Abstract
Malignant pleural effusion (MPE), persistently generated by thorax tumor cells at the advanced stage, remains a major challenge for cancer therapy. Herein, we develop an ultra-sensitive piezoelectric nano-system by doping ytterbium in metal-organic framework (O3P@LPYU), which can be triggered by physiological intrapleural pressure during breath. Under the gently alterative pressure, the piezoelectric nanoparticles with notable peroxidase-like activity effectively produce a burst of reactive oxygen species and induce immunogenic cell death by catalysis of carried ozone as well as peroxide in interstitial fluid. A clear and sustained biodistribution is observed in thorax effusion and tumors upon intrapleural administration of particle. Remarkably, due to the abundant substrates in oxygen-rich environment of pleural cavity, O3P@LPYU particle provides a potent reduction of MPE volume and durable inhibition of tumor growth in thorax. Our work not only develops a bio-responsive piezoelectric nano-system, but also provides a strategy for persistent suppression of MPE in clinics.
Similar content being viewed by others
Introduction
Malignant pleural effusion (MPE) is a severe condition indicative of terminal malignancy, associated with a uniformly poor prognosis. The effusion arises primarily from the direct invasion or metastasis of malignant tumors into the thoracic cavity, leading to the formation of metastatic lesions on the pleura. This is most commonly seen in lung cancer and breast cancer1,2. Tumor cells release cytokines such as vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β), increasing vascular permeability and obstructing lymphatic drainage, thereby resulting in the accumulation of pleural fluid. This fluid contains a complex microenvironment comprising numerous white blood cells, tumor cells, and proteins. Additionally, factors within the microenvironment, including cytokines, growth factors, and extracellular matrix components, influence tumor cell survival and proliferation3,4.
Current therapeutic approaches for MPE include thoracentesis, pleurodesis, intrapleural chemotherapy, and biological therapy. Thoracentesis provides temporary relief from symptoms caused by fluid accumulation, but it does not prevent recurrence and may lead to infection or lung injury2. Pleurodesis, which involves the use of sclerosing agents, can reduce the recurrence rate of pleural effusion, but it may be ineffective for certain types of tumors and can cause severe chest pain and fever5. Intrapleural chemotherapy aims to control the growth of metastatic tumors, but its effectiveness in managing pleural effusion is limited, resulting in the recurrent production of pleural fluid6. Biological therapies, including immunotherapy and targeted therapy, can reduce tumor burden and improve prognosis, but their efficacy varies by individual, and the potential side effects are limitations7. Due to the high recurrence rate of MPE, the limited efficacy in treating refractory tumors (such as intrathoracic metastases), and the complexity of the microenvironment, there is an urgent need for more precise treatment strategies and the development of new drugs.
MPE presents a unique pathophysiological environment characterized by continuous pressure changes and an oxygen-rich microenvironment8. This dynamic setting poses significant challenges for conventional therapies, which often struggle to achieve sustained efficacy. However, it also offers unique opportunities for innovative treatment approaches. In normal human respiration, the intrapleural pressure (IPP) fluctuates between −3 mmHg (at the end of normal exhalation) and −10 mmHg (at the end of normal inhalation)9. Utilizing the pressure variation to achieve physiological pressure-controlled piezoelectric catalysis therapy presents a promising and innovative treatment strategy for MPE10,11. Piezoelectric nanomaterials have shown promising results in anti-tumor therapy by generating reactive oxygen species (ROS) under mechanical stimulation, while gas therapies like ozone treatment have demonstrated success in directly inducing oxidative stress in tumor cells12,13,14. The combination of these approaches holds significant potential, as ozone can serve as a substrate for piezo-catalysis, augmenting ROS generation and thereby achieving a more potent anti-tumor effect. Besides, the oxygen-rich microenvironment further enhances ROS generation, making piezoelectric catalysis a highly efficient method for addressing the persistent and recurrent nature of MPE.
Piezoelectric materials generate electric potential in response to mechanical stress and are classified into ceramics, polymers, and composites, each with unique strengths. They are widely used in sensors, actuators, and energy harvesting, and have significant biomedical applications, including ultrasound imaging and targeted drug delivery15,16,17. Recently, metal-organic frameworks (MOFs) have emerged as advanced piezoelectric materials with enhanced properties. MOFs feature a porous structure that can adsorb gas molecules, serving as substrates for piezoelectric catalysis. This characteristic makes them particularly effective in biomedical applications, including drug delivery, biosensing, and piezo-catalysis18. However, to achieve piezoelectric catalysis in response to physiological IPP stimulation, enhancing the piezoelectric coefficient of the piezoelectric MOFs is crucial. Among various methods, doping modification is one of the most effective approaches to improve piezoelectric properties19.
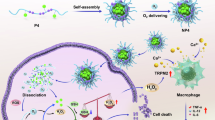
In this study, we address the challenges of MPE treatment by designing a therapeutic strategy that leverages the piezoelectric properties of rare-earth element Ytterbium (Yb) doped MOFs (UIO-66). The rare-earth element Yb was chosen for its ability to enhance electron transfer and inhibit electron-hole recombination, thereby improving the piezoelectric performance of UIO-66. The resulting material, Yb-doped modified UIO-66 encapsulated in liposomes loaded with ozone (O3P@LPYU), exhibits enhanced piezoelectric effects under physiological IPP, generating an internal electric field that promotes electron-hole separation and catalyzes the production of ROS from the initial substrate ozone and the MPE microenvironment O2 and H2O. This innovative approach aims to reduce MPE volume, inhibit tumor growth in the pleural cavity and lung parenchyma, and prolong survival in MPE-bearing mice. Furthermore, piezoelectric catalysis is expected to induce anti-tumor immunological effects by reprogramming the immune microenvironment. This work highlights the significance of doping engineering in optimizing piezoelectric materials and offers a new direction for the use of piezoelectric materials in the bio-responsive treatment of MPE (Fig. 1).
NPs nanoparticles, IPP intrapleural pressure, NH2-BDC 2-amino-1,4-benzenedicarboxylic acid, DMF dimethylformamide, PFD perfluorodecalin, VB valence band, CB conduction band, ROS reactive oxygen species. The figure was created in BioRender (https://BioRender.com/c31v614).
Results
Synthesis and characterization of Yb-UIO-66 nanoparticles
In this study, porous Yb-UIO-66 nanoparticles (NPs) were synthesized using a standard solvothermal method (Fig. 2a). Initially, a water/dimethylformamide (DMF) solution containing hafnium (IV) chloride (HfCl4), ytterbium (III) chloride (YbCl3), and 2-amino-1,4-benzenedicarboxylic acid (NH2-BDC) was placed in a Teflon-lined tube and heated to 120 °C for 18 h. The resulting product was a porous Yb-doped UIO-66 MOF. Transmission electron microscopy (TEM) images confirmed the successful formation of UIO-66, which exhibited a cubic structure with a diameter of approximately 40 nm. The particle size and morphology remained unchanged after Yb doping (Fig. 2b). Size distribution analysis further supported these findings (Fig. 2c). Additionally, Yb doping did not significantly alter the zeta potential of UIO-66 (Fig. 2d). Elemental mapping displayed the presence and uniform distribution of N, O, Hf, and Yb within the composite (Fig. 2e). Spherical aberration-corrected scanning transmission electron microscopy (STEM) image displayed a distinct lattice fringe with a crystal spacing of 0.884 nm, corresponding to the (111) diffraction plane of Yb-UIO-66 (Fig. 2f). Notably, the STEM image of Yb-UIO-66 displayed disordered lattice defects unlike UIO-66 (Supplementary Fig. 1), owing to the surface vacancies and defects caused by doping. X-ray diffraction (XRD) analysis confirmed the successful crystallization of the MOF, with the high crystallinity of UIO-66 maintained post-Yb doping (Fig. 2g). The UV-vis spectrum showed no significant change in absorption profile after Yb incorporation (Fig. 2h). The high-resolution X-ray photoelectron spectroscopy (XPS) in Fig. 2i revealed the appearance of characteristic peak associated with Yb in the spectrum of Yb-UIO-66 NPs, as compared to UIO-66, further confirming that the rare-earth element Yb has been successfully integrated into the UIO-66 NPs. Thermogravimetric analysis of both UIO-66 and Yb-UIO-66 NPs indicated good thermal stability (Supplementary Fig. 2). As shown in Fig. 2j–l, the doping of Yb affected the specific surface area (SBET) of UIO-66. Figure 2j, k shows the isothermal nitrogen adsorption-desorption curves of the samples and the Barrett–Joyner–Halenda (BJH) models. The isotherms of both sets of samples exhibited steep initial adsorption at low relative pressures (P/P₀), indicating monolayer adsorption on microporous materials, followed by a platform with minimal hysteresis, corresponding to a Type I hysteresis loop in the International Union of Pure and Applied Chemistry chemical nomenclature (IUPAC) classification, which is typical for microporous materials. The Brunauer–Emmett-Teller (BET) method calculated the specific surface areas of UIO-66 and Yb-UIO-66 to be 526.8756 m²/g and 558.0228 m²/g, respectively. The average pore diameters obtained from the BJH model were 2.0867 nm and 2.158 nm, with changes in pore volume observed to varying degrees (Fig. 2l).
a Schematic illustration of the synthesis process for porous Yb-UIO-66 NPs. The figure was created in BioRender (https://BioRender.com/u22w123). b TEM images depicting the morphology of UIO-66 and Yb-UIO-66 NPs. Scale bar = 50 nm. c Size distribution analysis, and d Zeta potential measurements for UIO-66 and Yb-UIO-66 NPs. Data is represented as mean ± SD (n = 3 biologically independent experiments). e EDS elemental mapping of UIO-66 and Yb-UIO-66 NPs. Scale bar = 50 nm. f STEM image of Yb-UIO-66. Scale bar = 5 nm. g XRD patterns, h UV-vis absorption spectra (inset: photographs of UIO-66 and Yb-UIO-66 NPs), and i XPS spectra of UIO-66 and Yb-UIO-66 NPs. j Nitrogen adsorption-desorption curves and BJH models of UIO-66 and k Yb-UIO-66, showing the BET surface area and properties of pores of each sample. l Textural parameters of UIO-66 and Yb-UIO-66. NH2-BDC 2-amino-1,4-benzenedicarboxylic acid, DMF dimethylformamide, PFD perfluorodecalin, NPs nanoparticles, TEM transmission electron microscopy, EDS energy dispersive spectroscopy, STEM spherical aberration-corrected scanning transmission electron microscopy, XRD X-ray diffraction, UV-vis absorption spectra Ultraviolet-Visible absorption spectra, XPS X-ray photoelectron spectroscopy, BJH Barrett–Joyner–Halenda, BET Brunauer–Emmett–Teller.
