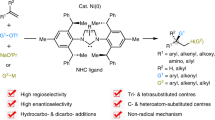
Abstract
Mono-metallic catalysts dominate in homogeneous catalysis, wherein all the element steps generally occur on one metal site. Inspired from bimetallic active sites in both enzymes and heterogeneous catalysts, the development of binuclear catalysis can offer the potential to induce novel intermediates, reactivity, and selectivity. Metal-catalyzed hydroarylation of alkynes generally leads to one alkyne incorporated products and alkyne dimerization-hydrocarbofunctionalization is rather challenging via conventional mono-metallic intermediates. Herein, a highly selective dimerization-hydrocarbofunctionalization of internal alkynes is achieved via dinickel catalysis, leading to the formation of synthetically challenging pentasubstituted 1,3-dienes. Mechanistic studies suggest that each Ni site can promote distinct elementary steps of two alkynes to generate a di-vinyl di-Ni intermediate. Such a mode of “binuclear convergent catalysis” is fundamentally different from the traditional mono-metallic catalysis and may provide new understanding on binuclear synergistic effects at atomic and molecular level.
Similar content being viewed by others
Introduction
Bimetallic active sites in both enzymes and heterogeneous catalysts are well known to be effective in mediating challenging and highly valuable reactions, which is largely attributed to synergistic effects between metals in close proximity1,2,3,4. However, achieving a fundamental understanding on the bifunctional role at the atomic and molecular level is very challenging due to the structural complexity of the catalytic sites in metallic surfaces and metalloenzyme. Over the last several decades, many efforts have been devoted to modelling these multimetallic activation processes for discovery of novel catalysts, primarily using dinuclear metal complexes in homogenous catalysis5,6,7,8,9,10,11,12,13,14. In this context, several types of bimetallic interaction models (e.g., co-activation of a single reactant by ligation with both metal nuclei, and synergistic activation of both reactants on distinct metal centers etc) have been proposed to account for the significant binuclear synergistic effects. Compared to the classical mono-metallic catalysis wherein all the element steps generally occur on one metal site (Fig. 1a, left), binuclear metallic catalysis offers a unique approach wherein two metal sites may independently promote distinct elementary steps in a parallel way during the initial stage of the catalytic cycle, resulting the formation of new binuclear catalytic intermediates, which thus may lead to unusual reactivity unattainable with a typical mononuclear catalyst (Fig. 1a, right). This would be highly desirable for the development of new reactions and further exploration of novel binuclear catalytic mechanisms15,16,17.
Alkynes are one of the most readily available feedstocks and versatile synthons18,19,20,21,22,23. Transition metal-catalyzed hydrocarbofunctionalization of alkynes using organometallic reagents has emerged as one of the most reliable strategies to access a wide range of highly substituted olefins (Fig. 1b)24,25,26,27,28. However, the reactions developed so far generally result in products with only a single equivalent of alkyne incorporated, and dimerization-hydrocarbofunctionalization of unsymmetrical internal alkynes with organometallic reagents remains elusive (mainly as side-reactions or giving a mixture of isomers)29,30,31,32,33,34,35, even though this may constitute an attractive strategy for quick access to synthetically versatile yet hardly prepared poly-substituted 1,3-dienes36,37,38,39,40,41,42,43. Controlling the degree of alkyne insertion as well as regio- and stereoselectivity constitutes nontrivial challenges, especially for an unsymmetrical internal alkyne via a general vinyl-metal intermediate or metallacyclopentadiene intermediate44,45,46. Thus, novel catalytic modes involving distinct intermediates from the classic mono-metallic species may need to be invoked for alkyne dimerization-hydrocarbofunctionalization processes, which eventually allow for efficient access to highly substituted 1,3-dienes.
Herein, we report dimerization and hydroarylation/hydroalkylation of unsymmetrical internal alkynes with organoborons using a dinuclear nickel complex as the catalyst, providing a series of pentasubstituted 1,3-dienes with excellent regio- and stereoselectivities (Fig. 1c). The versatile synthetic utility, broad functional group compatibility, and excellent chemo- and regioselectivity are demonstrated by substrate scope evaluation and product derivatization. In addition, the protocol can be applied to a one-pot synthesis of valuable yet complicated bridged bicyclic scaffolds that are otherwise difficult to prepare. Notably, control experiments using several mononuclear Ni catalysts only afford low yields of the corresponding hydroarylation products with a single equivalent of alkyne being incorporated, demonstrating the unique reactivity and distinct selectivity pattern of the current dinuclear Ni catalyst. Mechanistic studies suggest that two vinyl Ni moieties are generated intramolecularly on two Ni atoms, respectively, followed by a reductive elimination to form the final product. By analogy to “convergent strategies in total syntheses”47,48, it is tempting to tentatively name this catalytic model as “binuclear convergent catalysis” in homogeneous catalysis, since binuclear core enables parallel substrates activation via distinct elementary steps on two metals and cooperative intermediate transfer across dual metal centers to deliver the final product, whereas monometallic catalysis is generally confined to sequential reaction pathways mediated solely through a single active site.
