to two or three doses (Fig. 5A). Approximately 35% of girls who previously received one dose of 4vHPV had detectable IgG and IgA1 to at least one non-vaccine HPV type, as opposed to 81-84% of girls who received two or three doses. While we detected cross-reactive IgM and IgA2 among unvaccinated girls, the concentrations were generally low (Supplementary Figs. 3–7). For IgG subclasses, cross-reactive IgG1 responses mirrored total IgG, whereas cross-reactive IgG2, IgG3 and IgG4 were less commonly detected among girls in all dosage groups after 6 years (Fig. 5A). Cross-reactive responses to FcγR were more prominent for FcγR2A than FcγR2B or FcγR3A, and were lower in girls who previously received one dose compared to two or three doses of 4vHPV vaccine (Fig. 5A). Overall, cross-reactive HPV antibody responses after 6 years comprised largely of IgG1 and bound FcγR2A.
Stacked bar plots represent the percentage of individuals in each of 0-dose (0D, n = 20), 1-dose (1D, n = 20), 2-dose (2D, n = 21) and 3-dose (3D, n = 19) 4vHPV vaccine dosage groups after six years (A), and following 2vHPV (B) with detectable responses to any of five non-vaccine genotypes (HPV31, HPV33, HPV45, HPV52, HPV58) for each antibody feature. Detectable antibody responses defined as having MFI > mean + 2 standard deviations of BPV MFI.
Following 2vHPV vaccine, we found increases in cross-reactivity to multiple HPV genotypes across most antibody features including IgG, IgM, IgA and all three FcγR, as well as IgG1 and IgG3 (Fig. 5B). There was minimal antibody cross-reactivity for IgG2 and IgG4. IgG, IgG1 and FcγR2A responses to all five measured non-vaccine HPV genotypes were detectable in at least 70% of girls previously vaccinated with 4vHPV vaccine. We observed increases in the percentage of previously vaccinated girls with non-vaccine HPV-specific IgA1, IgA2, IgG3, FcγR2B and FcγR3A. In general, cross-reactive responses were similar between girls who previously received one, two or three doses of 4vHPV vaccine following the 2vHPV booster. Overall, these results suggest that one dose of 4vHPV vaccine can induce cross-reactive antibody responses with highly functional antibody features such as IgG, IgG1, IgG3 and FcγR binding, but to fewer non-vaccine HPV genotypes compared to two or three doses of vaccine. The distribution of antibody features induced by each of the five non-vaccine HPV genotypes are shown in Supplementary Fig. 8.
PCA was used to visualise the antibody profile against non-vaccine HPV genotypes (HPV31, 33, 45, 52, 58). Cross-reactive antibody responses were generally weak six years following the last dose of 4vHPV vaccine, as PCA could not distinguish between unvaccinated and vaccinated girls (Supplementary Fig. 9). Greater separation was observed following 2vHPV vaccine and was driven primarily by HPV58-specific IgG and HPV31-specific IgG1, with little difference between girls who were previously vaccinated with one, two or three doses of 4vHPV vaccine. Similar to what was observed for HPV16 and HPV18, cross-reactive antibody responses to non-vaccine HPV genotypes had a bias towards total IgG, particularly IgG1 (Supplementary Fig. 9). However, both models have relatively low variance due to the large diversity of responses.
Discussion
Previously, we evaluated the immune response in terms of cellular immunity and neutralizing antibodies to HPV16/18 and HPV genotypes not included in the 4vHPV vaccine in the same cohort of Fijian girls17,23,24. As an extension of the original study, we next evaluated the serum antibody response in a randomised subset of individuals using systems serology. Here, we found that one dose of 4vHPV vaccine induced similar HPV vaccine type antibody profiles to that of two or three doses, but with lower magnitude after six years. This serological HPV antibody signature was characterised by highly functional antibody isotypes and subclasses including IgG, IgG1 and FcγR2A binding, as well as IgG3 and IgA1, that correlated strongly with ADCP function. Moreover, a dose of 2vHPV vaccine resulted in enhanced HPV16/18-specific antibody concentrations in girls who previously received one dose of 4vHPV vaccine, indicative of immune memory, that were maintained for at least six years. Most HPV serology studies to date have focused on the measurement of total IgG or neutralising antibody concentration, which is only one aspect of vaccine-induced antibody immunity. To our knowledge, this is the first study to examine multiple antibody features induced by one dose of HPV vaccine.