DFT theoretical calculation analysis
Density functional theory (DFT) calculations further provide valuable supplementary insights. First, molecular models of UIO-66 and Yb-UIO-66 (Hf:Yb = 4:2) were constructed (Fig. 3a), with additional models for various doping ratios and rare-earth elements shown in Supplementary Fig. 3. Generalized Gradient Approximation (GGA)-Perdew, Burke and Ernzerhof (PBE) limitations in predicting elastic constants, due to vdW interaction inaccuracies, were addressed by excluding outliers, yielding a strong correlation (R2 = 0.75) between calculated and experimental d33 values. Based on the results, the systems were categorized into high (Yb1, Yb2), medium (La, Tb, Y), and low (Hf, Sm) piezoelectric responses (Fig. 3b). Mechanical properties indicate Yb doping increases flexibility, reflected in lower Cij values (Fig. 3c) due to significant changes in Yb–O bond lengths (up to 0.236 Å) and angles (up to 8.433°). Overall, for the same UIO-66 structure, Yb was the rare-earth element inducing the greatest flexibility (Supplementary Tables 1–9). This enhanced flexibility stemmed from van der Waals radius adjustments, improving resistance to shear. Furthermore, the Born Effective Charge (BEC) of Yb-UIO-66 was exceptionally high, exceeding nominal atomic charges, indicating orbital hybridization and charge redistribution (Fig. 3d). Additionally, Yb-UIO-66 exhibited a maximum dipole moment of 73.64 Debye along the z-axis, driven by asymmetric strain, resulting in stronger polarization and enhanced piezoelectric response (Fig. 3e). On top of this theoretical proof, we experimentally synthesized UIO-66 materials with different Hf:Yb doping ratios and various rare-earth element dopants using the solvothermal method (Supplementary Fig. 4a). Piezoelectric Force Microscopy (PFM) was then employed to measure the d33 values of the materials (Fig. 3f and Supplementary Fig. 4b). The results revealed that Yb-UIO-66 with a Hf:Yb doping ratio of 4:2 exhibited the best piezoelectric performance, consistent with the theoretical calculations.
a Structure of UIO-66 and Yb-UIO-66. b Calculated parameters piezoelectric coefficients d33, c elastic module, d Born effective charge, and e dipole moments in the x, y, and z axes of the investigated rare-earth-doped MOFs. f Amplitude vs bias voltage plot of UIO-66 and Yb-UIO-66 NPs. g Displacement response of UIO-66 and Yb-UIO-66 NPs. h Nyquist plots, and i PL spectra of UIO-66 and Yb-UIO-66 NPs. j Open-circuit voltage and k short-circuit current of the UIO-66@PDMS PENG (10 wt%) and Yb-UIO-66@PDMS PENG (10 wt%). l The average output power of Yb-UIO-66@PDMS PENG (10 wt%) with varied resistances. m ESR spectra of •OH, and n 1O2 in the control, UIO-66, and Yb-UIO-66 NPs. NPs nanoparticles, MOFs metal-organic frameworks, PL spectra photoluminescence spectra, PDMS polydimethylsiloxane, PENG piezoelectric nanogenerator, ESR electron spin resonance.
Besides, gas adsorption primarily occurs within MOF pores. In this work, the N2 molecules were placed in the pores near the Yb metal centers in constructing the adsorption model. The charge density difference plot shows electron transfer between the crystal and N₂, with charges concentrated around oxygen atoms, indicating weak interactions with metal ions (Supplementary Fig. 5a). The adsorption energy of Hf-UIO-66 with N₂ is −0.1365 eV, increasing to −1.3133 eV after Yb doping, aligning with BET results. Supplementary Table 10 details adsorption energy and free energy for N₂@Yb-UIO-66 at different doping ratios. Partial Density of States (PDOS) analysis confirms physical adsorption without chemical bonds, with Yb doping bringing oxygen p-orbitals closer to the Fermi energy, enhancing adsorption (Supplementary Fig. 5b).
Piezoelectricity of Yb-UIO-66 NPs
The piezoelectric properties of Yb-UIO-66 NPs were assessed using PFM (Supplementary Figs. 6, 7). Under a 10 V applied voltage, typical amplitude vs bias voltage butterfly loops and phase hysteresis curves were observed for UIO-66 and Yb-UIO-66 NPs (Fig. 3f, g). Analysis of the amplitude butterfly loops revealed that the d33 values for UIO-66 and Yb-UIO-66 NPs were 67 and 256 pm V−1, respectively (Fig. 3f). Yb doping enhanced the piezoelectric properties of UIO-66 by increasing the charge distribution imbalance within the material. Additionally, the separation, migration, and recombination of electron-hole pairs are crucial for piezoelectric catalytic performance. The electrochemical impedance spectra in Fig. 3h demonstrated the low electron transfer resistance of Yb-UIO-66 NPs. Furthermore, photoluminescence (PL) spectroscopy was used to assess the recombination rate of electron-hole pairs. The results showed that Yb doping significantly reduced the PL intensity of UIO-66 (Fig. 3i), suggesting its effective inhibition of electron-hole pair recombination.
To further investigate the piezoelectric performance of Yb-UIO-66, we constructed a Yb-UIO-66@Polydimethylsiloxane (PDMS) piezoelectric nanogenerator (PENG) (10 wt%) and conducted systematic tests. A high electric field of 40 kV/cm was applied to ensure complete polarization of all PENGs, followed by an evaluation of their output characteristics under periodic pressing (10 N stress at 10 Hz frequency). As shown in Fig. 3j, the open-circuit voltage of Yb-UIO-66@PDMS PENG (10 wt%) was significantly higher than that of UIO-66@PDMS PENG (10 wt%), with the optimal voltage reaching 33.48 V for Yb-UIO-66@PDMS PENG (10 wt%). Asymmetric positive and negative peak signals were observed, attributed to differences in strain rates during the application and release of pressure. Similarly, as shown in Fig. 3k, the short-circuit current followed the same trend, increasing to 3.12 µA for Yb-UIO-66@PDMS PENG (10 wt%). To investigate and compare the energy output performance of PENGs, the average power output of UIO-66@PDMS PENG (10 wt%) and Yb-UIO-66@PDMS PENG (10 wt%) was assessed across various external resistance values. As shown in Fig. 3l and Supplementary Fig. 8, the peak voltage, current, and instantaneous power of the PENGs were analyzed as the load resistance increased. The maximum instantaneous power was 0.31 μW for PDMS PENG, 1.63 μW for UIO-66@PDMS PENG (10 wt%), and 28.75 μW for Yb-UIO-66@PDMS PENG (10 wt%), respectively.
ROS generation ability study
The ROS production efficiency of piezoelectric MOFs is crucial for their effectiveness in piezoelectric catalytic therapy. To verify the generation of 1O2 and •OH, electron spin resonance (ESR) measurements were conducted, using 2,2,6,6-tetramethylpiperidine (TEMP) to trap 1O2 and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) for •OH. Compared to the control group and UIO-66, Yb-UIO-66 NPs exhibited significantly stronger signals for both •OH (1:2:2:1) and 1O2 (1:1:1) (Fig. 3m, n).
After verifying the piezoelectric properties of Yb-UIO-66 NPs, we synthesized composite liposomes (O3P@LPYU) using the thin film hydration method to enhance the biocompatibility of the nanoparticles and their ozone-carrying capacity. In this structure, the nanoparticles are encapsulated within the liposomes, while the phospholipid bilayer carries perfluorodecalin (PFD, a potent ozone carrier) (Fig. 2a). TEM revealed that O3P@LPYU had a ring-like structure with a particle size of approximately 150 nm, which was further confirmed by particle size analysis (Fig. 4a and Supplementary Fig. 9). The encapsulation efficiency of PFD within O3P@LPYU was determined using gas chromatography. Using the standard PFD as a reference, the encapsulated PFD in O3P@LPYU, showing a peak at 2.7571, was estimated to be 2 μg/ml (Fig. 4b). Building on the successful PFD encapsulation, we proceeded to evaluate the ozone-carrying capacity of O3P@LPYU. The ozone release profile was plotted by measuring the ozone concentration in the solution over a period of 48 h, with ozone-saturated H2O serving as a control. As shown in Fig. 4c, ozone release in the H2O solution exhibited a sharp increase followed by a rapid decline, with ozone levels dropping to 0 mg/L within 5 h. In contrast, ozone loaded in O3P@LPYU was released slowly, displaying a prolonged plateau phase, and maintained a detectable concentration for up to 24 h. Next, we analyzed the piezo-catalytic dye degradation abilities of UIO-66, Yb-UIO-66, and O3P@LPYU. First, to simulate pleural cavity pressure in the in vitro model, we used a reciprocating plunger pump to mimic the pressure variation pattern of the pleural cavity and pressurize the piezoelectric material. The working principle is demonstrated in Supplementary Movies 1–3. Additionally, we observed that O3P@LPYU NPs exhibited high stability in various solvents (Supplementary Fig. 10a, b). To prevent photolysis, the entire degradation experiment was conducted in darkness. As Fig. 4d and Supplementary Fig. 11 showed, only an inappreciable deterioration can be detected after ten minutes of cyclic mechanical pressure treatment in the UIO-66 group. After allowing Yb-UIO-66 NPs and methylene blue (MB)/rhodamine B (RhB) molecules to reach adsorption-desorption equilibrium overnight, the intensity significantly decreased when subjected to mechanical pressure. Additionally, liposomes encapsulating and providing ozone as a catalytic substrate further significantly enhanced the piezoelectric catalytic effect of Yb-UIO-66. Furthermore, as the duration of cyclic mechanical pressure increases or the mechanical pressure intensifies, the catalytic efficiency of O3P@LPYU in decomposing MB and RhB becomes stronger (Fig. 4e, f and Supplementary Figs. 12, 13).