Results
Reaction development
Bis pyridyl diimine (PDI) ligands49,50,51 based binuclear metal complexes have been used as catalysts in olefin polymerizations52 and studied in activation of small molecules53,54. Inspired by the striking potency of binuclear metallic catalysis5,6,7,8,9,10,11,12,13,14, our group has developed a macrocyclic bi-PDI based dicobalt complex-catalyzed stereodivergent semi-reduction of alkynes55 and a dinickel complex-catalyzed Z-selective 1,4-hydroarylation of 1,3-dienes56. As our continuous interest in developing new reactions using binuclear metal complexes as catalysts, we aimed to investigate the coupling of organoboronic acids with alkynes using binuclear Ni complexes as the catalysts. The study was initiated using unsymmetrical internal alkyne, 1-phenyl-1-propyne 1a and phenylboronic acid 2a as the model substrates.
After a systematic screening of the reaction parameters (for details, see Supplementary Tables 1–10), we found that a mono-ene 3a and a pentasubstituted 1,3-diene product 4a were obtained in 9% and 84% GC yields, respectively, in the presence of the dinickel complex A, NaBHEt3 and t-BuONa in a mixed solvent of MeOH and DMF at 80 °C (Table 1, entry 1). Lower efficiency was observed when the reaction was carried out at 60 °C (entry 2). Only trace amount of the target product 4a was detected without MeOH, suggesting that the alcohol is crucial for the reaction (entry 3). Of note, the length of methylene linkers (where n = 2–4) of the catalyst proved to be a critical factor for the reaction efficiency. Compared to dinickel complex A (n = 3), the reaction using either dinickel complexes B (n = 2) or C (n = 4) led to the formation of 1,3-diene product 4a in significantly lower yields (entries 4 and 5), suggesting that the Ni-Ni distances of the binuclear Ni complexes may influence the reaction outcomes, both in terms of activities and selectivities. Moreover, the catalytic performance of the dinuclear Ni complex was evaluated in comparison with a series of mono-nickel complexes bearing structurally related N-donor ligands, such as PDI (D-G), bipyridine (L1), bis-oxazoline (L2), and pyridine bis-oxazoline (L3). In these cases, though varying degrees of alkyne consumption was observed, no significant amount of 1,3-diene product 4a was detected (entries 6–12). These results further attested the unique catalytic activity and distinct selectivity of the dinuclear catalysts.
Reaction scope
With the optimized reaction conditions in hand, we set out to explore the generality of this methodology, by applying various aryl, alkyl-disubstituted internal alkynes in the reaction with phenylboronic acid 2a using dinickel complex A as the catalyst. The effect of substituents on the aromatic ring on the reaction outcomes was shown in Fig. 2a. Both electron-rich (methyl 1b-1c, tert-butyl 1d, methoxy 1e and methylthio 1f) and electron-deficient (fluoro 1g-1h, chloro 1i, bromo 1j and ester 1k) alkynes were well compatible with the protocol, to deliver the corresponding conjugated diene products (4b-4k) in 60–79% yields. It should be noted that the reaction of ortho-substituted aryl alkyne with large steric hindrance also proceeded smoothly to give the desired product 4h in 49% yield. Moreover, multi-substituent alkynes (1l-1n) and 2-naphthyl alkyne 1o underwent the hydroarylation reaction smoothly, resulting in the formation of 1,3-diene products 4l-4o in 52–83% yields. Importantly, this method was found to be compatible with an array of heteroaromatic alkynes, including indole, benzofuran, thiophene, pyrazole and ferrocene, furnishing the corresponding products (4p−4t) in good yields. Subsequently, the reaction scope with respect to the alkyl moieties of the aryl-alkyl acetylenes was also evaluated. Alkyne substrates bearing different linear alkyl substituents, including Et- and nPr-, and cyclic substituent, cyclopropyl, were well compatible in the reaction, albeit with moderate yields of the corresponding products (4u-4w). Of particular interest is that some functional groups on the alkyl chain, such as ester, chloro, phthalimide and free hydroxyl were well tolerated in the reaction, furnishing penta-substituted diene products 4x-4aa in good yields. In addition, diphenylacetylene was also proved to be a suitable substrate for this transformation, giving the product 4ab in 77% yield, with an olefin bond configuration different from that of the mono-Ni catalyzed transformation33. Under the standard conditions, the reaction of dialkyl acetylenes only led to mono hydroarylation products (for failed examples, see Supplementary Information Page S35). The structure of 4a was unambiguously confirmed by X-ray analysis.
a Scope of alkynes. b Scope of arylboronic acids. Reaction conditions A: alkyne 1 (0.40 mmol), arylboronic acid 2 (0.36 mmol), dinickel complex A (5.0 mol%), NaBHEt3 (20 mol%) and t-BuONa (30 mol%) in MeOH (1.0 mL)/DMF (2.0 mL) at rt for 1.0 h and then 80 °C for 12 h. Yields shown are of isolated products. For 4h and 4w, the reactions were conducted at 100 °C. PMP p-methoxyphenyl, NPhth N-Phthalimido, TMS trimethylsilyl.