Our findings are consistent with previous studies reporting IgG1 as the dominant IgG subclass induced by HPV16 and HPV18 following HPV vaccination, followed by IgG3 with minimal IgG2 and IgG425,26. Natural infection with HPV16 or HPV18 also appears to induce an IgG1-skewed antibody response with varying levels of IgG3 (HPV16: 20%, HPV18: 6%) and only 1-2% of total IgG being of the IgG2 or IgG4 subclass25. These findings are not unexpected as IgG1 is the most abundant IgG subclass in serum and is preferentially induced in response to viral infection and protein antigens27. IgG3 is particularly potent at inducing Fc-mediated effector functions such as ADCP, ADCC and complement activation, likely due to having a higher affinity for FcR and an extended hinge region compared to other IgG subclasses28. However, IgG3 has a short half-life in human serum, with concentrations peaking up to four weeks post-infection and then replaced by IgG129,30. IgG4 is the least prevalent IgG subclass in serum and are typically generated in response to allergens, or following long-term exposure to antigen27. Unlike other IgG subclasses, the bispecific structure of IgG4 renders it unable to form immune complexes with antigen in circulation and hence has limited capacity for antibody effector functions31. IgG2 antibodies are produced as a result of T cell-independent responses to glycolipid or polysaccharide antigens27. In addition, IgG2 is highly expressed on CD27+IgG+ memory B cells which accumulate with age and repeated immune responses to antigen, at the expense of IgG1 and IgG3 class-switching32. Neither IgG2 nor IgG4 are likely to have significant contributions to immune protection against HPV, as complement-mediated cytolysis is only detectable with high concentrations of IgG2 and is undetectable for IgG433.
We found that HPV16 was highly immunogenic and induced a more heterogeneous antibody response consisting of both IgG1 and IgG3 subclasses after six years and one month following a 2vHPV booster, unlike HPV18 which induced lower IgG concentrations and an IgG1-dominant response. Similar antibody characteristics were observed for genotypes related to HPV16 (HPV31, 33, 52, 58) and HPV18 (HPV45). As HPV16 and HPV18 belong to two different clades, α9 and α7 respectively, genomic or structural factors may contribute to the different antibody profiles observed in this study. Furthermore, the HPV18 VLP used in the 4vHPV is known to be intrinsically less immunogenic than HPV16 VLPs34. Our findings are consistent with other studies that have reported lower HPV18 antibody responses compared to HPV16 following HPV vaccination16,17,25. Despite this, HPV vaccines have been shown to provide strong protection against both HPV16 and HPV18, and long-term follow up of one-dose recipients are crucial since one dose of HPV vaccine generates lower antibody concentrations than multiple doses35. However, the clinical relevance of this is unknown as there is no established minimum level of antibody required for protection. A retrospective cohort study of pregnant women in Fiji who received 4vHPV vaccine in 2008/9 as part of the same cohort in this study, found uniformly low HPV16/18 DNA detection rates between one (2.5%), two (0%) and three doses (1.6%) eight years following vaccination, suggesting that these lower antibody concentrations induced by one dose of 4vHPV vaccine are protective17,36. Indeed, these findings are supported by other studies in Costa Rica37 and India6 that have found similarly low HPV16/18 prevalence after 7-10 years following one dose of 2vHPV or 4vHPV vaccine.
It is important to note that a heterologous vaccination schedule (4vHPV followed by 2vHPV vaccine) was used in this study, and whether a similar profile is observed following homologous vaccination is unknown. A heterologous 4vHPV and 2vHPV vaccination schedule was evaluated in this study to reflect the inclusion of 2vHPV vaccine in the Fiji national immunization schedule in 2013, following the completion of the 4vHPV vaccination program for girls aged 9-12 years in 2008-200917. Hence, this provided a unique opportunity to examine the immune response in girls who received less than the recommended 3 doses of 4vHPV vaccine, and evaluate the effect of a booster dose of 2vHPV vaccine in these same girls. Heterologous HPV vaccine schedules are currently used in Quebec (9vHPV followed by 2vHPV vaccine), which has been shown to be highly immunogenic with almost 100% seroconversion following one dose of 9vHPV in 9-10 year old boys and girls38,39. Differences in antibody responses induced by different HPV vaccines have been observed in previous studies, with the 2vHPV vaccine consistently inducing stronger cross-protection and higher antibody concentrations, including IgG3, than 4vHPV vaccine16,40. This is likely due to the difference in adjuvants in the 2vHPV and 4vHPV vaccines. The 2vHPV vaccine has AS04 adjuvant, which is a potent inducer of Th1 responses resulting in both IgG1 and IgG3 antibodies41. In contrast, the 4vHPV vaccine is formulated with aluminium salt and instead drives a Th2 response characterised by IgG142. Indeed, other studies evaluating the antibody subclass distribution following three doses of 2vHPV vaccine have found HPV18 IgG3 proportions of total IgG to be similar to those of HPV16, and significantly higher following 2vHPV vaccine compared to 4vHPV or 9vHPV vaccine16,25,43.