a TEM images depicting the morphology of O3P@LPYU NPs. Scale bar = 100 nm. b PFD content detection by gas chromatography. c Ozone concentration released curves of O3P@LPYU. d Degradation of MB under mechanical pressure in the presence of UIO-66, Yb-UIO-66, and O3P@LPYU NPs. e Degradation of MB under mechanical pressure in the presence of O3P@LPYU at different treatment times (1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 min), and f different pressure level (1, 2, 4, 6, 8, and 10 kPa). g The reaction-time curves of the TMB colorimetric reaction catalyzed by O3P@LPYU NPs. Data is represented as mean ± SD (n = 3 biologically independent experiments). h, i The reaction-time curves of the OPD and ABTS colorimetric reactions catalyzed by O3P@LPYU NPs. Data is represented as mean ± SD (n = 3 biologically independent experiments). j, k The reaction-time curves of the TMB colorimetric reaction with different concentrations of TMB catalyzed by O3P@LPYU NPs and the corresponding Michaelis–Menten curves fitted by ordinary Least squares. Data is represented as mean ± SD (n = 3 biologically independent experiments). l, m The reaction-time curves of the TMB colorimetric reaction with different concentrations of H2O2 catalyzed by O3P@LPYU NPs and the corresponding Michaelis–Menten curves fitted by ordinary Least squares. Data is represented as mean ± SD (n = 3 biologically independent experiments). NPs nanoparticles, TEM transmission electron microscopy, PFD perfluorodecalin, IPP intrapleural pressure, US ultrasound, MB methylene blue, TMB 3,3′,5,5′-tetramethylbenzidine, OPD o-phenylenediamine, ABTS 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
Peroxidase-like activities of O3P@LPYU NPs
Then, we tested the peroxidase-like activities of O3P@LPYU NPs by the oxidation of 3,3′,5,5′-Tetramethylbenzidine (TMB) with H2O2 as an oxidant. The oxidized TMB (oxTMB) product, identifiable by its blue color, displayed a distinct absorption peak at 652 nm. Figure 4g presents the reaction-time profiles for TMB oxidation catalyzed by O3P@LPYU NPs. The O3P@LPYU NPs demonstrated significantly enhanced peroxidase-mimicking activity compared to UIO-66 and Yb-UIO-66, showing a markedly faster reaction rate. In addition to TMB, the catalytic oxidation of other peroxidase substrates, such as o-phenylenediamine (OPD) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), was also examined using O3P@LPYU NPs. In Fig. 4h, i, O3P@LPYU NPs also exhibited much higher peroxidase-like activity than UIO-66 and Yb-UIO-66 during the oxidation of OPD and ABTS. Similarly, the Yb-UIO-66 NPs also had superior peroxidase-like activity than UIO-66 NPs, demonstrating the enhancement of the catalytic properties of UIO-66 by Yb doping. Figure 4j, k depicts the analysis of kinetic parameters for the oxidation of peroxidase substrate TMB catalyzed by O3P@LPYU NPs, performed by varying TMB concentrations (0–2 mM). Using the Michaelis–Menten equation (Fig. 4k), the maximum reaction rate (Vmax) and the Michaelis constant (Km) for TMB were calculated as 1.878 × 10-5 M/min and 0.6041 mM, respectively. Similarly, Fig. 4l, m shows the kinetic evaluation for H2O2 under varying concentrations (0–10 mM). The corresponding Vmax and Km for H2O2 were determined to be 1.514 × 10-5 M/min and 1.251 mM, as shown in Fig. 4m.
In vitro cytotoxicity of O3P@LPYU NPs
To investigate the cellular distribution of O3P@LPYU in LLC and 4T1 cells, hydrophobic dye FITC was encapsulated in the lipid layer to trace the particles. The results of cellular confocal microscopy showed that the FITC-labeled O3P@LPYU NPs were clearly internalized by LLC and 4T1 cells after 24 h incubation (Fig. 5a). The internalized FITC-labeled nanoparticles were quantified by flow cytometry (Fig. 5b). The results demonstrated that the internalization of particle began as early as 1 h after incubation, peaked at 24 h, and then gradually decreased.
a Confocal laser scanning microscopy images display the internalization of FITC-labeled O3P@LPYU (green) within cells. Scale bar = 20 μm. b Flow cytometry analysis of FITC-labeled O3P@LPYU endocytosis in LLC and 4T1 cells. c Short-term cytotoxicity of pressure-responsive UIO-66/Yb-UIO-66/O3P@LPYU assessed by CCK8 assay. Data is represented as mean ± SD (n = 3 biologically independent experiments). d Bright-field microscopy shows LLC cell morphology, proliferation, and viability across treatments. Scale bar = 100 μm. e Long-term cytotoxic effects of pressure-sensitive UIO-66/Yb-UIO-66/O3P@LPYU on LLC cells. f Calcein AM/PI staining of LLC cells following various treatments. Scale bar = 50 μm. g VEGF and h TNF-α release in LLC cell supernatants across different treatment groups. Data is represented as mean ± SD (n = 3 biologically independent experiments). Two-tailed Student’s t-test. VEGF: UIO-66 IPP− vs. IPP+, p = 0.0068 (**); Yb-UIO-66 IPP− vs. IPP+, p = 0.0016 (**); O3P@LPYU IPP− vs. IPP+, p < 0.0001 (****); IPP+ Yb-UIO-66 vs. O3P@LPYU, p = 0.0144 (*); TNF-α: UIO-66 IPP− vs. IPP+, p = 0.0252 (*); Yb-UIO-66 IPP− vs. IPP+, p = 0.0064 (**); O3P@LPYU IPP− vs. IPP+, p = 0.0007 (***); IPP+ Yb-UIO-66 vs. O3P@LPYU, p = 0.0018 (**). i DCFH-DA staining of LLC cells subjected to PBS, UIO-66, Yb-UIO-66, and O3P@LPYU, with or without IPP treatment. Scale bar = 50 μm. j Apoptosis in LLC cells, evaluated by 7AAD and Annexin V staining through flow cytometry. k Overview of the ICD mechanism in tumor cells. The figure was created in BioRender (https://BioRender.com/l05l925). l CRT membrane translocation in LLC cells, observed via confocal laser scanning microscopy. Scale bar = 50 μm. m HMGB1 and n ATP release in LLC cell supernatants after treatment. Data is represented as mean ± SD (n = 5 biologically independent experiments). Two-tailed Student’s t-test. HMGB1: Yb-UIO-66 IPP− vs. IPP+, p = 0.0003 (***); O3P@LPYU IPP− vs. IPP+, p < 0.0001 (****); IPP+ Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****). ATP: UIO-66 IPP− vs. IPP+, p = 0.0413 (*); Yb-UIO-66 IPP− vs. IPP+, p < 0.0001 (****); O3P@LPYU IPP− vs. IPP+, p < 0.0001 (****); IPP+ Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****). IPP intrapleural pressure, US ultrasound.
The antineoplastic activity of our nano-system was assessed by cell counting kit-8 (CCK8) assay (Fig. 5c). Without mechanical pressure triggering, UIO-66, Yb-UIO-66, and O3P@LPYU exhibited slight cytotoxicity, likely due to the disturbance of cell growth caused by the nanoparticles. Mechanical pressure stimulation significantly enhanced cytotoxicity, especially in the presence of ozone, with marked cell contraction observed in the IPP + O3P@LPYU treatment group (Fig. 5d and Supplementary Fig. 14). A similar anti-tumor trend was also observed in the long-term colony formation assay (Fig. 5e and Supplementary Fig. 15). To visualize the effects of piezoelectric catalysis on cell viability, live/dead cell staining assay was carried out by Calcein-AM and propidium iodide (PI) staining. A significant number of dead cells were clearly visible in the IPP + O3P@LPYU group, demonstrating severe cellular toxicity induced by piezo-catalytic therapy (Fig. 5f and Supplementary Fig. 16).
The formation of MPE is primarily caused by the direct invasion of malignant tumors into the pleura, leading to increased vascular permeability. The presence of tumor cells stimulates pleural fluid secretion while compressing lymphatic vessels, obstructing lymphatic drainage, and contributing to fluid accumulation in the pleural cavity. Additionally, tumor-secreted cytokines, such as VEGF and TNF-α, enhance vascular permeability, promoting plasma component leakage into the pleural cavity. Following the confirmation of the direct inhibitory effect of O3P@LPYU NPs on tumor cells under mechanical pressure, we further examined their impact on tumor cell secretion of cytokines regulating vascular permeability. As shown in Fig. 5g, h, under mechanical pressure, O3P@LPYU NPs significantly reduced the secretion of VEGF and TNF-α by tumor cells.
Anticancer mechanism of O3P@LPYU NPs
As designed, the primary cause of cytotoxicity is the ROS produced through piezoelectric catalysis. To prove this mechanism, ROS levels were measured using the fluorescent probe 2′,7′-dichlorodihydrofluorescein (DCFH) (Fig. 5i and Supplementary Fig. 17). Mechanical pressure stimulation significantly improved the production of ROS, and the ROS level of cells treated with IPP + O3P@LPYU was significantly the highest. The results of flow cytometry also showed that the ROS levels in LLC and 4T1 cells treated with IPP + O3P@LPYU were much higher than those in the control group (Supplementary Fig. 18a, b).
Given that intracellular ROS can trigger programmed cell death, we proceeded to assess cell apoptosis across the treatment groups. As expected, the observed apoptosis trends were closely similar to the level of ROS (Fig. 5j and Supplementary Fig. 19). After confirming that pressure-responsive nanoparticles induce apoptosis, we explored whether these piezoelectric particles could effectively trigger immunogenic cell death (ICD) (Fig. 5k). To this end, the evaluation of ICD was carried out using the following three methods: (1) immunofluorescence (IF) staining to visualize calreticulin (CRT) expression (Fig. 5l and Supplementary Fig. 20); (2) ELISA to measure the secretion of high mobility group box 1 protein (HMGB1) (Fig. 5m and Supplementary Fig. 21a); and (3) firefly luciferase assays to quantify extracellular ATP (Fig. 5n and Supplementary Fig. 21b). The results indicated that in the absence of mechanical pressure stimulation, ICD was barely detectable in LLC and 4T1 cells treated with UIO-66, Yb-UIO-66, or O3P@LPYU NPs. However, with mechanical pressure stimulation, both Yb-UIO-66 and O3P@LPYU NPs clearly induced ICD, with the IPP + O3P@LPYU treatment leading to a significantly higher level of ICD compared to IPP + Yb-UIO-66.