Encouraged by these results, we further examined the scope of arylboronic acids (Fig. 2b). The reactions of a series of para- and meta-substituted arylboronic acids, such as methyl, tert-butyl, trimethylsilyl, fluoro, chloro and ester, all afforded the desired 1,3-diene products 4ac-4ai in satisfactory yields with excellent regio- and stereoselectivity. In addition to monosubstituted arylboronic acids, polysubstituted arylboronic acids were also compatible with the procedure to give the corresponding products (4aj, 66% and 4ak, 33%). It is worth mentioning that boronic acids derived from several heterocycles, such as furan, benzofuran, dibenzo[b,d]thiophene, and carbazole, were also competent coupling partners, affording the corresponding pentasubstituted diene products 4al-4ao in moderate yields (42–62%).
To further expand the use of this methodology, we proceeded to examine the reaction of trialkylborane with alkyne using dinickel complex A as the catalyst. Under modified conditions, the reaction of 1-phenyl−1-propyne 1a and triethylborane 5a led to the desired ethyl-substituted 1,3-diene product 6a in 70% isolated yield (for details, see Supplementary Tables 11–14). Next, the substrate scope with regards to alkynes was examined (Fig. 3). Various aryl alkyl alkynes were amenable to this transformation, giving rise to the corresponding pentasubstituted diene products 6b-6m in good yields with high regio- and stereoselectivities. Thereafter, the scope of this reaction with other organoboranes was further explored. Commercially available tri-n-butylborane reacted well to furnish 6n in 54% yield. Additionally, the reactions using unpurified trialkylboranes (prepared from 1-hexene and 1-octene with BH3·SMe2) also gave 6o and 6p smoothly.
Reaction conditions B: alkyne 1 (0.40 mmol), trialkylborane 5 (0.40 mmol), dinickel complex A (5.0 mol%), NaBHEt3 (30 mol%) and t-BuOLi (50 mol%) in MeOH (10 equiv)/DMF (0.30 mL) at rt for 1.0 h and then 80 °C for 12 h. Yields shown are of isolated products. For 6n-6p, B2Pin2 (10 mol%) was used instead of NaBHEt3.
This strategy was subsequently applied into the functionalization of some alkynes bearing biologically relevant skeletons (Fig. 4a). The reactions of a range of internal alkynes derived from (-)-borneol, carbofuran phenol, canagliflozin, dapagliflozin, hymecromone, L-tyrosine or estrone with phenylboronic acid 2a proceeded smoothly to give the corresponding pentasubstituted diene products 4ap-4av in good yields with excellent regio- and stereoselectivity, respectively. All these results further underscored the good functional group compatibility of the reaction, attesting the robustness and practicality of the titled reaction.
Furthermore, a series of synthetic elaboration of the products 4a and 6a were conducted to showcase the synthetic versatility of the methodology (Fig. 4b). For examples, 4a was quantitatively converted to substituted indene derivative 7 by methanesulfonic acid (MeSO3H)-mediated intramolecular Friedel-Crafts cyclization, while Diels-Alder reaction of 4a with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and 4-phenyl-1,2,4-triazoline-3,5-dione delivered bicyclic products 8 and 9 in 80% and 92% yields, respectively. In addition, epoxidation of 6a with varying amounts of 3-chloroperbenzoic acid (m-CPBA) gave mono-epoxide 10 or di-epoxide 11, respectively, in moderate yields. It is noteworthy that sequential hydroarylation and double intramolecular Friedel-Crafts cyclization of two equivalents of alkyne 1 can be successfully achieved in one-pot via di-Ni catalysis followed by a treatment with trifluoromethanesulfonic acid (TfOH), leading to a series of biologically interesting dibenzobicyclo-[3.2.1]octadienes 12 in remarkable efficiency (Fig. 4c).
Experimental and theoretical mechanistic studies
To gain some insight into the reaction mechanism, a series of experiments were conducted (Fig. 5). The use of 2,6-di-tert-butyl-4-methylphenol (BHT, 1.0 equiv), a radical scavenger, in the reaction of alkyne 1a and phenylboronic acid 2a under the standard conditions showed almost no adverse effect on the formation of the desired product 4a, suggesting that the reaction was most likely not a radical-based process (Fig. 5a). Next, deuterium-labeling experiment was carried out to probe the origin of the hydrogen source for the hydroarylation. As shown in Fig. 5b, submission of 1a and arylboroxine 13 to the reaction using CH3OD resulted in 4a-D with 90% deuterium incorporation at the olefinic carbon position, indicating that methanol proton acts as the hydrogen source in the reaction. Moreover, kinetic isotope effect (KIE) was measured on the reaction of 1a and 2a in MeOH and MeOD, respectively, to deliver a kH/kD value of 1.3 (Fig. 5c), suggesting that proton transfer is unlikely to be the rate-determining step. Additionally, a competitive reaction of two electronically reversed alkynes, 1e and 1k, with 2a was run under the standard conditions (Fig. 5d). In this case, the homo-coupling products 4e and 4k were isolated in 17% and 45% yields, respectively, along with the formation of a 34% yield of cross-coupling product 4aw. These results suggested that alkyne with electron-deficient substituent on the phenyl ring is more favorable in the competition reaction. Then, kinetic orders of the reaction were studied under the standard reaction conditions (Fig. 5e). The rate data show a linear dependence on the concentration of the dinuclear nickel catalyst A and alkyne 1a, but a zero-order dependence is observed for arylboronic acid 2a. These results suggested the involvement of one catalyst molecule and one alkyne molecule in the rate-determining step, and the binuclear Ni structure remains largely intact during the catalytic process. Furthermore, Hammett studies were performed to investigate the electronic effect of substituents appended on the alkynes and arylboronic acids, respectively (Fig. 5f). A linear correlation between log(kX/kH) and the Hammett constant (σpara) with a slightly positive slope of 0.157, indicating that the effect of electronic effects of alkyne on the reaction rate is not significant. Moreover, a ρ value of −1.455 was obtained for a series of para-substituted arylboronic acids, suggesting that the presence of an electron-rich group on arylboronic acid is favorable for the reaction.