An interesting observation from this study was the detection of HPV16-specific IgA1 in serum. While this was maintained at low concentrations after six years, it was enhanced following 2vHPV vaccine, indicative of an immune memory response. Unlike mucosal IgA which persists in dimeric form and is equally distributed between IgA1 and IgA2, serum IgA antibodies at steady state are predominantly monomeric IgA1, and its function has been relatively understudied44,45. Whether serum IgA1 has antiviral functions and can transudate to the mucosa to elicit mucosal immunity is unclear, as only small amounts of HPV16/18-specific cervical IgA appear to originate from the circulation after vaccination46. Nevertheless, as cervical IgA concentrations correlate well with serum IgA up to 2 years post-vaccination, the role of serum IgA antibodies in mediating local protection against HPV warrants further investigation46.
Antibody isotypes and subclasses have different Fc-mediated effector functions. We found that one dose of HPV vaccine induced antibodies with capacity to bind FcγR (FcγR2A, FcγR2B and FcγR3A) and trigger ADCP to a similar capacity as two or three doses. Evidence of the importance of ADCP following HPV vaccination was best described in a murine HPV cervicovaginal model. Passive transfer of sera from mice with high concentrations of HPV16/18 antibodies prevented HPV pseudovirions from binding to the basement membrane and abrogated HPV16 infection in naïve mice, consistent with neutralisation21. However, at low HPV16/18 antibody concentrations, HPV16 pseudovirions could still bind to the basement membrane, but were subsequently found to be associated with cellular aggregates consisting of neutrophils and were cleared from the site of infection. We therefore focused our study on ADCP as the most biologically relevant effector function.
While we also found robust FcγR3A binding, typically associated with ADCC, following 4vHPV vaccination, we did not measure ADCC in vitro as its relevance in mediating protection against HPV is less clear (and somewhat controversial). Currently available HPV vaccines are prophylactic and are not thought to not clear existing lesions or infection47. Previous studies have found increased expression of natural killer (NK) cell receptors, as well as activation of NK cell degranulation (CD107a) and chemokine MIP1b secretion following HPV vaccination in healthy women16,48. However, further research is needed to fully understand the role of other Fc-mediated functions in HPV vaccine immune protection. Similarly, while the complement system has known roles in opsonisation and lysis of pathogens, whether HPV-antibody complexes can also activate complement is unknown. Nevertheless, we and others have found that HPV vaccines can induce antibodies that bind FcγR3A16, which is expressed mainly on NK cells but also γδ T cells, macrophages, neutrophils and eosinophils, and is typically associated with ADCC49,50. In contrast with FcγR2A and Fcγ3A, FcγR2B is an inhibitory receptor with roles in B cell selection and limiting FcγR signalling to prevent inappropriate IgG-mediated inflammation51,52. In fact, as most myeloid cells and effector lymphocytes co-express activating and inhibitory FcγR, the outcome of IgG-mediated Fc effector function is determined by the opposing signalling of these FcγRs53,54.
The breadth of immunity to non-vaccine genotypes is an important consideration for the 4vHPV and 2vHPV vaccines. Three doses of either vaccine has been shown to generate cross-reactive antibodies against non-vaccine but phylogenetically related oncogenic genotypes including HPV31, 33, 45, 52 and 58, albeit at lower concentrations than vaccine types HPV16 and HPV1823,55. Our study extends this observation to one and two doses, with cross-reactive antibody profiles largely driven by total IgG and IgG1 much like for vaccine types HPV16 and HPV18. Considering that we found that one dose of 4vHPV vaccine generated weaker cross-reactive responses after six years, two doses may be needed to generate cross-reactive antibody responses to HPV31/33/45/52/58, although this needs to be interpreted with caution because of the use of the mixed vaccine schedule in our study. Despite the lower cross-reactive antibody concentrations compared to multiple doses, one dose of 2vHPV or 4vHPV vaccine has been shown to induce cross-protection against HPV31/33/45 incident infections for up to 10 years56,57,58. This is particularly important for LMICs considering introducing HPV vaccine, since 9vHPV is substantially more expensive than the 4vHPV or 2vHPV vaccines. Larger studies with longer follow up are needed to confirm the cross-protection offered by a single dose of HPV vaccine6,37.