Biodistribution of O3P@LPYU NPs in the MPE model
Two mouse MPE models were established by intrapleural injection of lung cancer (LLC) and breast cancer (4T1) cells. Diffuse tumor fluorescence signals in the pleural cavity were detected using an In Vivo Imaging System (IVIS) 14 days post-injection (Fig. 6a). Furthermore, pleural effusion and multifocal tumors on the pleural surface were clearly observed both in vivo using ultrasound and during autopsy (Fig. 6b, c). The above results indicated the successful establishment of the MPE animal model. Based on the established MPE model, a microscale manometer was used to measure the IPP in mice under different conditions (Fig. 6d, e). In healthy mice, the maximum IPP at the end of normal expiration was approximately -3 mmHg, while the minimum IPP at the end of normal inspiration reached −7 mmHg (IPP range: −3 mmHg to −7 mmHg). As the disease progressed in MPE mice, the end-inspiration IPP in the early stage decreased to −8 mmHg (IPP range: −3 mmHg to −8 mmHg). In the late stage of the disease, the end-inspiration IPP further decreased to as low as −10 mmHg (IPP range: −3 mmHg to −10 mmHg). To analyze the biodistribution and the duration of sustained biological activity (half-life) of the nanoparticles after intrapleural injection, we used the IVIS imaging system to continuously monitor the fluorescence signal distribution and intensity of O3P@LPYU NPs labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR). The results showed that after intrapleural injection of DiR-O3P@LPYU, the fluorescence signal was confined to the thoracic region. The fluorescence intensity persisted and decreased to half of its initial intensity by day 5, indicating a half-life of 5 days (Fig. 6f). Ex vivo IVIS further confirmed the presence of DiR-O3P@LPYU in the MPE, pleural tumors, lungs, liver, and spleen (Fig. 6g).
a Bioluminescence analysis on day 14 after pleural injection of LLC-LUC cells into C57BL/6 mice and 4T1-LUC cells into BALB/c mice; b Ultrasound detection of metastatic tumors and pleural effusion in the pleural cavity; c Representative images of pleural cavity metastatic tumors and effusion; d, e. Measurement of IPP in healthy mice and LLC- and 4T1-MPE models at different disease stages (early and late stages). Data is represented as mean ± SD (n = 3 biologically independent animals per group). Two-tailed Student’s t-test. LLC: Normal end-expiration vs. end-inspiration, p < 0.0001 (****); early stage end-expiration vs. end-inspiration, p < 0.0001 (****); advanced stage end-expiration vs. end-inspiration, p < 0.0001 (****); end-inspiration early stage vs. advanced stage, p = 0.0003 (***); 4T1: Normal end-expiration vs. end-inspiration, p < 0.0001 (****); early stage end-expiration vs. end-inspiration, p < 0.0001 (****); advanced stage end-expiration vs. end-inspiration, p < 0.0001 (****); end-inspiration early stage vs. advanced stage, p < 0.0001 (****). f IVIS imaging over time post-intrapleural DiR-O3P@LPYU NPs. Data is represented as mean ± SD (n = 3 biologically independent animals per group). g Representative ex vivo IVIS imaging of major organs from MPE mice receiving intrapleural DiR-O3P@LPYU NPs. L left, R right, MFI mean fluorescence intensity, MPE malignant pleural effusion, T tumor, H heart, Li liver, Sp spleen, Lu lung, K kidney, B brain, St stomach, I intestine, IPP intrapleural pressure, IVIS in vivo imaging system.
In vivo MPE treatment with O3P@LPYU NPs
After analyzing the in vivo biodistribution of the intrapleural injection of the nanoparticles, we further evaluated the therapeutic efficacy of the nano-system on the LLC-MPE mouse model (Fig. 7a). Through in vivo imaging and ultrasound detection for continuous monitoring of tumor and pleural effusion changes, we found that intrapleural administration of Yb-UIO-66 delayed tumor growth and the progression of MPE, while treatment with O3P@LPYU led to an even greater reduction in MPE volume and pleural tumor burden (Fig. 7b, c). Anatomical images and statistical analysis of pleural effusion volume and tumor weight further confirmed these results (Fig. 7d–f). For the overall condition of MPE mice, the O3P@LPYU treatment also significantly delayed weight loss and extended survival (Fig. 7g, h). Moreover, the symptoms and survival of MPE mice were also related to the involvement of adjacent vital organs, primarily the lungs and heart. Similar to previous results, MPE mice in the O3P@LPYU treatment group exhibited significantly reduced involvement of the lungs and heart (Fig. 7i, j). Additionally, the proliferation ability of the tumors was notably diminished (Fig. 7k, l). Similar anti-tumor therapeutic efficacy of the O3P@LPYU treatment was also observed in the 4T1-MPE mouse model (Fig. 8a–k).
a Treatment schedule of O3P@LPYU-based piezoelectric catalytic therapy. The figure was created in BioRender (https://BioRender.com/h74k184). b Bioluminescence and c ultrasound images of LLC-MPE mice from different treatment groups were captured every other day after the start of treatment. d Representative images of pleural effusions and tumors in LLC-MPE mice with the indicated treatment. e Measurement of MPE volume on day 22 in LLC-MPE mice (n = 5 biologically independent animals per group). Data are expressed as the mean ± SD. One-way ANOVA with Tukey’s multiple comparisons tests: PBS vs. Yb-UIO-66, p = 0.0001 (***); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****). f Measurement of pleural tumor weight on day 22 in LLC-MPE mice (n = 5 biologically independent animals per group). Data are expressed as the mean ± SD. One-way ANOVA with Tukey’s multiple comparisons tests: PBS vs. Yb-UIO-66, p = 0.0331 (*); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p = 0.0026 (**). g Body weight changes in LLC-MPE mice receiving the indicated treatments (n = 5 biologically independent animals per group). Data are presented as mean ± SD. Two-way ANOVA with Tukey’s multiple comparison test. h Kaplan–Meier survival analysis of LLC-MPE mice subjected to different treatments (n = 5 biologically independent animals per group); statistical analysis using the two-sided log-rank test: Yb-UIO-66 vs. O3P@LPYU, p = 0.0197 (*). i Representative images of lungs and heart in LLC-MPE mice following the indicated treatments. j Representative histological examinations of the lung and heart with hematoxylin and eosin staining. Scale bar = 500 μm for lung; Scale bar = 200 μm for heart. k Full-section fluorescence scan and local magnification of TUNEL and DAPI staining in pleural tumor tissues. Scale bar (full-section scan) = 1 mm. Scale bar (local magnification) = 200 μm. l Pleural tumors were analyzed by IHC for Ki67 expression. Scale bar = 200 μm. IVIS in vivo imaging system, US ultrasound, MPE malignant pleural effusion.
a Bioluminescence and b ultrasound images of 4T1-MPE mice from different treatment groups were captured every other day after the start of treatment. c Representative images of pleural effusions and tumors in 4T1-MPE mice with the indicated treatment. d Measurement of MPE volume on day 22 in 4T1-MPE mice (n = 5 biologically independent animals per group). Data are expressed as the mean ± SD. One-way ANOVA with Tukey’s multiple comparisons tests: PBS vs. Yb-UIO-66, p < 0.0001 (****); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****). e Measurement of pleural tumor weight on day 22 in 4T1-MPE mice (n = 5 biologically independent animals per group). Data are expressed as the mean ± SD. One-way ANOVA with Tukey’s multiple comparisons tests: PBS vs. Yb-UIO-66, p = 0.0013 (**); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****). f Body weight changes in 4T1-MPE mice with different treatments (n = 5 biologically independent animals per group). Data are presented as mean ± SD. Two-way ANOVA with Tukey’s multiple comparison tests: PBS vs. O3P@LPYU, p = 0.0393 (*). g Kaplan–Meier survival analysis of 4T1-MPE mice subjected to various treatments (n = 5 biologically independent animals per group); statistical analysis using the two-sided log-rank test: UIO-66 vs. Yb-UIO-66, p = 0.0143 (*); Yb-UIO-66 vs. O3P@LPYU, p = 0.0018 (**). h Representative images of lungs and heart in 4T1-MPE mice with the indicated treatment. i Representative histological examinations of the lung and heart with hematoxylin and eosin staining. Scale bar = 500 μm for lung; Scale bar = 200 μm for heart. j Full-section fluorescence scan and local magnification of TUNEL and DAPI staining in pleural tumor tissues. Scale bar (full-section scan) = 1 mm. Scale bar (local magnification) = 200 μm. k Pleural tumors were analyzed by IHC for Ki67 expression. Scale bar = 200 μm. MPE malignant pleural effusion.
Next, we assessed the safety of all experimental regimens through blood cell detection, blood biochemical analysis, and hematoxylin-eosin (H&E) staining of vital organs. All routine parameters in biochemical analysis remained within the normal range (Supplementary Fig. 22a, b). After intrapleural administration of O3P@LPYU in healthy C57BL/6 mice, H&E-stained sections of major organs revealed no significant damage (Supplementary Fig. 23). These findings confirmed the safety of all regimens.
Immune characters of MPE treated with O3P@LPYU NPs
To comprehensively investigate the molecular and cellular mechanisms underlying tumor growth inhibition induced by various treatments, we performed transcriptome profiling of tumors across all treatment groups. Comparative analysis identified 791, 761, and 219 differentially expressed genes (DEGs) in MPE mice treated with UIO-66, Yb-UIO-66, and O3P@LPYU, respectively, compared to the PBS control group (Fig. 9a). Notably, Gene Set Enrichment Analysis (GSEA) revealed distinct activation profiles unique to O3P@LPYU treatment, including T cell activation, T cell-mediated immunity, activation of innate immune response, among others (Fig. 9b, c). In agreement with our GSEA analysis, the estimated relative abundances of cytotoxic CD8+ T cells, CD4+ T cells, and antigen-presenting dendritic cells were significantly higher in the O3P@LPYU group compared to other groups (Fig. 9d). In contrast, immune-cold tumors in the control group showed an enrichment of Treg cells. These findings suggested that the pressure-responsive O3P@LPYU activated anti-tumor immune responses by enhancing the presentation of tumor-specific antigens and promoting T cell activation, distinguishing it from the other treatments evaluated. To further elucidate the specific pathways activated by O3P@LPYU, we performed Gene Set Variation Analysis (GSVA) (Fig. 9e). As anticipated, pathways associated with T cell activation, differentiation, and cytotoxic activity were significantly up-regulated in the O3P@LPYU group. Additionally, ICD score and ROS biosynthesis score were increased in the O3P@LPYU group, while the MPE signature was decreased (Fig. 9f). These results highlighted the strong connection between O3P@LPYU treatment and oxidative-stress-induced ICD, further underscoring its potential to effectively engage the immune system in combating tumors. As proved in vitro, ROS generated by piezo-catalysis could effectively induce ICD in lung cancer and breast cancer cell lines. We wonder if this phenomenon persists in the microenvironment of MPE and successfully activates anti-tumor immunity. Immunohistochemistry (IHC) staining for CRT and HMGB1 was used to evaluate ICD intensity (Fig. 10a, b). Without ozone loading, Yb-UIO-66 significantly induced ICD, while ozone-loaded O3P@LPYU more effectively enhanced HMGB1 release and CRT re-localization. To validate the changes in the tumor microenvironment of MPE mice with different treatments, we tested the canonical markers of various immunocytes, including CD8+ T cells, CD4+ T cells, DCs, Tregs, myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAM) by flow cytometry (Fig. 10c–j and Supplementary Figs. 24, 25). A similar immune cell distribution pattern was observed, consistent with the sequencing analysis results. Collectively, these results suggested that O3P@LPYU treatment exerted potent tumor-eliminating effects by inducing a robust immune response.