To further unveil the mechanistic details of this multicomponent reaction, density functional theory (DFT) calculations were further performed at SMD-M06L-D3/def2-TZVP//M06L/def2-TZVP/def2-SVP level of theory (see detailed introduction in Supplementary Information). A formally reduced [(PDI)Ni0]2 ([Ni2]) is firstly generated via in situ reduction of the catalyst precursor A (Fig. 6)56. Based on our previous work, herein the formal [(PDI)Ni0]2 can be described more appropriately as [(PDI−)NiI]2, a dinuclear Ni(I) complex in ground state of triplet56, which facilitates the activation of alkyne 1a (1INT1A, −22.7 kcal/mol) rather than methanol (3INT1C, −13.3 kcal/mol, see detailed mechanism of Path C in Supplementary Fig. 15). Oxidative cyclization of the alkyne π bond across the two Ni centers forms a four-membered bimetallic intermediate 1INT1A with the C1−C2 bond length being elongated to 1.34 Å, which is close to a C=C double bond of alkenes (1.33 Å)45. This similar reactivity and coordination model was disclosed on the addition of diphenylacetylene to a neutral bis-pyridyldiimine dicobalt complex by Tomson group57. In addition, we failed to locate the side-on mode (µ-η2:η2) of alkyne with the C≡C triple bond perpendicular to the Ni-Ni direction58,59, which would spontaneously transfer to the end-on mode (µ-η1:η1) 1INT1A, possibly due to the steric linkage (-C3H6-) of PDI ligands. Therefore, the reaction may be initiated by the oxidative addition of the first alkyne triple bond across the dinuclear Ni core (1INT1A). The viability of synergistic oxidative cyclometallation of two alkynes on 3[Ni2] to form 3INT2B was also assessed as Path B58,59, however, it was found unfavorable due to the much higher energy barrier (∆G‡ = 32.2 kcal/mol, Supplementary Fig. 16). More pathways can be envisioned starting from 1INT1A to generate the intermediate 3INT4A or 3INT4B, depending on the order of protonation, transmetalation and alkyne insertion (Paths A1–A4 in Fig. 6). The protonation of 1INT1A with MeOH to give 1INT2A would be a pathway more favorable than the alkyne insertion of 1INT1A with the second alkyne (1a) on di-Ni core to 3INT2B in Path A1 (see detailed mechanism in Supplementary Fig. 14). After the formation of intermediate 1INT2A, the direct transmetalation to form 1INT3A needs to overcome an energy barrier of +21.9 kcal/mol, which is more accessible than the second alkyne (1a) insertion to form 3INT3B (Path A2, 41.8 kcal/mol via 3TS2B in Supplementary Fig. 14). However, the following alkyne insertion into the Nia-C2 bond of 1INT3A as depicted in Path A3 is associated with a rather high energy barrier (32.5 kcal/mol), suggesting that the butadienyl intermediate 3INT4B might not be accessible. Alternatively, the second alkyne (1a) can be selectively inserted into the Nib-Cph bond at the C4 site with a favorable energy barrier (∆G‡ = 28.7 vs 33.1 kcal/mol at C3 site, path A4), resulting in two different vinyl-Ni moieties within intermediate 3INT4A and finally generates the diene products through a bimetallic reductive elimination. Detailed free energy profiles were depicted in Fig. 7.
The Positive values (kcal/mol) are free energy barriers, and the negative one (kcal/mol) is the free energy change of the reaction. NPA charges are shown in purple and bond length (Å) in black. IGMH analysis of 3INT4A with δginter = 0.004. For DFT calculation results on unfavored pathways, see Supplementary Figs. 14–16.
See unfavored pathways of singlet states in Supplementary Fig. 17; NPA charges are shown in purple; Spin population in green and bond length (Å) in black. IGMH analysis of 3TS3A with δginter = 0.004. MECP the minimum energy crossing points.