A strength of this study is our unique cohort of adolescent Fijian girls which allowed us to examine the humoral immune response after six years and also recall response to a booster dose as a proxy for immunological memory after one dose of HPV vaccine. We also were able to generate a comprehensive profile of the antibody response to one dose of HPV vaccine, measuring 8 antibody subclasses, 3 FcR and ADCP to 8 antigens for each individual sample assayed. Despite our small sample size, our results were robust in demonstrating HPV antibody signatures including breadth of immunity and recall response following one dose of HPV vaccine. Other limitations of our study include the lack of HPV infection data from study participants, although HPV16/18 DNA prevalence was very low (0-2.5%) and similar between Fijian pregnant women vaccinated with one, two or three doses of 4vHPV vaccine eight years earlier36. We also did not evaluate other Fc-mediated effector functions as their biological relevance to HPV immunity is less certain. Another limitation is that our data, except for HPV16/18 are reported in MFI, which may not be directly comparable to other studies. The WHO International Standards have only just been developed for HPV6, HPV11, HPV31, HPV33, HPV45, HPV52 and HPV58 for reporting in International Units (IU/mL)59.
In summary, we described the antibody features of HPV vaccination following one, two or three doses of 4vHPV vaccine and the subsequent responses to a booster dose of 2vHPV vaccine. One dose of 4vHPV vaccine generated similar immune memory and antibody features as two or three doses of 4vHPV vaccine. HPV antibody features were characterised by IgG1, as well as IgG3 and IgA1 antibodies following 2vHPV vaccine, and strong Fc receptor binding that correlated well with ADCP function. Different antibody profiles and magnitude of antibody response were found for HPV16 and HPV18. These results indicate that one dose of HPV vaccine can induce long-term immunogenicity and ADCP with similar profiles to two and three doses, which advances our understanding of immunity induced by one dose of HPV vaccine.
Methods
Study samples
The serum samples used in this project were derived from a retrospective cohort study (ClinicalTrials.gov identifier: NCT02276521) conducted in 2015, as described previously17. In 2008-2009, Fiji implemented a 4vHPV vaccination campaign targeting girls aged 9-12 years through a school-based immunization program. Recruitment was conducted in February-March 2015 from immunization records obtained from the Fiji Ministry of Health and Medical Services. A total of 200 Fijian girls aged 15-19 years living in the Greater Suva area (Suva, Nasinu, Nausori) who were immunised six years prior with one, two or three doses of 4vHPV vaccine, as well as girls who had not received any HPV vaccine were recruited for the study. One dose of 2vHPV vaccine was given at day 0 to all participants to evaluate immunological memory, and blood samples were collected to assess HPV-specific immune responses six years following the last dose of 4vHPV vaccine, and 28 days following 2vHPV immunisation.
For this analysis, 80 (0-dose n = 20, 1-dose n = 20, 2-dose n = 21, 3-dose n = 19, per timepoint) of the original 200 serum samples collected during the study at each timepoint were used due to logistical limitations and sample availability. Samples were assigned a new study identification number and randomly allocated to be used in this analysis in a single-blind manner, with an equal distribution of ethnicity (Indigenous Fijians; iTaukei and Fijians of Indian Descent).