a Multiple volcanic maps illustrating significantly dysregulated genes in MPE mice treated with UIO-66, Yb-UIO-66, and O3P@LPYU compared to PBS. Top 10 up-regulated DEGs are labeled. Red dots indicate significantly up-regulated DEGs; the blue dots indicate significantly down-regulated DEGs; and the gray dots indicate no significance. b GSEA analysis demonstrating distinct pathway enrichment signatures across three comparison groups. Enriched signatures are visualized through color-coded comparison groups. The size of the circles represents the number of genes belonging to that pathway. c Representative enriched signatures in the O3P@LPYU group compared to the PBS group. Statistical significance was assessed using a two-sided p-value, and multiple testing correction was applied using the Benjamini–Hochberg method. d Relative abundances of the immune cell types identified in individual MPE model mice. Box plots depicting the infiltration levels of immune cells, with median values shown as a horizontal line within the box, whiskers representing the range, and quartiles indicated by the box bounds. n = 3 biologically independent animals per group. e Heatmaps visualization of normalized GSVA scores for hallmark gene signatures derived from the Molecular Signatures Database (MSigDB). Significant activated signatures in the O3P@LPYU condition are indicated. f Comparative analysis of ICD score, ROS biosynthesis score, and MPE score across all treatment groups. MPE malignant pleural effusion, DEGs differentially expressed genes, GSEA gene set enrichment analysis, GSVA, gene set variation analysis, ROS reactive oxygen species, ICD immunogenic cell death.
a, b CRT and HMGB1 stain of LLC- and 4T1-MPE model, evaluated by immunohistochemistry. Scale bar = 200 μm. c, d Representative contour plots and summary of frequencies of CD8+ T cells and CD4+ T cells in LLC- and 4T1-MPE model. LLC-CD8+ T cells: PBS vs. Yb-UIO-66, p = 0.0025 (**); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****); LLC-CD4+ T cells: PBS vs. Yb-UIO-66, p = 0.0237 (*); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****); 4T1-CD8+ T cells: PBS vs. Yb-UIO-66, p = 0.0012 (**); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****); 4T1-CD4+ T cells: PBS vs. O3P@LPYU, p = 0.0003 (***); Yb-UIO-66 vs. O3P@LPYU, p = 0.01 (*). e, f Representative contour plots and summary of frequencies of Tregs in LLC- and 4T1-MPE model. LLC-Tregs: PBS vs. Yb-UIO-66, p = 0.0005 (***); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p = 0.0013 (**); 4T1-Tregs: PBS vs. Yb-UIO-66, p = 0.0048 (**); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p = 0.0005 (***). g, h Representative contour plots and summary of frequencies of MDSC and TAM in LLC- and 4T1-MPE model. LLC-TAM: PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****); 4T1-TAM: PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p < 0.0001 (****). i, j Representative contour plots and summary of frequencies of DCs in LLC- and 4T1-MPE model. LLC-DC: PBS vs. Yb-UIO-66, p = 0.0116 (*); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p = 0.042 (*); 4T1-DC: PBS vs. Yb-UIO-66, p = 0.0011 (**); PBS vs. O3P@LPYU, p < 0.0001 (****); Yb-UIO-66 vs. O3P@LPYU, p = 0.0025 (**). n = 5 biologically independent animals per group. Data are expressed as the mean ± SD. One-way ANOVA with Tukey’s multiple comparisons test. ICD immunogenic cell death, MPE malignant pleural effusion, MDSCs myeloid-derived suppressor cells, TAMs tumor-associated macrophages, DCs dendritic cells.
Discussion
The treatment of MPE remains a significant challenge in oncology, given its association with advanced cancer stages and poor prognosis. Conventional treatment strategies often fall short due to issues like insufficient efficacy, severe side effects, recurrence, and progression1,4. In this study, we proposed an innovative approach to address these challenges by leveraging the continuous pressure changes and oxygen-rich physiological characteristics of the pleural cavity. This approach involves a novel therapeutic system, namely piezoelectric catalysis using a physiological IPP-sensitive piezoelectric MOF (O3P@LPYU). Our findings demonstrate substantial improvements in the management of MPE, showcasing both the innovation and breakthroughs of our approach.
Nanozymes, which are nanomaterials with enzyme-like properties, have recently attracted considerable interest for their promising applications across diverse biomedical areas20,21,22. They are typically categorized based on their catalytic mechanisms, including photodynamic, chemokinetic, and sonodynamic nanozymes. Each of these types has unique characteristics and advantages. Photodynamic nanozymes are activated by light, chemokinetic nanozymes by chemical reactions, and sonodynamic nanozymes by ultrasound waves23,24. However, these conventional nanozyme therapies often require external activation conditions, such as light or ultrasound, which may pose certain limitations. For instance, photodynamic and sonodynamic therapies necessitate specific equipment and controlled environments, limiting their applicability and accessibility. Additionally, the need for repeated treatments can increase the risk of treatment-related adverse effects and patient discomfort. Frequent administrations are often required to maintain therapeutic efficacy, further complicating treatment regimens25.
In contrast, physiologically responsive nanozymes offer several advantages over these externally activated systems. These nanozymes are designed to respond to physiological stimuli, such as pH changes, redox conditions, or mechanical pressures within the body26,27,28. This intrinsic responsiveness eliminates the need for external activation conditions, making the treatment process more straightforward and less invasive. Furthermore, physiologically responsive nanozymes can provide more precise and timely responses to pathological conditions, potentially extending the intervals between treatments and reducing the frequency of drug administration. This can also minimize treatment-related adverse effects, thereby enhancing patients' compliance and comfort.
In the context of treating MPE, the unique physiological environment of the pleural cavity—characterized by continuous pressure changes and an oxygen-rich microenvironment—presents an ideal scenario for the application of piezoelectric materials8. Piezoelectric nanomaterials, such as the O3P@LPYU system utilized in this study, respond to physiological mechanical pressure to generate catalytic activity. This piezoelectric catalysis harnesses the mechanical energy from the IPP fluctuations to induce therapeutic reactions without the need for external stimuli. The application of piezoelectric nanozymes for treating MPE has several notable advantages. First, the continuous pressure variations in the pleural cavity provide a consistent and reliable stimulus for activating the piezoelectric nanozymes. Second, the oxygen-rich environment enhances the generation of ROS, which is crucial for the therapeutic efficacy of the treatment. This method ensures a more targeted and sustained therapeutic effect, reducing the likelihood of recurrence and progression of pleural effusions.
However, traditional piezoelectric materials often face limitations in biomedical applications due to their relatively weak piezoelectric properties, which result in suboptimal therapeutic effects in vivo. To overcome these limitations, doping modifications have become a crucial strategy for enhancing the piezoelectric characteristics of these materials29,30. Among various doping elements, rare-earth elements have shown significant potential in improving the performance of piezoelectric materials31,32. In our study, we utilized rare-earth element Yb doping to modify the MOF UIO-66 to enhance its piezoelectric properties. The incorporation of Yb into UIO-66 significantly improved its piezoelectric coefficient, which is critical for its catalytic performance under physiological conditions. This enhancement is attributed to the increased charge distribution imbalance and the superior stability provided by the rare-earth doping, making the Yb-doped UIO-66 a highly effective piezoelectric nanomaterial. Furthermore, to amplify the therapeutic efficacy of our initial treatment, we introduced ozone as a piezoelectric catalytic substrate. Ozone’s high reactivity and ability to generate ROS make it a considerable candidate for enhancing the catalytic activity of piezoelectric materials33. When combined with the Yb-UIO-66 NPs, the ozone enhances the production of ROS under mechanical stress, leading to increased oxidative stress and effective tumor cell killing.
The biocompatibility and biosafety of piezo-catalytic therapy for MPE were systematically evaluated using the established MPE model. The treatment demonstrated encouraging biocompatibility, with the piezo-catalytic nanoparticles primarily localized within the pleural cavity, minimizing systemic adverse effects. No significant toxicity or inflammatory injury was observed in major organs, confirming the localized nature of the therapy, which highlights the safety advantage of nanomaterials in local therapy10,34,35. Furthermore, the sustained in vivo activity of the nanoparticles, with a half-life of 5 days, highlights their prolonged therapeutic efficacy. This extended duration allows for longer dosing intervals, effectively reducing the frequency of administration and minimizing potential side effects, thus improving the overall safety profile of the treatment36.
This innovative approach of combining Yb-doped UIO-66 with ozone in piezoelectric catalysis offers a robust and efficient method for enhancing ROS generation and tumor cytotoxicity. The improved piezoelectric properties of the Yb-UIO-66 ensure that even subtle physiological mechanical pressures can trigger significant catalytic activity. Our constructed bio-responsive piezoelectric catalytic nano-platform utilizes the initial substrate ozone, along with the abundant O2 and H2O in the MPE environment, to achieve initial shock and sustained treatment, ultimately improving the overall efficacy of physiological response piezoelectric catalytic therapy for MPE.
Methods
Materials
Hafnium (IV) chloride (HfCl4, ≥ 98%, CAS: 13499-05-3), 2-amino-1,4-benzenedicarboxylic acid (NH2-BDC, ≥98%, CAS: 10312-55-7), N,N-Dimethylformamide (DMF, ≥99%, CAS: 68-12-2), Ytterbium (III) chloride (YbCl3, ≥99.9%, CAS: 10361-91-8), and SYLGARD® 184 Silicone Elastomer Kit (S466341) were purchased from Aladdin. Indium tin oxide-polyethylene terephthalate (ITO-PET, 639303), 3,3′,5,5′-tetramethylbenzidine (TMB, T2885, ≥98%, CAS: 54827-17-7), o-phenylenediamine (OPD, P9029, ≥98%, CAS: 95-54-5), and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, A3219) were purchased from Sigma. Perfluorodecalin (PFD, 95%, CAS: 306-94-5) was purchased from J&K Scientific Ltd. Methylene blue (MB, 95%, CAS: 30354-18-8) and rhodamine B (RhB, 99%, CAS: 81-88-9) were sourced from Rhawn. The NileRed fluorescence probe (98.08%, CAS: 7385-67-3) and Calcein AM (99.08%, CAS: 148504-34-1) were obtained from MedChemExpress. The Annexin V-PE/7AAD apoptosis detection kit (A213-02) was provided by Vazyme. ATP Assay Kit (S0027), Reactive Oxygen Species Assay Kit (S0033M), and One Step TUNEL Apoptosis Assay Kit (C1088) were purchased from Beyotime. Mouse VEGF/VEGFA ELISA Kit (EK0541) and Mouse TNF Alpha/TNFA ELISA Kit (EK0527) were purchased from Boster Bio. The ELISA kit for HMGB1 (E-EL-M0676) was acquired from Elabscience, while D-Luciferin Potassium Salt D (40902ES) and DAPI fluoromount-G mounting medium (36308ES) were supplied by YEASEN. APC/Cy7 anti-mouse CD45 (147718), PerCP/Cy5.5 anti-mouse CD3 (100218), FITC anti-mouse CD4 antibody (100406), BV510 anti-mouse CD8 (100752), APC anti-mouse CD11b (101212), BV605 anti-mouse F4/80 (123133), PE anti-mouse CD11c (117308), ALEXA FLUOR 647 anti-mouse CD25 (102020), AF700 anti-mouse MHCII (107622), PE anti-mouse FOXP3 (126404), and PE/Cy7 anti-mouse CD206 antibody (141720) were purchased from Biolegend. BV786 anti-mouse GR1 (740850) and BD Horizon™ Fixable Viability Stain 620 (564996) were purchased from BD Biosciences. HMGB1 (ab79823), CRT (ab92516), and Ki67 (ab15580) were purchased from Abcam. DMEM, RPMI medium, and Fetal bovine serum were purchased from Gibco.