The calculated pathway with the most favorable energy profile was found to consist of species mostly occurring in the triplet state, and spin populations of Nia and Nib in the relevant intermediates and transition states may vary throughout the reaction course, and some of intermediates were found to adopt singlet as their ground states (See unfavored pathway of singlet states in Supplementary Fig. 17). Oxidative addition of alkyne (1a) with two Ni centers would result in the formation of a bimetallic intermediate 1INT1A via a MECP with a barrierless process (see Supplementary Fig. 18 for further discussion), which could react with MeOH via a binuclear concerted protonation process to give 1INT2A. NPA charges of 1a and 1INT1A indicated that binuclear Ni2 core acts as an electron-donor to achieve two-electron transfer to the triple bond of 1a, and hence the accumulated electron density on C1 rather than C2 in 1INT1A would favor the capture the proton of MeOH by C1 (20.4 kcal/mol in 3TS1A vs 21.9 kcal/mol in 3TS1A’), which is consistent with the regioselective protonation of C1 site as revealed by the deuterium labelling experiment (Fig. 5b). Upon generation of 1INT2A, the methoxy group in 1INT2A readily associates with phenylboronic acid 2a, which undergoes transmetalation on Nib (21.9 kcal/mol in 3TS2A) under the triplet state, which is more stable than the corresponding singlet state species (40.3 kcal/mol in 1TS2A, see Supplementary Fig. 17). After release of MeOB(OH)2 in 1INT3A, the following reductive elimination of the aryl and alkenyl on Nia and Nib, respectively, has to overcome a relatively high energy barrier of 30.2 kcal/mol, which would prevent the formation of the hydroarylation product 3a with incorporation of only one alkyne (for details, see Supplementary Fig. 19). Alternatively, through the ligand exchange of the second alkyne 1a with MeOB(OH)2 ligand of 1INT3A, the second alkyne insertion of Nib-Cph bond proceeds via transition state 3TS3A to form intermediate 3INT4A featured by π-π interactions between styrenyl-Nia and the phenyl group from the second alkyne as well as the π-π interactions between PDI and the phenyl group from phenylboronic acid (IGMH analysis in Fig. 6). The activation barrier for the migratory insertion of the phenyl group to C4 site was 4.4 kcal/mol lower than that for C3 site (28.7 kcal/mol in 3TS3A vs 33.1 kcal/mol in 3TS3A’), which is consistent with the regioselectivity observed in the reaction. Closer inspections of 3TS3A revealed that the electronic interaction between aryl group and alkyne substrate is the key to account for this regioselectivity of the insertion step along with the π–π stabilization of two aryls, which was further supported by the energy decomposition analysis (EDA, in Supplementary Fig. 20) and IGMH of 3TS3A in Fig. 7. This is also in agreement with the Hammett studies that electron-rich arylboronic acid is favorable for the reaction. For the following reductive elimination step, we failed to locate the seemingly plausible concerted bimetallic transition state (Nia-C2-C3-Nib) from intermediate 3INT4A. Instead, calculations indicated that the reductive elimination would occur on a single Ni site (Nia) of 3INT5A, formed by migration of the diphenyl substituted alkenyl group from Nib to the low-coordinated Nia center in 3INT4A, a process facilitated by the dissociation of one N-Nia coordination bond in PDIa (Nia-N1 = 3.21 Å). The activated Nia of 3INT5A favors the acceptance of electrons by its empty d orbitals to facilitate reductive elimination of two alkenyl groups via a three-membered ring transition state 3TS4A (26.2 kcal/mol). Finally, upon the ligand exchange with substrate 1a would release the product 4a and regenerate the active 1INT1A for the next catalytic cycle. Over the catalytic cycle, the two Ni centers of the dinuclear nickel complex can mediate the individual steps of transformation of two alkynes, respectively, and ultimately achieve double alkynes incorporation and hydroarylation efficiently with high regioselectivity in a “bimetallic convergent catalysis” manner. DFT calculation suggested that the insertion of the second molecule of alkyne should be the rate-limiting step, which is consistent with the experimentally determined first-order kinetic dependence of 1a together with the Hammett studies. Alkyne with electron-deficient substituent on the phenyl ring is more favorable in the competition experiment (Fig. 5d), which might be due to its favorable coordination with low-valent di-Ni core during the generation of the intermediate 1INT1A.
Based on the afore mentioned mechanistic studies, a plausible mechanism was depicted in Fig. 8. After reduction of di-Ni complex A with NaBHEt3, the resulting binuclear complex 3[Ni2] activates the first alkyne via oxidative cyclization to produce a bimetallacycle intermediate 1INT1A, allowing for a binuclear concerted protonation by MeOH to the C1 position of 1a. The formed intermediate 1INT2A then undergoes transmetalation with PhB(OH)2 on Nib and the second alkyne insertion to generate the di-vinyl di-Ni intermediate 3INT4A rather than the direct hydroarylation with one alkyne. Finally, a migration of the diphenyl substituted alkenyl group occurs to generate 3INT5A for the next reductive elimination, delivering the product 4a. Benefited from the binuclear core, the catalytic cycle can be regarded as a manner of “binuclear convergent catalysis”, wherein two alkynes are activated and transferred to the corresponding alkenyl-Ni on two different Ni atom: Nia is responsible for the activation and transfer of the first alkyne to alkenyl-Nia, and arylation of the second alkyne occurs on the Nib to generate the other alkenyl-Nib. Then the reductive elimination of these two “activated moieties” on binuclear Ni core generates the coupling product. The arylation of the second alkyne on Nib plays a key role in the current dimerization and hydrocarbofunctionalization of alkynes over that of mono-alkyne. The electronic effect of the alkyne insertion with aryl group from boronic acid and the π–π interaction of two aryls regulated the activity and regioselectivity.