Multiplex assay for measuring antibody features
The bead-based multiplex immunoassay for measuring HPV antibody responses has been described previously18. Briefly, HPV VLPs (HPV16, 18, 31, 33, 45, 52, 58, BPV negative control antigen) conjugated to carboxylated magnetic beads were diluted to a final concentration of 20 beads/μL in 0.1% BSA-PBS assay buffer in a 96-well optical bottom plate. Serum samples diluted 1:50 or 1:100 in assay buffer and assay controls (samples known to have negative/low to high type-specific antibody concentrations) were included in each plate at 50 μL and incubated overnight at 2-8 °C on a plate shaker. The following day, the plate was incubated with 50 μL of R-phycoerythrin (PE)-conjugated anti-human secondary antibody diluted to 1.3 μg/mL in assay buffer for 2 h at RT. Serum samples were evaluated for a total of 8 antibody isotypes/subclasses, including IgG (SouthernBiotech, US, cat no. 9040-09), IgM (SouthernBiotech, US, cat no. 9020-09), IgA1 (SouthernBiotech, US, cat no. 9130-09), IgA2 (SouthernBiotech, US, cat no. 9140-09), IgG1 (SouthernBiotech, US, cat no. 9052-09), IgG2 (SouthernBiotech, US, cat no. 9070-09), IgG3 (SouthernBiotech, US, cat no. 9210-09) and IgG4 (SouthernBiotech, US, cat no. 9200-09). For FcR analyses, the plate was instead incubated with either FcγR2A (H131), FcγR2B or FcγR3A (V158) dimer at RT60, then resuspended in streptavidin-PE for 1 h at RT. Finally, the plate was washed twice followed by resuspension in 100 µL of Luminex sheath fluid on a plate shaker for 10 min. Quantitative analysis including correction for background was performed on the Bio-Plex 200 (Bio-Rad, US) using Bio-Plex Manager 6.0 software. IgM, IgA1, IgA2, IgG1, IgG2, IgG3, IgG4 and FcγR binding levels were quantified as MFI. HPV16 and HPV18-specific IgG levels were measured in Luminex Units (LU/mL) and calibrated with the WHO HPV16 (Product no: 05/134) and HPV18 (Product no: 10/140) International Standards to report in International Units (IU/mL), with a conversion factor of 1 LU/mL equal to 0.055 IU/mL for HPV16, or 0.021 IU/mL for HPV18. The multiplex assay has been validated by comparison with the pseudovirion-based neutralization assay (PBNA), with IgG MFI strongly correlating with neutralizing antibodies measured by PBNA as demonstrated in ref. 18. Any responses above the median of BPV were considered to be positive, and below the median to be negative.
Antibody-dependent cellular phagocytosis assay
A flow cytometry-based assay using opsonised HPV VLP-coated beads to assess antibody-dependent phagocytosis (ADCP) in vitro in a THP-1 monocyte cell line was adapted for HPV using the method previously described in ref. 61. THP-1 monocytes were obtained from the American Type Culture Collection (ATCC, US; Product TIB-202) and passaged according to manufacturer instructions. Briefly, THP-1 cells were thawed by gentle agitation in a 37°C water bath, transferred to a 15 mL tube containing 9 mL R10 media (RPMI-1640 medium supplemented with 10% FBS, 10 mM HEPES, 1% penicillin-streptomycin) and pelleted by centrifugation at 125 x g for 5 mins at RT. Cells were then transferred to a T75 filter flask in 9 mL R10 media and kept in a 37°C/5% CO2 incubator. THP-1 cells were maintained every 2-3 days at 2-3×105 cells/mL, for up to 3 times before use in the ADCP assay.
For the ADCP assay, NeutrAvidin® labelled 1 μm fluorescent beads (Thermo Fisher Scientific, AU) were coupled overnight at 4 °C with biotinylated HPV16 or HPV18 L1/L2 VLP, at a ratio of 15 μg biotinylated HPV per 3 μL of beads used, then washed twice the following day with 2% BSA-PBS buffer to remove unbound antigen. HPV VLP-coated beads at 10 μL were incubated with 10 μL of serum samples diluted 1:100 in a 96-well round bottom plate for 2 h at 37 °C. A HPV seronegative serum was run on every plate as an internal control to determine the baseline level of phagocytosis in THP-1 cells. Subsequently, 1×105 THP-1 cells in 200 μL of RF10 media were added per well and incubated for 16 h in a 37 °C5% CO2 incubator.
Following overnight incubation, the THP-1 cells were stained for 30 min at RT with 50 μL of CD32-BV510 antibody (BD Biosciences, cat. no. 744254, clone 3D3) diluted 1:200 in PBS to examine FcγR2 expression. The cells were washed with cold PBS and fixed with 100 μL of BD Cytofix™ (4.21% formaldehyde w/w, BD Biosciences, US) for 20 min on ice, then resuspended in 100 μL of FACS buffer (heat-inactivated FBS diluted 1:70 in PBS) for analysis by flow cytometry. Cells were acquired on the Cytek Aurora spectral flow cytometer, recording 20,000 events per sample (Cytek Biosciences, US). A phagocytic score was determined by gating on CD32 + THP-1 cells with bead uptake using FlowJo™ software (FlowJo LLC, US) and calculated as follows: (% bead-positive cells x geometric MFI of bead positive cells)/104. The gating strategy used to quantify bead-positive THP-1 cells is shown in Supplementary Fig. 1.