Cell lines and animals
The LLC (Catalog number: TCM47) and 4T1 (Catalog number: TCM32) cell lines, purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China), were cultured using a nutrient-rich mixture of DMEM and RPMI medium, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. These cells were grown in a controlled incubator set to 37 °C with a 5% CO2 atmosphere.
Female C57BL/6 (6–8 weeks) and BALB/c (6–8 weeks) mice were procured from BEIJING HFK BIOSCIENCE CO., LTD (Beijing, China). Mice were housed in a standard feeding environment with a 12:12 light-dark cycle, a temperature of 25 ± 2 °C, and a humidity of 60 ± 10%. All animal procedures were conducted in strict compliance with protocols approved by the Ethics Review Committee of Animal Experimentation at Sichuan University (No. 20240402004). These procedures were conducted in accordance with the guidelines set forth in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and in compliance with the principles of the Animal Welfare Act.
Synthesis of O3P@LPYU NPs
Synthesis of Yb-UIO-66 NPs: 0.137 g of HfCl4 and 0.155 g of BDC-NH2 were combined with 50 mL of DMF and 140 μL of deionized water in a 100 mL pear-shaped flask. The mixture was then stirred at room temperature for 1 h to ensure that all solid components were fully dissolved. Then, 0.12 g of YbCl3 was added to the above mixture and stirred at room temperature for 30 min. Next, the mixture was transferred to an autoclave reactor lined with a polytetrafluoroethylene tube and heated it in an oil bath at 120 °C for 18 h. Finally, the product was obtained by centrifugation, followed by three rounds of washing with DMF and ethanol, and then vacuum-dried at 60 °C for 6 h. Different Yb-doping content UIO-66 was obtained using the same procedure by varying the Yb/Hf molar ratio. For comparison, bare UIO-66 was also synthesized under the same conditions without the addition of Yb.
Preparation of PFD@LP-Yb-UIO-66: 10 mg of Yb-UIO-66 was thoroughly dispersed in 10 ml of PBS under ultrasound and stirring conditions to form a stable suspension. Cholesterol (50 mg) and egg yolk lecithin (150 mg) were dissolved in 10 ml of chloroform, followed by the addition of 400 μL of PFD. The mixture was stirred at room temperature for 30 min. Subsequently, the mixture was transferred into a 100 ml pear-shaped flask and evaporated using a vacuum rotary evaporator at 40 °C for 1 h to form a lipid film. Once the chloroform had evaporated, the PBS solution containing Yb-UIO-66 NPs was added to the flask, followed by low-temperature hydration at 40 °C for 1 h, resulting in the formation of PFD@LP-Yb-UIO-66.
Ozone saturation (O3P@LPYU): The ozone saturation process follows our previous methodology33. In short, ozone was introduced into 1 mg of PFD@LP-Yb-UIO-66 suspended in 1 mL PBS at a flow rate of 0.5 NL/min for 2 min, leading to the creation of O3P@LPYU.
Physical and chemical characterization
Sample morphology was analyzed using TEM and High-Resolution TEM (HRTEM) on an FEI Talos F200X equipped with a 200 kV field emission gun. The particle size and zeta potential of the nanoparticles, before and after doping modification, were determined via Mastersizer. To identify the phase composition and structure of the nanoparticles, XRD patterns were recorded over a 2θ range of 5° to 80° at a scanning rate of 0.05° s⁻¹ using a Bruker D8 Advance diffractometer (Cu Kα = 1.5404 Å). The optical band gap of the nanoparticles was assessed via UV-Vis diffuse reflectance spectroscopy (UV-3600, SHIMADZU) and calculated using Tauc plot analysis. XPS was conducted on a VG MultiLab 2000 spectrometer (VG Inc.) with monochromatic Al Kα radiation as the excitation source, under a vacuum of 2 × 10-6 Pa. The binding energy was charge-corrected using the C 1s peak at 284.80 eV. The specific surface area, pore volume and pore size were determined from the BET analyzer.
Theoretical calculation methods
DFT calculations were carried out using the Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA) exchange-correlation function to estimate the piezoelectric tensor and BEC37. The Vienna Ab Initio Simulation Package (VASP) was employed to apply density functional perturbation theory (DFPT)38,39. A relatively high plane-wave energy cutoff of 500 eV was adopted in the calculations of structural relaxation, elastic tensor, and piezoelectric coefficient tensor. A 1 × 1 × 1 Monkhorst-Pack k-point grid was employed to sample the Brillouin zone. A tight energy convergence criterion of 1E-5 eV was set in all calculations, which is an important consideration for performing DFPT calculations. Additionally, six finite strains were applied to the lattice with the finite difference method, obtaining the elastic constants from the stress-strain relationship to effectively evaluate the flexibility of the system. The \({d}_{33}\) piezoelectric coefficient is an important parameter widely used, being a common indicator to evaluate piezoelectric performance. Since the induced polarization depends on the applied stress, the piezoelectric coefficients \({{{{\rm{d}}}}}_{{{{\rm{ij}}}}}\) are determined by the combination of the piezoelectric coefficient tensor \({e}_{{ij}}\) and the elastic coefficients \({C}_{{ij}}\), as shown in the formula 1:
Generally, the materials with large \({e}_{{ij}}\) or low \({C}_{{ij}}\) values are expected to exhibit reasonably large piezoelectric coefficients \({{{{\rm{d}}}}}_{{{{\rm{ij}}}}}\).
Furthermore, in the calculations of the nitrogen adsorption model, three parameters, including adsorption energy, charge density difference, and density of states, were used to characterize molecular adsorption performance. Here, all data post-processing was performed using the VASPKIT code40. The formulas for the adsorption energy (\({{{{\rm{E}}}}}_{{{{\rm{ad}}}}}\)) and charge density difference (∆ρ) are as follows:
Piezoelectric characterization
PFM was carried out using a Bruker Dimension ICON atomic force microscope (AFM) integrated with a ferroelectric test system; UIO-66 or Yb-UIO-66 suspensions were spin-coated onto a Pt-coated SrTiO3 substrate. During PFM measurements, the AC bias for amplitude and phase image formation was set to 0 V, and the resonance frequency used for the butterfly-shaped amplitude curve and hysteresis loop analysis was 275.5 kHz. Electrochemical evaluations were conducted using an Autolab (PGSTAT302N) workstation in a standard three-electrode cell configuration, with Pt sheets serving as both the working and counter electrodes, and a saturated calomel electrode as the reference. Dispersions for these tests were prepared by mixing 30 mg of UIO-66 or Yb-UIO-66 NPs in 30 mL of 0.5 M Na2SO4 electrolyte. ROS were detected using electron paramagnetic resonance (EPR) spectroscopy on a Bruker A200-9.5/12 instrument.
Fabrication of Yb-UIO-66@PDMS PENG devices
First, ground Yb-UIO-66 was added to 500 μL of n-hexane and stirred for 1 h to create a uniform suspension. Simultaneously, PDMS was prepared by mixing the base and curing agents in a 10:1 (w/w) ratio. The Yb-UIO-66 suspension was then incorporated into the PDMS elastomer at a concentration of 10%, followed by thorough stirring at room temperature to achieve a homogeneous mixture. This mixture was poured into a 2 × 2 cm² polytetrafluoroethylene (PTFE) mold, spread evenly using a scraper, and cured at 60 °C for 5 h to produce composite films approximately 1 mm thick. The Yb-UIO-66@PDMS PENG was assembled using a standard contact-separation method. A 2 × 2 cm² ITO-PET sheet served as the flexible substrate, with sponge layers attached to both substrates for buffering. Double-sided conductive aluminum tape was used as electrodes. The Yb-UIO-66@PDMS composite film was affixed to the bottom electrode, and copper wires were connected to the metal layers to complete the Yb-UIO-66@PDMS-based triboelectric nanogenerator, with an effective area of 2 × 2 cm². The preparation method for UIO-66@PDMS PENG was the same as described above, except that no nanoparticles were added during the process for the blank control PDMS PENG.
Simulated intrapleural pressure device in vitro
To simulate cyclic changes in IPP in vitro, we designed and constructed a reciprocating plunger pump pressurization device. To better replicate the in vivo environment, we set the parameters (including pressure variation and frequency) to exactly match the physiological values of MPE mice. First, we calibrated the system to match the physiological range of IPP changes using an electronic pressure gauge and a cerebrospinal fluid manometer (approximately −3 mmHg to −10 mmHg). We then adjusted the peristaltic pump frequency to 100 strokes per minute to match the breathing rate of the MPE mice. After determining these parameters, piezoelectric nanomaterials were placed in the conduit system for pressurization treatment. Subsequent in vitro simulation experiments were conducted based on this setup, maintaining parameters consistent with those of MPE mice. Please refer to Supplementary Movies 1–3 for the schematic diagram.
Functional characterization
The ozone concentration in O3P@LPYU was determined through the following process: Ozone was continuously injected into 1 mL of the material and ultrapure water at a flow rate of 0.5 NL/min until saturation was achieved. The ozone-saturated solution was then dispersed into 30 mL of ultrapure water. The ozone concentration in the solution was measured at specified intervals using a pen-type detector (Clean Instruments Co., Ltd., Taiwan, China).
To detect the generated singlet oxygen (1O2) and hydroxyl radicals (•OH), RhB and MB probes were utilized. In a light-protected environment, 30 μL of RhB (5 mg/mL) or 10 μL of MB (10 mg/mL), along with 30 μL of UIO-66, Yb-UIO-66, or O3P@LPYU (30 mg/mL), were added to a 3 mL ultrapure water system. The mixture was placed in the IPP simulation device and continuously treated under the following parameters: pressure variation range of −3 mmHg to −10 mmHg and a frequency of 100 cycles per minute. Finally, the absorbance spectra of the samples were measured across the 300–700 nm range using a multifunctional microplate reader (SYNERGY H1).