Discussion
In summary, we have developed an efficient dimerization and hydrocarbofunctionalization of alkynes with organoborons using a bis-PDI dinickel complex as the catalyst, affording a wide spectrum of pentasubstituted 1,3-dienes with excellent regio- and stereoselectivities. This protocol exhibits high functional group tolerance and broad substrate scope, and the resulting products are amenable to a variety of transformations, especially for the efficient synthesis of bridged bicyclic compounds. Experimental and theoretical mechanistic studies suggested that each Ni site can facilitate distinct elementary steps of two alkynes to generate a key di-vinyl di-Ni intermediate, followed by a reductive elimination to release the final product, which is substantially different from classic mono-metallic catalysis. This work may stimulate the development of novel reactions via binuclear catalysis and provides an improved understanding of the binuclear catalytic mechanisms in industrial / biocatalytic systems.
Methods
Procedure for dimerization-hydroarylation of alkynes
Under argon atmosphere, arylboronic acid (0.36 mmol, 1.8 equiv), dinuclear nickel complex A (10.8 mg, 5.0 mol%) and tBuONa (5.7 mg, 30 mol%) were added to an oven-dried 10 mL Schlenk tube equipped with a magnetic stir bar. Then the Schlenk tube was moved to a nitrogen-filled glovebox, alkyne (0.40 mmol, 2.0 equiv), MeOH (1.0 mL), DMF (2.0 mL) and NaBHEt3 (40 μL, 20 mol%, 1.0 M in THF) were added sequentially. After that, the tube was sealed and moved out of the glovebox. The reaction mixture was stirred at room temperature for 1.0 h, then heated to 80 °C and stirred for 12 h. After cooling to room temperature, saturated brine was added, and the reaction mixture was extracted with ethyl acetate. The organic layer was concentrated in vacuum and the residue was purified by chromatography on silica gel to give the product.
Procedure for dimerization-hydroalkylation of alkynes
Under argon atmosphere, dinuclear nickel complex A (10.8 mg, 5.0 mol%) and tBuOLi (8.0 mg, 50 mol%) were added to an oven-dried 10 mL Schlenk tube equipped with a magnetic stir bar. Then the Schlenk tube was moved to a nitrogen-filled glovebox, alkyne (0.40 mmol, 2.0 equiv), trialkylborane (400 μL, 2.0 equiv, 1.0 M in THF), MeOH (81 μL, 10 equiv), DMF (0.30 mL) and NaBHEt3 (60 μL, 30 mol%, 1.0 M in THF) were added sequentially. After that, the tube was sealed and moved out of the glovebox. The reaction mixture was stirred at room temperature for 1.0 hour, then heated to 80 °C and stirred for 12 h. After cooling to room temperature, saturated brine was added, and the reaction mixture was extracted with ethyl acetate. The organic layer was concentrated in vacuum and the residue was purified by chromatography on silica gel to give the product.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and Supplementary Information files, and are also available from the corresponding author upon request. Crystallographic data coordinates for structures reported in this article has been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 2364647 (product 4a), CCDC 2364651 (product 8), CCDC 2364652 (product 12a) and CCDC 2364654 (product 4ax). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Cartesian coordinates of the calculated structures are available from Supplementary Data.
References
Sträter, N., Lipscomb, W. N., Klabunde, T. & Krebs, B. Two-metal ion catalysis in enzymatic Acyl- and phosphoryl-transfer reactions. Angew. Chem. Int. Ed. 35, 2024–2055 (1996).
Lindahl, P. A. Metal-metal bonds in biology. J. Inorg. Biochem. 106, 172–178 (2012).
Shi, J. On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev. 113, 2139–2181 (2013).
Pan, Y., Zhang, C., Liu, Z., Chen, C. & Li, Y. Structural regulation with atomic-level precision: from single-atomic site to diatomic and atomic interface. Catal. Matter 2, 78–110 (2020).
Powers, D. C. & Ritter, T. Bimetallic redox synergy in oxidative palladium catalysis. Acc. Chem. Res. 45, 840–850 (2012).
Eisenhart, R. J., Clouston, L. J. & Lu, C. C. Configuring bonds between first-row transition metals. Acc. Chem. Res. 48, 2885–2894 (2015).
Mankad, N. P. Selectivity effects in bimetallic catalysis. Chem. Eur. J. 22, 5822–5829 (2016).
Farley, C. M. & Uyeda, C. Organic reactions enabled by catalytically active metal–metal bonds. Trends Chem. 1, 497–509 (2019).
Campos, J. Bimetallic cooperation across the periodic table. Nat. Rev. Chem. 4, 696–702 (2020).
Nath, B. D., Takaishi, K. & Ema, T. Macrocyclic multinuclear metal complexes acting as catalysts for organic synthesis. Catal. Sci. Technol. 10, 12–34 (2020).
Xu, W., Li, M., Qiao, L. & Xie, J. Recent advances of dinuclear nickel- and palladium-complexes in homogeneous catalysis. Chem. Commun. 56, 8524–8536 (2020).
Fricke, C., Sperger, T., Mendel, M. & Schoenebeck, F. Catalysis with palladium(I) dimers. Angew. Chem. Int. Ed. 60, 3355–3366 (2021).
Zhou, Y.-Y. & Uyeda, C. Catalytic reductive [4 + 1]-cycloadditions of vinylidenes and dienes. Science 363, 857 (2019).