Statistical analysis
The participant characteristics of the different dosage groups were compared using the one-way analysis of variance test for continuous variables or compared using Fisher’s exact test for ethnicity. The magnitude of HPV type-specific antibody subclass and FcR responses were quantified as median fluorescence intensity (MFI) or International Units (IU/mL) for IgG, averaged across duplicates and corrected for background fluorescence. Statistically significant differences between dosage groups and between timepoints was determined using the two-tailed Kruskal-Wallis test and corrected for multiple comparisons with Dunn’s test. Data was visualised in GraphPad Prism 9.1.1 (GraphPad Software, US), with error bars shown as median ± interquartile range (IQR). Polar plots (Fig. 4) were generated in R Studio 2024.04.0 with the ggplot2 package, and the correlation heatmap (Supplementary Fig. 2) with the corrplot package. A p-value of less than 0.05 was considered to be statistically significant for all relevant analyses.
To determine the minimal set of features (antibody isotypes, FcγR, ADCP) needed to characterise 4vHPV vaccine dosage groups using principal component analysis (PCA), LASSO was applied using MATLAB 2023a statistical software (Mathworks, US) with PLS_Toolbox_90. Raw antibody data was first normalised using a log10 transformation, then z-scored. A sequential step-forward algorithm was used to determine the frequency of which each feature that corresponds to the minimum cross-validated mean squared error (MSE) was selected across 100 model iterations. Model prediction was assessed at each step using 4-fold cross-validation. PCA, an unsupervised machine learning method, was used with the most important variables that explained the variance in the dataset, as identified by LASSO feature selection, to visualise the difference between 4vHPV dosage groups. PCA plots and bar plots showing contribution to PC1 incorporating log10-transformed LASSO-selected features were generated in R Studio 2024.04.0 using the mixOmics, factoextra and factoMineR packages.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used to support the findings of this study have been deposited in the Zenodo database under accession code 14848069.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem (World Health Organization, 2020).
Toh, Z. Q. et al. Recombinant human papillomavirus nonavalent vaccine in the prevention of cancers caused by human papillomavirus. Infect. Drug Resist. 12, 1951–1967 (2019).
Kreimer, A. R. et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 16, 775–786 (2015).
Kreimer, A. R. et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT Trial. J. Natl Cancer Inst. 112, 1038–1046 (2020).
Basu, P. et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. 22, 1518–1529 (2021).
Barnabas, R. V. et al. Efficacy of single-dose human papillomavirus vaccination among young African women. NEJM Evid. 1, EVIDoa2100056 (2022).
Watson-Jones, D. et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. Lancet Glob. Health 10, e1473–e1484 (2022).
Joshi, S. et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine 41, 236–245 (2023).
Quang, C., Chung, A. W., Frazer, I. H., Toh, Z. Q. & Licciardi, P. V. Single-dose HPV vaccine immunity: is there a role for non-neutralizing antibodies? Trends Immunol. 43, 815–825 (2022).
Suscovich, T. J. et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci. Transl. Med. 12, eabb4757 (2020).
Aitken, E. H. et al. Developing a multivariate prediction model of antibody features associated with protection of malaria-infected pregnant women from placental malaria. Elife 10, e65776 (2021).
Spencer, D. A. et al. Phagocytosis by an HIV antibody is associated with reduced viremia irrespective of enhanced complement lysis. Nat. Commun. 13, 662 (2022).
Neidich, S. D. et al. Antibody Fc effector functions and IgG3 associate with decreased HIV-1 risk. J. Clin. Investig. 129, 4838–4849 (2019).
Meyer, M. et al. Ebola vaccine-induced protection in nonhuman primates correlates with antibody specificity and Fc-mediated effects. Sci. Transl. Med. 13, eabg6128 (2021).
Roy, V. et al. Differences in HPV-specific antibody Fc-effector functions following Gardasil® and Cervarix® vaccination. npj Vaccines 8, 39 (2023).