Peroxidase-like activities
The peroxidase-like activity of O3P@LPYU was evaluated by measuring the absorbance of oxTMB at 652 nm using a multifunctional microplate reader. In a standard experiment, 1 mg/mL of the nanomaterial was added to a solution containing 1.0 mM TMB and 5.0 mM H₂O₂ in acetate buffer (pH 5.0), and the absorbance was monitored over time. Kinetic studies were conducted by varying TMB concentrations (0–2.0 mM) with a fixed H₂O₂ concentration (5.0 mM) or altering H₂O₂ concentrations (0–10.0 mM) with a fixed TMB concentration (1.0 mM) in the presence of 1 mg/mL O3P@LPYU. Lineweaver-Burk plots were derived from Michaelis–Menten curves for the analysis. Unless specified otherwise, all tests were performed at room temperature. For OPD and ABTS oxidation, the absorbance of the oxidized products was measured at 450 nm and 420 nm, respectively.
Cellular uptake
The LLC and 4T1 cell lines were seeded into 24-well glass-bottom culture plates at a density of 10⁴ cells per well. After overnight incubation, FITC-labeled O3P@LPYU at a concentration of 50 μg/mL was added to each well and thoroughly mixed. Following an additional 24 h incubation, the cells were washed with PBS to remove any excess nanoparticles. The cells were then fixed with cold methanol, permeabilized with Triton X-100, and stained with WGA 647 antibody at 4 °C for 20 s. After washing off the antibody, a DAPI fluoromount-G mounting medium was applied to seal the slides. This entire process was conducted under light-protected conditions. Images were captured using confocal laser scanning microscopy (CLSM, Nikon C2) and processed with NIS Viewer software.
To evaluate cellular internalization, flow cytometry was utilized. After following the same treatment protocol as described, the cells were directly harvested for flow cytometric analysis at various time points post O3P@LPYU exposure.
Cytotoxicity, ROS level, and apoptosis detection
Cytotoxicity was evaluated using the CCK8 assay. In brief, LLC and 4T1 cell lines were plated at a density of 1500 cells per well in 96-well plates. After 24 h, varying concentrations of UIO-66, Yb-UIO-66, and O3P@LPYU were added to the wells. For the mechanical pressure stimulation group, the piezoelectric materials were subjected to pressurization using the IPP simulation device for 10 min prior to addition, with parameters set to a pressure variation range of −3 mmHg to −10 mmHg and a frequency of 100 cycles per minute. After an additional 24 h of incubation, 10 μL of CCK8 assay reagent was introduced into each well, followed by a 2 h incubation. The absorbance at 450 nm was then measured for each well using a multifunctional microplate reader.
The clonogenic assay was performed to assess the long-term effects of piezoelectric catalysis treatment. LLC and 4T1 cells were seeded at a density of 5000 cells per well in 6-well plates and incubated overnight. The following day, the cells were exposed to different treatment conditions following the protocol described in the CCK8 assay. The growth medium was refreshed every 2 days to maintain optimal cell growth conditions. After 14 days, the cells were carefully washed with PBS, fixed with 4% paraformaldehyde, and then stained with 0.05% crystal violet.
To evaluate ROS levels after various treatments, the DCFH-DA probe was employed. LLC and 4T1 cells were plated on cell slides within 24-well plates and incubated overnight to promote growth. The cells were then subjected to different treatment protocols, following the same procedures as in the CCK8 assay. After 4 h, the cells were washed with PBS and incubated with DCFH-DA (5 μM) for 30 min. Post-incubation, the cells were rinsed three times with PBS, fixed with 4% paraformaldehyde for 30 min, and sealed with DAPI fluoromount-G mounting medium. ROS signals were observed using confocal laser scanning microscopy with 488 nm excitation.
For the apoptosis assay, LLC and 4T1 cells were seeded at a density of 105 cells per well in 12-well plates and incubated overnight to allow for attachment and growth. The cells were then subjected to different treatment protocols, following the same procedures as in the CCK8 assay. After 4 h of incubation, cells were collected, stained with Annexin V-PE for 15 min, followed by 7AAD staining, and analyzed by flow cytometry.
ICD and cytokine secretion evaluation in vitro
The detection of ICD was evaluated through three key aspects: CRT membrane re-localization, HMGB1 secretion, and ATP release. CRT membrane re-localization was examined using IF. Briefly, LLC and 4T1 cells were seeded onto cell slides placed in 24-well plates and incubated overnight. The following day, after treating the cells according to the specified protocol, they were fixed with cold methanol and incubated overnight at 4 °C in the dark with the CRT antibody. The cells were then washed with PBS, followed by the addition of a fluorescent secondary antibody, and incubated at room temperature in the dark for 1 h. Finally, the slides were mounted with an anti-fade mounting medium, and images were captured using a confocal microscope.
For the evaluation of HMGB1 secretion induced by treatment, the collected supernatants are analyzed using HMGB1 ELISA kits (E-EL-M0676, Elabscience), following the manufacturer’s prescribed protocols.
An ATP assay kit (S0027, Beyotime) was used to quantitatively analyze extracellular ATP levels. Briefly, LLC and 4T1 cells were seeded at a density of 1 × 105 cells per well in 6-well plates and incubated overnight. The following day, after treating the cells according to the specified protocol for 2 h, the supernatant was collected for analysis. Extracellular ATP levels were quantified according to the instructions provided by the ATP assay kit.
For evaluation of the secretion of cytokines (VEGF and TNF-α) affecting vascular permeability induced by the treatment, the collected supernatants were analyzed using VEGF (EK0541, Boster Bio) and TNF-α (EK0527, Boster Bio) ELISA kits, following the manufacturer’s prescribed protocols.
MPE model construction and biodistribution
The surgical procedure for establishing the MPE model was conducted as follows:
A hole was drilled at the bottom of a 50 ml centrifuge tube, and sufficient clean cotton was inserted from the orifice of the tube to plunge the hole at the bottom. The tube should be used for anesthesia of mice and must be sterilized before use. An operating table, sterilized using ultraviolet light, was prepared. The mouse was positioned near the orifice of the prepared tube with its head facing the opening. Upon gently pulling its tail, the mouse naturally entered the tube. The mouse was retained inside the tube, and 300 μL of isoflurane was injected into the cotton through the hole in the tube using a syringe (followed mice need just 100 μL per one to keep the amount of isoflurane in the cotton). The mice were rapidly anesthetized and then transferred onto the surgery table. During surgery, the tube kept covering the head of the mice to maintain its anesthesia. The hair on the mouse’s anterior chest wall was shaved to fully expose the skin. The puncture site (the right chest wall) was disinfected with an alcohol swab. A 26G intravenous catheter was inserted into the right pleural cavity by puncturing through the 10th or 11th intercostal space along the midaxillary line. Upon successful entry into the pleural cavity, the needle core was carefully removed, and 50 μL of LLC-Luc (1.2 × 105) or 4T1-Luc (1.2 × 105) cell suspension was administered through the catheter using a sterile syringe. After the injection, the catheter was withdrawn, and local pressure was applied to stop bleeding and prevent leakage. MPE progression was tracked using bioluminescence imaging or ultrasound.
Based on the established MPE model, we used a pressure sensor to measure the IPP in mice under different conditions (normal, early-stage MPE, and late-stage MPE). Subsequently, we conducted a biodistribution study of O3P@LPYU based on the above MPE animal model. Twelve days after implantation, Dir-O3P@LPYU (0.5 mg/kg) was administered into the pleural cavity of the mice. The in vivo distribution of O3P@LPYU was monitored at various time points—1 h, 3 h, 6 h, 12 h, 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, and 7 d post-injection—using the IVIS Spectrum. To further investigate the specific distribution of nanomaterials in key organs in vivo, 24 h after intrapleural injection of DiR-O3P@LPYU, the mice were euthanized, and organs including the heart, liver, spleen, lungs, kidneys, brain, stomach, intestines, tumors, and MPE were collected for ex vivo imaging.
Experimental protocol in vivo
The in vivo experimental procedure to evaluate the treatment efficacy of piezoelectric catalysis is described as follows. Based on the above animal model, once intrathoracic metastases and MPE formed, the animals were randomly assigned to different treatment groups. Under isoflurane anesthesia, mice were injected into the pleural cavity with 50 μL of nanomaterial suspension (UIO-66, Yb-UIO-66, or O3P@LPYU NPs, 10 mg/ml) every 5 days, with the control group receiving an equivalent volume of PBS. To evaluate the status of pleural tumors and MPE, IVIS imaging and ultrasound examination were performed every other day on mice in all groups after the start of treatment. Throughout the treatment period, the mice’s diet, weight, and survival status were closely monitored.
After 22 days, the mice were euthanized using CO2 asphyxiation. The abdominal wall was then carefully opened, and the internal organs were retracted to expose the diaphragm. Pleural fluid was gently drawn out using a 3 ml syringe, and its volume was measured with a 1000 μL pipette. Tumor tissues from the pleural cavity and major organs were collected and fixed in 4% paraformaldehyde.
Immunohistochemistry and immunofluorescence staining
To assess damage-associated molecular patterns in tumor tissues, IHC techniques were employed. Tumor tissues were initially fixed in formalin for 24 h before being placed into embedding cassettes for dehydration and paraffin embedding. Sections of the tumor, 4 μm in thickness, were then affixed to glass slides. After deparaffinization, the IHC staining procedures were performed.
The slides underwent several steps: first, an endogenous peroxidase blocking buffer was applied and incubated for 15 min in a light-protected environment. Next, a blocking step with a 5% BSA-PBS solution was carried out at room temperature for 1 h. The slides were then incubated overnight at 4 °C with primary antibodies specific to HMGB1 and CRT. Following this, the slides were rinsed with PBS and incubated with species-specific HRP-conjugated secondary antibodies designed for IHC. After further PBS rinses, the slides were treated with a DAB visualization solution. Finally, the slides were gently rinsed with tap water, counterstained with hematoxylin, dehydrated with ethanol, and mounted with neutral balsam. All image acquisition was performed using the VS200 slide scanner.
Flow cytometry
On day 22, the mice were sacrificed, and solid tumors from the pleural surface, mediastinum, and mediastinal lymph nodes were collected for further analysis. Flow cytometry was conducted using a BD Fortessa cytometer, with data processed in FlowJo software (v.10.8.1). Details of the antibodies used can be found in the “Materials” section, and all mouse flow cytometry antibodies were diluted 1:100. Doublets and dead cell debris were excluded prior to applying specific gating strategies (Supplementary Fig. 26, 27). Gating and quadrant settings were determined based on isotype control staining.
Transcriptome sequencing and bioinformatic analysis
Total RNA was extracted and purified using the RNeasy Plus Mini Kit (Qiagen, Hilden Germany) according to the manufacturer’s instructions. The quality and integrity of the RNA were evaluated using the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA) and agarose gel electrophoresis. For library preparation, 1 μg of RNA from each sample was processed using the TruSeq Stranded Total RNA LT Kit (Illumina Inc., San Diego, CA). Paired-end sequencing (PE150) was conducted on the Illumina platform.