Hueffel, J. A. et al. Accelerated dinuclear palladium catalyst identification through unsupervised machine learning. Science 374, 1134–1140 (2021).
Ying, Y., Luo, X., Qiao, J. & Huang, H. “More is different:” synergistic effect and structural engineering in double-atom catalysts. Adv. Funct. Mater. 31, 2007423 (2021).
Ro, I. et al. Bifunctional hydroformylation on heterogeneous Rh-WOx pair site catalysts. Nature 609, 287–292 (2022).
Hai, X. et al. Geminal-atom catalysis for cross-coupling. Nature 622, 754–760 (2023).
Trost B. M. & Li, C.-J. (eds) Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations (Wiley, 2014).
Omae, I. Three characteristic reactions of alkynes with metal compounds in organic synthesis. Appl. Organomet. Chem. 22, 149–166 (2008).
Chen, L., Chen, K. & Zhu, S. Transition-metal-catalyzed intramolecular nucleophilic addition of carbonyl groups to alkynes. Chem 4, 1208–1262 (2018).
Liu, M., Sun, J. & Engle, K. M. Recent advances in the generation and functionalization of C(alkenyl)–Pd species for synthesis of polysubstituted alkenes. Tetrahedron 103, 132513 (2022).
Lei, J. et al. Recent advances of catalytic processes on the transformation of alkynes into functional compounds. Chem. Eng. Sci. 171, 404–425 (2017).
Ramani, A., Desai, B., Patel, M. & Naveen, T. Recent advances in the functionalization of terminal and internal alkynes. Asian J. Org. Chem. 11, e202200047 (2022).
Flynn, A. B. & Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 107, 4698–4745 (2007).
Murakami, K. & Yorimitsu, H. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein J. Org. Chem. 9, 278–302 (2013).
Corpas, J., Mauleón, P., Arrayás, R. G. & Carretero, J. C. Transition-metal-catalyzed functionalization of alkynes with organoboron reagents: new trends, mechanistic insights, and applications. ACS Catal. 11, 7513–7551 (2021).
Bhakta, S. & Ghosh, T. Nickel-catalyzed hydroarylation reaction: a useful tool in organic synthesis. Org. Chem. Front. 9, 5074–5103 (2022).
Haro, T. & Nevado, C. Hydroarylation reactions. In Comprehensive Organic Synthesis, 2nd edn, (eds Knochel, P. & Molander, G. A.), Vol. 5, 1621–1659 (Elsevier, 2015).
Shirakawa, E., Takahashi, G., Tsuchimoto, T. & Kawakami, Y. Nickel-catalysed hydroarylation of alkynes using arylboron compounds: selective synthesis of multisubstituted arylalkenes and aryldienes. Chem. Commun. 2688–2689 https://doi.org/10.1039/b107866f (2001).
Neaţu, F. et al. Arylation of alkynes over hydrotalcite docked Rh-m-TPPTC complex. Catal. Today 247, 155–162 (2015).
Green, N. J., Willis, A. C. & Sherburn, M. S. Direct cross-couplings of propargylic diols. Angew. Chem. Int. Ed. 55, 9244–9248 (2016).
Liu, Y., Wang, L. & Deng, L. Selective double carbomagnesiation of internal alkynes catalyzed by iron-N-Heterocyclic carbene complexes: a convenient method to highly substituted 1,3-Dienyl magnesium reagents. J. Am. Chem. Soc. 138, 112–115 (2016).
Liu, Y., Zhang, G. & Huang, H. Ni-catalyzed dimerization and arylation of diarylacetylenes with arylboronic acids. Org. Lett. 19, 6674–6677 (2017).
Zhou, Z., Chen, J., Chen, H. & Kong, W. Stereoselective synthesis of pentasubstituted 1,3-dienes via Ni-catalyzed reductive coupling of unsymmetrical internal alkynes. Chem. Sci. 11, 10204–10211 (2020).
Wang, C., Wu, C., Yang, Y., Xing, J. & Dou, X. Rhodium-catalyzed formal [2 + 2 + 1] annulation of arylboronic acids with alkynes. Org. Chem. Front. 9, 6915–6919 (2022).
De Paolis, M., Chataigner, I. & Maddaluno, J. Recent advances in stereoselective synthesis of 1,3-dienes. Top. Curr. Chem. 327, 87–146 (2012).
Hubert, P., Seibel, E., Beemelmanns, C., Campagne, J. M. & Figueiredo, R. M. Stereoselective construction of (E,Z)-1,3-dienes and its application in natural product synthesis. Adv. Synth. Catal. 362, 5532–5575 (2020).
Soengas, R. G. & Rodríguez-Solla, H. Modern synthetic methods for the stereoselective construction of 1,3-dienes. Molecules 26, 249 (2021).
Dumonteil, G. & Berteina-Raboin, S. Synthesis of conjugated dienes in natural compounds. Catalysts 12, 86–111 (2022).
Herrmann, N., Vogelsang, D., Behr, A. & Seidensticker, T. Homogeneously catalyzed 1,3-Diene functionalization – A success story from laboratory to miniplant scale. ChemCatChem 10, 5342–5365 (2018).