Toh, Z. Q. et al. Sustained antibody responses 6 years following 1, 2, or 3 doses of quadrivalent human papillomavirus (HPV) vaccine in adolescent Fijian girls, and subsequent responses to a single dose of bivalent HPV vaccine: a prospective cohort study. Clin. Infect. Dis. 64, 852–859 (2017).
Quang, C. et al. Development of a human papillomavirus (HPV) multiplex immunoassay to profile HPV antibodies. J. Med. Virol. 96, e29732 (2024).
Wieland, A. & Ahmed, R. Fc receptors in antimicrobial protection. in Fc Mediated Activity of Antibodies: Structural and Functional Diversity (eds Ravetch, J. V. & Nimmerjahn, F.) 119–150 (Springer International Publishing, 2019).
Wang, J. W. et al. Roles of Fc ___domain and exudation in L2 antibody-mediated protection against human papillomavirus. J. Virol. 92, e00572–18 (2018).
Day, P. M. et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 8, 260–270 (2010).
de Sanjose, S. et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 11, 1048–1056 (2010).
Toh, Z. Q. et al. Selective persistence of HPV cross-neutralising antibodies following reduced-dose HPV vaccine schedules. Vaccines 7, 1–13 (2019).
Toh, Z. Q. et al. Cellular immune responses 6 years following 1, 2, or 3 doses of quadrivalent HPV vaccine in Fijian girls and subsequent responses to a dose of bivalent HPV vaccine. Open Forum Infect. Dis. 5, ofy147 (2018).
Scherpenisse, M. et al. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS ONE 8, e74797 (2013).
Harro, C. D. et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl Cancer Inst. 93, 284–292 (2001).
Vidarsson, G., & Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520 (2014).
Damelang, T., Rogerson, S. J., Kent, S. J. & Chung, A. W. Role of IgG3 in infectious diseases. Trends Immunol. 40, 197–211 (2019).
Yates, N. L. et al. Multiple HIV-1-specific IgG3 responses decline during acute HIV-1: implications for detection of incident HIV infection. AIDS 25, 2089–2097 (2011).
Dugast, A.-S. et al. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. Eur. J. Immunol. 44, 2925–2937 (2014).
Uversky, V. N., Redwan, E. M., Makis, W. & Rubio-Casillas, A. IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 spike protein. Vaccines 11, 991 (2023).
de Jong, B. G. et al. Human IgG2- and IgG4-expressing memory B cells display enhanced molecular and phenotypic signs of maturity and accumulate with age. Immunol. Cell Biol. 95, 744–752 (2017).
Michaelsen, T. E., Garred, P. & Aase, A. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 21, 11–16 (1991).
Schiller, J. T. & Lowy, D. R. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J. Infect. Dis. 200, 166–171 (2009).
Sankaranarayanan, R. et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 36, 4783–4791 (2018).
Reyburn, R. et al. A single dose of quadrivalent HPV vaccine is highly effective against HPV genotypes 16 and 18 detection in young pregnant women eight years following vaccination: an retrospective cohort study in Fiji. Lancet Reg. Health West. Pac. 37, 100798 (2023).
Kreimer, A. R. et al. Evidence for single-dose protection by the bivalent HPV vaccine—review of the Costa Rica HPV vaccine trial and future research studies. Vaccine 36, 4774–4782 (2018).
Quebec Gd. Human papillomavirus (HPV) immunization program. https://www.quebec.ca/en/health/advice-and-prevention/vaccination/human-papillomavirus-hpv-vaccination-program (2024).
Gilca, V. et al. Immunogenicity and safety of a mixed vaccination schedule with one dose of nonavalent and one dose of bivalent HPV vaccine versus two doses of nonavalent vaccine—a randomized clinical trial. Vaccine 36, 7017–7024 (2018).
Einstein, M. H. et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum. Vaccines 5, 705–719 (2009).
Didierlaurent, A. M. et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 183, 6186–6197 (2009).
Ciabattini, A. et al. Modulation of primary immune response by different vaccine adjuvants. Front. Immunol. 7, 427 (2016).
Pasmans, H. et al. Characterization of the early cellular immune response induced by HPV vaccines. Front. Immunol. 13, 863164 (2022).
Davis, S. K., Selva, K. J., Kent, S. J. & Chung, A. W. Serum IgA Fc effector functions in infectious disease and cancer. Immunol. Cell Biol. 98, 276–286 (2020).