After quality control and the trimming of low-quality reads and adapters with TrimGalore (v0.6.10) (https://github.com/FelixKrueger/TrimGalore), the clean reads were aligned to the GRCm39 primary assembly genome using STAR (v2.7.3a)41. Gene expression quantification was performed using Salmon (v1.10.2)42. The R package ‘DESeq2’ (v1.42.1)43 was utilized to identify differentially expressed genes. The resulting P-values were adjusted using Benjamini and Hochberg’s approach. Genes with absolute values of log2fold change > 1 and P-adjust <0.05 were considered to be significant. GSEA was performed using the compareCluster function implemented in the clusterProfiler R package (v4.10.1)44,45, with a significance threshold of adjusted P-value < 0.05. To comprehensively characterize pathway-level alterations, we employed GSVA (v1.50.5)46 using the canonical hallmark gene sets from the Molecular Signatures Database (MSigDB). Pathway activation scores were calculated for each treatment condition, with statistical significance determined by adjusted p-values < 0.05. ICD score, ROS biosynthesis score, and MPE score were calculated with the GSVA algorithm. The relevant gene sets are provided in Supplementary Table 11. CIBERSORT algorithm (v1.03)47, along with the input of a pre-established reference signature matrix encompassing 25 immune cell types48, was employed to estimate the infiltrating immune cells in mouse tissues.
Statistical analysis
Statistical analyses are conducted using GraphPad Prism 9.0 software. The quantitative data were expressed as mean ± SD. Statistical differences were calculated using unpaired student’s t-test or one-way analysis of variance (ANOVA) when comparing two or multiple groups, respectively. The survival curve was analyzed by the Log-rank test. Statistical significance is determined using the following thresholds: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, with non-significant findings labeled as NS (not significant).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data generated in this study are provided in the Supplementary Information/Source Data file. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (GSA) database of the National Genomics Data Center (NGDC) with the accession number CRA022400. Source data are available for Figs. 2–10 and Supplementary Figs. 2, 4, 8–13, 21, 22, 24, and 25 in the associated source data file. Source data are provided with this paper.
Code availability
Our studies use well-established computational and statistical analysis tools, with full details provided in the main text and Methods section. All tools utilized in this research are publicly accessible.
References
Maskell, N. A. Treatment options for malignant pleural effusions: patient preference does matter. JAMA 307, 2432–2433 (2012).
Gayen, S. Malignant pleural effusion: presentation, diagnosis, and management. Am. J. Med. 135, 1188–1192 (2022).
Gonnelli, F. et al. Malignant pleural effusion: current understanding and therapeutic approach. Respir. Res. 25, 47 (2024).
Egan, A. M., McPhillips, D., Sarkar, S. & Breen, D. P. Malignant pleural effusion. QJM 107, 179–184 (2014).
Thomas, R. et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: The AMPLE Randomized Clinical Trial. JAMA 318, 1903–1912 (2017).
Figlin, R., Mendoza, E., Piantadosi, S. & Rusch, V. Intrapleural chemotherapy without pleurodesis for malignant pleural effusions. LCSG Trial 861. Chest 106, 363S–366S (1994).
Donnenberg, A. D., Luketich, J. D., Dhupar, R. & Donnenberg, V. S. Treatment of malignant pleural effusions: the case for localized immunotherapy. J. Immunother. Cancer. 7, 110 (2019).
DeBiasi, E. M. & Feller-Kopman, D. Physiologic basis of symptoms in pleural disease. Semin. Respir. Crit. Care Med. 40, 305–313 (2019).
Hall, J. E. Guyton and Hall Textbook of Medical Physiology 13th edn (Elsevier, 2016).
Xiang, Z. et al. Tumor microenviroment-responsive self-assembly of barium titanate nanoparticles with enhanced piezoelectric catalysis capabilities for efficient tumor therapy. Bioact. Mater. 33, 251–261 (2024).
Yang, J. et al. Employing piezoelectric Mg(2+)-doped hydroxyapatite to target death receptor-mediated necroptosis: a strategy for amplifying immune activation. Adv. Sci. 11, e2307130 (2024).
Wang, Q. et al. Bimetallic organic frameworks of high piezovoltage for sono-piezo dynamic therapy. Adv. Mater. 35, e2301784 (2023).
Truong, H. Q. et al. Piezocatalytic 2D WS(2) nanosheets for ultrasound-triggered and mitochondria-targeted piezodynamic cancer therapy synergized with energy metabolism-targeted chemotherapy. Adv. Mater. 35, e2300437 (2023).
Chen, M. et al. Nanotechnology based gas delivery system: a “green” strategy for cancer diagnosis and treatment. Theranostics 14, 5461–5491 (2024).
Chen, S. et al. Piezocatalytic medicine: an emerging frontier using piezoelectric materials for biomedical applications. Adv. Mater. 35, e2208256 (2023).
Chorsi, M. T. et al. Piezoelectric biomaterials for sensors and actuators. Adv. Mater. 31, e1802084 (2019).
Yuan, X. et al. Piezoelectricity, pyroelectricity, and ferroelectricity in biomaterials and biomedical applications. Adv. Mater. 36, e2308726 (2024).
Zhang, C. et al. Piezo-photocatalysis over metal-organic frameworks: promoting photocatalytic activity by piezoelectric effect. Adv. Mater. 33, e2106308 (2021).
Cai, L. et al. Piezoelectric metal-organic frameworks based sonosensitizer for enhanced nanozyme catalytic and sonodynamic therapies. ACS Nano 17, 7901–7910 (2023).
Zhang, L., Wang, H. & Qu, X. Biosystem-inspired engineering of nanozymes for biomedical applications. Adv. Mater. 36, e2211147 (2024).
Ren, X. et al. Nanozymes-recent development and biomedical applications. J. Nanobiotechnol. 20, 92 (2022).
Manoharan, D., Wang, L. C., Chen, Y. C., Li, W. P. & Yeh, C. S. Catalytic nanoparticles in biomedical applications: exploiting advanced nanozymes for therapeutics and diagnostics. Adv. Healthc. Mater. 13, e2400746 (2024).
Zhang, Y. et al. The applications of nanozymes in cancer therapy: based on regulating pyroptosis, ferroptosis and autophagy of tumor cells. Nanoscale 15, 12137–12156 (2023).
Fu, Q., Wei, C. & Wang, M. Transition-metal-based nanozymes: synthesis, mechanisms of therapeutic action, and applications in cancer treatment. ACS Nano 18, 12049–12095 (2024).
Jiang, Z., Han, X., Zhao, C., Wang, S. & Tang, X. Recent advance in biological responsive nanomaterials for biosensing and molecular imaging application. Int. J. Mol. Sci. 23, 1923 (2022).
Ding, H. et al. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 348, 206–238 (2022).
Li, Z. et al. Hydrogel transformed from nanoparticles for prevention of tissue injury and treatment of inflammatory diseases. Adv. Mater. 34, e2109178 (2022).
Cai, P. et al. Biomechano-interactive materials and interfaces. Adv. Mater. 30, e1800572 (2018).
Tian, B. et al. Doping engineering to modulate lattice and electronic structure for enhanced piezocatalytic therapy and ferroptosis. Adv. Mater. 35, e2304262 (2023).
Xu, Q. et al. Multifunctional nanoplatform based on tetragonal BaTiO(3)-Au@polydopamine for computed tomography imaging-guided photothermal synergistic and enhanced piezocatalytic cancer therapy. J. Colloid Interface Sci. 658, 597–609 (2024).
Liu, Y., Li, Q., Qiao, L., Xu, Z. & Li, F. Achieving giant piezoelectricity and high property uniformity simultaneously in a relaxor ferroelectric crystal through rare-Earth element doping. Adv. Sci. 9, e2204631 (2022).
Li, F. et al. Giant piezoelectricity of Sm-doped Pb(Mg(1/3)Nb(2/3))O(3)-PbTiO(3) single crystals. Science 364, 264–268 (2019).
Song, L. et al. Improvement of TNBC immune checkpoint blockade with a microwave-controlled ozone release nanosystem. J. Control. Release 351, 954–969 (2022).
Wang, C. et al. Ferritin-based targeted delivery of arsenic to diverse leukaemia types confers strong anti-leukaemia therapeutic effects. Nat. Nanotechnol. 16, 1413–1423 (2021).
Zhao, C. et al. Shape designed implanted drug delivery system for in situ hepatocellular carcinoma therapy. ACS Nano 16, 8493–8503 (2022).
Li, H. et al. Fully bioabsorbable capacitor as an energy storage unit for implantable medical electronics. Adv. Sci. 6, 1801625 (2019).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter. 47, 558–561 (1993).
Baroni, S., de Gironcoli, S., Dal Corso, A. & Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001).
Wang, V., Xu, N., Liu, J., Tang, G. & Geng, W. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7 (2013).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Chen, Z. et al. Inference of immune cell composition on the expression profiles of mouse tissue. Sci. Rep. 7, 40508 (2017).
Acknowledgements
The authors thank Fei Fu and Yijing Long (Core Facility of West China Hospital, Sichuan University) for providing the gas chromatography equipment and technical support. This work was supported by (1) the National Natural Science Foundation of China (No. 82404672 to Z.X.; No. 82172634 to H.S.; No. 82104212 to P.D.); (2) the National Key Research and Development Program of China (2022YFA1207300 to J.J.); (3) Key Program of the Science and Technology Bureau of Sichuan (No. 2021YFSY0007 to H.S.; No. 2024YFFK0352 to D.Z.); (4) 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD23028 to H.S.). (5) the China Postdoctoral Science Foundation (No. 2023M742486 to Z.X.); (6) the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (No. GZC20231826 to Z.X.).
Author information
Authors and Affiliations
Contributions
H.S., J.J., and Z.X. conceived the project. Z.X. designed experiments, analyzed data, and compiled the figures. Y.G., X.H., L.T., and J.H. provided the bioinformatic analysis. Yx.Z., L.S., T.X., M.C., X.W., and S.C. helped with animal experiments. L.S., Yj.Z., P.D., H.M., Y.L., and F.L. helped with imaging experiments. Z.X. and H.S. wrote the manuscript. H.S., J.J., and W.Z. supervised the project. All authors contributed to revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All animal procedures were conducted in strict compliance with protocols approved by the Ethics Review Committee of Animal Experimentation at Sichuan University (No. 20240402004). These procedures were conducted in accordance with the guidelines set forth in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and in compliance with the principles of the Animal Welfare Act.
Peer review
Peer review information
Nature Communications thanks Chaochao Zhao and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Z., He, X., Gui, Y. et al. Intrapleural pressure-controlled piezo-catalytic nanozyme for the inhibition of malignant pleural effusion. Nat Commun 16, 3194 (2025). https://doi.org/10.1038/s41467-025-58354-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58354-9