Liu, J., Yang, J., Baumann, W., Jackstell, R. & Beller, M. Stereoselective synthesis of highly substituted conjugated dienes via Pd-catalyzed carbonylation of 1,3-Diynes. Angew. Chem. Int. Ed. 58, 10683–10687 (2019).
Chen, S. et al. Chemo-, regio- and stereoselective access to polysubstituted 1,3-dienes via Nickel-catalyzed four-component reactions. Nat. Commun. 15, 5479–5492 (2024).
Ogata, K., Murayama, H., Sugasawa, J., Suzuki, N. & Fukuzawa, S.-I. Nickel-catalyzed highly regio- and stereoselective cross-trimerization between triisopropylsilylacetylene and internal alkynes leading to 1,3-Diene-5-ynes. J. Am. Chem. Soc. 131, 3176–3177 (2009).
Joó, F. Dimerization, oligomerization and polymerization of alkenes and alkynes. In Aqueous Organometallic Catalysis (Springer Netherlands, 2001).
Temkin, O. N. “Golden Age” of homogeneous catalysis chemistry of alkynes: dimerization and oligomerization of alkynes. Kinet. Catal. 60, 689–732 (2019).
Ma, W. et al. Metallacyclopentadienes: synthesis, structure and reactivity. Chem. Soc. Rev. 46, 1160–1192 (2017).
Urabe, D., Asaba, T. & Inoue, M. Convergent strategies in total syntheses of complex terpenoids. Chem. Rev. 115, 9207–9231 (2015).
Gao, Y. & Ma, D. In pursuit of synthetic efficiency: convergent approaches. Acc. Chem. Res. 54, 569–582 (2021).
Gibson, V. C., Redshaw, C. & Solan, G. A. Bis(imino)pyridines: surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 107, 1745–1776 (2007).
Chirik, P. J. & Wieghardt, K. Radical ligands confer nobility on base-metal catalysts. Science 327, 794 (2010).
Flisak, Z. & Sun, W.-H. Progression of diiminopyridines: from single application to catalytic versatility. ACS Catal. 5, 4713–4724 (2015).
Khoshsefat, M., Ma, Y. & Sun, W.-H. Multinuclear late transition metal catalysts for olefin polymerization. Coord. Chem. Rev. 434, 213788 (2021).
Wang, Q., Brooks, S. H., Liu, T. & Tomson, N. C. Tuning metal-metal interactions for cooperative small molecule activation. Chem. Commun. 57, 2839–2853 (2021).
Thierer, L. M. et al. Macrocycle-induced modulation of internuclear interactions in homobimetallic complexes. Inorg. Chem. 61, 6263–6280 (2022).
Chen, K. et al. Dinuclear cobalt complex-catalyzed stereodivergent semireduction of alkynes: switchable selectivities controlled by H2O. ACS Catal. 11, 13696–13705 (2021).
Chen, K. et al. Switch in selectivities by dinuclear nickel catalysis: 1,4-hydroarylation of 1,3-dienes to Z-olefins. J. Am. Chem. Soc. 145, 24877–24888 (2023).
Spentzos, A. Z. et al. Reactivity profile of a formally dicobalt(0) complex bound by a redox-active macrocycle. Organometallics 43, 1425–1437 (2024).
Chung, Y. K. Transition metal alkyne complexes: the Pauson–Khand reaction. Coord. Chem. Rev. 188, 297–341 (1999).
Uyeda, C., Steiman, T. & Pal, S. Catalytically active Nickel–Nickel bonds using redox-active ligands. Synlett 27, 814–820 (2016).
Acknowledgements
The authors acknowledge financial support from the National Key R&D Program of China (No. 2022YFA1503200 (X.W.), 2021YFA1500100 (Q.P.)), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB1180000; XDB0610000 (X.W.)), the National Natural Science Foundation of China (92256303 (X.W.), 22171278 (X.W.), 92156017 (Q.P.), 22403053 (H.Z.)), the Shanghai Science and Technology Committee (23ZR1482400 (X.W.)), the Natural Science Foundation of Ningbo (2023J034 (X.W.)), Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (X.W.), the Research Funds of Hangzhou Institute for Advanced Study, UCAS (2024HIAS-p003 (X.W.)), the Natural Science Foundation of Tianjin (24JCZDJC00750 (Q.P.)), “Frontiers Science Center for New Organic Matter”, Nankai University (No. 63181206 (Q.P.)), Haihe Laboratory of Sustainable Chemical Transformation of Tianjin (No. 24HHWCSS00019 (Q.P.)), the “Fundamental Research Funds for the Central Universities”, Nankai University (63241203 (Q.P.)), and the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (Grant No. GZC20240750 (H.Z.)) for generous financial support.
Author information
Authors and Affiliations
Contributions
K.C., X.W., and K.D. directed the project and designed the experiments; K.C. and S.J. performed all the experiments and analyzed all the data with X.W.; Q.P. directed the project by the theoretical study and wrote the related manuscript with H.Z. who completed the DFT calculations. K.C. and X.W. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Cristina Tejel, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, K., Zhu, H., Jiang, S. et al. Alkyne dimerization-hydroarylation to form pentasubstituted 1,3-dienes via binuclear nickel catalysis. Nat Commun 16, 3077 (2025). https://doi.org/10.1038/s41467-025-58398-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58398-x