Steffen, U. et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 11, 120 (2020).
Scherpenisse, M. et al. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Hum. Vaccin. Immunother. 9, 314–321 (2013).
Yang, A., Farmer, E., Wu, T. C. & Hung, C. F. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 23, 75 (2016).
Colmenares, V. et al. Human papillomavirus immunization is associated with increased expression of different innate immune regulatory receptors. Clin. Vaccin. Immunol. 19, 1005–1011 (2012).
Gogesch, P., Dudek, S., van Zandbergen, G., Waibler, Z. & Anzaghe, M. The role of Fc receptors on the effectiveness of therapeutic monoclonal antibodies. Int. J. Mol. Sci. 22, 8947 (2021).
Cooper, M. A., Fehniger, T. A. & Caligiuri, M. A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
Samuelsson, A., Towers, T. L. & Ravetch, J. V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291, 484–486 (2001).
Bournazos, S. & Ravetch, J. V. Fcγ receptor function and the design of vaccination strategies. Immunity 47, 224–233 (2017).
Ackerman, M. E. et al. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J. Virol. 87, 5468–5476 (2013).
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005).
Mariz, F. C. et al. Peak neutralizing and cross-neutralizing antibody levels to human papillomavirus types 6/16/18/31/33/45/52/58 induced by bivalent and quadrivalent HPV vaccines. npj Vaccines 5, 14 (2020).
Tsang, S. H. et al. Durability of cross-protection by different schedules of the bivalent HPV vaccine: the CVT Trial. J. Natl Cancer Inst. 112, 1030–1037 (2020).
Wheeler, C. M. et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 13, 100–110 (2012).
Li, C., Hall, T. G., Hall, J. J. & He, W. Q. Effectiveness of quadrivalent HPV vaccination in reducing vaccine-type and nonvaccine-type high risk HPV infection. Epidemiol. Infect. 151, e37 (2023).
Kemp, T. J. et al. WHO International Standards for antibodies to HPV6 HPV11 HPV31 HPV33 HPV45 HPV52 and HPV58. npj Vaccines 9, 165 (2024).
Wines, B. D. et al. Dimeric FcγR ectodomains as probes of the Fc receptor function of anti-influenza virus IgG. J. Immunol. 197, 1507–1516 (2016).
Selva, K. J. et al. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 12, 2037 (2021).
Acknowledgements
We thank Serene Yeow (Murdoch Children’s Research Institute) and Troy Kemp (Frederick National Laboratory for Cancer Research) for technical advice and assistance with the production of HPV VLPs used in the multiplex immunoassay. This work was also supported in part by the Victorian Government’s Operational Infrastructure Support Program. C.Q. is supported by the Research Training Program Scholarship (RTP) from the Australian Commonwealth Government and the University of Melbourne. J.A. and A.W.C. are supported by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant.
Author information
Authors and Affiliations
Contributions
The study was conceived by P.V.L. and Z.Q.T. in conjunction with A.W.C. T.R., E.T., I.H.F., S.M.G., F.M.R., R.R., R.D. and K.M. were investigators of the original retrospective cohort study and provided important intellectual contributions. B.W. and P.M.H. provided expertise related to the FcγR studies. J.A. contributed important expertise and input on the ADCP assays. C.Q. acquired and interpreted the data and drafted this manuscript. All authors made significant contributions to revising the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
S.M.G. has received grants from Merck, GlaxoSmithKline, CSL and the Commonwealth Department of Health; has received nonfinancial support from Merck; and has delivered lectures and received speaking fees from MSD and Sanofi Pasteur MSD for work performed in her personal time. All other authors report no potential conflicts.
Inclusion and ethics
Ethics approval for use of the serum samples in this analysis has previously been obtained from the Fiji National Research Ethics Review Committee, Fiji National Research Committee (2014.5.FNRERC.5.SU), and the Royal Children’s Hospital Human Research Ethics Committee, Melbourne, Australia (34239 A). Informed consent or assent was received from participants in this study.
Peer review
Peer review information
Nature Communications thanks Alex Vorsters, who co-reviewed with Laura Téblick; Simon Beddows; and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Quang, C., Anderson, J., Russell, F.M. et al. Systems serology analysis shows IgG1 and IgG3 memory responses six years after one dose of quadrivalent HPV vaccine. Nat Commun 16, 2130 (2025). https://doi.org/10.1038/s41467-025-57443-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57443-z